Abstract
The acetylcholinesterase (AChE) inhibitor donepezil is used as a therapy for Alzheimer's disease and has been recommended as a treatment for enhancing attention and memory after traumatic brain injury (TBI). Although select clinical case studies support the use of donepezil for enhancing cognition, there is a paucity of experimental TBI studies assessing the potential efficacy of this pharmacotherapy. Hence, the aim of this pre-clinical study was to evaluate several doses of donepezil to determine its effect on functional outcome after TBI. Ninety anesthetized adult male rats received a controlled cortical impact (CCI; 2.8 mm cortical depth at 4 m/sec) or sham injury, and then were randomly assigned to six TBI and six sham groups (donepezil 0.25, 0.5, 1.0, 2.0, or 3.0 mg/kg, and saline vehicle 1.0 mL/kg). Treatments began 24 h after surgery and were administered i.p. once daily for 19 days. Function was assessed by motor (beam balance/walk) and cognitive (Morris water maze) tests on days 1–5 and 14–19, respectively. No significant differences were observed among the sham control groups in any evaluation, regardless of dose, and therefore the data were pooled. Furthermore, no significant differences were revealed among the TBI groups in acute neurological assessments (e.g., righting reflex), suggesting that all groups received the same level of injury severity. None of the five doses of donepezil improved motor or cognitive function relative to vehicle-treated controls. Moreover, the two highest doses significantly impaired beam-balance (3.0 mg/kg), beam-walk (2.0 mg/kg and 3.0 mg/kg), and cognitive performance (3.0 mg/kg) versus vehicle. These data indicate that chronic administration of donepezil is not only ineffective in promoting functional improvement after moderate CCI injury, but depending on the dose is actually detrimental to the recovery process. Further work is necessary to determine if other AChE inhibitors exert similar effects after TBI.
Key words: AChE inhibitor, behavior, CCI, functional recovery, learning and memory, Morris water maze, TBI
Introduction
Each year in the United States, ∼1,500,000–2,000,000 individuals sustain a traumatic brain injury (TBI), ranging from mild concussions often associated with loss of consciousness or amnesia to severe trauma or even death. TBI is a contributing factor to almost one third of injury-related deaths (∼50,000).1–7 Direct medical expenses and indirect costs of TBI, such as loss of productivity, intense rehabilitation programs, or costs incurred by family members caring for the patients are estimated to exceed $60 billion per year in the United States.8 In addition, more than 120,000 TBI patients every year are reported to develop a constellation of long-term disabilities, especially motor and cognitive symptoms.3,4,6,9 The most common cognitive impairments among patients with TBI are deficits in learning and memory, consisting of memory loss and the inability to acquire or store new information.10 Additionally, many individuals with TBI have difficulty engaging in regular daily activities and may be unable to return to the workforce for weeks or months. Another striking characteristic of TBI epidemiology is that more than one third of the victims are children and young adults, predominantly males, which substantiates the long-term social, economic, and psychological consequences of this condition.11 Therefore, identifying specific pharmacotherapies targeting neurochemical, motor, and cognitive recovery after TBI is of significant priority in both pre-clinical and clinical settings.
Considerable advances have been made in recent decades in understanding the complex mechanisms of damaging biochemical events and neurophysiological basis of secondary neuronal injury after brain trauma. Still, the mechanistic heterogeneity and individual characteristics of TBI in addition to inconsistent randomized controlled clinical trials have led to a surprising lack of mainstream pharmacological treatments and limited translational applicability of findings from experimental TBI into clinical practice.12–15 Clinical and pre-clinical evidence has linked biochemical disruptions involving the cholinergic system to the pathology and symptoms of TBI.13,16–20 Specifically, brain regions known to play a pivotal role in attention, spatial learning and memory, storage and retrieval of salient information, which also receive rich cholinergic innervations, such as the hippocampus and frontal cortex, are often disrupted in clinical or experimental TBI.13,18,21–24 Experimental TBI studies suggest that acetylcholine (ACh) neurotransmission is chronically decreased after TBI,25–27 which may therefore, at least partially, contribute to both motor and memory impairments in animals and patients with TBI.24,28–31 Prevention or reversal of these deficits is an ongoing challenge for the management of TBI, and improving cholinergic transmission has become an increasingly attractive approach in animal models, as well as in recent studies involving TBI patients.
The acetylcholinesterase inhibitor (AChEI) donepezil (Aricept®) increases the availability of acetylcholine at postsynaptic receptors by inhibiting its breakdown in the central nervous system, and is approved by the Food and Drug Administration to treat symptoms of Alzheimer's disease. Pre-clinical studies aimed at better understanding therapeutic effects and time windows for drug intervention found that repeated donepezil administration for 15 days improved spatial learning memory in the Morris water maze (MWM) in aged rats,32 and reversed working memory deficits in scopolamine-treated mice.33 Recent studies30,31 have reported that low doses of chronic steady-state physostigmine treatment after cerebral cortex impact injury reversed spatial memory and learning impairments and attenuated TBI-induced deficits in locomotor function in the accelerating rotarod test, whereas higher doses induced progressive deterioration of performance. The aim of the current study was to investigate the therapeutic potential of a range of donepezil doses provided chronically to adult rats with CCI injury-induced motor and cognitive impairments. Furthermore, because of its clinical applicability, considerably higher tolerability, and significantly fewer cardiovascular and autonomic side effects than other cholinergic drugs,13,34 donepezil may prove to be a valuable therapy if it is shown to reverse detrimental behavioral effects in pre-clinical models of TBI.
Methods
Animals
A total of 90 adult male Sprague–Dawley rats (Harlan, Indianapolis, IN) were housed in standard steel-wire mesh cages and maintained in a temperature (21±1°C) and 12/12 h light/dark cycle (lights on at 0700 h) controlled environment with food and water available ad libitum. They were allowed to acclimate to the housing facility for 1 week before use in any experimental or surgical procedures. After the acclimatization period, rats underwent a single day of beam walk training as a baseline measure of motor function, which consisted of 3–5 trials to traverse the beam (60 sec per trial with an inter-trial interval of 30 sec). All experiments were performed during the light portion of the cycle, between 0700 and 1900 h. All procedures were conducted in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals (National Academy Press, 2010), and were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. All efforts were made to minimize animal pain, suffering, or discomfort, and to minimize the number of rats used.
Surgery
On the day of surgery, rats weighing 300–325 g were randomly assigned to either CCI or sham injury groups, and surgical procedures were performed as previously published.35–41 Briefly, animals were placed under isoflurane gaseous anesthesia at concentrations of 4% and 2%, respectively, in 2:1 N2O:O2 in a vented anesthesia chamber. Rats were subsequently intubated endotracheally and secured in a stereotaxic frame with mechanical ventilation. A heating blanket was used to maintain core temperature at 37±0.5°C, which was measured with a rectal probe throughout surgery. Using aseptic procedures, a craniectomy was performed in the right hemisphere with a handheld trephine. A TBI of moderate severity was then produced by advancing the impacting rod into the exposed right parietal cortex to a depth of 2.8 mm tissue deformation at 4 m/sec. After the impact, anesthesia was discontinued and the incision was promptly sutured. The rats were extubated and assessed for acute neurological outcome. Sham injury rats were not subjected to the cortical impact, but otherwise underwent similar surgical procedures.
Acute neurological evaluation
Following cessation of anesthesia, hindlimb reflexive ability was assessed by briefly squeezing the rats' paw every 5 sec, and the time to elicit a withdrawal response was recorded. Return of the righting reflex also was determined by recording the average time required to turn from the supine to the prone position.
Drug administration
After surgery, TBI and sham injured rats were randomly distributed among groups that were to receive varying doses of donepezil hydrochloride (Ivy Fine Chemicals, Cherry Hill, NJ) dissolved in physiological saline, which also was used as the vehicle. Donepezil (0.25 mg/kg, 0.5 mg/kg, 1.0 mg/kg, 2.0 mg/kg, or 3.0 mg/kg) or a comparable volume of vehicle (1.0 mL/kg) was administered via intraperitoneal injection beginning 24 h after cortical impact or sham injury, and then made fresh and injected once daily for a total of 19 days. The doses of donepezil and route of administration were selected based on multiple studies using this drug.30–32,42,43
Motor function: beam balance and beam walk
Motor function was evaluated using well-validated beam tests.35–38,40,44,45 In the beam balance task, rats were placed individually on an elevated narrow wooden beam (1.5 cm wide, 90 cm height from floor) and the time they remained on it was recorded for a maximum of 60 sec. In the beam walk task, a modified version from that originally developed by Feeney and colleagues,46 rats learned based on a negative reinforcement paradigm to escape bright light and white noise by traversing an elevated narrow wooden beam (2.5 cm wide, 100 cm long, 90 cm height from floor) and entering a darkened goal box at the opposite end. The termination of the aversive stimuli upon entering the goal box served as reinforcement (reward) for completing the task. Beam balance and beam walk ability were assessed by recording the time rats remained on the beam, as well as time elapsed while traversing the beam and distance travelled, respectively.36,37,41 As mentioned, rats were tested for motor function in these tasks prior to surgery to establish a baseline measure, as well as on postoperative days 1–5. Three trials of 60 sec each with a 30 sec inter-trial interval were provided daily on each task, and the average daily scores for each subject were used in statistical analyses. If the rat was unable to traverse the entire length of the beam, the maximum allowed time of 60 sec was recorded.
Cognitive function: acquisition of spatial learning
A MWM task47 that is sensitive to alterations in cognitive function following TBI37,38,40,48,49 was used to assess acquisition of spatial learning. The maze consisted of a plastic pool (180 cm diameter; 60 cm height) filled with water (26±1°C) to a depth of 28 cm and was situated in a room with salient visual cues that were maintained constant throughout the experiments. A clear Plexiglas platform stand (10 cm diameter, 26 cm high) was placed 26 cm from the maze wall in the southwest maze quadrant, and maintained in a constant position for each rat. Acquisition of spatial learning began on postoperative day 14, and each rat was to locate the platform, which was submerged 2 cm below the water surface. Rats were subjected to a block of four daily trials (120 sec maximum, 4 min inter-trial interval) for 5 consecutive days (i.e., days 14–18 post-surgery). During each block of four daily trials, rats were placed in the pool facing the wall in each maze quadrant (north, east, south, west) in a randomized fashion. The time required for the rat to climb onto the platform was recorded during each trial, or until 120 sec had elapsed, whichever occurred first. Rats that failed to locate the platform within the allotted time were manually guided to it. After each trial, rats remained on the platform for 30 sec before being placed in a heated incubator during the inter-trial time interval. The average time of the four daily trials for each rat was used in the statistical analyses. One day after the final acquisition training session (i.e., day 19), rats were given a single probe trial to assess memory retention. During this test phase, the platform was removed from the pool and the rats were placed in the maze from the location point most distal to the quadrant where the platform was previously situated (i.e., “target quadrant”) and allowed to freely explore the pool for 30 sec. The rationale is that rats that have learned the specific location of the escape platform exhibit a spatial bias and spend significantly more time in the target quadrant. The percent time spent in the target quadrant was used in the statistical analysis. A spontaneous motor activity recording and tracking (SMART) system (San Diego Instruments, San Diego, CA) was used to record the behavioral performance data.
Statistical analysis
Data were collected by observers blinded to treatment conditions, and statistical analyses were performed using StatView 5.0.1 software (Abacus Concepts, Inc., Berkeley, CA). The acute neurological and core body temperature data were analyzed by one way analysis of variance (ANOVA) tests. The motor and cognitive data were analyzed by two way repeated-measures ANOVA tests, with drug dose as the between-subject factor, and day post-injury as the within-subject repeated measure factor. If a significant effect was revealed by the overall ANOVAs, the Bonferroni post-hoc test was further employed to determine specific group differences. Data are expressed as mean values±standard error of the mean (SEM). Statistical significance was set at p≤0.05 or as determined by the Bonferroni corrections for multiple comparisons.
Results
Statistical analyses were performed on a total of 87 rats, as 3 were excluded from the study (2 from the TBI+donepezil [0.25 mg/kg] group and 1 from the TBI+donepezil [2.0 mg/kg] group) after being unsuccessful in locating the visible platform, which may indicate impaired visual acuity. There were no significant differences in any outcome measures among the sham control groups, regardless of treatment or dose, and, therefore, the data were pooled and analyzed as one group (designated as “SHAM”).
Acute neurological evaluation
There were no significant differences among TBI groups with respect to hindlimb reflex withdrawal latency in response to a brief paw pinch administered to either limb (left range=180.7±4.2 sec to 192.4±6.6 sec, p>0.05; right range=174.1±4.6 sec to 188.3±6.7 sec, p>0.05) following termination of anesthesia. Also, no significant differences were detected among TBI groups for the return to righting ability (range 357.8±21.7 sec to 430.4±16.7 sec, p>0.05). The lack of significant differences with these acute neurological indices indicates that all TBI groups experienced similar levels of injury and anesthesia.
Motor function: beam-balance
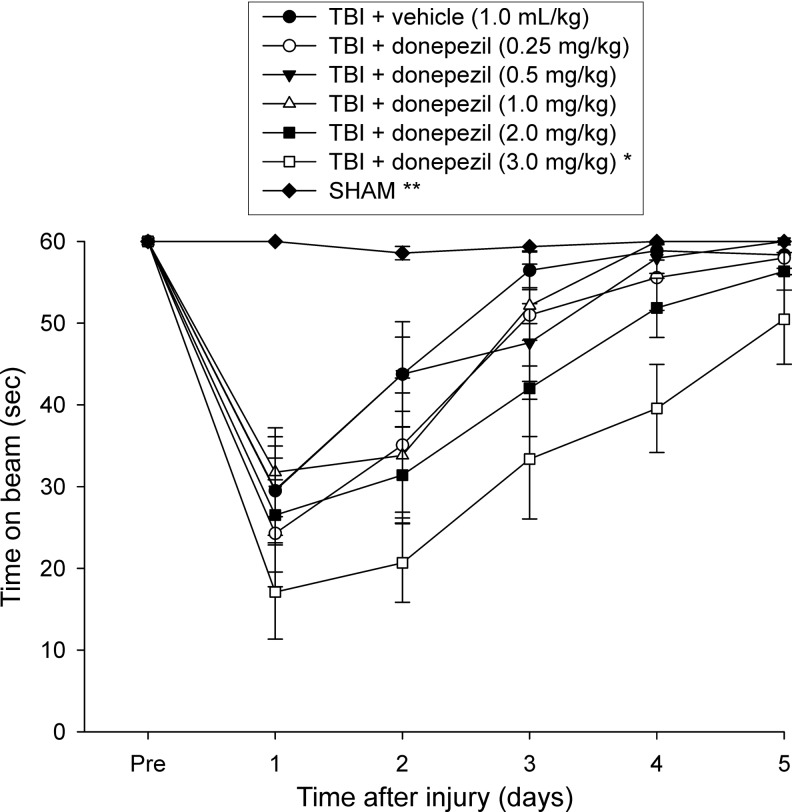
There were no pre-surgical differences among groups, as all rats were capable of balancing on the beam for the allotted 60 sec on each of the three trials (Fig. 1). Following the CCI, all TBI rats were significantly impaired compared with the SHAM group, which was able to maintain pre-surgical balancing ability for the entire 60 sec. The ANOVA revealed significant overall group (F6,80=17.234, p<0.0001) and day (F5,400=103.674, p<0.0001) differences, as well as a significant group x day interaction (F30,400=7.346, p<0.0001), which was primarily because of the SHAMS performing significantly better than all TBI groups (p<0.0001). Beam balance ability improved gradually in the TBI groups in a similar fashion, except for the animals in the group administered the highest dose of donepezil (TBI+donepezil [3.0 mg/kg]), which performed worse than the TBI+vehicle group (p<0.0001), as well as other TBI+donepezil groups (0.25 mg/kg, p=0.0003; 0.5 mg/kg, p<0.0001; 1.0 mg/kg, p<0.0001), with a trend of performing worse than the TBI+donepezil group (2.0 mg/kg, p=0.0051; required p=0.0024 by the Bonferroni/Dunn statistic after adjusting for multiple comparisons) (Fig. 1). No other significant comparisons were revealed among the drug groups.
FIG. 1.
Mean (±SEM) time (sec) balancing on an elevated narrow beam prior to, and after, traumatic brain injury (TBI) or sham injury. All TBI+donepezil groups were significantly impaired relatively to the SHAM group (**p<0.0001). Additionally, beam balance ability improved similarly across 5 testing days in the TBI groups regardless of drug dose or vehicle administration, except for the TBI+donepezil (3.0 mg/kg) group, which performed significantly worse than the TBI+vehicle group, as well as other TBI+donepezil groups (*p<0.0005 vs.TBI+vehicle and TBI+donepezil: 0.25 mg/kg, 0.5 mg/kg, 1.0 mg/kg).
Motor function: beam walk (time to traverse)
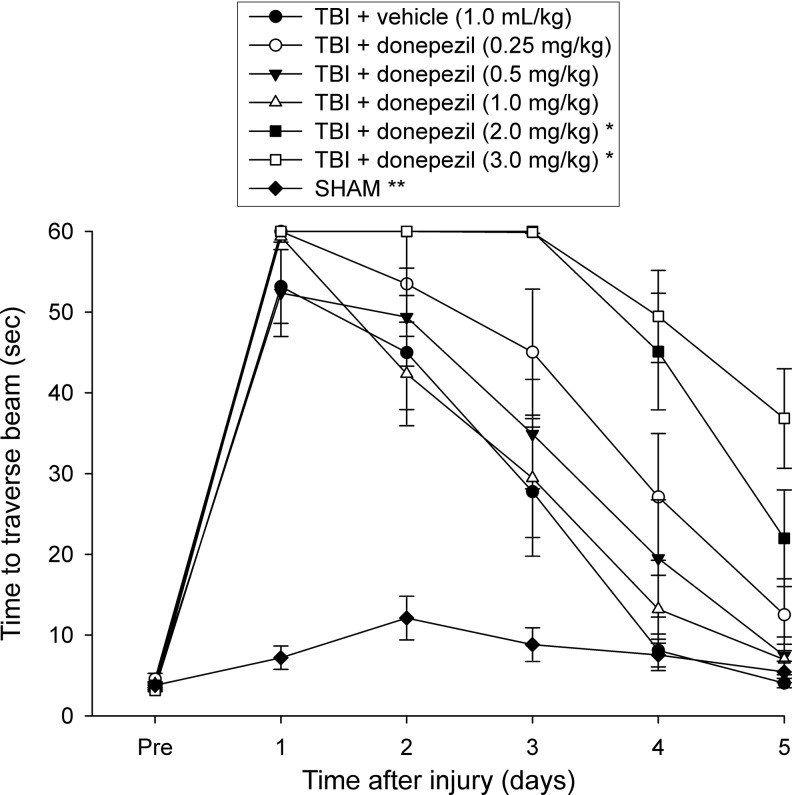
Similar to the beam balance results, there were no differences among groups prior to surgery, as all rats proficiently traversed the entire length of the beam to reach the goal box (Fig. 2). Following TBI, there was a significant increase in beam walking time for all injured groups compared with SHAM controls. The ANOVA revealed significant overall group (F6,80=37.328, p<0.0001) and day (F5,400=239.43, p<0.0001) differences, as well as a significant group x day interaction (F30,400=15.42, p<0.0001), which was attributed to all TBI groups performing significantly worse than the SHAM animals (p<0.0001). Furthermore, post-hoc tests also showed a significantly slower recovery in beam walk ability for the groups receiving the two highest doses of donepezil (TBI+donepezil [2.0 mg/kg] and TBI+donepezil [3.0 mg/kg]) compared with TBI+vehicle, TBI+donepezil (0.5 m/kg) and TBI+donepezil (1.0 mg/kg) groups (all p<0.0001 except p=0.0004 for TBI+donepezil [0.5 mg/kg] vs. TBI+donepezil [2.0 mg/kg]). The statistical analyses also revealed a trend for rats from the TBI+donepezil (3.0 mg/kg) to also display impaired beam walking ability compared with the TBI+donepezil (0.25 mg/kg) group (p=0.0057, required p=0.0024). Also, the beam walk time for the TBI+donepezil (2.0 mg/kg) and TBI+donepezil (3.0 mg/kg) groups did not appear to return to baseline levels by the last day of testing, suggesting a slower rate of recovery with the two highest drug doses (Fig. 2). No other group comparisons were significant.
FIG. 2.
Mean (±SEM) walking ability as measured by time (sec) to traverse an elevated wooden beam prior to, and after, traumatic brain injury (TBI) or sham injury. All TBI+donepezil groups were significantly impaired relatively to the SHAM group (**p<0.0001). At the two highest doses of chronic donepezil (TBI+donepezil: 2.0 and 3.0 mg/kg), rats displayed significantly slower recovery in beam walk ability during the 5 testing days compared with TBI+vehicle, TBI+donepezil (0.5 m/kg), and TBI+donepezil (1.0 mg/kg) (*p<0.0005, Bonferroni post-hoc tests).
Cognitive function: acquisition of spatial learning (time to platform)
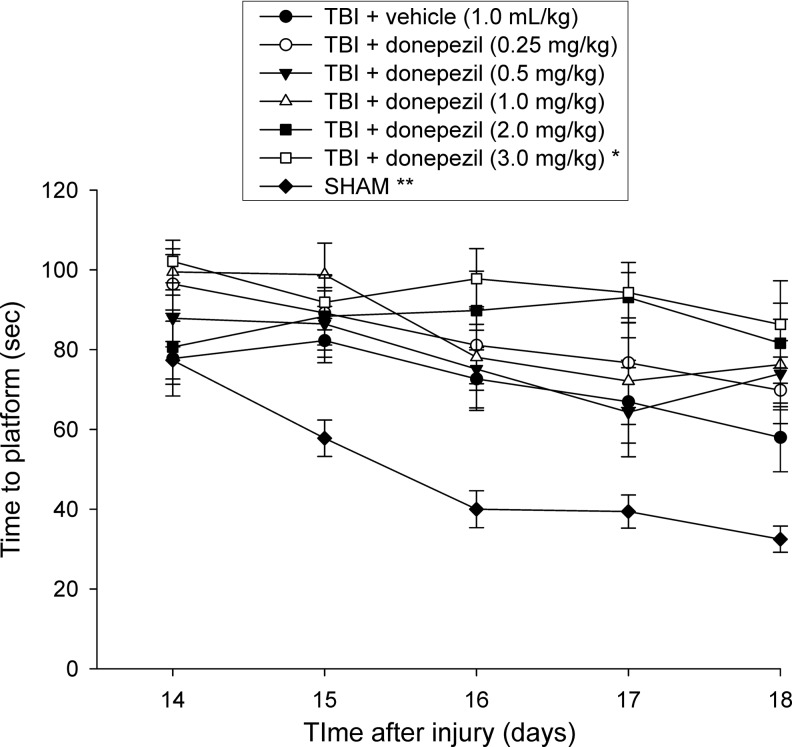
During acquisition of spatial learning in the MWM test on days 14–18 post-TBI or sham surgery, the ANOVA revealed significant group (F6,80=12.9, p<0.0001), day (F4,320=12.884, p<0.0001) and group x day (F24, 320=1.963, p<0.01) differences, effects suggesting substantial TBI-induced water maze performance deficits in all TBI groups compared with SHAM controls (p<0.0001) (Fig. 3). Post-hoc Bonferroni analyses further revealed no particular beneficial effects of donepezil administered to TBI rats in locating the submerged platform over time. On the contrary, injured groups became progressively better at locating the escape platform in a similar fashion, regardless of whether they received chronic administration of vehicle or the lower doses of donepezil (0.25 mg/kg, 0.5 mg/kg, and 1 mg/kg, p<0.0024, Fig. 3), although they were still significantly impaired relative to the SHAM rats, which were able to learn the task at a faster rate. Moreover, rats receiving the highest drug dose, TBI+donepezil (3.0 mg/kg) displayed a slower recovery of cognitive performance while training to locate the submerged platform over 5 test days, which was significantly worse compared with the TBI+vehicle group (p<0.0001) and the TBI+donepezil (0.5 mg/kg) group (p<0.0021). A statistical trend was found for animals from the next highest drug dose, TBI+donepezil (2.0 mg/kg), to also perform worse than the TBI+vehicle group at learning the location of the escape platform over time, although it did not reach statistical significance (p=0.0072; required p=0.0024 by the Bonferroni/Dunn statistic after adjusting for multiple comparisons).
FIG. 3.
Mean (±SEM) time (sec) to locate a hidden (submerged) platform in the Morris water maze test. There were substantial traumatic brain injury (TBI)-induced water maze performance deficits in all TBI groups compared with SHAM controls (**p<0.0001). At the highest donepezil dose (3.0 mg/kg), TBI rats displayed significantly slower recovery rates of spatial learning abilities compared with the TBI+vehicle group (*p<0.0001) and the TBI+donepezil (0.5 mg/kg) group (p<0.0021).
Cognitive function: probe trial and swim speed
Analysis of the probe (memory retention) behavioral data on the day following water maze spatial memory acquisition training (i.e., day 19) revealed a significant group effect (F6,80=10.627, p<0.0001). Specifically, the SHAM group spent a significantly greater percentage of the 30 sec allotted time in the target quadrant compared with all other TBI groups, regardless of whether they received vehicle or donepezil (Sham uninjured controls: 41.7±1.6%; TBI groups range: 24.2±1.8% to 31.2±4.6%, p<0.0001 as described by the Bonferroni post-hoc individual analyses) (data not shown). No other probe comparisons were significant, and neither beneficial nor detrimental effects of drug administration on memory retention were detected in TBI rats, albeit neither group demonstrated intact memory retention comparable to uninjured animals. Additionally, no significant differences in swim speed (range: 28.7±0.6 cm/sec to 32.8±1.5 cm/sec) were observed among any of the groups (F6,80=1.201, p>0.05) (data not shown).
Discussion
The purpose of the present study was to determine whether motor and cognitive functions, which are dramatically altered in a CCI model of TBI, would be improved by a delayed and chronic post-injury administration of donepezil, a pharmacotherapy approved to treat Alzheimer's disease symptomatology, but relatively novel to the TBI field. Donepezil is a mixed competitive, reversible, and potent inhibitor of AChE; therefore, administration of this drug in vivo abolishes the action of degrading cholinesterase enzymes, enhancing the life of the neurotransmitter ACh in the synaptic cleft, and, presumably, enhancing overall brain cholinergic neurotransmission.50 Donepezil displays considerably higher tolerability and significantly fewer cardiovascular and autonomic side effects than do other cholinergic drugs,13,34 and, therefore may be a valuable therapy if shown to reverse detrimental behavioral effects in pre-clinical models of TBI.
However, our data revealed that donepezil administration for 19 days (0.25–3.0 mg/kg) starting the day after TBI did not attenuate injury-induced motor or cognitive impairments. Moreover, the highest doses of donepezil (2.0 and 3.0 mg/kg) led to further performance deterioration compared with TBI+vehicle or TBI followed by the low doses of donepezil. This effect was not a result of confounding factors influencing the accurate assessment of place learning, such as drug-related motor impairments or visual disparities, especially in the water maze test, as probe trial performance and swim speed parameters were comparable among groups.
The doses used in this study are well within the range previously shown to display significant effects on altering AChE activity and ACh release. Liang and Tang reported maximal increases of ACh levels in the cerebral cortex 30 min after systemic donepezil administration (i.e., ∼0.8, 1.6, and 3.2 mg/kg). In parallel, donepezil (4 μmol/kg) attenuated cortical AChE activity by 12% compared with baseline levels.51 When injected 30 min before testing, donepezil (0.3 mg/kg and 1.0 mg/kg) significantly attenuated scopolamine-induced increases in escape latency in the MWM,52 but in a different study, donepezil (2.0 mg/kg and 3.0 mg/kg) failed to reverse spatial learning deficits induced by scopolamine.53 Chronic donepezil regimens have also been shown to modulate ACh neurotransmission via neurotrophic effects and reinvigorating cholinergic availability in the synapse. For example, chronic intragastric donepezil (5 mg/kg/day) had no effects on whole-brain AChE protein levels, but it did increase levels of choline acetyltransferase (ChAT), the rate-limiting enzyme for the synthesis of acetylcholine, and it reversed spatial learning deficits in aged mice.54 Similarly, we reported an attenuation of CCI-induced ChAT(+) medial septal cell loss at 3 weeks post-injury that correlated with improved cognitive performance.40 Furthermore, Pike and Hamm showed an attenuation of fluid percussion (FP) injury-induced reduction of basal forebrain ChAT immunoreactivity after chronic administration of Lu 25-109-T, a partial M1 muscarinic receptor agonist and presynaptic M2 autoreceptor antagonist.55
The lack of behavioral effects with our full dose response profile of donepezil suggests a fairly narrow dose range, which has also been seen with other AChEI drugs. Specifically, chronic administration of low-dose physostigmine (1.6 and 3.2 μmol/kg/day) improved outcome in the accelerating rotarod test, but higher doses (6.4 and 12.8 μmol/kg/day) resulted in progressive performance deterioration after CCI.30,31 Support for this idea could also be inferred from studies such as that by Rezvani et al., in which acute subcutaneous donepezil administration displayed inverted U-shaped dose-response patterns in an operant visual signal detection task.56 When administered 30 min prior to testing, a low dose of donepezil (0.01 mg/kg) successfully reversed detrimental effects on attention induced by the N-methyl-d-aspartate (NMDA) glutamate receptor antagonist dizocilpine, whereas higher doses (0.1 and 1 mg/kg) also induced significant effects on dizocilpine-induced attentional impairments, although the effect was less than a full reversal. We cannot exclude the possibility that higher doses of an AChEI may exert nonspecific effects, therefore limiting the effectiveness of the drug. For example, larger doses of physostigmine exert noncompetitive blockade effects at the nicotinic acetylcholine receptor-ion channel (nAChR) complex of skeletal muscles,57 and it has been reported that antagonism of nAChR in the brain results in detrimental effects on working memory.58
An earlier study with the first commercial AChEI, tetrahydroaminoacridine (tacrine), reported that daily tacrine administration starting at 24 h after moderate fluid percussion (FP) injury in rats resulted in a dose-related impairment of water maze performance for both TBI-injured and sham-operated animals.59 The authors concluded that chronic tacrine administration may not be an effective treatment for cognitive impairments after TBI, as it resulted in further worsening of performance. In our study, chronic donepezil treatment did not affect behavioral performance of sham subjects, therefore suggesting that the drug effects in TBI rats could be the result of injury-induced alterations in brain cholinergic neurotransmission. Specifically, chronic treatment with a cholinesterase inhibitor may concomitantly induce sustained tonic stimulation of M1 muscarinic postsynaptic receptors, as well as inhibition of presynaptic ACh release by activating presynaptic M2 autoreceptors.59 As a result, a possible hypothesis could be that donepezil-induced neurotoxic or detrimental effects in TBI animals may occur via M1 muscarinic receptor sensitization or upregulation caused by injury. To the best of our knowledge, this has not been directly investigated using our model. However, reductions in binding to M2 muscarinic- type receptors in the hippocampal formation and adjacent cortex have been observed after FP injury,60 albeit a number of studies did not detect changes in M1 type muscarinic receptor in rats60 or humans.17 Future studies specifically targeting cholinergic transmission via direct stimulation of M1-type muscarinic receptors or enhancement of presynaptic ACh release by blockade of M2 autoreceptors are, therefore, warranted. In one such study, chronic, but not acute, subcutaneous administration of the M2 autoreceptor antagonist, BIBN 99, successfully attenuated spatial learning deficits in the MWM after FP injury.61
Another alternative explanation regarding the lack of beneficial effects and drug-induced worsening of performance involves putative antagonist properties of donepezil at the NMDA receptor. Tacrine can act as an NMDA receptor antagonist,62 and it has been proposed that NMDA antagonists worsen water maze deficits in rats after TBI.63 Interestingly, co-administration of donepezil (2.5–10 mg/kg) and the NMDA antagonist, memantine, in adult rats resulted in significantly greater neurotoxic effects and subsequent neuronal injury than the memantine alone group.64 Donepezil also induced voltage-dependent blockade of responses of recombinant NMDA receptors expressed in Xenopus oocytes,65 although the authors suggested that given its low potency characteristics, NMDA receptor blockade likely does not contribute to the therapeutic actions of this drug.
Considering that low subthreshold AChEI doses may often prove ineffective in TBI models, and higher doses seem to be associated with negative side effects, an interesting approach may be a combinatorial pharmacotherapy of AChEIs, such as donepezil, and drugs affecting other brain neurotransmitters known to play a role in cognitive function. For example, Wise and colleagues42 reported that combined subthreshold doses of donepezil (0.1 mg/kg) and the cannabinoid 1 receptor antagonist rimonabant (0.3 mg/kg) significantly enhanced memory function in a rat delay radial-arm maze task. Promising results have also been seen in a touchscreen-based two choice visual discrimination cognitive task following a combined, but not individual, regimen of donepezil (0.3 mg/kg) and FK962 (1 mg/kg), a compound considered as a potential treatment for Alzheimer's disease.66 Similarly in a recent report, patients with mild-to-moderate Alzheimer's disease significantly benefited from a 20 week administration regimen of donepezil (5–10 mg/day) and natural hirudin, a specific thrombin inhibitor isolated from the salivary gland of the medicinal leech, as measured across a variety of psychometric tests.67 In addition to pharmacological therapies, our laboratory has shown that environmental enrichment provides benefits after TBI and, therefore, combining that paradigm with donepezil may result in positive effects after CCI injury.
During the past decade, promising cognitive recovery with donepezil or other AChEIs has been reported either during acute rehabilitation therapy68 or in chronic TBI patients after 3 months of treatment.69 Similar reports described improved neuropsychological scores in short-term memory and sustained attention in post-acute TBI patients taking donepezil or placebo for 10 weeks each in a within-subject design,70 as well as improved vigilance and attention in chronic TBI patients receiving donepezil or other AChEIs, such as galantamine or rivastigmine.71 A positive effect could also be seen for both immediate and delayed visual memory functioning after 6 months of donepezil treatment in TBI survivors at a dose of 10 mg/kg/day, but not at lower doses.72 On the other hand, Courtney et al.73 reported “below minimally relevant threshold” effects of donepezil in Alzheimer's disease patients. Overall, the data regarding the efficacy of AChEIs after TBI are mixed and, therefore, continued pre-clinical studies addressing potential beneficial effects and neurobiological targets of chronic AChEI administration across a range of feasible doses, with or without other adjunct clinically-relevant classes of drugs, require further evaluation.
Acknowledgment
This work was supported, in part, by National Institutes of Health (NIH) grants NS060005 and HD069620 (to Dr. Kline)
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Centers for Disease Control and Prevention (CDC) National Center for Injury Prevention and Control (2003) Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Centers for Disease Control and Prevention; Atlanta: [Google Scholar]
- 2.Moore E.L. Terryberry-Spohr L. Hope D.A. Mild traumatic brain injury and anxiety sequelae: a review of the literature. Brain Inj. 2006;20:117–132. doi: 10.1080/02699050500443558. [DOI] [PubMed] [Google Scholar]
- 3.Selassie A.W. Zaloshnja E. Langlois J.A. Miller T. Jones P. Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 2008;23:123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- 4.Summers C.R. Ivins B. Schwab K.A. Traumatic brain injury in the United States: an epidemiologic overview. Mt. Sinai J. Med. 2009;76:105–110. doi: 10.1002/msj.20100. [DOI] [PubMed] [Google Scholar]
- 5.Bales J.W. Wagner A.K. Kline A.E. Dixon C.E. Persistent cognitive dysfunction after traumatic brain injury: a dopamine hypothesis. Neurosci. Biobehav. Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faul M. Xu L. Wald M.M. Coronado V.G. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta: 2010. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. [Google Scholar]
- 7.Garcia A.N. Shah M.A. Dixon C.E. Wagner A.K. Kline A.E. Biologic and plastic effects of experimental traumatic brain injury treatment paradigms and their relevance to clinical rehabilitation. P.M. R. 2011;3:S18–27. doi: 10.1016/j.pmrj.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkelstein E.A. Corso P.S. Miller T.R. and Associates. The Incidence and Economic Burden of Injuries in the United States. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 9.Corrigan J.D. Selassie A.W. Orman J.A. The epidemiology of traumatic brain injury. J. Head Trauma Rehabil. 2010;25:72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 10.Horneman G. Emanuelson I. Cognitive outcome in children and young adults who sustained severe and moderate traumatic brain injury 10 years earlier. Brain Inj. 2009;23:907–914. doi: 10.1080/02699050903283239. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) National Center for Injury Prevention and Control (1999) Centers for Disease Control and Prevention; Atlanta: Traumatic Brain Injury in the United States—A Report to Congress. [Google Scholar]
- 12.Doppenberg E.M. Choi S.C. Bullock R. Clinical trials in traumatic brain injury: lessons for the future. J. Neurosurg. Anesthesiol. 2004;16:87–94. doi: 10.1097/00008506-200401000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Tenovuo O. Cholinergic treatment of traumatic brain injury. Curr. Drug Ther. 2006:187–209. [Google Scholar]
- 14.Flanagan S.R. Cantor J.B. Ashman T.A. Traumatic brain injury: future assessment tools and treatment prospects. Neuropsychiatr. Dis. Treat. 2008;4:877–892. doi: 10.2147/ndt.s1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menon D.K. Unique challenges in clinical trials in traumatic brain injury. Crit. Care Med. 2009;37:S129–135. doi: 10.1097/CCM.0b013e3181921225. [DOI] [PubMed] [Google Scholar]
- 16.Bornstein M.B. Presence and action of acetylcholine in experimental brain trauma. J. Neurophysiol. 1946;9:349–366. doi: 10.1152/jn.1946.9.5.349. [DOI] [PubMed] [Google Scholar]
- 17.Dewar D. Graham D.I. Depletion of choline acetyltransferase activity but preservation of M1 and M2 muscarinic receptor binding sites in temporal cortex following head injury: a preliminary human postmortem study. J. Neurotrauma. 1996;13:181–187. doi: 10.1089/neu.1996.13.181. [DOI] [PubMed] [Google Scholar]
- 18.Murdoch I. Perry E.K. Court J.A. Graham D.I. Dewar D. Cortical cholinergic dysfunction after human head injury. J. Neurotrauma. 1998;15:295–305. doi: 10.1089/neu.1998.15.295. [DOI] [PubMed] [Google Scholar]
- 19.Shao L. Ciallella J.R. Yan H.Q. Ma X. Wolfson B.M. Marion D.W. Dekosky S.T. Dixon C.E. Differential effects of traumatic brain injury on vesicular acetylcholine transporter and M2 muscarinic receptor mRNA and protein in rat. J. Neurotrauma. 1999;16:555–566. doi: 10.1089/neu.1999.16.555. [DOI] [PubMed] [Google Scholar]
- 20.Griffin S.L. van Reekum R. Masanic C. A review of cholinergic agents in the treatment of neurobehavioral deficits following traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2003;15:17–26. doi: 10.1176/jnp.15.1.17. [DOI] [PubMed] [Google Scholar]
- 21.Sarter M. Hasselmo M.E. Bruno J.P. Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res. Brain Res. Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Cardenas D.D. McLean A., Jr. Farrell–Roberts L. Baker L. Brooke M. Haselkorn J. Oral physostigmine and impaired memory in adults with brain injury. Brain Inj. 1994;8:579–587. doi: 10.3109/02699059409151010. [DOI] [PubMed] [Google Scholar]
- 23.Gorman L.K. Fu K. Hovda D.A. Murray M. Traystman R.J. Effects of traumatic brain injury on the cholinergic system in the rat. J. Neurotrauma. 1996;13:457–463. doi: 10.1089/neu.1996.13.457. [DOI] [PubMed] [Google Scholar]
- 24.Arciniegas D. Adler L. Topkoff J. Cawthra E. Filley C.M. Reite M. Attention and memory dysfunction after traumatic brain injury: cholinergic mechanisms, sensory gating, and a hypothesis for further investigation. Brain Inj. 1999;13:1–13. doi: 10.1080/026990599121827. [DOI] [PubMed] [Google Scholar]
- 25.Dixon C.E. Bao J. Long D.A. Hayes R.L. Reduced evoked release of acetylcholine in the rodent hippocampus following traumatic brain injury. Pharmacol. Biochem. Behav. 1996;53:679–686. doi: 10.1016/0091-3057(95)02069-1. [DOI] [PubMed] [Google Scholar]
- 26.Dixon C.E. Ma X. Marion D.W. Reduced evoked release of acetylcholine in the rodent neocortex following traumatic brain injury. Brain Res. 1997;749:127–130. doi: 10.1016/s0006-8993(96)01310-8. [DOI] [PubMed] [Google Scholar]
- 27.Ciallella J.R. Yan H.Q. Ma X. Wolfson B.M. Marion D.W. DeKosky S.T. Dixon C.E. Chronic effects of traumatic brain injury on hippocampal vesicular acetylcholine transporter and M2 muscarinic receptor protein in rats. Exp. Neurol. 1998;152:11–19. doi: 10.1006/exnr.1998.6831. [DOI] [PubMed] [Google Scholar]
- 28.Murdoch I. Nicoll J.A. Graham D.I. Dewar D. Nucleus basalis of Meynert pathology in the human brain after fatal head injury. J. Neurotrauma. 2002;19:279–284. doi: 10.1089/08977150252807018. [DOI] [PubMed] [Google Scholar]
- 29.Salmond C.H. Chatfield D.A. Menon D.K. Pickard J.D. Sahakian B.J. Cognitive sequelae of head injury: involvement of basal forebrain and associated structures. Brain. 2005;128:189–200. doi: 10.1093/brain/awh352. [DOI] [PubMed] [Google Scholar]
- 30.Holschneider D.P. Guo Y. Roch M. Norman K.M. Scremin O.U. Acetylcholinesterase inhibition and locomotor function after motor-sensory cortex impact injury. J. Neurotrauma. 2011;28:1909–1919. doi: 10.1089/neu.2011.1978. [DOI] [PubMed] [Google Scholar]
- 31.Scremin O.U. Norman K.M. Roch M. Holschneider D.P. Scremin A.M. Acetylcholinesterase inhibition interacts with training to reverse spatial learning deficits after cortical impact injury. J. Neurotrauma. 2012;29:2457–2464. doi: 10.1089/neu.2012.2465. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez C.M. Gearhart D.A. Parikh V. Hohnadel E.J. Davis L.W. Middlemore M.L. Warsi S.P. Waller J.L. Terry A.V., Jr. Comparison of galantamine and donepezil for effects on nerve growth factor, cholinergic markers, and memory performance in aged rats. J. Pharmacol. Exp. Ther. 2006;316:679–694. doi: 10.1124/jpet.105.093047. [DOI] [PubMed] [Google Scholar]
- 33.Bontempi B. Whelan K.T. Risbrough V.B. Lloyd G.K. Menzaghi F. Cognitive enhancing properties and tolerability of cholinergic agents in mice: a comparative study of nicotine, donepezil, and SIB-1553A, a subtype-selective ligand for nicotinic acetylcholine receptors. Neuropsychopharmacology. 2003;28:1235–1246. doi: 10.1038/sj.npp.1300150. [DOI] [PubMed] [Google Scholar]
- 34.Taverni J.P. Seliger G. Lichtman S.W. Donepezil medicated memory improvement in traumatic brain injury during post acute rehabilitation. Brain Inj. 1998;12:77–80. doi: 10.1080/026990598122881. [DOI] [PubMed] [Google Scholar]
- 35.Cheng J.P. Aslam H.A. Hoffman A.N. Zafonte R.D. Kline A.E. The neurobehavioral benefit conferred by a single systemic administration of 8-OH-DPAT after brain trauma is confined to a narrow therapeutic window. Neurosci. Lett. 2007;416:165–168. doi: 10.1016/j.neulet.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng J.P. Hoffman A.N. Zafonte R.D. Kline A.E. A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav. Brain Res. 2008;194:79–85. doi: 10.1016/j.bbr.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng J.P. Shaw K.E. Monaco C.M. Hoffman A.N. Sozda C.N. Olsen A.S. Kline A.E. A relatively brief exposure to environmental enrichment after experimental traumatic brain injury confers long-term cognitive benefits. J. Neurotrauma. 2012;29:2684–2688. doi: 10.1089/neu.2012.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kline A.E. Yu J. Massucci J.L. Zafonte R.D. Dixon C.E. Protective effects of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin against traumatic brain injury-induced cognitive deficits and neuropathology in adult male rats. Neurosci. Lett. 2002;333:179–182. doi: 10.1016/s0304-3940(02)01101-1. [DOI] [PubMed] [Google Scholar]
- 39.Kline A.E. Wagner A.K. Westergom B.P. Malena R.R. Zafonte R.D. Olsen A.S. Sozda C.N. Luthra P. Panda M. Cheng J.P. Aslam H.A. Acute treatment with the 5-HT(1A) receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav. Brain Res. 2007;177:186–194. doi: 10.1016/j.bbr.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kline A.E. McAloon R.L. Henderson K.A. Bansal U.K. Ganti B.M. Ahmed R.H. Gibbs R.B. Sozda C.N. Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma. 2010;27:2021–2032. doi: 10.1089/neu.2010.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yelleswarapu N.K. Tay J.K. Fryer W.M. Shah M.A. Garcia A.N. Cheng J.P. Kline A.E. Elucidating the role of 5-HT(1A) and 5-HT(7) receptors on 8-OH-DPAT-induced behavioral recovery after experimental traumatic brain injury. Neurosci. Lett. 2012;515:153–156. doi: 10.1016/j.neulet.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wise L.E. Iredale P.A. Stokes R.J. Lichtman A.H. Combination of rimonabant and donepezil prolongs spatial memory duration. Neuropsychopharmacology. 2007;32:1805–1812. doi: 10.1038/sj.npp.1301297. [DOI] [PubMed] [Google Scholar]
- 43.Haug K.H. Bogen I.L. Osmundsen H. Walaas I. Fonnum F. Effects on cholinergic markers in rat brain and blood after short and prolonged administration of donepezil. Neurochem. Res. 2005;30:1511–1520. doi: 10.1007/s11064-005-8828-6. [DOI] [PubMed] [Google Scholar]
- 44.Kline A.E. Massucci J.L. Dixon C.E. Zafonte R.D. Bolinger B.D. The therapeutic efficacy conferred by the 5-HT(1A) receptor agonist 8-Hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J. Neurotrauma. 2004;21:175–185. doi: 10.1089/089771504322778631. [DOI] [PubMed] [Google Scholar]
- 45.Kline A.E. Olsen A.S. Sozda C.N. Hoffman A.N. Cheng J.P. Evaluation of a combined treatment paradigm consisting of environmental enrichment and the 5-HT(1A) receptor agonist buspirone after experimental traumatic brain injury. J. Neurotrauma. 2012;29:1960–1969. doi: 10.1089/neu.2012.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feeney D.M. Gonzalez A. Law W.A. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 47.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 48.Hamm R.J. Dixon C.E. Gbadebo D.M. Singha A.K. Jenkins L.W. Lyeth B.G. Hayes R.L. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- 49.Olsen A.S. Sozda C.N. Cheng J.P. Hoffman A.N. Kline A.E. Traumatic brain injury-induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT(1A)-receptor agonist buspirone. J. Neurotrauma. 2012;29:1898–1907. doi: 10.1089/neu.2012.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seltzer B. Donepezil: a review. Expert Opin. Drug Metab. Toxicol. 2005;1:527–536. doi: 10.1517/17425255.1.3.527. [DOI] [PubMed] [Google Scholar]
- 51.Liang Y.Q. Tang X.C. Comparative effects of huperzine A, donepezil and rivastigmine on cortical acetylcholine level and acetylcholinesterase activity in rats. Neurosci. Lett. 2004;361:56–59. doi: 10.1016/j.neulet.2003.12.071. [DOI] [PubMed] [Google Scholar]
- 52.Chen Z. Xu A.J. Li R. Wei E.Q. Reversal of scopolamine-induced spatial memory deficits in rats by TAK-147. Acta Pharmacol. Sin. 2002;23:355–360. [PubMed] [Google Scholar]
- 53.Lindner M.D. Hogan J.B. Hodges D.B., Jr. Orie A.F. Chen P. Corsa J.A. Leet J.E. Gillman K.W. Rose G.M. Jones K.M. Gribkoff V.K. Donepezil primarily attenuates scopolamine-induced deficits in psychomotor function, with moderate effects on simple conditioning and attention, and small effects on working memory and spatial mapping. Psychopharmacology (Berl) 2006;188:629–640. doi: 10.1007/s00213-006-0556-3. [DOI] [PubMed] [Google Scholar]
- 54.Su D. Zhao Y. Wang B. Xu H. Li W. Chen J. Wang X. Isoflurane-induced spatial memory impairment in mice is prevented by the acetylcholinesterase inhibitor donepezil. PLoS One. 2011;6:e27632. doi: 10.1371/journal.pone.0027632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pike B.R. Hamm R.J. Chronic administration of a partial muscarinic M1 receptor agonist attenuates decreases in forebrain choline acetyltransferase immunoreactivity following experimental brain trauma. Exp. Neurol. 1997;147:55–65. doi: 10.1006/exnr.1997.6582. [DOI] [PubMed] [Google Scholar]
- 56.Rezvani A.H. Cauley M.C. Johnson E.C. Gatto G.J. Levin E.D. Effects of AZD3480, a neuronal nicotinic acetylcholine receptor agonist, and donepezil on dizocilpine-induced attentional impairment in rats. Psychopharmacology (Berl) 2012;223:251–258. doi: 10.1007/s00213-012-2712-2. [DOI] [PubMed] [Google Scholar]
- 57.Shaw K.P. Aracava Y. Akaike A. Daly J.W. Rickett D.L. Albuquerque E.X. The reversible cholinesterase inhibitor physostigmine has channel-blocking and agonist effects on the acetylcholine receptor-ion channel complex. Mol. Pharmacol. 1985;28:527–538. [PubMed] [Google Scholar]
- 58.Besheer J. Bevins R.A. Acetylcholine: II. Nicotinic receptors, in: From Messenger to Molecules: Memories are Made of These. In: Riedel G., editor; Platt B., editor. Landes Bioscience; Austin: 2004. pp. 113–124. [Google Scholar]
- 59.Pike B.R. Hamm R.J. Temple M.D. Buck D.L. Lyeth B.G. Effect of tetrahydroaminoacridine, a cholinesterase inhibitor, on cognitive performance following experimental brain injury. J. Neurotrauma. 1997;14:897–905. doi: 10.1089/neu.1997.14.897. [DOI] [PubMed] [Google Scholar]
- 60.DeAngelis M.M. Hayes R.L. Lyeth B.G. Traumatic brain injury causes a decrease in M2 muscarinic cholinergic receptor binding in the rat brain. Brain Res. 1994;653:39–44. doi: 10.1016/0006-8993(94)90369-7. [DOI] [PubMed] [Google Scholar]
- 61.Pike B.R. Hamm R.J. Post-injury administration of BIBN 99, a selective muscarinic M2 receptor antagonist, improves cognitive performance following traumatic brain injury in rats. Brain Res. 1995;686:37–43. doi: 10.1016/0006-8993(95)00448-y. [DOI] [PubMed] [Google Scholar]
- 62.Albin R.L. Young A.B. Penney J.B. Tetrahydro-9-aminoacridine (THA) interacts with the phencyclidine (PCP) receptor site. Neurosci. Lett. 1988;88:303–307. doi: 10.1016/0304-3940(88)90228-5. [DOI] [PubMed] [Google Scholar]
- 63.Hamm R.J. Pike B.R. O'Dell D.M. Lyeth B.G. Traumatic brain injury enhances the amnesic effect of an NMDA antagonist in rats. J. Neurosurg. 1994;81:267–271. doi: 10.3171/jns.1994.81.2.0267. [DOI] [PubMed] [Google Scholar]
- 64.Creeley C.E. Wozniak D.F. Nardi A. Farber N.B. Olney J.W. Donepezil markedly potentiates memantine neurotoxicity in the adult rat brain. Neurobiol. Aging. 2008;29:153–167. doi: 10.1016/j.neurobiolaging.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maurice T. Meunier J. Feng B. Ieni J. Monaghan D.T. Interaction with sigma(1) protein, but not N-methyl-D-aspartate receptor, is involved in the pharmacological activity of donepezil. J. Pharmacol. Exp. Ther. 2006;317:606–614. doi: 10.1124/jpet.105.097394. [DOI] [PubMed] [Google Scholar]
- 66.McCarthy A.D. Owens I.J. Bansal A.T. McTighe S.M. Bussey T.J. Saksida L.M. FK962 and donepezil act synergistically to improve cognition in rats: potential as an add-on therapy for Alzheimer's disease. Pharmacol. Biochem. Behav. 2011;98:76–80. doi: 10.1016/j.pbb.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 67.Li D.Q. Zhou Y.P. Yang H. Donepezil combined with natural hirudin improves the clinical symptoms of patients with mild-to-moderate Alzheimer's disease: a 20-week open-label pilot study. Int. J. Med. Sci. 2012;9:248–255. doi: 10.7150/ijms.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker W. Seel R. Gibellato M. Lew H. Cornis–Pop M. Jena T. Silver T. The effects of donepezil on traumatic brain injury acute rehabilitation outcomes. Brain Inj. 2004;18:739–750. doi: 10.1080/02699050310001646224. [DOI] [PubMed] [Google Scholar]
- 69.Khateb A. Ammann J. Annoni J.M. Diserens K. Cognition-enhancing effects of donepezil in traumatic brain injury. Eur. Neurol. 2005;54:39–45. doi: 10.1159/000087718. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L. Plotkin R.C. Wang G. Sandel M.E. Lee S. Cholinergic augmentation with donepezil enhances recovery in short-term memory and sustained attention after traumatic brain injury. Arch. Phys. Med. Rehabil. 2004;85:1050–1055. doi: 10.1016/j.apmr.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 71.Tenovuo O. Central acetylcholinesterase inhibitors in the treatment of chronic traumatic brain injury–clinical experience in 111 patients. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:61–67. doi: 10.1016/j.pnpbp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Morey C.E. Cilo M. Berry J. Cusick C. The effect of Aricept in persons with persistent memory disorder following traumatic brain injury: a pilot study. Brain Inj. 2003;17:809–815. doi: 10.1080/0269905031000088586. [DOI] [PubMed] [Google Scholar]
- 73.Courtney C. Farrell D. Gray R. Hills R. Lynch L. Sellwood E. Edwards S. Hardyman W. Raftery J. Crome P. Lendon C. Shaw H. Bentham P. AD2000 Collaborative Group (2004). Long-term donepezil treatment in 565 patients with Alzheimer's disease (AD2000): randomised double-blind trial. Lancet. 363:2105–2115. doi: 10.1016/S0140-6736(04)16499-4. [DOI] [PubMed] [Google Scholar]