Abstract
Susceptibility-weighted imaging (SWI) is a technique that exploits the susceptibility difference between tissues to provide contrast for different regions of the brain. In essence, it uses the deoxygenated hemoglobin of veins, hemosiderin of hemorrhage, etc. as intrinsic contrast agents, allowing for much better visualization of blood and microvessels even without administration of an external contrast agent. It is a fast-evolving field that is being constantly improved and increasingly implemented with updates in relevant technology. Multiple studies have been done on the role of SWI in the management of various neurologic disorders and it is also being seen as a further step in the neuroradiologist’s goal of being able to noninvasively grade tumors in order to influence therapy. This article briefly reviews the evolution of SWI since its conception and provides the reader with a comprehensive summary of various studies that have been done on its application for detecting and grading intraaxial brain tumors, specifically gliomas. Other useful magnetic resonance techniques that have shown promise in grading gliomas are also discussed.
Keywords: Glioma grade, brain tumors, susceptibility-weighted imaging, neoangiogenesis
Introduction
Gliomas are the most common brain tumors and account for 70% of primary adult malignant brain tumors[1]. There have been reports that the incidence of gliomas has been increasing in recent years and some studies have inconclusively linked this to increased cell phone usage[2]. The World Health Organization (WHO) categorizes gliomas into grades I to IV, with grade I being benign and grade IV most malignant[3]. The importance of glioma grading lies in the fact that it is the most important prognostic factor for the patient. Benign tumors are often amenable to surgery, whereas malignant tumors have to be frequently managed with radiochemotherapy and indicate a low survival rate. Magnetic resonance (MR) imaging is the initial investigation of choice in patients with suspected glioma and plays a major role in the initial differential, but currently, no imaging features are considered in the confirmatory diagnosis or grading of gliomas; these are solely based on invasive stereotactic biopsies[4].
In the previous decade, MR imaging has become the mainstay diagnostic tool for the evaluation of suspected brain tumors preoperatively and follow-up after therapy. The strength of MR imaging lies in its vast array of sequences, which do not just anatomically delineate lesions (t1, t2) but also differentiate them physiologically[5]. The susceptibility-weighted imaging (SWI) sequence is a useful recent addition to this arsenal of MR imaging sequences; it has been designed to utilize the susceptibility difference between the deoxygenated blood in veins and the surrounding brain parenchyma to provide a high degree of contrast[6].
Information attained via structural MR imaging scans in patients with glioma, such as the accurate anatomic location and the relationship of the tumor with important surrounding brain structures, has major influences on the therapeutic decisions. However, more recently, increasing attention is being paid to the physiologic data gained from MR scans via novel sequences such as diffusion- and perfusion-weighted imaging (DWI and PWI) as well MR spectroscopy. This information is proving very valuable in limiting differentials of space-occupying lesions and in guiding biopsies to the most malignant areas of frequently encountered heterogeneous tumors, thereby leading to accurate histopathologic diagnoses. A lot of research has been done and is ongoing to demonstrate the usefulness of these methods in grading gliomas with varying degrees of success. These are briefly summed up in the final section of this article. Despite the potential, definitive noninvasive glioma grading still remains an unachieved goal for radiologists. However, with the addition of SWI to this list of MR sequences, some researchers now hope that MR imaging can play a more substantial role toward this goal.
Although a full assessment of the role of SWI has yet to be confirmed in the literature, it is fast becoming part of the standard brain tumor imaging protocol in most of the leading diagnostic centers worldwide. It is also under intensive study because it is largely felt that its full potential has yet to be realized.
SWI and its evolution
An excellent review by Haacke et al.[7] describes the relevant physics involved in creating SWI in detail. It is not the purpose of this article to go into the details of the physics, instead we have summarized it in simple terms. Routinely, most conventional MR sequences use magnitude information for sequencing. Even though phase data are generated each time and contain potentially very useful information, they cannot be put to clinical use because of the effects of background magnetic fields. The phase images are sensitive for showing changes in the local magnetic field, i.e. susceptibility, caused by blood and its various products along with other substances such as calcium. It was only in 1997 that a mechanism was developed to remove the unwanted artifacts while retaining the local phase of interest by passing the data through a high-pass Hanning filter. The remaining useful phase information was then combined with magnitude images multiplied several times and to produce SWI images. This pioneering work is due to Reichenbach et al.[6,8].
This novel technique was termed high-resolution blood oxygen level dependent (BOLD) venography by the early researchers, because it was due to the low oxygen levels that the veins could be visualized on SWI[9]. Among its first uses, it was successfully used to detect occult vascular malformations[10], which could only be diagnosed after they had bled on conventional MR sequences. Since then, several reviews have shown SWI to be a very useful adjunct technique in evaluating a wide range of neurologic disease processes including traumatic brain injury, cerebral amyloid angiopathy, stroke, neurodegenerative disorders, vascular malformations and brain tumors in adults[11,12]; it has also been studied in children by Tong et al.[13].
The generation of SWI images is now automated and is being supplied with consoles from the major manufacturers (GE, Siemens). The final SWI consists of four sets of images: magnitude images, filtered phase images, minimum intensity projections images and the final SWI images. It can be tedious to perform SWI because of its long acquisition time of over 8 min in a 1.5-T scanner, but with the widespread availability of 3 T scanners, this has been greatly reduced (<5 min). Other benefits of 3-T scanners versus the 1.5-T scanners include better signal to noise ratio (SNR) and better image resolution with smaller pixel size. Experimental studies have also been done using ultra-high magnetic field strength (7 T[14] and 8 T[15]) with largely positive results. Significant two-fold reductions in acquisition times without any loss of contrast were achieved with GRAPPA-based imaging at 7 T[16]. However, the use of these scanners is still in the experimental phase and more data can be expected once their use becomes mainstream.
Tumor neoangiogenesis, hemorrhage and the role of SWI
The strength of SWI lies in its ability to better distinguish blood from the surrounding tissue. Hence, it will be helpful for us to begin our discussion with a short insight into the life cycle of tumors and the role of blood. It has been shown that neoangiogenesis and neovascularization play a central role in the growth and spread of tumors[17], particularly for solid tumors such as oligodendrogliomas and glioblastomas. Neoangiogenesis is the process of vascularization of a tissue that includes the development of new vessels in order to feed the growing tumor cells. More aggressive tumors tend to have denser and faster growing blood vessels. These capillaries are tortuous and of wider caliber. They also differ from the normal capillaries by their immature walls and increased endothelial gaps. Hence, these neocapillaries tend to be leaky, leading to edema of the surrounding tissue and intratumoral hemorrhage. Increased recognition of this phenomenon has led to multiple trials being done to treat high-grade tumors with anti-angiogenic therapy[18,19], such as bevacizumab[20] for example, to halt tumor growth.
It has since been proposed that studying the mechanisms of vascular proliferation and quantifying tumor blood flow by various microvascular parameters, such as cerebral blood flow (CBF), cerebral blood volume (CBV), etc., measured via perfusion-weighted dynamic susceptibility contrast (DSC)-enhanced MR imaging could be of help in assessing and grading tumors[21,22]. It has been seen that relative CBV correlates well with angiographic and histologic parameters of tumor vascularity, and the expression of vascular endothelial growth factor[22] and tumors with high regional CBV (rCBV) tend to be solid tumors such as high-grade gliomas or glioblastomas[23].
The ability to visualize and analyze tumor vasculature can therefore play an essential part in the detection and evaluation of tumors. Various ways have been suggested to do this with MR imaging[24]. These include studying the signal changes associated with changes in oxygenation or response to vasomodulators[25]; steady state approaches to measurement of perfusion by arterial spin labeling tagging of the nuclear MR signal of water and contrast-enhanced approaches for direct detection of blood volume, vessel diameter, and permeability and using these as an indirect measure of expression of specific endothelial cell markers; and utilization of intrinsic venous contrast via SWI. SWI is arguably the simplest and the most cost-effective of these methods because it does not require administration of external substances or complicated post-imaging analysis.
Besides the evaluation of macro- and microvessels, it is also important to be able to recognize hemorrhage. Intratumoral hemorrhages are frequently in the form of microhemorrhages, which are very hard to detect on conventional MR sequences including T2* and contrast-enhanced T1. Areas identified as hemorrhage on conventional sequences can actually be areas of necrosis[26]. The hemorrhagic extravascular blood is constituted by magnetically susceptible substances such as intracellular deoxyhemoglobin and other broken down blood pigments such as hemosiderin and ferritin. These are seen as dark hypointense regions on SWI, whereas necrosis is usually not evident on SWI. It has since become the preferred MR modality to study microhemorrhages. Recent examples include the study of microhemorrhages that appear in response to anti-angiogenic therapy[27] as well as radiotherapy[28] on SWI performed at 7 T.
Hemorrhage can usually be separated from veins on SWI based on the tubular structure of veins and their continuation on multiple slices. However, their common hypointense appearance on SWI can sometimes lead to confusion. Sehgal et al.[29] suggested that this confusion can be resolved by taking SWI before and after contrast administration. Areas of blood flow enhance, whereas areas of inactive hemorrhage do not enhance. The use of contrast agents is also supported by a few other authors who found that contrast agent shortens the scan time and results in better image quality[30,31]. However, most authors maintain that noncontrast SWI produces results that are equivalent to contrast-enhanced SWI and the use of contrast agent is not necessary[32].
Mass differential and tumor diagnosis
Early and accurate tumor diagnosis is paramount in neuroimaging. In a recent study, SWI was shown to be useful for early detection and tracking of rare basal ganglia germinomas[33]. Multiple studies have shown SWI to be better than almost any other widely used sequence for detecting blood and blood products[21,26]. In an interclass sequence comparison, SWI was found to be the best for visualizing intratumoral architecture including small vessels and microhemorrages[34]. This property is due to the fact that gradient-recalled echo (GRE) sequences, in general, are more sensitive than spin-echo (SE) sequences to blood[35] and SWI, with its additional phase information post-processing, is more sensitive than T2* GRE[26]. Better detection of microbleeds and tortuous vessels, common occurrences in cancer, leads to better diagnosis.
Another property of SWI that could prove to be a major asset in the differential diagnoses of intraaxial masses is its ability to identify calcium. As the presence or absence of calcium holds major clues to the true pathologic nature of a lesion, the lack of ability to reliably identify it on MR imaging frequently leads to adjunct computed tomography (CT) scans. Statistically, a calcified intraaxial supratentorial tumor mass is most likely to be an oligodendroglioma, ependymymoma or low-grade astrocytoma[1]. Thus, although histopathology is mandatory for the histologic diagnosis of a tumor, the presence of calcium can help limit the differential.
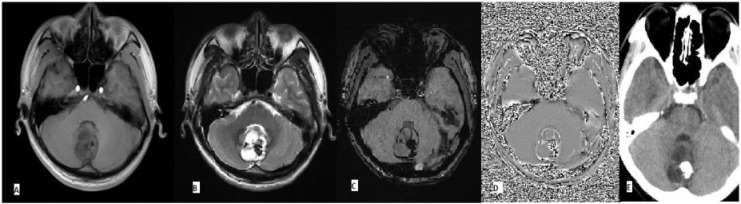
Conventional MR sequences have repeatedly been proved to be inferior to CT in the identification of calcium. SWI has been shown to have better ability to detect calcium compared with the conventional sequences, although it is currently thought of as unlikely to replace CT as the preferential imaging modality for this criterion[36]. On SWI, calcium, a diamagnetic substance, is represented with the same dark signal intensity as the other paramagnetic substances such as deoxygenated heme. But, the two groups of molecules show opposite signal intensities on the filtered phase images. In a right-handed system (such as that used by GE), diamagnetic substances appear bright and paramagnetic substances appear dark, and the vice versa for left-handed system (Siemens)[7]. Hence, with information on the manufacturer’s choice of the system, a comparison between the SWI and phase images can be used to identify calcium with MR imaging (Fig. 1).
Figure 1.
Grade 1 astrocytoma. Nonspecific findings of a tumor mass on (A) T1- and (B) T2-weighted images. Comparison of hypointensity on SWI (C) and hyperintensity on phase image (D) is suggestive of calcification, which was confirmed on CT (E). The presence of calcification and lack of hemorrhage is also suggestive of a low-grade tumor.
Tumor parenchyma is better differentiated with SWI compared with conventional MR images including contrast-enhanced T1. In the study by Seghal et al.[21], it was proposed that SWI could prove helpful in tumor characterization. In this study, three independent radiologists reviewed images of 44 patients with brain masses and compared SWI with conventional sequences (T1, T1 postcontrast, T2, proton density, fluid attenuated inversion recovery (FLAIR) and DWI at 1.5 T). It was found that SWI provided complementary or better information than conventional sequences regarding tumor visibility, boundary definition (3/37), blood products (25/37), venous vasculature (32/37), tumor architecture (12/37) and edema. This means that SWI could add a 70% increment to more accurate detection of tumors. The authors concluded that for any given lesion, a single SWI sequence can adequately detect the tumor, depict surrounding edema with FLAIR-like contrast, accurately assess the effects on the surrounding brain anatomy, and detect blood products with superlative contrast while showing increased internal detail.
Another group of authors explored the benefits of SWI in the differential diagnosis of solitary enhancing brain lesions (SELs) via the assessment of intratumoral susceptibility signals (ITSSs) observed on SWI[37]. SELs detected on conventional sequences could indicate a highly varied pathology ranging from non-tumorous lesions (tumefactive multiple sclerosis and inflammatory granuloma) to anaplastic astrocytomas and glioblastoma multiforme (GBMs), and their value in the differential diagnosis remains poor. The study included retrospective evaluation of 64 SELs. Two consensus reviews were performed, one with only conventional images and another with added high-resolution SWI, which included ITSS. The benefits of evaluating ITSSs were obvious. ITSSs, which were constituted by low signal intensity structures depicting blood products, calcifications and venous vasculature, were seen in all of the GBMs and were absent in all the non-tumorous lesions and lymphomas. The specificity of differentiating non-tumorous lesions from GBMs (25/25) was 100% with the addition of high-resolution SWI.
Differentiating predominantly necrotic GBMs from abscesses is a frequent clinical dilemma encountered in routine practice. Both these lesions are seen as hyperintense space-occupying lesions with an enhancing hypointense rim on T2-weighted images. The rim of brain abscesses is thought to represent the abscess capsule. When evaluated on SWI, it was found to have a negative phase value with certain characteristic features. The rims of brain abscesses compared with GBMs were found to be smoother (P < 0.001) and more complete (P < 0.001). Seventy-five percent of abscesses (n = 12) were also seen to have a dual rim sign (inner hyper and outer hypointensity rims), whereas none of the GBMs exhibited this sign[38].
Tumor grading and SWI
The accurate grading of brain tumors has important prognostic and therapeutic implications, because high-grade lesions are handled differently from low-grade lesions. Patients with resectable and unresectable high-grade lesions receive either radiation therapy or combined radiochemotherapy. Low-grade gliomas (WHO grades I and II) are amenable to surgical resection with curative intent, and adjuvant radiochemotherapy is only recommended for patients with incompletely resected grade II tumors or for patients older than age 40 years regardless of the extent of resection[39]. In standard practice, a stereotactic biopsy of the brain is considered the gold standard for grading tumors. Of all the parameters evaluated on a biopsy, it was found that only vascular proliferation differentially predicted both short- and long-term survival of patients with astrocytomas[40]. Detection of neovascularization in the tumor places it in WHO grade IV (Figs. 2 and 3).
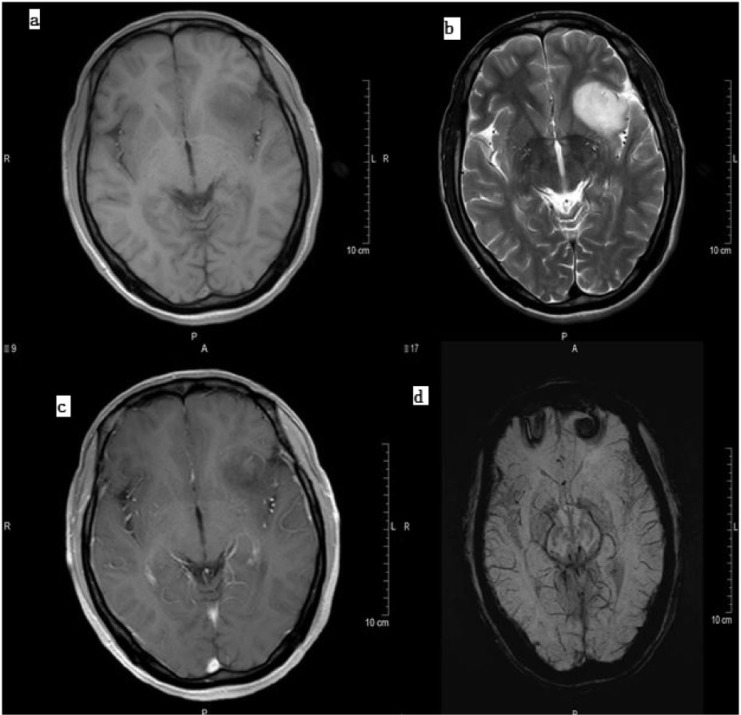
Figure 2.
Grade 2 astrocytoma as seen on (a) T1-weighted image, (b) T2-weighted image, (c) contrast-enhanced T1 and (d) SWI. Note the lack of susceptibility signals in the low-grade tumor on SWI.
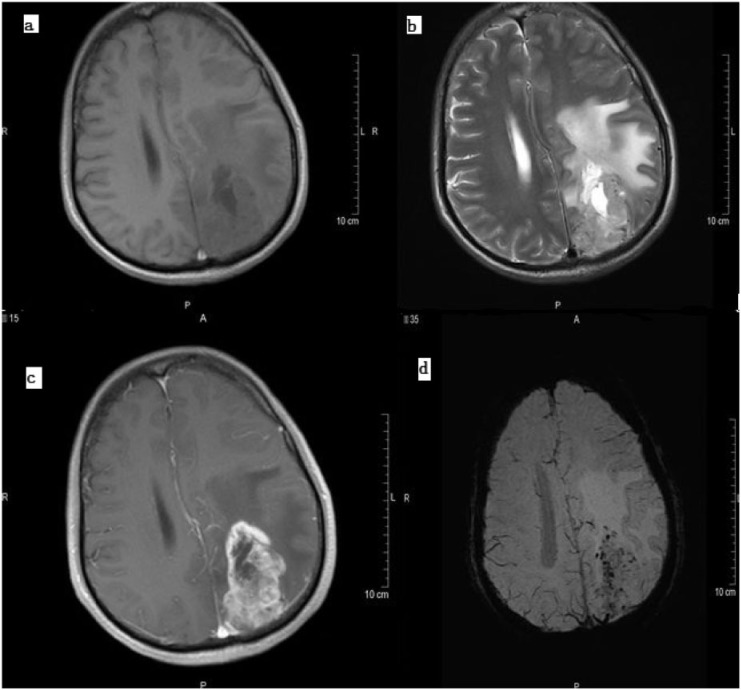
Figure 3.
High-grade astrocytoma (GBM) on (a) a T1-weighted image, (b) a T2-weighted image, (c) contrast-enhanced T1 and (d) SWI. The increased number of vessels and microhemorrhages on SWI suggested a high tumor grade, which was confirmed on pathology. Also note the high contrast with which surrounding edema is visible on SWI.
It has been more than a decade since the use of susceptibility effects was recommended for preoperatively grading tumors to better influence therapy and estimate prognosis[41]. Low SNR and poor contrast and resolution, however, proved to be major roadblocks. With recent improvement in both software and hardware, a lot of those issues have been resolved to a great extent. The long acquisition time with 1.5-T scanners has been overcome with 3 T, and it has now become feasible to include SWI sequencing in every MR imaging scan of suspected brain neoplasms. Higher strength of magnetization also means less signal loss and higher SNR with better pixel resolution. Because of these developments and the apparent usefulness of assessing intratumoral vascularity for grading tumors, various studies have been done to establish methods to assess its usefulness. These studies have attempted to grade tumors based on specific criteria.
For example, Hori et al.[42] evaluated magnetic susceptibility artifacts in lesions on MR images for grading gliomas. This was based on the theory that intratumoral hemorrhage and microvascularity correlated with tumor grade. The tumors were analyzed based on three grading criteria: (1) the presence of hypointensity on SWI; (2) the ratio of the area of hypointensity in the tumor; and (3) the dominant structure of hypointensity in the tumor. Detection of hypointense signals and visualization of vessels within the tumor were taken as signs of neovascularization, which are indicators of a growing tumor. This directly correlates with tumor grade. In this study, only the mean grading scores for the ratio of the area of hypointensity in the tumor were statistically significant and higher for high-grade tumors than for low-grade tumors. An additional finding that correlated strongly with higher grade was that of positive abnormal enhancement around the tumor rim on postcontrast-enhanced SWI. This is thought to be due to the breakdown of the blood–brain barrier.
Among gliomas, astrocytomas account for the majority of tumors; glioblastoma multiforme is the most common. Methods have been proposed to grade astrocytomas specifically. More or less the same theory of small vessel proliferation and microhemorrhage correlation with tumor grade was further assessed by Li et al.[34]. The authors first compared the number of small vessels and microhemorrhage volume seen in conventional MR sequences to those seen in SWI in order to establish the dominance of SWI. Their results for this criterion were consistent with other studies. SWI was found to be better than T2-weighted imaging and T2 FLAIR, which in turn were found to be better T1-weighted and contrast-enhanced T1-weighted imaging for the visualization of vascular structures. Next, comparison of SWI images of tumors resulted in positive findings of increased vessels and hemorrhages with high-grade tumors and less evidence of blood in low-grade tumors. SWI sequences displayed on average 17.7 ± 12.71 small vessels in high-grade astrocytomas, whereas low-grade astrocytomas showed an average of only 7.9 ± 7.62. As far as microhemorrhages were concerned, high-grade astrocytomas had a mean volume of 4.84 ± 3.51 cm3, and low-grade astrocytomas displayed a mean volume of 2.19 ± 3.35 cm3 in SWI sequences. Microhemorrhages and small vessels, visualized on SWI, can be taken as an indirect measure of neoangiogenesis. Neoangiogenesis, as discussed earlier, is an important factor in determining the grade of the tumor. Hence, an inference that small vessels and microhemorrhages could be used to grade astrocytomas was made.
The position that SWI enjoys in terms of being the best at demonstrating blood and vascular structures is further bolstered by the development of high-resolution SWI at 3 T. This technique enables visualization of very small vessels of millimeter thickness with a high degree of contrast. High-resolution SWI is seen to better portray microvasculature and various other susceptibility effects. In a study done by Pinker et al.[43], susceptibility effects seen on high-resolution contrast-enhanced SWI correlated with the areas of increased blood flow on positron emission tomography (PET) scanning. Also, good correlation was seen between the tumors with increased and decreased susceptibility effects on high-resolution SWI and their grade, which was found to be high and low grade, respectively, on histopathologic sections done later. A statistically significant correlation between the frequency of intralesional susceptibility effects and tumor grade was seen as determined by both PET and histopathology. This technique was found to be even better than dynamic gadolinium scanning, which only showed diffuse postcontrast hyperintensity due to increased rCBF in areas with blood and could not demonstrate small vessels per se. Conglomerates of vascular proliferation were seen on histopathology in areas of high intralesional susceptibility effects. Also, because it is common practice to biopsy the most accessible region of the tumor (which may not always be the best indicator of pathology in a heterogeneous tumor like GBM), the authors noted that high-resolution SWI should be better used to guide biopsies to regions of high intratumoral vascularity so that an accurate histopathologic diagnosis can be made.
In a similar study, Park et al.[44] proposed that high susceptibility effects in high-grade gliomas not only reflect tumor vascularity but also indicate considerable susceptibility in areas of macro- and micronecrosis in higher-grade gliomas. To make their point, they compared areas of ITSSs seen on high-resolution SWI with areas of rCBV on DSC. In good agreement with Kim et al.[37], ITSSs were seen in all the samples of GBM and were not seen in any of the low-grade (less than grade II) tumors. In their comparisons, they found that the degree of ITSS showed significant correlation with the value of rCBVmax in the same tumor segments. Fine linear and dotlike ITSSs did partly correspond with regions of visual rCBVmax on coregistered images of high-resolution SWI and rCBV maps. However, areas of highest ITSS did not accurately correspond with the same regions of visual rCBVmax supposedly because ITSSs not only showed susceptibility associated with increased tumor vascularity but also showed susceptibility from microhemorrhages and tumor necrosis. The authors found that the diagnostic performance of high-resolution SWI was comparable with that of DSC and suggested a noncontrast brain imaging protocol for patients with contraindications to contrast agents. However, some authors believe that the use of contrast agents leads to shortening of scan time and better contrast of intratumoral parenchyma.
Other MR methods and tumor grading
Besides SWI, there is substantial material available in the current literature on the use of other physiologic MR sequences for grading gliomas, namely DWI, proton MR spectroscopy (1HMRS) and PWI. Each has its own advantages and shortcomings. A brief introduction into the present state of research of these different methods is provided in this section. DWI is usually part of the routine MR imaging protocol performed in the preoperative assessment of patients with glioma. Apparent diffusion coefficient (ADC) values obtained by DWI indicate the amount of movement of water molecules in a particular tissue of interest. Generally speaking, areas of cytotoxic edema tend to have more restricted movement of water molecules and hence, a lower ADC value. Solid components of gliomas are also frequently seen to have low ADC values, which is thought to be due to higher cell density and a higher nuclear to cytoplasmic ratio in highly mitotic cells and hence a higher-grade glioma[45]. However, a study involving confirmation of ADC values with stereotactic biopsies failed to find a significant correlation between low ADC and high cell density (Fig. 4)[36]. Hence, although low ADC values are frequently seen in high-grade neoplasms, the specificity of this method for grading gliomas remains low[46,47].
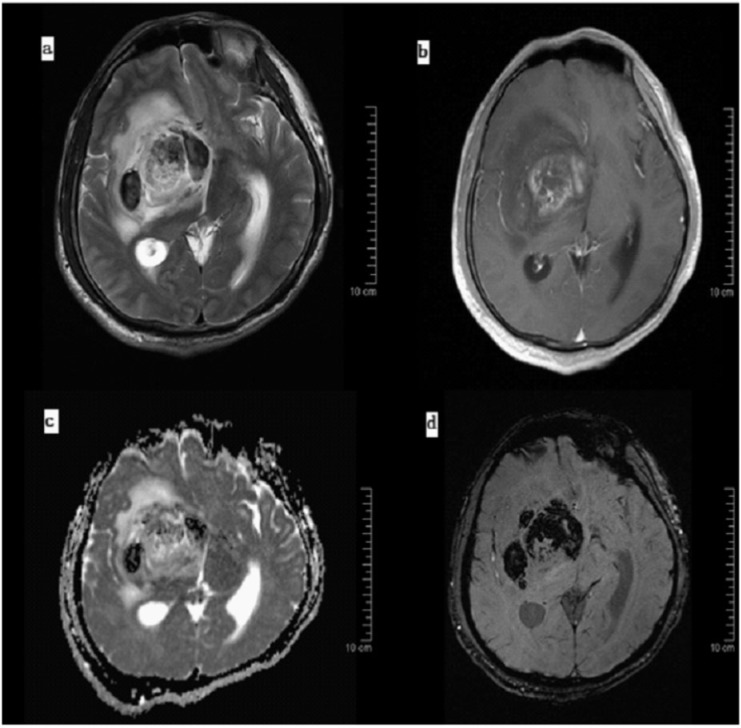
Figure 4.
Histopathologically confirmed grade IV GBM. (a) T2-weighted Image shows extensive necrosis and edema with mid-line shift. (b) Contrast-enhanced T1-weighted Image shows fair enhancement of the lesion. (c) Low ADC values are seen in the diffusion-weighted image, and (d) extensive areas of hemorrhage are seen on the susceptibility-weighted image indicating a higher tumor grade.
1HMRS imaging is another useful method and was among the first to gain notoriety for its potential to help grade gliomas[48]. It enables noninvasive measurement of various chemical metabolites in the lesion of interest, which can then be interpreted to assess the nature of the lesion. High specificity is achieved when using increased choline (Cho) and low N-acetylaspartate levels to differentiate neoplastic from nonneoplastic lesions[49,50]. Despite its potential, histologic grading with 1HMRS remains controversial. Human gliomas tends to be very heterogeneous, with low-grade and high-grade regions sometimes coexisting within the same tumor. Some authors have suggested limiting the area of 1HMRS data to hyperperfused regions of the tumor, as identified by PWI, to reliably differentiate high-grade from low-grade tumors[51]. A high Cho/creatine ratio has been found to be a reliable marker for high-grade gliomas in multiple studies[51–53].
The argument made for the using SWI for grading tumors, i.e. neovascularization is a good indicator for tumor grade, also holds for PWI because the values of CBV, CBF, etc. measured by this method are an indirect measure of neovascularization. Perfusion studies involve administration of a bolus of contrast medium. Many tumor patients tend to have concomitant nephropathies or allergies, making it impractical to perform the study because of the associated toxicity of contrast administration. However, rCBV measured with DSC-weighted imaging is considered to be a good indicator not just for microvascular density but also for cellular density[36] and has shown good correlation with tumor grades in many studies[47,53,54]. The issue of frequent instances of low-grade tumors exhibiting high levels of rCBV and rCBF[55] remains to be solved.
Although the above discussion does not do justice to any of the MR methods, it is clear that no individual method is sufficient to confidently grade gliomas on its own. Studies that have combined structural and physiologic MR sequences have had higher accuracy in this regard[42]. SWI has a simplistic approach and can be very easily fused with the conventional MR sequences, including DWI, providing the radiologist with an estimate of the vascularity of the tumor. Despite the potential of MRS, the associated time, expertise and expense make it impractical for routine use in high-volume centers. Different sequences exhibit different unique aspects of gliomas, many of which need consideration, if an MR protocol to confidently grade gliomas is to be developed.
Conclusion
The theory that susceptibility signals show microvasculature that correlates with tumor grade has been well validated with the help of various studies. However, the cons of SWI lie within the technique itself. Small tweaks made in imaging parameters lead to varying subjective results. This lack of standardization of the SWI technique remains an obstacle in its integration into mainstream grading of gliomas. SWI for now plays an important role in detecting gliomas and guiding biopsies. The goal of noninvasive accurate grading of tumors is yet to be realized. Further studies with greater sample size and better collaborations are warranted in this regard.
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Emblem KE, Scheie D, Due-Tonnessen P, et al. Histogram analysis of MR imaging-derived cerebral blood volume maps: combined glioma grading and identification of low-grade oligodendroglial subtypes. AJNR Am J Neuroradiol. 2008;29:1664–1670. doi: 10.3174/ajnr.A1182. . PMid:18583405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levis AG, Minicuci N, Ricci P, Gennaro V, Garbisa S. Mobile phones and head tumours. The discrepancies in cause-effect relationships in the epidemiological studies - how do they arise? Environ Health. 2011;10:59. doi: 10.1186/1476-069X-10-59. . PMid:21679472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Ieva A, Göd S, Grabner G, et al. Three-dimensional susceptibility-weighted imaging at 7 T using fractal-based quantitative analysis to grade gliomas. Neuroradiology. 2013;55:35–40. doi: 10.1007/s00234-012-1081-1. . PMid:22903580. [DOI] [PubMed] [Google Scholar]
- 4.Fuller GN, Scheithauer BW. The 2007 revised World Health Organization (WHO) classification of tumours of the central nervous system: newly codified entities. Brain Pathol. 2007;17:304–307. doi: 10.1111/j.1750-3639.2007.00084.x. . PMid:17598822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts TP, Mikulis D. Neuro MR: principles. J Magn Reson Imaging. 2007;26:823–837. doi: 10.1002/jmri.21029. . PMid:17685415. [DOI] [PubMed] [Google Scholar]
- 6.Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology. 1997;204:272–277. doi: 10.1148/radiology.204.1.9205259. PMid:9205259. [DOI] [PubMed] [Google Scholar]
- 7.Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. 2009;30:19–30. doi: 10.3174/ajnr.A1400. . PMid:19039041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reichenbach JR, Barth M, Haacke EM, Klarhöfer M, Kaiser WA, Moser E. High-resolution MR venography at 3.0 Tesla. J Comput Assist Tomogr. 2000;24:949–957. doi: 10.1097/00004728-200011000-00023. . PMid:11105717. [DOI] [PubMed] [Google Scholar]
- 9.Higano S, Yun X, Kumabe T, et al. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006;241:839–846. doi: 10.1148/radiol.2413051276. . PMid:17032910. [DOI] [PubMed] [Google Scholar]
- 10.Lee BC, Vo KD, Kido DK, et al. MR high-resolution blood oxygenation level-dependent venography of occult (low-flow) vascular lesions. AJNR Am J Neuroradiol. 1999;20:1239–1242. PMid:10472978. [PMC free article] [PubMed] [Google Scholar]
- 11.Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009;30:232–252. doi: 10.3174/ajnr.A1461. . PMid:19131406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson RJ, Bhuta S. Susceptibility-weighted imaging of the brain: current utility and potential applications. J Neuroimaging. 2011;21:e189–204. doi: 10.1111/j.1552-6569.2010.00516.x. . PMid:21281380. [DOI] [PubMed] [Google Scholar]
- 13.Tong KA, Ashwal S, Obenaus A, Nickerson JP, Kido D, Haacke EM. Susceptibility-weighted MR imaging: a review of clinical applications in children. AJNR Am J Neuroradiol. 2008;29:9–17. doi: 10.3174/ajnr.A0786. . PMid:17925363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burrell JS, Walker-Samuel S, Baker LC, et al. Evaluation of novel combined carbogen USPIO (CUSPIO) imaging biomarkers in assessing the antiangiogenic effects of cediranib (AZD2171) in rat C6 gliomas. Int J Cancer. 2012;131:1854–1862. doi: 10.1002/ijc.27460. . PMid:22290271. [DOI] [PubMed] [Google Scholar]
- 15.Lee EJ, terBrugge K, Mikulis D, et al. Diagnostic value of peritumoral minimum apparent diffusion coefficient for differentiation of glioblastoma multiforme from solitary metastatic lesions. AJR Am J Roentgenol. 2011;196:71–76. doi: 10.2214/AJR.10.4752. . PMid:21178049. [DOI] [PubMed] [Google Scholar]
- 16.Lupo JM, Banerjee S, Hammond KE, et al. GRAPPA-based susceptibility-weighted imaging of normal volunteers and patients with brain tumor at 7 T. Magn Reson Imaging. 2009;27:480–488. doi: 10.1016/j.mri.2008.08.003. . PMid:18823730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. . PMid:1688381. [DOI] [PubMed] [Google Scholar]
- 18.de Bouard S, Guillamo JS. Angiogenesis and anti-angiogenic strategies for glioblastoma. Bull Cancer. 2005;92:360–372. PMid:15888393. [PubMed] [Google Scholar]
- 19.Sawlani RN, Raizer J, Horowitz SW, et al. Glioblastoma: a method for predicting response to antiangiogenic chemotherapy by using MR perfusion imaging–pilot study. Radiology. 2010;255:622–628. doi: 10.1148/radiol.10091341. . PMid:20413772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabner G, Nöbauer I, Elandt K, et al. Longitudinal brain imaging of five malignant glioma patients treated with bevacizumab using susceptibility-weighted magnetic resonance imaging at 7 T. Magn Reson Imaging. 2012;30:139–147. doi: 10.1016/j.mri.2011.08.004. . PMid:21982163. [DOI] [PubMed] [Google Scholar]
- 21.Sehgal V, Delproposto Z, Haddar D, et al. Susceptibility-weighted imaging to visualize blood products and improve tumor contrast in the study of brain masses. J Magn Reson Imaging. 2006;24:41–51. doi: 10.1002/jmri.20598. . PMid:16755540. [DOI] [PubMed] [Google Scholar]
- 22.Zulfiqar M, Dumrongpisutikul N, Intrapiromkul J, Yousem DM. Detection of intratumoral calcification in oligodendrogliomas by susceptibility-weighted MR imaging. AJNR Am J Neuroradiol. 2012;33:858–864. doi: 10.3174/ajnr.A2862. . PMid:22268093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brasch R, Turetschek K. MRI characterization of tumors and grading angiogenesis using macromolecular contrast media: status report. Eur J Radiol. 2000;34:148–155. doi: 10.1016/S0720-048X(00)00195-9. . PMid:10927157. [DOI] [PubMed] [Google Scholar]
- 24.Hayashida Y, Hirai T, Morishita S, et al. Diffusion-weighted imaging of metastatic brain tumors: comparison with histologic type and tumor cellularity. AJNR Am J Neuroradiol. 2006;27:1419–1425. PMid:16908550. [PMC free article] [PubMed] [Google Scholar]
- 25.Rauscher A, Sedlacik J, Barth M, Haacke EM, Reichenbach JR. Nonnvasive assessment of vascular architecture and function during modulated blood oxygenation using susceptibility weighted magnetic resonance imaging. Magn Reson Med. 2005;54:87–95. doi: 10.1002/mrm.20520. . PMid:15968657. [DOI] [PubMed] [Google Scholar]
- 26.Löbel U, Sedlacik J, Sabin ND, et al. Three-dimensional susceptibility-weighted imaging and two-dimensional T2*-weighted gradient-echo imaging of intratumoral hemorrhages in pediatric diffuse intrinsic pontine glioma. Neuroradiology. 2010;52:1167–1177. doi: 10.1007/s00234-010-0771-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Ieva A, Matula C, Grizzi F, Grabner G, Trattnig S, Tschabitscher M. Fractal analysis of the susceptibility weighted imaging patterns in malignant brain tumors during antiangiogenic treatment: technical report on four cases serially imaged by 7 T magnetic resonance during a period of four weeks. World Neurosurg. 2012;77:785.e11–21. doi: 10.1016/j.wneu.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Lupo JM, Chuang CF, Chang SM, et al. 7-Tesla susceptibility-weighted imaging to assess the effects of radiotherapy on normal-appearing brain in patients with glioma. Int J Radiat Oncol Biol Phys. 2012;82:e493–500. doi: 10.1016/j.ijrobp.2011.05.046. . PMid:22000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sehgal V, Delproposto Z, Haacke EM, et al. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005;22:439–450. doi: 10.1002/jmri.20404. . PMid:16163700. [DOI] [PubMed] [Google Scholar]
- 30.Lin W, Mukherjee P, An H, et al. Improving high-resolution MR bold venographic imaging using a T1 reducing contrast agent. J Magn Reson Imaging. 1999;10:118–123. doi: 10.1002/(SICI)1522-2586(199908)10:2<118::AID-JMRI2>3.0.CO;2-V. . PMid:10441013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinker K, Noebauer-Huhmann IM, Stavrou I, et al. High-field, high-resolution, susceptibility-weighted magnetic resonance imaging: improved image quality by addition of contrast agent and higher field strength in patients with brain tumors. Neuroradiology. 2008;50:9–16. doi: 10.1007/s00234-007-0298-x. . PMid:17876570. [DOI] [PubMed] [Google Scholar]
- 32.Hori M, Ishigame K, Kabasawa H, et al. Precontrast and postcontrast susceptibility-weighted imaging in the assessment of intracranial brain neoplasms at 1.5 T. Jpn J Radiol. 2010;28:299–304. doi: 10.1007/s11604-010-0427-z. . PMid:20512548. [DOI] [PubMed] [Google Scholar]
- 33.Lou X, Ma L, Wang FL, et al. Susceptibility-weighted imaging in the diagnosis of early basal ganglia germinoma. AJNR Am J Neuroradiol. 2009;30:1694–1699. doi: 10.3174/ajnr.A1696. . PMid:19581340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Ai B, Li Y, Qi H, Wu L. Susceptibility-weighted imaging in grading brain astrocytomas. Eur J Radiol. 2010;75:e81–85. doi: 10.1016/j.ejrad.2009.08.003. . PMid:19726149. [DOI] [PubMed] [Google Scholar]
- 35.Atlas SW, Mark AS, Grossman RI, Gomori JM. Intracranial hemorrhage: gradient-echo MR imaging at 1.5 T. Comparison with spin-echo imaging and clinical applications. Radiology. 1988;168:803–807. doi: 10.1148/radiology.168.3.3406410. PMid:3406410. [DOI] [PubMed] [Google Scholar]
- 36.Sadeghi N, D'Haene N, Decaestecker C, et al. Apparent diffusion coefficient and cerebral blood volume in brain gliomas: relation to tumor cell density and tumor microvessel density based on stereotactic biopsies. AJNR Am J Neuroradiol. 2008;29:476–482. doi: 10.3174/ajnr.A0851. . PMid:18079184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HS, Jahng GH, Ryu CW, Kim SY. Added value and diagnostic performance of intratumoral susceptibility signals in the differential diagnosis of solitary enhancing brain lesions: preliminary study. AJNR Am J Neuroradiol. 2009;30:1574–1579. doi: 10.3174/ajnr.A1635. . PMid:19461062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toh CH, Wei KC, Chang CN, et al. Differentiation of pyogenic brain abscesses from necrotic glioblastomas with use of susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2012;33:1534–1538. doi: 10.3174/ajnr.A2986. . PMid:22422181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stupp R, Pica A, Mirimanoff RO, Michielin O. [A practical guide for the management of gliomas] Bull Cancer. 2007;94:817–822. PMid:17878102. [PubMed] [Google Scholar]
- 40.Weber MA, Henze M, Tüttenberg J, et al. Biopsy targeting gliomas do functional imaging techniques identify similar target areas? Invest Radiol. 2010;45:755–768. doi: 10.1097/RLI.0b013e3181ec9db0. . PMid:20829706. [DOI] [PubMed] [Google Scholar]
- 41.Bagley LJ, Grossman RI, Judy KD, et al. Gliomas: correlation of magnetic susceptibility artifact with histologic grade. Radiol. 1997;202:511–516. doi: 10.1148/radiology.202.2.9015082. [DOI] [PubMed] [Google Scholar]
- 42.Hori M, Mori H, Aoki S, et al. Three-dimensional susceptibility-weighted imaging at 3 T using various image analysis methods in the estimation of grading intracranial gliomas. Magn Reson Imaging. 2010;28:594–598. doi: 10.1016/j.mri.2010.01.002. . PMid:20233645. [DOI] [PubMed] [Google Scholar]
- 43.Pinker K, Noebauer-Huhmann IM, Stavrou I, et al. High-resolution contrast-enhanced, susceptibility-weighted MR imaging at 3T in patients with brain tumors: correlation with positron-emission tomography and histopathologic findings. AJNR Am J Neuroradiol. 2007;28:1280–1286. doi: 10.3174/ajnr.A0540. . PMid:17698528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park MJ, Kim HS, Jahng GH, Ryu CW, Park SM, Kim SY. Semiquantitative assessment of intratumoral susceptibility signals using non-contrast-enhanced high-field high-resolution susceptibility-weighted imaging in patients with gliomas: comparison with MR perfusion imaging. AJNR Am J Neuroradiol. 2009;30:1402–1408. doi: 10.3174/ajnr.A1593. . PMid:19369602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9:53–60. doi: 10.1002/(SICI)1522-2586(199901)9:1<53::AID-JMRI7>3.0.CO;2-2. . PMid:10030650. [DOI] [PubMed] [Google Scholar]
- 46.Castillo M, Smith JK, Kwock L, Wilber K. Apparent diffusion coefficients in the evaluation of high-grade cerebral gliomas. AJNR Am J Neuroradiol. 2001;22:60–64. PMid:11158889. [PMC free article] [PubMed] [Google Scholar]
- 47.Rizzo L, Crasto SG, Moruno PG, et al. Role of diffusion- and perfusion-weighted MR imaging for brain tumour characterisation. Radiol Med. 2009;114:645–659. doi: 10.1007/s11547-009-0401-y. . PMid:19430732. [DOI] [PubMed] [Google Scholar]
- 48.Negendank WG, Sauter R, Brown TR, et al. Proton magnetic resonance spectroscopy in patients with glial tumors: a multicenter study. J Neurosurg. 1996;84:449–458. doi: 10.3171/jns.1996.84.3.0449. PMid:8609557. [DOI] [PubMed] [Google Scholar]
- 49.Hourani R, Brant LJ, Rizk T, Weingart JD, Barker PB, Horská A. Can proton MR spectroscopic and perfusion imaging differentiate between neoplastic and nonneoplastic brain lesions in adults? AJNR Am J Neuroradiol. 2008;29:366–372. doi: 10.3174/ajnr.A0810. . PMid:18055564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Möller-Hartmann W, Herminghaus S, Krings T, et al. Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology. 2002;44:371–381. doi: 10.1007/s00234-001-0760-0. [DOI] [PubMed] [Google Scholar]
- 51.Chawla S, Wang S, Wolf RL, et al. Arterial spin-labeling and MR spectroscopy in the differentiation of gliomas. AJNR Am J Neuroradiol. 2007;28:1683–1689. doi: 10.3174/ajnr.A0673. . PMid:17893221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim JH, Chang KH, Na DG, et al. 3T 1H-MR spectroscopy in grading of cerebral gliomas: comparison of short and intermediate echo time sequence. AJNR Am J Neuroradiol. 2006;27:1412–1418. PMid:16908549. [PMC free article] [PubMed] [Google Scholar]
- 53.Fayed N, Dávila J, Medrano J, Olmos S. Malignancy assessment of brain tumours with magnetic resonance spectroscopy and dynamic susceptibility contrast MRI. Eur J Radiol. 2008;67:427–433. doi: 10.1016/j.ejrad.2008.02.039. . PMid:18442889. [DOI] [PubMed] [Google Scholar]
- 54.Hu LS, Eschbacher JM, Dueck AC, et al. Correlations between perfusion MR imaging cerebral blood volume, microvessel quantification, and clinical outcome using stereotactic analysis in recurrent high-grade glioma. AJNR Am J Neuroradiol. 2012;33:69–76. doi: 10.3174/ajnr.A2743. . PMid:22095961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu CH, Fang XM, Hu XY, Cui L. Analysis of the mismatched manifestation between rCBF and rCBV maps in cerebral astrocytomas. Clin Imaging. 2009;33:417–423. doi: 10.1016/j.clinimag.2009.01.014. . PMid:19857800. [DOI] [PubMed] [Google Scholar]