Abstract
The existence of adult β-cell progenitors remains the most controversial developmental biology topic in diabetes research. It has been reported that β-cell progenitors can be activated by ductal ligation–induced injury of adult mouse pancreas and apparently act in a cell-autonomous manner to double the functional β-cell mass within a week by differentiation and proliferation. Here, we demonstrate that pancreatic duct ligation (PDL) does not activate progenitors to contribute to β-cell mass expansion. Rather, PDL stimulates massive pancreatic injury, which alters pancreatic composition and thus complicates accurate measurement of β-cell content via traditional morphometry methodologies that superficially sample the pancreas. To overcome this potential bias, we quantified β-cells from the entire pancreas and observed that β-cell mass and insulin content are totally unchanged by PDL-induced injury. Lineage-tracing studies using sequential administration of thymidine analogs, rat insulin 2 promoter–driven cre-lox, and low-frequency ubiquitous cre-lox reveal that PDL does not convert progenitors to the β-cell lineage. Thus, we conclude that β-cells are not generated in injured adult mouse pancreas.
Controversy about the origin of adult β-cells has engaged scientists for more than 100 years (1–5). Several mechanisms have been invoked to explain adult β-cell mass expansion, including neogenesis from pancreatic ducts or hematopoietic tissues, replication of specialized β-cell progenitors, and self-renewal by β-cells. Studies now indicate that normal β-cell growth in mice primarily occurs by self-renewal of mature β-cells—not by replication of specialized progenitors (6–8).
A recent study powerfully challenged prevailing consensus regarding the origins of new β-cells and described how β-cells are abundantly generated from endogenous progenitors in injured adult mouse pancreas (9). The authors used PDL to induce pancreatic injury, which resulted in acinar cell death and ductal proliferation. β-Cell mass doubled within a week, with an associated 10-fold increase in β-cell proliferation. PDL also induced neurogenin 3 (Ngn3) expression. The study has been heralded as providing convincing evidence for multipotent endocrine progenitors in adult pancreas (10–12). But subsequent studies indicate that ductal-derived progenitors do not contribute to the doubling of β-cell mass after pancreatic injury, leaving open the question as to where the PDL-induced newly generated β-cells come from if not ducts (2,13–16).
We reexamined β-cell neogenesis after PDL, reasoning that quantitative imaging and lineage tracing would reveal the source and amount of new β-cells. As expected, PDL-induced injury stimulates massive acinar death and ductal proliferation. Surprisingly, β-cell mass and insulin content is unaltered by PDL. Moreover, β-cell proliferation is not increased by PDL. Using sequential labeling with thymidine analogs, cre-lox lineage tracing driven by the insulin promoter, or low-frequency ubiquitous cre-lox lineage tracing, we found that progenitors do not contribute to the β-cell lineage in response to PDL. Therefore, β-cells are not generated in PDL-injured adult mouse pancreas.
RESEARCH DESIGN AND METHODS
Experiments were performed according to the Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee. Male F1 hybrid B6129SF1/J and BALB/cByJ mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The Jackson Laboratory Rosa YFP mice [B6.129 × 1-Gt(Rosa)26Sortm(EYFP)Cos/J] were crossed with Ins2-CreERT (6) (a gift from D. Melton, Harvard University and Howard Hughes Medical Institute, via B. Stanger, University of Pennsylvania Perelman School of Medicine) and UBC-CreERT2 mice (17) (a gift from E. Brown, University of Pennsylvania Perelman School of Medicine). Glucose homeostasis, histomorphometry, and gene expression were performed as previously described (7). Ins2-CreERT mice were orally treated with tamoxifen or vehicle for 5 days (8 mg/day).
PDL was performed as previously described (9,13,18,19). The splenic segment of the pancreatic duct was ligated immediately distal to the confluence of the splenic and common bile duct. Partial pancreatectomy performed was as previously described (7).
RT-PCR.
Pancreas head and tail were dissected, weighed, flash frozen, and pulverized with a liquid nitrogen–cooled mortar and pestle. RNA extraction and cDNA synthesis were performed as previously described (20).
Total pancreatic insulin content.
Pancreata were placed in acid-ethanol solution, incubated overnight at −20°C, homogenized with a PowerGen 125 tissue homogenizer (category no. FTH-115; Fisher Scientific), incubated overnight at −20°C, and centrifuged at 2,000 rpm for 15 min at 40°C. Supernatant was neutralized with 1:1 1 mol/L Tris pH 7.5, diluted ×1 PBS containing 0.25% BSA (1:100, 500, 1000, 5,000, 10,000, and 15,000), and measured in triplicate using a mouse insulin RIA kit (Linco Research). Samples were normalized (i.e., multiplied by the dilution factor) and reported as micrograms for the total pancreatic organ. No other normalization was performed for other factors (e.g., protein input), as such factors might be differentially present within injured pancreas.
Thymidine analog lineage tracing.
We performed sham or PDL operations in mice followed by continuous labeling via drinking water, with CldU for the first half and then IdU (both 1 mg/mL) as previously described (7).
Statistics.
Results are reported as means ± SEM, compared with independent t tests (unpaired and two-tailed), and reported as P values.
RESULTS
PDL injures pancreas in a stereotypic manner.
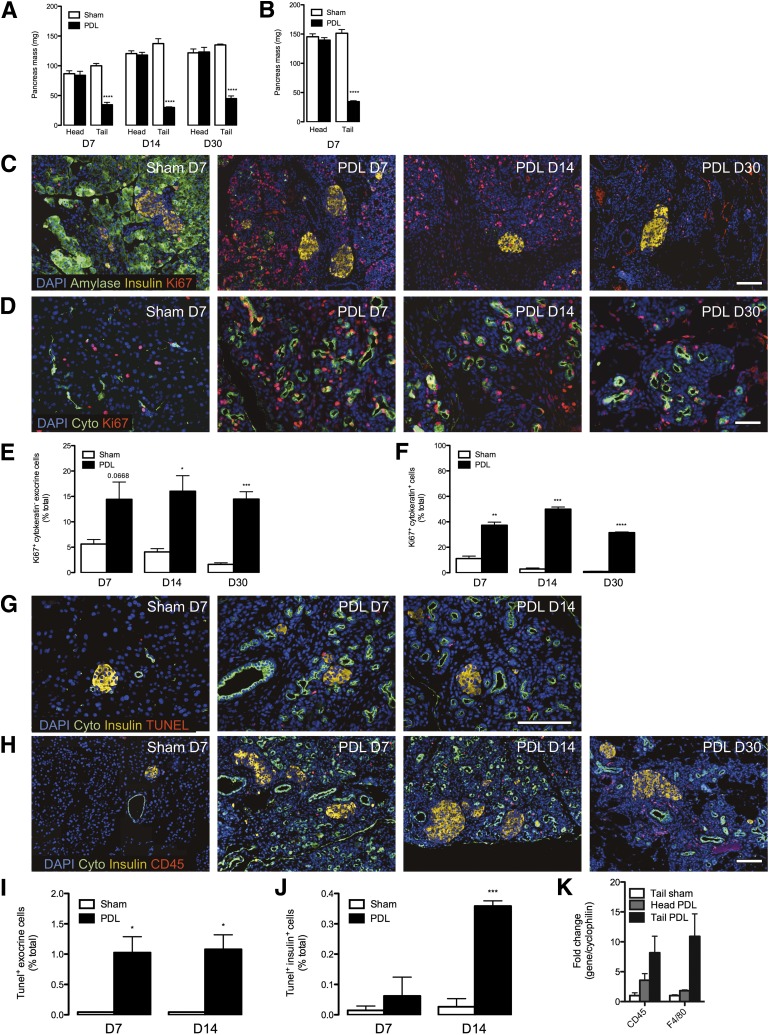
PDL has been performed by many groups using a standard protocol without reported variation in acinar cell atrophy or ductal proliferative response (1,9,13–16,18,19,21–36). We performed PDL on mixed genetic background and inbred mice (Supplementary Fig. 1A). PDL was well tolerated, with unaltered body weight and glycemia (Supplementary Fig. 1B–E and H–J and Supplementary Tables 1–4). PDL resulted in atrophy of the tail of the pancreas, leaving the head unaffected (Fig. 1A and B). PDL-injured pancreas had reduced amylase expression but had numerous small ducts visible (Fig. 1C and D and Supplementary Fig. 2A and B).
FIG. 1.
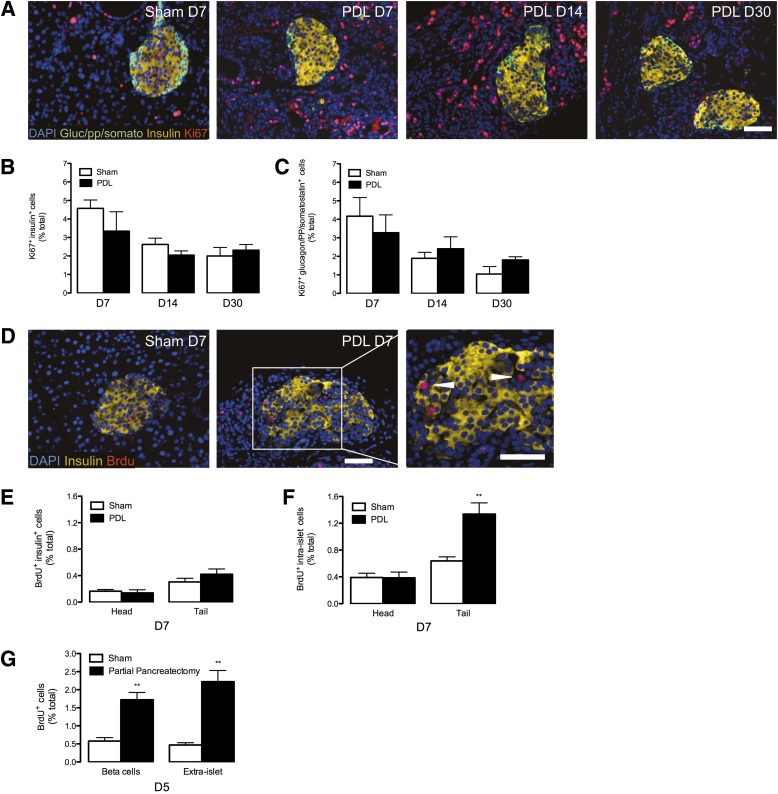
Ligation of the main pancreatic duct induces stereotypic reorganization throughout the tail of the pancreas, with acinar apoptosis and ductal proliferation. The splenic branch of the pancreatic duct was ligated in 6- to 7-week-old mixed genetic background mice (F1 hybrid B6129SF1/J) and in 8-week-old Balb/c mice, leaving the head of the pancreas intact. A and B: Mass of the pancreatic tail is severely reduced by ductal ligation. Quantitative analysis of pancreas mass in mixed genetic background at 7, 14, and 30 days (A) and in Balb/c mice at 7 days (B). Data are means ± SEM; 3–5 animals per group. C: Pancreatic acinar tissue is replaced by highly proliferative cells after PDL injury. Images from 7-day sham-operated (control) and PDL tail pancreas. DAPI (blue), amylase (green), insulin (yellow), and ki67 (red). D: PDL induces highly proliferative duct cells. DAPI (blue), pancytokeratin (green), and ki67 (red). E: Quantitative analysis of extraislet nonduct proliferation (ki67+ cytokeratin− exocrine cells) from sham-operated and PDL tail pancreas. F: Quantitative analysis of ductal proliferation (ki67+ pancytokeratin+ cells) from sham-operated and PDL tail pancreas. G: PDL induces massive death of cells within the exocrine pancreas. Images from sham-operated (control) and PDL tail pancreas. DAPI (blue), pancytokeratin (green), insulin (yellow), and TUNEL (red). H: PDL recruits lymphocytes (CD45+) to the pancreas. Images from sham-operated (control) and PDL tail pancreas. DAPI (blue), pancytokeratin (green), insulin (yellow), and CD45 (red). I: Quantitative analysis of extraislet death (TUNEL+ cells) from sham-operated and PDL tail pancreas. J: Quantitative analysis of intraislet death (TUNEL+ insulin+ cells) from sham-operated and PDL tail pancreas. K: PDL recruits cells with markers of lymphocytes (CD45+ cells) and macrophages (F4/80+ cells) to the pancreas. Gene expression analysis of total RNA from sham-operated and PDL pancreas at day 7. Scale bars: 100 μm. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ligated vs. unligated pancreas tail. D, day.
PDL induces characteristic changes in pancreatic histology.
Ki67+ cells were vastly increased by PDL in tail pancreata at 7, 14, or 30 days compared with controls (Fig. 1C–F). PDL induced ductal proliferation (Fig. 1D and F), and acinar cells had reduced amylase expression and extensive apoptosis, with increased TUNEL+ cells (Fig. 1C, G, and I). TUNEL+ cells were also increased in islets, indicating that PDL-induced injury extended to islets (Fig. 1J). Injury was associated with CD45+ lymphocytic cells (a pan-lymphocyte marker) (Fig. 1H). CD45 and F4/80 mRNAs were induced, indicating lymphocytes and macrophages, respectively (Fig. 1K).
Ngn3 expression is induced in PDL-injured pancreas.
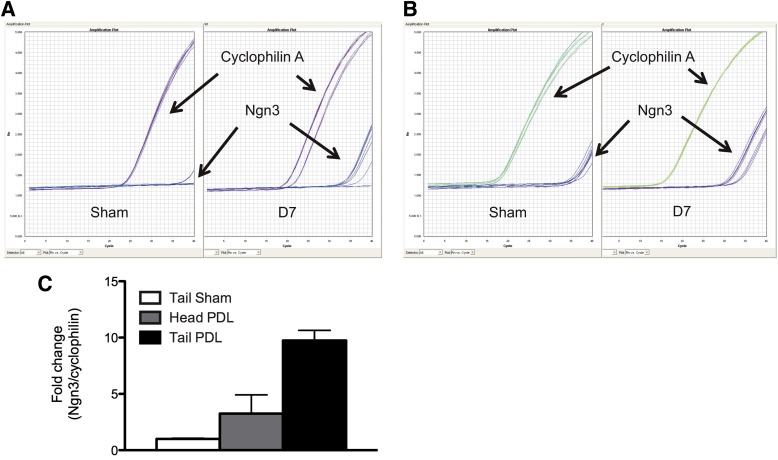
We quantified Ngn3 mRNA in pancreas using flash freezing to limit autodigestion. Ngn3 mRNA was readily detected in PDL-injured pancreas, with threshold amplification cycles (Ct) from ∼33 to 36 (Fig. 2A). In contrast, Ngn3 was only occasionally detected in controls (Fig. 2A). These results could imply that Ngn3 was massively induced by PDL (more than one million–fold). However, many control Ngn3 Ct values were 39 or 40 (Fig. 2A) and beyond the reliable detection limit of the quantitative PCR assay. Consequently, we repeated our studies with much more mRNA input (a whole mouse pancreas divided up into just a few quantitative PCRs) (see research design and methods). As expected, increased pancreatic cDNA resulted in much earlier Ct detection of Ngn3 in PDL samples (Ct 29–33) (Fig. 2B). We also consistently detected Ngn3 mRNA in controls (Ct 33–36) (Fig. 2B). Other endocrine mRNAs were readily detected, such as insulin, glucagon, GLUT2, and prohormone convertase 2 (PCSK2) (Supplementary Fig. 3). In summary, our studies confirm that Ngn3 is powerfully induced by PDL (Fig. 2C) and suggest that Ngn3 mRNA is also present in uninjured mouse pancreas as previously described (9,16,37,38).
FIG. 2.
Ngn3 expression is induced in PDL-injured pancreas. A and B: Concentrated cDNA samples are necessary to accurately and consistently amplify Ngn3 transcript in sham pancreas samples using quantitative real-time PCR. A: Representative quantitative PCR curves from samples with traditional (smaller) quantities of pancreatic cDNA input from PDL samples (right panel) or sham-operated control tail samples (left panel) to amplify Ngn3 or cyclophilin A. B: Representative quantitative PCR curves from samples with revised (larger) quantities of pancreatic cDNA input from PDL samples (right panel) or sham-operated control tail samples (left panel) to amplify Ngn3 or cyclophilin A. C: Gene expression analysis of total RNA from sham or PDL pancreas at day (D) 7. Mean ± SEM; 3 animals per group.
PDL does not generate new β-cells.
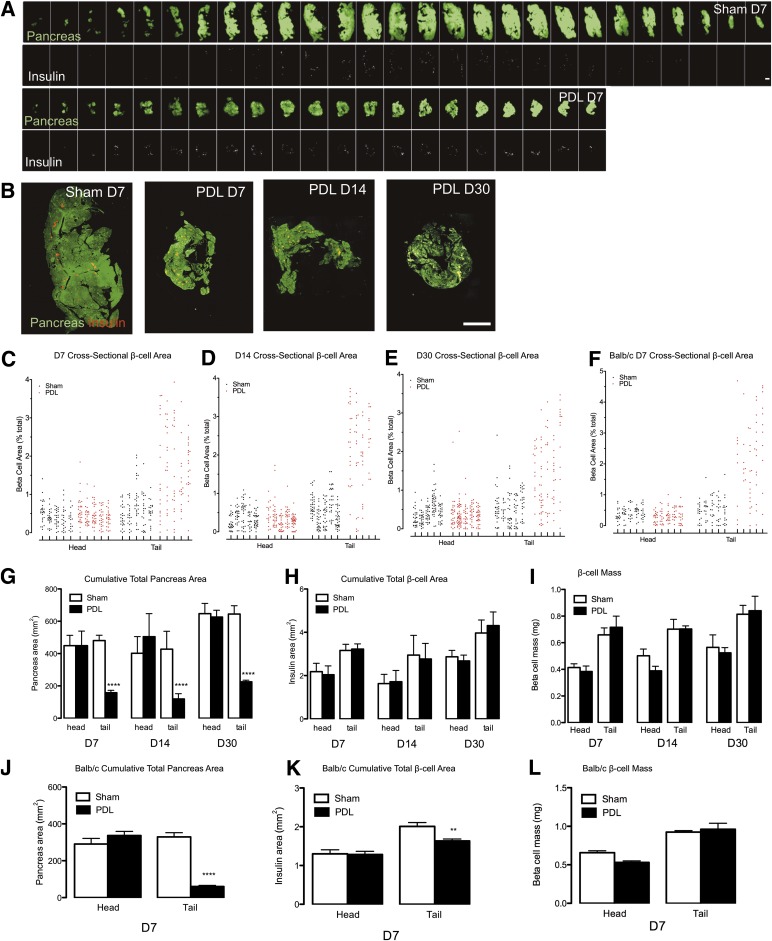
To directly test whether β-cells are generated by PDL, we quantified β-cells by morphometric analysis. Traditionally, only a small percentage of the total depth of the pancreas is analyzed for mass. PDL-injured pancreata are thinner (∼2 mm) than control pancreata (∼4 mm). Superficial depth sampling of uninjured pancreas could bias measurements by preferentially sampling islets on the periphery (where there are few islets). In contrast, superficial sampling in PDL-injured pancreas would yield a high density of islets. To prevent bias, we quantified β-cells from the entire pancreas in both sham and PDL, cutting 5-μm tissue sections every 95 µm. This strategy resulted in a large number of sections (30–40 from sham and 15–25 from PDL) (Fig. 3A and B, Supplementary Fig. 4A–C, and Supplementary Tables 1–4). We also analyzed β-cell area in Balb/c mice 7 days after PDL with sections every 160 μm. β-Cell area relative to pancreas area was increased in PDL tail compared with controls (Fig. 3C–F and Supplementary Fig. 5A and B). The cumulative total pancreas area was reduced by ∼70% (Fig. 3G and J), equivalent to the ∼70% reduction in pancreatic mass after PDL (Fig. 1A and B). Because we measured β-cell density through the entire thickness of the pancreas, we were able to compare cumulative total β-cell area of PDL samples to control pancreas. Surprisingly, the cumulative total β-cell area was not altered by PDL. Similarly, β-cell mass was totally unchanged by PDL (Fig. 3H and I). Cumulative total sectional β-cell area was slightly decreased in the tail pancreata of Balb/c mice 7 days after PDL. However, β-cell mass was not altered by PDL compared with control (Fig. 3K and L). Islets were slightly smaller in Balb/c mice 7 days after PDL (Supplementary Fig. 5C), perhaps as a consequence of their distorted morphology. In summary, PDL did not increase total cross-sectional β-cell area or β-cell mass.
FIG. 3.
β-Cells are not generated in PDL-injured pancreas. Sham operation or PDL was performed in mixed genetic background mice after they were killed at 7, 14, or 30 days (A–E and G–I). Five-micrometer sections were obtained from pancreata every 95 μm followed by detection of β-cell and total pancreas area. Total pancreas was detected with amylase antisera and autofluorescence. Equivalent studies were also performed in Balb/c mice after they were killed at 7 days, with sections obtained every 160 μm (F and J–L). A: Image sequences through the entire pancreas of sham-operated and PDL tail samples. Pancreas (green), insulin (white). B: Images from sham-operated and PDL tail at 7, 14, or 30 days. Pancreas (green), insulin (red). C–F: Morphometric analysis of cross-sectional β-cell area at day 7 (C and F), 14 (D), or 30 (E). β-Cell area for each longitudinal pancreas section is plotted as points within a vertical column representing individual mice, expressed as % total pancreas area per section. G–L: Quantitative analysis of cumulative total pancreas area, expressed as micrometers squared. G and J: PDL results in a drastic reduction in total cross-sectional pancreas area. H and K: Cumulative total β-cell area is unchanged by PDL, expressed as micrometers squared. I and L: β-Cell mass is unchanged by PDL, expressed as milligrams. Data are means ± SEM; 4–5 animals per group. Scale bars: 2 mm. **P < 0.01, ****P < 0.0001, ligated vs. unligated pancreas tail. D, day.
PDL has minimal effects on pancreatic insulin content.
We also tested whether pancreatic insulin content is increased by PDL. It was previously reported that insulin content was doubled by 14 days after PDL (9). However, insulin content was only increased by a tiny amount (∼20%) in tail PDL pancreas at 7 and 14 days and unchanged at 30 days (Supplementary Fig. 6). Similarly, insulin content was unaltered in control head PDL and sham pancreata (Supplementary Fig. 6).
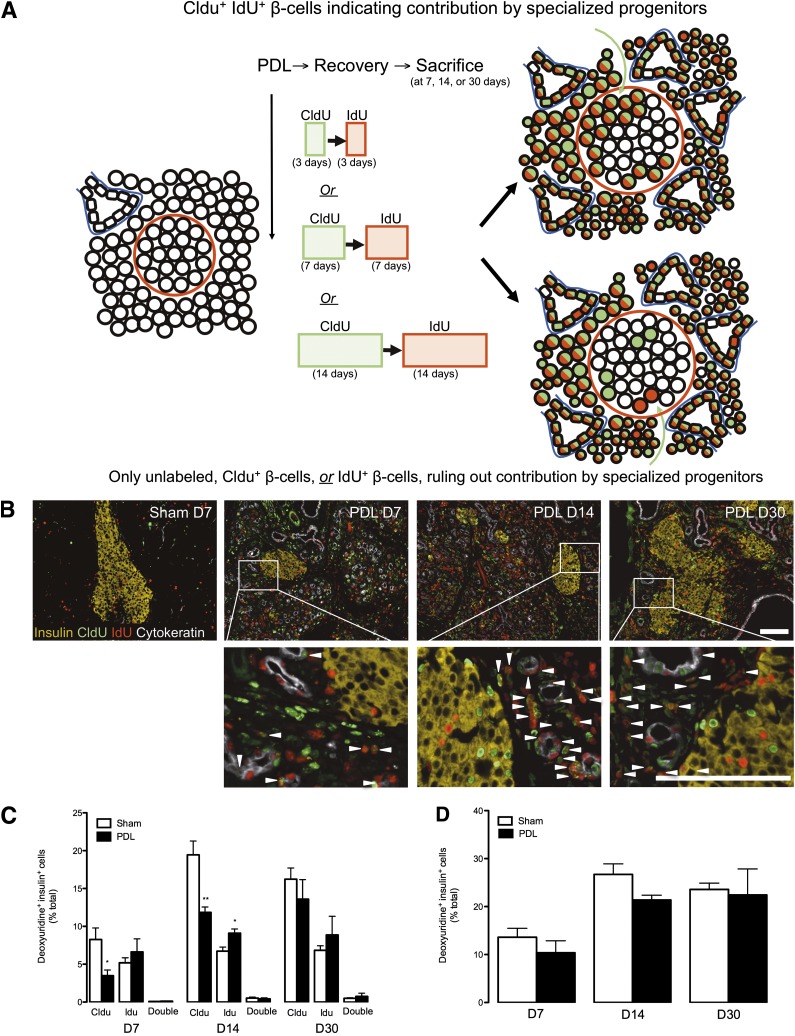
PDL induces serial proliferation in duct cells but not in β-cells or their adult progenitors.
We performed studies to define contribution to the β-cell lineage by serial proliferating cells (those that divide multiple times with a given period of study) after PDL. We used sequential administration of two different halogen-substituted thymidine analogs. This strategy can detect contribution of highly proliferative progenitors to a tissue of interest, thus inferring contribution by a lineage mechanism that includes transit-amplifying cells and possibly stem cells (7,39) (see Fig. 4A for a schematic). We carried out PDL or sham followed by continuous labeling with CldU for 3 days and then IdU for 3 days in the drinking water (mice killed at day 7) (Fig. 4A). Similar studies were performed with CldU labeling for 7 days and IdU for 7 days (killed at day 14) or CldU labeling for 14 days and IdU for 14 days (killed at day 30). As expected, PDL resulted in islets with irregular borders (Fig. 4B and Supplementary Fig. 7A). PDL-induced duct-like cells (pancytokeratin+) were highly proliferative and frequently CldU+IdU+ copositive (Fig. 4B and Supplementary Fig. 4A). PDL also induced serial proliferation of other unidentified small cells in pancreatic parenchyma (Fig. 4B and Supplementary Fig. 7A). β-Cells were almost never CldU+/IdU+ copositive in PDL-injured pancreata (Fig. 4B and C and Supplementary Fig. 7A). Moreover, total β-cell proliferation was not altered at any time point after PDL-induced pancreatic injury despite continuous labeling with thymidine analogs (Fig. 4D). Thus, the PDL-stimulated, highly proliferative duct cells do not appear to enter the β-cell lineage in substantial quantities.
FIG. 4.
PDL induces serial proliferation of cells in duct cells but not in β-cells or their adult progenitors. A: Labeling scheme used to assess the replicative origin of β-cells after PDL. PDL or sham operation was performed in 6-week-old mixed genetic background mice, followed by sequential labeling with CldU and then IdU before they were killed. B: Sequential proliferation within ductal structures but not islets after PDL. Images from tail pancreas from sham-operated and PDL pancreas at 7, 14, or 30 days (D). Insulin (yellow), CldU (green), IdU (red), and pancytokeratin (white). Arrow heads indicate CldU+ IdU+ copositive cells. Scale bars: 100 μm. C: Quantitative analysis of β-cell proliferation after thymidine analog labeling, as measured by insulin+ cells that contained CldU+, IdU+, or both. Data are means ± SEM; 4–5 animals per group. D: Unaltered cumulative β-cell proliferation during the weeks after PDL, as measured by thymidine analog incorporation (CldU+ or IdU+) within insulin+ cells. *P < 0.05, **P < 0.01, ligated vs. unligated pancreas tail.
PDL does not increase β-cell proliferation.
To verify our observation of unchanged thymidine incorporation after PDL, we quantified β-cell proliferation with ki67 staining. ki67+ insulin+ cells were not increased after PDL (Fig. 5A and B and Supplementary Tables 1–3). Moreover, proliferation of non-β-cell islet endocrine cells was unchanged by PDL (Fig. 5A and C and Supplementary Tables 1–3). We further quantified β-cell proliferation within our other cohort (Balb/c) with BrdU injected 1 h before the mice were killed. BrdU+ insulin+ cells were unchanged by PDL in Balb/c mice on recovery day 7 (Fig. 5D and E and Supplementary Table 4). To verify that the Balb/c mice were capable of responding to β-cell regenerative stimuli, we also carried out parallel pancreatic injury studies with partial pancreatectomy. As expected, BrdU+ insulin+ cells and extra islet BrdU+ cells were increased threefold by partial pancreatectomy (Fig. 5G). Thus, β-cell proliferation was unchanged by PDL but powerfully stimulated by partial pancreatectomy. We also quantified total intraislet proliferation defined as any DAPI+ cells within the capsule of the islet. This could theoretically include endothelial, epithelial, endocrine, neural, and glial cells. In the short-term BrdU labeling experiment, PDL increased the quantity of intraislet BrdU+ cells in the tail pancreata compared with controls (Fig. 5F). However, in 14-day CldU/IdU-labeling studies, PDL increased intraislet IdU+ cells (administered days 7–14) but not intraislet CldU+ cells (administered days 1–7) in the injured tail pancreas compared with control (Supplementary Fig. 7B–E). While the BrdU experiment shows that there are a greater number of proliferating intraislet cells on recovery day 7, the CldU and IdU experiment further reveals that intraislet cell replication/turnover in response to PDL is variable during the recovery period after injury. Taken together, these studies indicate that PDL does not increase the proliferation of β-cells or other islet endocrine cells.
FIG. 5.
PDL does not stimulate β-cell proliferation. A: PDL stimulates proliferation of cells within the duct cells but not β-cells. Images from tail pancreas from sham-operated (control) and PDL pancreas at 7, 14, or 30 days. Insulin (yellow), glucagon (Gluc)/pancreatic polypeptide (pp)/somatostatin (somato) (green), ki67 (red), and DAPI (blue). B and C: Quantitative analysis of islet proliferation in sham and ligated pancreas tail. Data are means ± SEM; 5 animals per group. B: Unaltered β-cell proliferation (insulin+ ki67+) after PDL. C: Unaltered proliferation of other islet endocrine cells (glucagon/pancreatic polypeptide/somatostatin+ ki67+). D–F: PDL increases intraislet proliferation without altering β-cell proliferation in the Balb/c cohort. D: Images from tail pancreas from sham-operated (control) and PDL pancreas at 7 days (D). DAPI (blue), insulin (yellow), BrdU (red). Triangles indicate BrdU+ cells that do not express insulin. E: Quantitative analysis of β-cell proliferation (insulin+ BrdU+). F: Quantitative analysis of total intraislet BrdU+ proliferation. Data are means ± SEM; 5 animals per group. Scale bars: 50 μm. **P < 0.01, ligated vs. unligated pancreas tail. G: Partial pancreatectomy stimulates β-cell proliferation in the Balb/c cohort. Quantitative analysis of proliferation in β-cells (insulin+ BrdU+) and extraislet cells. Data are means ± SEM; 5 animals per group.
PDL does not convert non-β-cells to β-cells.
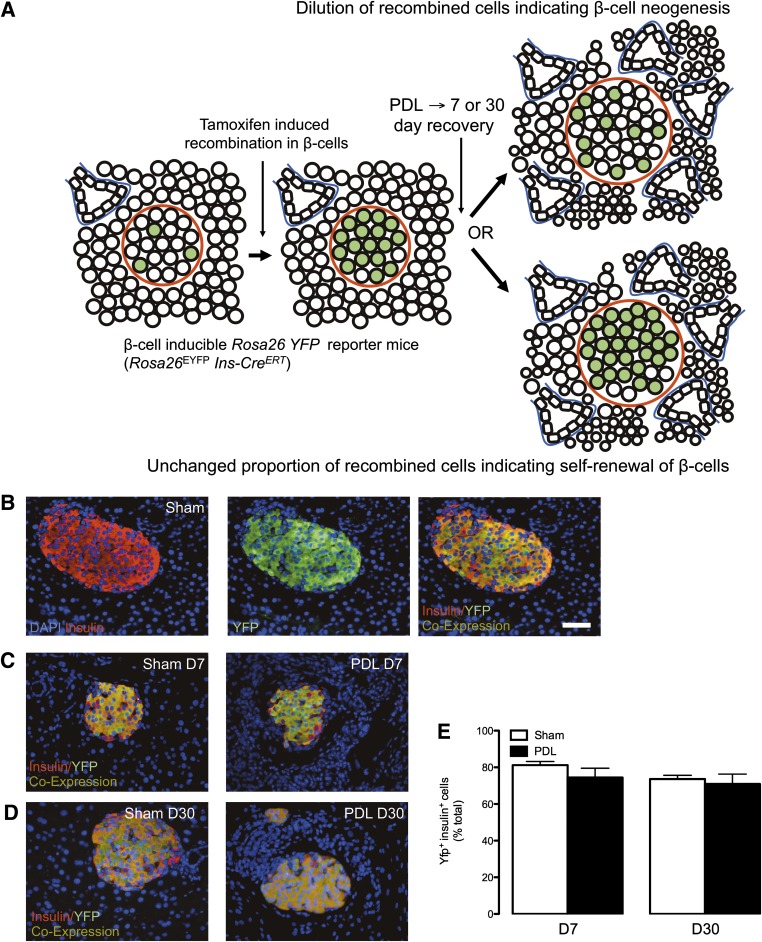
To further investigate the lineage of β-cells after PDL-induced pancreatic injury, we carried out insulin promoter–based lineage tracing. This strategy uses the insulin promoter to drive a tamoxifen-inducible cre (CreERT) in a loxP reporter mouse; tamoxifen induction of cre permanently marks adult β-cells and their progeny with yellow fluorescent protein (YFP) expression (Fig. 6A). Contribution by non–insulin-containing progenitors to the adult β-cell lineage would be indicated by an increase in β-cells that are not marked by tamoxifen-induced YFP expression. PDL-induced β-cell neogenesis would result in reduced proportions of tamoxifen-marked β-cells. We derived insulin 2 CreERT ROSA26 lox-stop–lox YFP reporter mice (Ins2-CreERT ROSA reporter mice) (6,40) and induced recombination with tamoxifen at 5–6 weeks of age, followed by PDL at 8–9 weeks of age; mice were killed 7 or 30 days thereafter (Fig. 6A). We achieved very high efficiency baseline recombination in β-cells with the Ins2-CreERT ROSA reporter mice (Fig. 6B and E). Nevertheless, tamoxifen-induced recombination rates were unchanged by PDL-induced injury at both 7 and 30 days (Fig. 6C–E). Thus, unmarked β-cell progenitors did not dilute the adult β-cell lineage after PDL. This surprising result demonstrates that non–insulin-containing progenitors do not significantly contribute to the adult β-cell lineage after PDL-induced pancreatic injury.
FIG. 6.
PDL does not convert non-β-cells to the β-cell lineage. A: Genetic lineage tracing scheme used to assess the cellular origin of β-cells after PDL. B: Highly efficient induction of recombination within β-cells after tamoxifen treatment in control mice. C and D: Images of tail pancreas from tamoxifen-treated sham-operated control mice. DAPI (blue), insulin (red), YFP (green). Unchanged proportion of recombined β-cells after PDL at 7 days (C) and 30 days (D). E: Quantitative analysis of β-cell recombination. Data are means ± SEM; 4–5 animals per group. Scale bars: 50 μm. D, day.
While characterizing Ins2-CreERT ROSA reporter mice, we noticed some YFP+ β-cells in untreated mice, indicating that β-cells undergo spontaneous recombination in the absence of tamoxifen (Supplementary Fig. 8A and C). Ongoing spontaneous recombination could complicate interpretation of lineage-tracing studies in Ins2-CreERT ROSA reporter mice. Consequently, we measured YFP+ β-cells in untreated mice as a function of time. Spontaneous recombination progressed in an extremely slow and predictable manner with age (Supplementary Fig. 8A). Moreover, YFP+ β-cells in control pancreata were present in the same proportions over 30 days, which strongly suggests that spontaneous recombination did not significantly complicate our studies (Supplementary Fig. 8D–F).
The presence of such spontaneous recombinants presented an opportunity to further test the lineage mechanism of β-cells within PDL-injured pancreas without the use of tamoxifen. We analyzed recombination rates in non–tamoxifen-treated PDL and control cohorts (Supplementary Fig. 8B). PDL did not alter the proportion of YFP+ β-cells after 7 days (Supplementary Fig. 8D–F). Similarly, PDL did not alter the proportion of YFP+ β-cells in non–tamoxifen-treated mice after 30 days (Supplementary Fig. 8E and F). Notably, β-cell proliferation was unaltered by tamoxifen treatment in both PDL and sham mice (Supplementary Fig. 8G). These results further support the conclusion that PDL does not convert non-β-cells to contribute to the β-cell lineage.
PDL does not convert multipotent progenitors to β-cells.
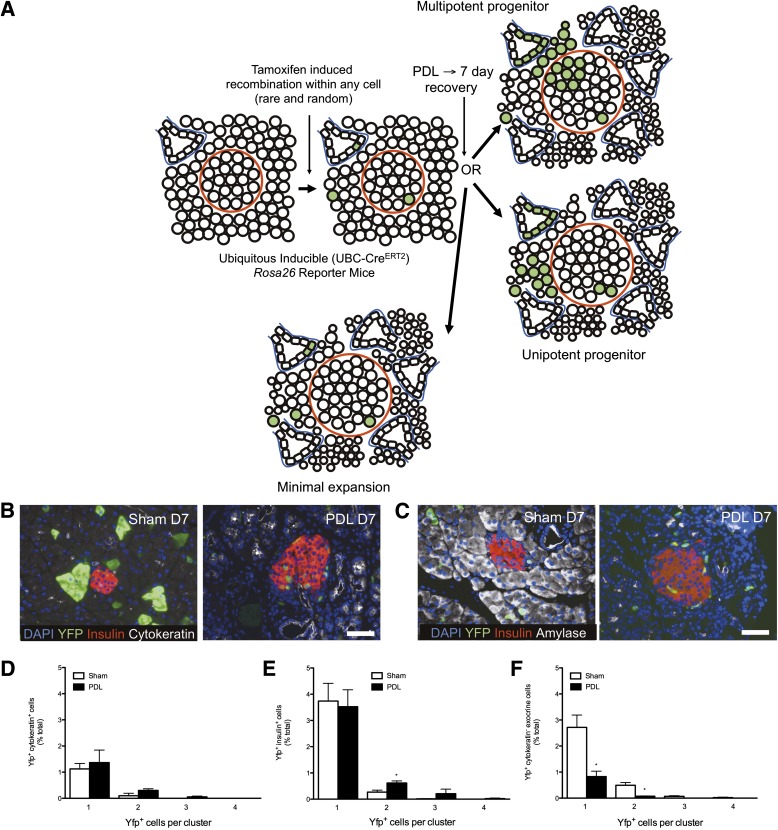
We tested whether PDL-induced pancreatic injury involved clonal, multipotent pancreatic progenitors. We induced YFP expression within a small percentage of all pancreatic cell types and quantified clonal expansion after PDL or sham in various types of cells. We envisioned three possible outcomes (schematic in Fig. 7A): β-Cell neogenesis might lead to clonal expansion across multiple cell types, with YFP-expressing cells directly extending from duct cells in the pancreas to islets. Alternatively, clonal expansion could only be detected within expanding populations of single cell types. Finally, solitary recombined cells might be observed, implying minimal expansion. We used a ubiquitously expressed promoter to drive low-frequency inducible cre-lox recombination. We crossed reporter mice with transgenic mice with the human ubiquitin C promoter (UBC) driving CreERT2 (17). The resulting UBC CreERT2 ROSA26 lox-stop–lox YFP mice (UBC-CreERT2 ROSA reporter mice) exhibited dose-dependent recombination without any leakiness in a variety of pancreatic tissues including β-cells, α-cells, and ductal and acinar cells with varying rates of recombination (Supplementary Fig. 9). We treated mice with a single small dose of tamoxifen (0.015 g/kg) followed by PDL or sham (mice killed at 7 days). Surprisingly, recombined cells were mostly solitary in PDL, with rare two-cell clusters and even fewer three-cell clusters (Fig. 7B–F). YFP clusters within duct-like cells (cytokeratin+) or other extraislet nonduct cells (insulin− and cytokeratin−) never extended into the β-cell lineage after PDL, and two-cell clusters were never comprised of more than one cell type. In summary, PDL does not induce rapid clonal expansion of individually labeled β-, α-, ductal, or acinar cells or clonal expansion from a non-β-cell to β-cell lineage.
FIG. 7.
PDL does not convert multipotent progenitors to the β-cell lineage. A: Lineage-tracing scheme used to assess the cellular origin of β-cells after PDL. B and C: Tamoxifen-dependent recombination. Images of tail pancreas from mice treated with a single dose of tamoxifen (0.015 g/kg) followed by sham operation or PDL (mice killed at 7 days). B: DAPI (blue), YFP (green), insulin (red), pancytokeratin (white). C: DAPI (blue), YFP (green), insulin (red), amylase (white). D–F: Quantitative analysis of multicellular clones, assessed by the presence of ductal clusters of YFP+ pancytokeratin+ cells (D), β-cell clusters of YFP+ insulin+ cells (E), and pancreatic acinar cell clusters of YFP+ amylase+ cells (F). Scale bars: 50 μm. *P < 0.05, ligated vs. unligated pancreas tail. D, day.
DISCUSSION
PDL induces rapid loss of acinar cells and a strong inflammatory response, which causes a 70% reduction in tail pancreas mass. Ngn3 mRNA is more readily detected after PDL-induced injury, and PDL induces highly proliferative ductal and nonductal cells within the pancreatic parenchyma. However, substantial β-cells are not generated by PDL, β-cell proliferation is not increased by PDL, and the highly proliferative cells of the pancreatic parenchyma do not enter the β-cell lineage. Moreover, the β-cell lineage is not diluted by non-β-cell–derived progenitors after PDL, and multipotent progenitors do not enter the β-cell lineage.
Although confounding factors could conceivably reconcile our observation that β-cells are not generated after PDL with published results (9), none seem likely. For example, PDL might be theorized to produce variable pancreatic injury among different mouse strains or laboratories. However, we followed the PDL procedure as described by Xu et al. (9) and invariably achieved the expected exocrine phenotype: reduction of pancreatic mass by ∼70%, acinar cell death, ductal expansion, macrophage infiltration, and induction of Ngn3 expression. Our exocrine pancreas results also mirrored other reports (18,19,21–35). Thus, PDL is a highly robust procedure with a predictable phenotype, and our results were identical to the expected exocrine phenotype. Similarly, quantification of β-cell proliferation could be theorized to be inaccurate within injured pancreas. PDL causes pancreatitis, which could reduce oral intake and thus reduce thymidine analog consumption administered in drinking water. However, we found that β-cell thymidine analog incorporation was identical in the PDL and sham head pancreas, indicating that labeling efficiency is equivalent in PDL and sham mice. We also quantified proliferation with ki67, but ki67+ insulin+ cells and other islet endocrine cells were unchanged after PDL. Finally, we quantified β-cell proliferation by injecting mice with short-term BrdU, but BrdU+ insulin+ cells were unchanged by PDL. Importantly, duct cell proliferation was potently increased by PDL, consistent with the exocrine phenotype described by Xu et al. (9). Thus, our studies using multiple methods to detect pancreatic cell replication are internally consistent and highly accurate.
Inducible lineage-tracing studies could be complicated by tamoxifen persistence, as recently described (41). Tamoxifen persistence could cause increasing rates of recombination with time. However, recombination rates were the same in our 30-day shams compared with our 7-day shams. Moreover, we also performed studies on mice that had not received any tamoxifen. Thus, tamoxifen persistence did not complicate our studies. Insulin promoter–based lineage-tracing studies might fail to detect contribution by insulin+ progenitors. While insulin+ duct cells do exist in sham and PDL-injured pancreata, they are solitary and extremely rare. Given their paucity, insulin+ duct cells would undergo massive expansion through multiple rounds of replication to substantially increase β-cell mass. But CldU/IdU copositive β-cells were almost nonexistent at any time point after PDL. We further performed low-frequency lineage tracing. Thus, by multiple methods we find that PDL-induced proliferative duct cells do not enter the β-cell lineage. Quantitative tools to measure β-cells after PDL could theoretically be problematic. Insulin content could inaccurately assess β-cell content owing to variable insulin recovery from extracted pancreas. However, insulin content was very similar in our PDL and sham tail samples (increased by 20% after 7 days and unchanged at 30 days). Moreover, insulin content was also identical in head pancreas from PDL and sham, which serve as internal controls. Therefore, our insulin content measurements are internally consistent and highly robust. Similarly, β-cell mass measurements could be biased by injured pancreas. However, β-cell morphometry of the entire pancreas revealed β-cell mass to be unchanged in multiple cohorts. Similarly, β-cell mass in head pancreata was unchanged, indicating that β-cells were accurately measured. PDL responses could also be speculated to be intrinsically variable in different strains of mice. However, we carried out studies in several strains, including those used by Xu et al. (9).
To address the discordance between our results and those of Xu et al., we compared our PDL β-cell proliferation results with published values. Xu et al. (9) reported that PDL increased β-cell proliferation 10-fold. Notably, the control β-cell proliferation rates of Xu et al. were extremely low (e.g., day 7 sham tail ∼0.02 ± 0.01% per hour), which is 15-fold less than our controls in the same Balb/c strain (sham tail 0.30 ± 0.05% per hour). Importantly, the Xu et al. PDL tail β-cell proliferation rates perfectly matched our PDL tail results (∼0.36 ± 0.03% per hour vs. our values of 0.42 ± 0.07% per hour) (9). Wei and colleagues similarly reported β-cell proliferation rates from 1-month-old Balb/c mice (∼15.5% over 48 h or ∼0.32% per hour) (42). Similarly, Stoffers and colleagues reported total islet proliferation in 8- to 10-week-old Balb/c control mice and obtained results that are also close to ours (∼1.7% over 6 h or ∼0.28% per hour) (43). Thus, the ∼10-fold induction in PDL β-cell proliferation reported by Xu et al. can be largely explained by anomalously low β-cell proliferation in their controls (9).
Our study resolves a major outstanding controversy regarding putative β-cell generation after PDL-induced pancreatic injury (1–5). Such putative β-cell progenitors were assumed to be of ductal origin. However, recent lineage-tracing studies have excluded ducts as significant contributors to β-cell generation in injured pancreas (2,13–16,44,45). Therefore, our work reconciles competing theories by revealing that β-cells are not generated by PDL-induced pancreatic injury.
The notion that PDL stimulates β-cell mass expansion largely rests on studies by Heimberg, Bouwens, and colleagues (9,13,18,19). However, Jansson and colleagues reported that β-cell mass was unchanged 1 and 2 weeks post-PDL in rats, and only 50% increased by 4 weeks (34). Indeed, Bouwens and colleagues reported that pancreatic insulin content was unchanged by PDL in rats at 1 and 2 weeks (19). Nevertheless, the notion of PDL-induced doubling of β-cell mass has been ingrained in the field. Recent lineage-tracing studies have attempted to define a source for new β-cells without quantifying the effect of PDL on β-cell mass or β-cell proliferation (14,15,35). An additional study recently reported that PDL stimulated β-cell mass threefold but did not alter pancreatic insulin content, prompting the authors to speculate that the observed generation of β-cells after PDL might be due to a quantitative artifact when measuring β-cell mass within injured pancreas (16). Indeed, we hypothesize that variable recovery or quantification of pancreatic endocrine cells within uninjured versus injured pancreas could have contributed to many disparate observations regarding β-cell mass expansion after PDL. Thus, our studies resolve an outstanding controversy on the role of PDL-induced injury as a putative stimulus to expand β-cells.
Given that β-cells are not generated by PDL-induced injury, our study questions the entire premise of islet progenitors within the adult pancreas. Notably, Xu et al. observed that Ngn3-marked cells could give rise to various islet endocrine cell types when transplanted into cultured fetal tissue (9). This could imply that Ngn3+ cells represent pluripotent tissue stem cells of the pancreas. Our studies reveal that PDL-induced expression of Ngn3 and other islet endocrine markers can be disconnected from adult β-cell mass expansion. We remain unsure whether increased Ngn3 expression in PDL pancreata is the result of a bona fide induction of Ngn3 expression or some sort of artifact due to differences in RNA recovery from injured compared with uninjured pancreas. Regardless, the relevance of Ngn3+ cells to adult β-cell mass expansion is tenuous in light of our results, which concur with Sox9-mediated lineage-tracing studies by Sander and colleagues (16). Moreover, we note that the fetal pancreas could have had a dominant influence in the transformation of PDL-derived Ngn3+ cells into islet endocrine cells. Supporting this, Barrandon and colleagues have described that thymic epithelial cells can be reprogrammed by the dermal niche to become skin cells (46). Indeed, fetal mesenchymal tissues can induce islet differentiation when exposed to pancreatic ducts (47). Thus, it is difficult to assess the significance of Ngn3+ cells versus the recipient fetal tissues. We also find that Ngn3 transcript and protein are more abundant in the ligated pancreas. However, we find that Ngn3 is also expressed in normal adult pancreas in both endocrine+ and pancytokeratin+ cells, as have others (9,16,37,38). Our study reveals that adult β-cells are not derived from specialized progenitors after pancreatic injury. We show that β-cells are not generated in injured mouse pancreas. This observation is consistent with evidence showing that adult β-cells are derived by self-renewal (6–8).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (grants 1R01DK064101, 1R01AG040110, and P30DK079638), the JDRF, the Commonwealth of Pennsylvania (Center for Excellence in Regenerative Medicine Grant 4100043362), and Robert and Janice McNair Foundation.
J.A.K. serves on the scientific advisory board of Johnson & Johnson. P. Kushner of Olema Pharmaceuticals gave helpful comments on the manuscript. No other potential conflicts of interest relevant to this article were reported.
M.M.R. conceived and designed the experiments, performed the experiments, and analyzed data. C.J.W., K.R., E.J.S., and A.G. assisted with performing experiments. J.A.K. conceived and designed the experiments, performed the experiments, analyzed data, and wrote the manuscript. J.A.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank D. Martinez, N. Panackal, and S. Agrio of the pathology core at the Children’s Hospital of Philadelphia (CHOP) for their expert assistance with slides. The authors also thank M. Reasoner of the CHOP laboratory animal facility for her thoughtful supervision of the animal work in this study. Finally, the authors thank C. Blalock, K. Rogers, D. Kettlewell, B. Pekkattil, L. Herrera, S. Vale, and S. Ramirez, all of the Texas Children's Diabetes and Endocrinology Center, for administrative expertise and support.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0848/-/DC1.
REFERENCES
- 1.Granger A, Kushner JA. Cellular origins of beta-cell regeneration: a legacy view of historical controversies. J Intern Med 2009;266:325–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kushner JA, Weir GC, Bonner-Weir S. Ductal origin hypothesis of pancreatic regeneration under attack. Cell Metab 2010;11:2–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collombat P, Xu X, Heimberg H, Mansouri A. Pancreatic beta-cells: from generation to regeneration. Semin Cell Dev Biol 2010;21:838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. Beta-cell growth and regeneration: replication is only part of the story. Diabetes 2010;59:2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desgraz R, Bonal C, Herrera PL. β-cell regeneration: the pancreatic intrinsic faculty. Trends Endocrinol Metab 2011;22:34–43 [DOI] [PubMed] [Google Scholar]
- 6.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 7.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007;12:817–826 [DOI] [PubMed] [Google Scholar]
- 8.Brennand K, Huangfu D, Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biol 2007;5:e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, D’Hoker J, Stangé G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008;132:197–207 [DOI] [PubMed] [Google Scholar]
- 10.Dor Y, Melton DA. Facultative endocrine progenitor cells in the adult pancreas. Cell 2008;132:183–184 [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Habener JF. Alpha cells beget beta cells. Cell 2009;138:424–426 [DOI] [PubMed] [Google Scholar]
- 12.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science 2008;322:1490–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solar M, Cardalda C, Houbracken I, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell 2009;17:849–860 [DOI] [PubMed] [Google Scholar]
- 14.Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 2011;43:34–41 [DOI] [PubMed] [Google Scholar]
- 15.Kopinke D, Brailsford M, Shea JE, Leavitt R, Scaife CL, Murtaugh LC. Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development 2011;138:431–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopp JL, Dubois CL, Schaffer AE, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 2011;138:653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruzankina Y, Pinzon-Guzman C, Asare A, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 2007;1:113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rooman I, Lardon J, Bouwens L. Gastrin stimulates beta-cell neogenesis and increases islet mass from transdifferentiated but not from normal exocrine pancreas tissue. Diabetes 2002;51:686–690 [DOI] [PubMed] [Google Scholar]
- 19.Wang RN, Klöppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 1995;38:1405–1411 [DOI] [PubMed] [Google Scholar]
- 20.Kushner JA, Ye J, Schubert M, et al. Pdx1 restores beta cell function in Irs2 knockout mice. J Clin Invest 2002;109:1193–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hultquist GT, Joensson LE. Ligation of the Pancreatic Duct in Rats. Acta Soc Med Ups 1965;70:82–88 [PubMed] [Google Scholar]
- 22.Andersson A, Hallberg A, Hellerström C, Hultquist G, Jansson L. Release of insulin in vitro from normal and duct-ligated rat pancreas. Acta Pathol Microbiol Scand A 1979;87A:285–288 [DOI] [PubMed] [Google Scholar]
- 23.Pound AW, Walker NI. Involution of the pancreas after ligation of the pancreatic ducts. I: a histological study. Br J Exp Pathol 1981;62:547–558 [PMC free article] [PubMed] [Google Scholar]
- 24.Walker NI, Pound AW. An autoradiographic study of the cell proliferation during involution of the rat pancreas. J Pathol 1983;139:407–418 [DOI] [PubMed] [Google Scholar]
- 25.Watanabe S, Abe K, Anbo Y, Katoh H. Changes in the mouse exocrine pancreas after pancreatic duct ligation: a qualitative and quantitative histological study. Arch Histol Cytol 1995;58:365–374 [DOI] [PubMed] [Google Scholar]
- 26.Wang RN, Rehfeld JF, Nielsen FC, Klöppel G. Expression of gastrin and transforming growth factor-alpha during duct to islet cell differentiation in the pancreas of duct-ligated adult rats. Diabetologia 1997;40:887–893 [DOI] [PubMed] [Google Scholar]
- 27.Bertelli E, Bendayan M. Intermediate endocrine-acinar pancreatic cells in duct ligation conditions. Am J Physiol 1997;273:C1641–C1649 [DOI] [PubMed] [Google Scholar]
- 28.Yasuda H, Kataoka K, Ichimura H, et al. Cytokine expression and induction of acinar cell apoptosis after pancreatic duct ligation in mice. J Interferon Cytokine Res 1999;19:637–644 [DOI] [PubMed] [Google Scholar]
- 29.Scoggins CR, Meszoely IM, Wada M, Means AL, Yang L, Leach SD. p53-dependent acinar cell apoptosis triggers epithelial proliferation in duct-ligated murine pancreas. Am J Physiol Gastrointest Liver Physiol 2000;279:G827–G836 [DOI] [PubMed] [Google Scholar]
- 30.Page BJ, du Toit DF, Muller CJ, Mattysen J, Lyners R. An immunocytochemical profile of the endocrine pancreas using an occlusive duct ligation model. JOP 2000;1:191–203 [PubMed] [Google Scholar]
- 31.Okamura K, Watanabe S, Abe K, Kondo S, Katoh H. Autonomic nerve changes in the mouse pancreas after pancreatic duct ligation. Pancreas 2003;27:52–57 [DOI] [PubMed] [Google Scholar]
- 32.Li M, Miyagawa J, Moriwaki M, et al. Analysis of expression profiles of islet-associated transcription and growth factors during beta-cell neogenesis from duct cells in partially duct-ligated mice. Pancreas 2003;27:345–355 [DOI] [PubMed] [Google Scholar]
- 33.Nagaya M, Kubota S, Isogai A, Tadokoro M, Akashi K. Ductular cell proliferation in islet cell neogenesis induced by incomplete ligation of the pancreatic duct in dogs. Surg Today 2004;34:586–592 [DOI] [PubMed] [Google Scholar]
- 34.Jansson L, Bodin B, Källskog O, Andersson A. Duct ligation and pancreatic islet blood flow in rats: physiological growth of islets does not affect islet blood perfusion. Eur J Endocrinol 2005;153:345–351 [DOI] [PubMed] [Google Scholar]
- 35.Inada A, Nienaber C, Katsuta H, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA 2008;105:19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic β-cell neogenesis by direct conversion from mature α-cells. Stem Cells 2010;28:1630–1638 [DOI] [PubMed] [Google Scholar]
- 37.Dror V, Nguyen V, Walia P, Kalynyak TB, Hill JA, Johnson JD. Notch signalling suppresses apoptosis in adult human and mouse pancreatic islet cells. Diabetologia 2007;50:2504–2515 [DOI] [PubMed] [Google Scholar]
- 38.Wang S, Jensen JN, Seymour PA, et al. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc Natl Acad Sci USA 2009;106:9715–9720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuttle AH, Rankin MM, Teta M, et al. Immunofluorescent detection of two thymidine analogues (CldU and IdU) in primary tissue. J Vis Exp. 7 December 2010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinberg N, Ouziel-Yahalom L, Knoller S, Efrat S, Dor Y. Lineage tracing evidence for in vitro dedifferentiation but rare proliferation of mouse pancreatic beta-cells. Diabetes 2007;56:1299–1304 [DOI] [PubMed] [Google Scholar]
- 41.Reinert RB, Kantz J, Misfeldt AA, et al. Tamoxifen-Induced Cre-loxP Recombination Is Prolonged in Pancreatic Islets of Adult Mice. PLoS ONE 2012;7:e33529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng SW, Zhu LY, Chen M, et al. Heterogeneity in mitotic activity and telomere length implies an important role of young islets in the maintenance of islet mass in the adult pancreas. Endocrinology 2009;150:3058–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee CS, De León DD, Kaestner KH, Stoffers DA. Regeneration of pancreatic islets after partial pancreatectomy in mice does not involve the reactivation of neurogenin-3. Diabetes 2006;55:269–272 [PubMed] [Google Scholar]
- 44.Kopinke D, Murtaugh LC. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol 2010;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blaine SA, Ray KC, Anunobi R, Gannon MA, Washington MK, Means AL. Adult pancreatic acinar cells give rise to ducts but not endocrine cells in response to growth factor signaling. Development 2010;137:2289–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonfanti P, Claudinot S, Amici AW, Farley A, Blackburn CC, Barrandon Y. Microenvironmental reprogramming of thymic epithelial cells to skin multipotent stem cells. Nature 2010;466:978–982 [DOI] [PubMed] [Google Scholar]
- 47.Dudek RW, Lawrence IE, Jr, Hill RS, Johnson RC. Induction of islet cytodifferentiation by fetal mesenchyme in adult pancreatic ductal epithelium. Diabetes 1991;40:1041–1048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.