Abstract
Congenital hyperinsulinism causes persistent hypoglycemia in neonates and infants. Most often, uncontrolled insulin secretion (IS) results from a lack of functional KATP channels in all β-cells or only in β-cells within a resectable focal lesion. In more rare cases, without KATP channel mutations, hyperfunctional islets are confined within few lobules, whereas hypofunctional islets are present throughout the pancreas. They also can be cured by selective partial pancreatectomy; however, unlike those with a KATP focal lesion, they show clinical sensitivity to diazoxide. Here, we characterized in vitro IS by fragments of pathological and adjacent normal pancreas from six such cases. Responses of normal pancreas were unremarkable. In pathological region, IS was elevated at 1 mmol/L and was further increased by 15 mmol/L glucose. Diazoxide suppressed IS and tolbutamide antagonized the inhibition. The most conspicuous anomaly was a large stimulation of IS by 1 mmol/L glucose. In five of six cases, immunohistochemistry revealed undue presence of low-Km hexokinase-I in β-cells of hyperfunctional islets only. In one case, an activating mutation of glucokinase (I211F) was found in pathological islets only. Both abnormalities, attributed to somatic genetic events, may account for inappropriate IS at low glucose levels by a subset of β-cells. They represent a novel cause of focal congenital hyperinsulinism.
Congenital hyperinsulinism (CHI) is the major cause of severe and persistent hypoglycemia in neonates and infants and is a brain-damaging and potentially life-threatening condition (1–3). Excessive secretion of insulin by pancreatic β-cells has been linked to mutations in several genes.
Most cases are caused by inactivating mutations in ABCC8 (encoding sulfonylurea receptor 1) or KCNJ11 (encoding Kir6.2) (4,5), the two subunits of ATP-sensitive K (KATP) channels that mediate the effects of glucose on β-cell membrane potential (6,7). Histologically, two forms of KATP channel–related CHI have been identified (8,9). In diffuse forms inherited in an autosomal-recessive (rarely dominant) manner, all β-cells in all islets are affected, and subtotal pancreatectomy may be necessary. In focal forms, a localized adenomatous hyperplasia of abnormal β-cells is present in an otherwise normal pancreas, and its selective resection cures the patient (10,11). One important clinical feature of KATP channel–related CHI patients is their usual resistance to medical treatment with diazoxide (1–3). In vitro studies of islets or pancreatic fragments from operated patients have verified the following predictable consequences of this lack of functional KATP channels in β-cells: membrane depolarization; uncontrolled influx of Ca2+; increase in the cytosolic concentration of free Ca2+; and high rate of insulin secretion at low glucose levels (12–14). They further showed that drugs acting on KATP channels are unable to produce their normal stimulatory (tolbutamide) or inhibitory (diazoxide) effects on insulin secretion (14).
Less often CHI is caused by activating mutations in GCK (encoding glucokinase), GLUD1 (encoding glutamate dehydrogenase), or SLC16A1 (encoding monocarboxylate transporter 1) or by inactivating mutations in HADH (encoding 3-hydroxyacyl-CoA dehydrogenase), UCP2 (encoding mitochondrial uncoupling protein 2), HNF4A (encoding HNF4α), or HNF1A (encoding HNF1α) (15–21). It is unclear how mutations in HNF4A and HNF1A alter β-cell function. All the others affect distinct metabolic pathways in β-cells in such a way that too many otherwise normal KATP channels are closed at any glucose concentration. In these cases of accelerated β-cell metabolism, diazoxide retains its ability to open the channels, which explains the sensitivity of the patients to medical treatment with the drug (1–3). The islet features in KATP channel–unrelated, diazoxide-treatable CHI cases are not well-known because of the rarity and only exceptional surgical treatment of these cases.
A novel anatomopathological form of CHI was described recently in 16 patients (22). Its hallmark is a mosaicism of the islets. Morphologically hyperfunctional islets containing β-cells with large nuclei, abundant cytoplasm, and signs of intense proinsulin synthesis coexist with resting islets containing β-cells with small nuclei, shrunken cytoplasm, and signs of low proinsulin synthesis. Whereas hypoactive islets are present in the whole pancreas, hyperactive islets are located in one or in a few adjacent lobules. This concentration in a limited region of the gland explains why selective partial pancreatectomy often was curative (22). Despite some similarities, this pathological entity differs from KATP channel–related focal CHI by a lack of germinal mutation in ABCC8 or KCNJ11 and a clinical responsiveness of the cases to treatment with diazoxide (22).
Insulin secretion by pancreatic fragments from six of these patients could be investigated in vitro using the same methods as in our recent study of KATP channel–related CHI pancreas (14). We show that β-cells from the hyperactive region have functionally normal KATP channels, and that the inappropriate secretion of insulin can be attributed to an increased responsiveness to glucose that, on the basis of immunohistochemical or genetic analyses of the tissues, we attribute to undue expression of low-Km hexokinase I (HK-I) or to an activating mutation in GCK in hyperfunctional islets only.
RESEARCH DESIGN AND METHODS
Subjects and in vitro studies of pancreatic fragments.
The six cases of CHI included in the current study (here numbered 1–6) correspond to the last six consecutive cases (there numbered 11–16) of a recently reported series in which their clinical characteristics can be found (22). The six patients studied here were at least transiently sensitive to diazoxide treatment and were found negative for mutations in ABCC8, KCNJ11, or GCK (case 3 was not tested). However, they underwent surgery (two at 8–9 months and the others at 21–37 months) because of progressive loss of diazoxide efficacy (cases 2 and 6), distressing hypertrichosis, or suspicion of a focal form by pancreatic venous sampling (cases 1–3) (22). In all of them, lobules containing hyperfunctional islets were localized by intraoperative biopsies and resected with a rim of normal tissue. The pathologist sampled pathological and normal fragments for functional studies (no normal tissue was available from case 4). These fragments were transported in RPMI culture medium to the laboratory, where they were treated exactly as described, before measurements of insulin secretion and content (14). The study was conducted with the approval of and according to the regulations of the Commission d’Ethique Biomédicale of the University of Louvain, Faculty of Medicine. The requested informed consent was obtained from the parents.
Immunodetection of HK-I.
Formalin-fixed, paraffin-embedded sections (5-μm-thick) were first incubated for 75 min at 100°C in citrate buffer (pH 5.8) for antigen retrieval. They were then incubated overnight at room temperature with rabbit anti-HK-I (H-95, batch F0205, 1/90; Santa Cruz), which was detected by the EnVision+ system horseradish peroxidase–labeled polymer (Dako). The retrieval step that was necessary for HK-I detection made subsequent insulin detection impossible. Therefore, to assess the cellular localization of HK-I, insulin-containing cells were first immunostained with fluorescence detection (without retrieval step) and the section was photographed. The slice was then treated as described for HK-I detection and photographed again.
Search of glucokinase mutations in tissue.
In pancreatic fragments from case 6, regions containing resting or hyperfunctional islets were identified on formalin-fixed, paraffin-embedded sections, and the two zones were macrodissected separately (total of 15 sections of 5-μm thickness). Total RNA was then extracted with the recover all Total Nucleic Acid Isolation kit (Ambion) and reverse-transcribed with SuperScript III First-Strand Synthesis System (Invitrogen) with 165 ng oligo(dt)20 primer and 100 ng random primer. Then, 5 µL cDNA was amplified by PCR using primers chosen to cover the three regions of the glucokinase-transcript-variant1-NM _000162.2, in which mutations have been associated with CHI. Region 1 corresponds to AA 30–142 (forward: gga gga gga cct gaa gaa gg; reverse: ttg tcc agg aag tcg gag at). Region 2 corresponds to AA 180–246 (forward: gca gaa ggg aac aat gtc gt; reverse: cac att ctg cat ctc ctc ca). Region 3 corresponds to AA 402–464 (forward: gca gcg agg acg taa tgc; reverse: tca ctg gcc cag cat aca g). The products were sequenced using a BigDye terminator cycle sequencing kit and analyzed using 3130 XL Genetic Analyzer (Applied Biosystems).
RESULTS
Morphological aspect and sampling of tissue.
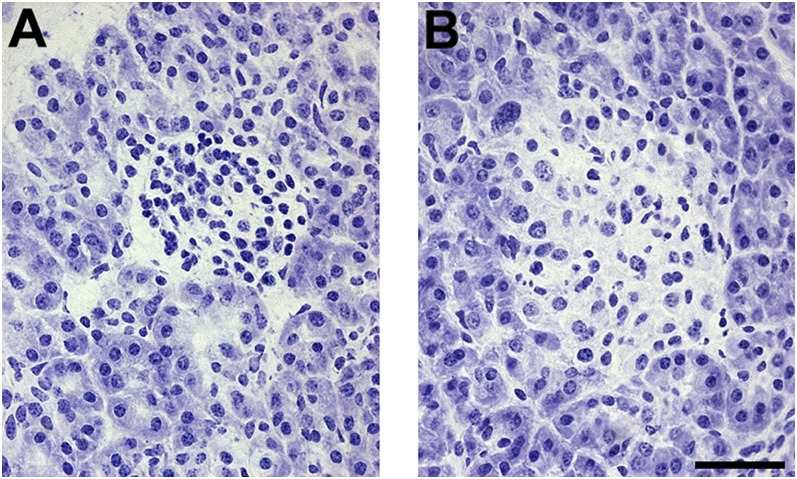
Intraoperative diagnosis was based on examination of frozen pancreatic biopsy specimens stained with toluidine blue (Fig. 1). The characteristic feature is the coexistence of two types of islets (mosaicism). In small hypoactive islets, β-cells are poor in cytoplasm and have small nuclei (Fig. 1A), whereas hyperactive islets contain cytoplasm-rich β-cells with some enlarged nuclei (Fig. 1B). As previously described, hyperactive islets are concentrated in a few lobules of the gland (22). This pathological region was resected with a rim of normal tissue, and fragments from both regions were saved for functional studies. Postoperative examination of fixed specimens confirmed the segregation of the two types of islets, but separation of pathological and normal regions was not always complete. In particular, samples labeled “normal” still contained some hyperfunctional islets in cases 3 and 5. A small proportion of hypofunctional islets also were often present in pathological samples.
FIG. 1.
Morphological mosaicism of the islets. Intraoperative diagnosis using frozen sections stained with toluidine blue is based on the coexistence of small islets containing β-cells with scanty cytoplasm (A) and hyperplastic islets containing cytoplasm-rich β-cells (B). Representative sections from the pancreas of case 3. Scale bar, 50 µm.
Insulin content.
The initial insulin content of the tissue was not directly measured but was estimated as previously described (14). The pathological region did not contain more insulin (22.5 ng/mg; range, 6.5–47.5) than the normal region (23.1 ng/mg; range, 10.9–43.1).
Effects of glucose, diazoxide, and tolbutamide on insulin secretion.
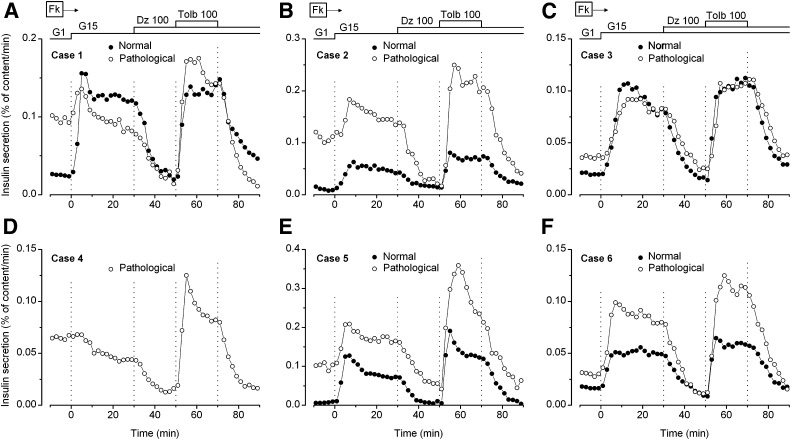
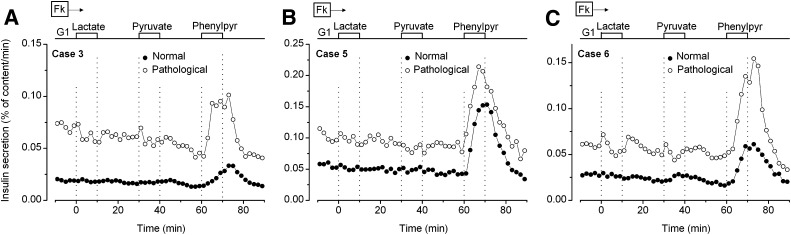
All experiments were performed in the presence of 1 µmol/L forskolin to increase β-cell cAMP levels. In normal pancreas (Fig. 2), an increase in the glucose concentration from 1 to 15 mmol/L stimulated insulin secretion several-fold. This stimulation was abolished by 100 µmol/L diazoxide and reversibly restored by 100 µmol/L tolbutamide. Except for a less pronounced biphasic pattern, this response is similar to that observed in normal adult islets (23) and in fragments of normal pancreas from infants (14). Figure 2 also shows responses of the pathological region from the same pancreas. In five of five cases (one comparison was not possible), insulin secretion rate in 1 mmol/L glucose was two-fold to many-fold higher than in adjacent normal pancreas. An increase in secretion was induced by 15 mmol/L glucose in five of six cases, and it was sustained in four cases. Strikingly, diazoxide inhibited insulin secretion to similar low levels as in normal pancreas, and tolbutamide consistently increased it in a reversible manner (Fig. 2). Altogether, these results indicate that KATP channels are functional in hyperactive islets.
FIG. 2.
Effects of glucose and drugs acting on KATP channels on insulin secretion by normal (closed circles) and pathological pancreas (open circles) from each of the six studied cases. Normal tissue was not available for case 4. The concentration of glucose (G) was increased from 1 to 15 mmol/L, and 100 μmol/L diazoxide (Dz) and 100 μmol/L tolbutamide (Tolb) were added, as indicated at the top of the figures. Forskolin (Fk, 1 μmol/L) was present throughout to activate cAMP formation.
Effects of stepwise increases in glucose on insulin secretion.
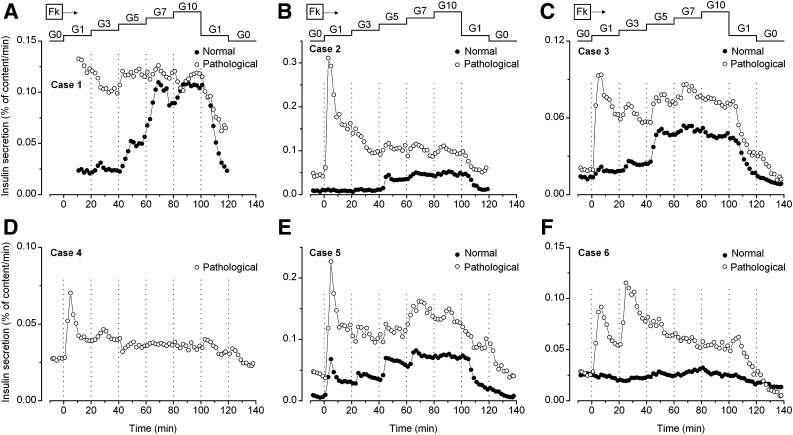
In normal pancreas, glucose-induced insulin secretion was concentration-dependent, with a maximum already reached at 7 mmol/L (Fig. 3). The lack of a response in case 6 is considered unreliable because of low amounts of insulin in the sample. This concentration dependency was markedly altered in pathological pancreas. When the test was started in the absence of glucose (five of six cases), addition of only 1 mmol/L glucose consistently induced a large peak of secretion, whereas subsequent increases had no or little further effect except in case 6 (Fig. 3). At the end of the experiments, switching from 1 to 0 mmol/L glucose (in four of six cases) was followed by a further decrease in secretion rate. Although the threshold for glucose-induced insulin secretion in normal adult islets or fragments of infant pancreas is at 3 mmol/L (14,23), an increase was observed in response to 1 mmol/L glucose in normal pancreas of cases 3 and 5. We attribute this premature response to the presence of a small proportion of abnormal β-cells in the sample. Another puzzling observation is that in three of four cases, insulin secretion rate was higher in pathological than in normal pancreas in absence of glucose.
FIG. 3.
Effects of stepwise increases and decreases in glucose concentration (G in mmol/L) on insulin secretion by normal (closed circles) and pathological pancreas (open circles) from each of the six studied cases. There was no period in G0 at the start or the end of the experiment with tissue from cases 1 and 2. Normal tissue was not available for case 4. The low insulin content of normal fragments from case 6 makes the results uncertain. Forskolin (Fk, 1 μmol/L) was present throughout.
Effects of various agents on insulin secretion.
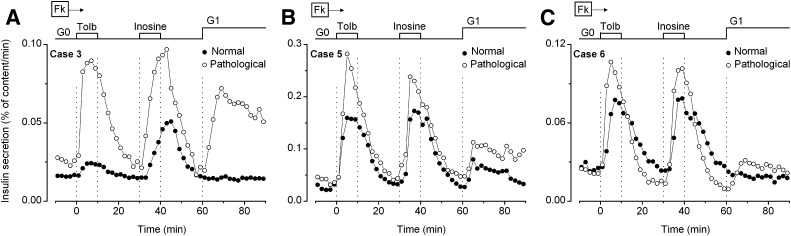
Enough tissue for additional tests was available in only three cases. In two of these, basal insulin secretion (no glucose) was again slightly higher in pathological than in normal tissue (Fig. 4). Tolbutamide (25 µmol/L) consistently increased secretion, with a similar or greater efficacy in pathological than in normal pancreas. In mouse islet cells, inosine is split to hypoxanthine, which has no effect on insulin secretion, and ribose-1-phosphate, the subsequent metabolism of which increases ATP levels (24). Inosine mimics most effects of glucose in mouse islets (25) and induces insulin secretion in human adult islets (23). It was effective in pathological and in normal pancreas (Fig. 4). Finally, stimulation with 1 mmol/L glucose increased insulin secretion in pathological pancreas of the three cases, had no effect in normal pancreas of two cases, and was slightly effective in normal pancreas of case 5. These observations back-up those shown in Fig. 3.
FIG. 4.
Effects of 25 μmol/L tolbutamide (Tolb) and 5 mmol/L inosine on insulin secretion by normal (closed circles) and pathological pancreas (open circles) from three cases. The experiments were started in the absence of glucose (G0), which was added at 1 mmol/L (G1) at 60 min. Forskolin (Fk, 1 μmol/L) was present throughout.
In a last series performed with 1 mmol/L glucose throughout, lactate and pyruvate were without effect on insulin secretion by pathological and normal pancreas (three of three) (Fig. 5), which indicates that the pathology is not underlain by abnormal transport of monocarboxylic acids into β-cells (17,26). In contrast, membrane-permeant phenylpyruvate increased secretion two-fold to three-fold in both pathological and normal pancreas. A similar stimulation occurs in rodent islets through acceleration of β-cell mitochondrial metabolism and direct inhibition of KATP channels (27).
FIG. 5.
Effects of 10 mmol/L lactate, pyruvate, and phenylpyruvate (Phenylpyr) on insulin secretion by normal (closed circles) and pathological pancreas (open circles) from three cases. The experiments were performed in the presence of 1 mmol/L glucose (G1). Forskolin (Fk, 1 μmol/L) was present throughout.
Expression of HK-I in hyperfunctional β-cells.
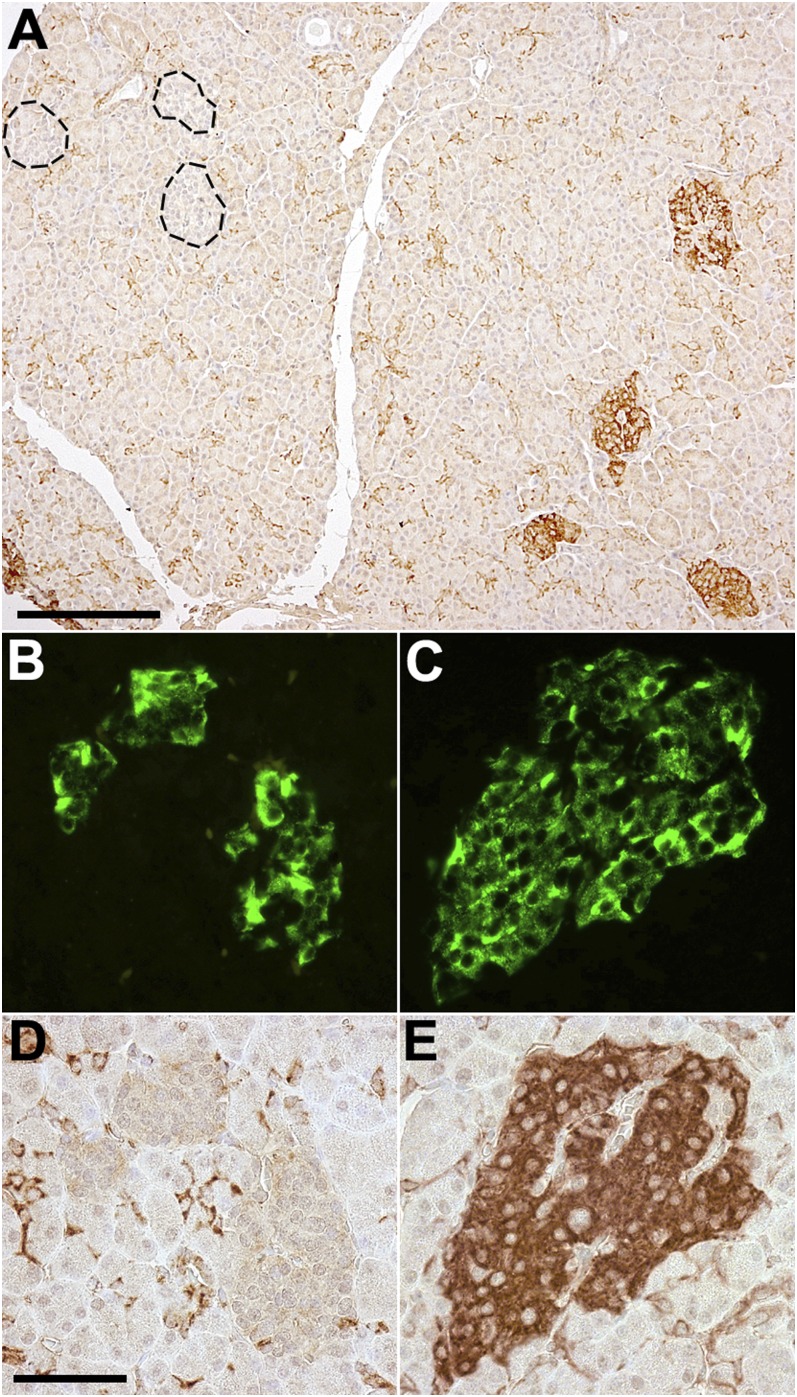
The stimulation of insulin secretion by as little as 1 mmol/L glucose in pathological fragments led us to search for the presence of a low-Km hexokinase in β-cells. Immunocytochemistry for HK-I was positive in morphologically hyperfunctional islets from five cases as follows: strongly in cases 1, 3, 4, and 5, and weakly in case 2. However, it was consistently negative in their hypofunctional islets. Figure 6A illustrates this striking difference in case 3, in which all islets were labeled in the pathological region in contrast to islets in a normal adjacent lobule. Figure 6 also shows insulin immunolabeling in hypofunctional (Fig. 6B) and hyperfunctional islets (Fig. 6C) from case 1. HK-I was clearly negative in β-cells of hypofunctional islets (Fig. 6D) and positive in β-cells from hyperfunctional islets (Fig. 6E). Glucokinase was present (immunodetection) in β-cells positive for HK-I (Supplementary Fig. 1A and B).
FIG. 6.
A: Presence of HK-I in islets of the pathological region in pancreas of case 3. Hyperfunctional islets were positive for HK-I immunostaining, whereas hypofunctional islets (highlighted by a dotted line) were negative in an adjacent normal lobule. Scale bar, 200 µm. Immunolabeling of insulin (B and C) and HK-I (D and E) in the same islets from the normal (B and D) and pathological (C and E) regions of the pancreas of case 1. HK-I is present in β-cells of pathological islets and is absent in β-cells of normal islets. Scale bar, 50 µm. Note that in the exocrine pancreas, centro-acinar cells and vessel walls are more strongly labeled than acinar cells (also see Supplementary Fig. 2).
Glucokinase mutation in hyperfunctional islets.
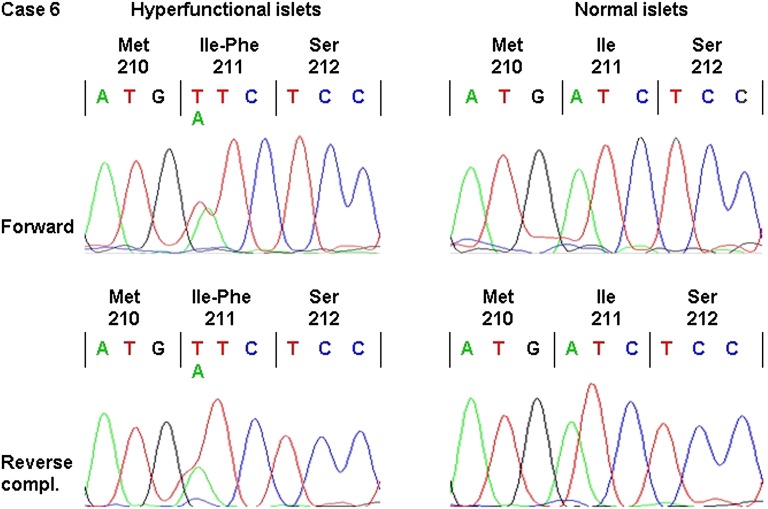
Because hyperfunctional islets from case 6 were negative for HK-I, we looked for a mutation in GCK after RNA extraction from sections of pathological and normal islets. As shown in Fig. 7, a mutation (I211F) was identified in the pathological region that also contained wild-type RNA. The presence of both abnormal and normal transcripts suggests that only one allele was mutated. No mutation was found in the normal region, which, together with the lack of GCK mutation in blood DNA of the patient (22), indicates a somatic mutation in hyperfunctional islets. No mutation in GCK was found in islets from two cases showing weak (case 2) or strong (case 3) HK-I labeling in their hyperfunctional β-cells.
FIG. 7.
Electropherograms identifying a GCK mutation in case 6. RNA was extracted from paraffin-embedded slices containing hyperfunctional or hypofunctional islets and was reverse-transcribed. cDNA was then amplified and sequenced in both forward and reverse directions as shown. Both wild-type and mutated cDNA (Ile211Phe) were found in the pathological region, whereas only wild-type cDNA was identified in the normal region.
DISCUSSION
Histologically, the pancreas from CHI patients can display two major aspects. In diffuse forms, all islets look alike because all β-cells are affected through inheritance of a recessive or dominant germinal mutation in various genes involved in the control of insulin secretion. In focal forms, only a subset of islets contains abnormal β-cells. These islets are concentrated in a restricted zone of the pancreas, referred to as the focal lesion. Thus, focal CHI is a topographical, not a mechanistic, characteristic of the disease. At least two types exist.
The most frequent and best-defined focal lesions result from two genetic events, inheritance of a recessive paternal mutation in ABCC8 or KCNJ11 and somatic deletion of the corresponding maternal segment of chromosome 11p15 in a clone of β-cells. This causes loss of heterozygosity in these β-cells (28,29) and their hyperplasia because of the lack of maternally expressed tumor-suppressor CDKN1C and H19 associated with the unrestrained expression of paternal IGF2 (28,30,31). The inactivating nature of the paternal mutation makes β-cells of these adenomatous focal lesions resistant to diazoxide that cannot inhibit insulin secretion in vivo (1–3) or in vitro (14). The second type of focal lesion is less sharply defined. It is characterized by a mosaicism of the islets, with hyperfunctional islets (without β-cell hyperplasia) preferentially localized in just a few lobules and resting islets in the rest of the pancreas. These patients have no mutation in KATP channel genes, have no loss of 11p15 heterozygosity in hyperfunctional β-cells, and are at least transiently sensitive to diazoxide treatment (22). The focal nature of the lesion is compatible with a somatic genetic event, but none has been identified so far.
The present in vitro study of the pancreas of six patients with a clinically diazoxide-sensitive focal pathology identified major differences with focal lesions linked to a mutation in ABCC8 or KCNJ11 genes. First, whereas the insulin concentration is ∼14-fold higher in KATP channel–related focal lesions than in the adjacent normal pancreas (14), there is no difference between pathological and normal regions in this series. This may seem surprising because of the larger size of islets and β-cells in the lesion. However, immunodensitometry of insulin labeling showed that β-cells in small islets within the normal region contain more insulin than large islets within the pathological region (22). The second difference is the presence of functional KATP channels in β-cells from both pathological and normal regions. Thus, diazoxide produced a similar inhibition of insulin secretion, and tolbutamide was able to reverse this inhibition and to stimulate secretion in the absence of glucose and diazoxide. A third difference is that glucose increased insulin secretion in the lesion, whereas it did so inconsistently in KATP channel-related focal lesions (14). However, the concentration dependency of the response to glucose was strikingly abnormal. When we studied our first case (case 1), the experiments were started with 1 mmol/L glucose and revealed a much higher insulin secretion rate in the lesion than in normal tissue, despite evidence of functional KATP channels. This intriguing observation led us to begin subsequent studies in the absence of glucose, which uncovered that 1 mmol/L glucose caused a rapid and large peak of insulin secretion in the five other cases. Further stepwise increases in glucose produced no or little additional stimulation, but an increase from 1 to 15 mmol/L was often effective.
Stimulation of insulin secretion by 1 mmol/L glucose suggested an anomaly in glucose metabolism. In rodent and human β-cells, glucose is phosphorylated by the high-Km glucokinase (32,33), whose properties set the threshold for stimulation of insulin secretion at ∼3 mmol/L in human islets (23,34). Normal β-cells express no or little low-Km HK (35). Using immunohistochemistry, we detected the presence of HK-I in islets from five of six of our cases. HK-I was localized in insulin-containing cells of hyperactive islets within the pathological region, whereas hypoactive islets were consistently negative for HK-I. We therefore propose that the abnormal insulin secretion in response to 1 mmol/L glucose is attributable to HK-I substituting to glucokinase for phosphorylating glucose. β-cells positive for HK-I also were positive for glucokinase. This may explain why some insulin response persisted on stimulation with glucose concentrations >1 mmol/L. Admittedly, no direct measurements of glucose metabolism are available, but our conclusion is compatible with experimental models. Thus, transgenic mice expressing the low-Km yeast HK-B in β-cells showed lower blood glucose levels with relative hyperinsulinemia. In vitro, their islets displayed increased responsiveness to glucose, with higher insulin secretion rates at basal (3 mmol/L) glucose (36). Forced expression of mammalian HK-I in mouse insulinoma (MIN-6) cells or rat islets also led to increases in glucose metabolism and augmentation of basal insulin secretion (37,38).
Unexpectedly, HK-I was not detected in hyperfunctional islets from case 6, in which no GCK mutation had been found in blood cell DNA (22). We therefore searched for a somatic mutation in GCK by sequencing RNA extracted from pancreatic sections. One mutation (I211F) was identified together with wild-type RNA in the pathological region but was not detected in the normal region. In vitro experiments have shown that the I211F variant is the most active human glucokinase identified to date (39), but it had not been reported in CHI patients. In our case 6, expression of the hyperactive glucokinase in a subset of β-cells is explainable by a somatic mutation during embryogenesis.
Activating mutations of GCK are a rare cause of generally autosomal-dominant CHI. Since the first description (15), 11 mutations have been reported (40). The phenotype ranges from asymptomatic to marked hypoglycemia, with the most severe cases being caused by the most activating mutations (40). Most cases are responsive to diazoxide, but the needed dose may be high when the activity of glucokinase is strongly increased (40). Interestingly, our case 6 was initially controlled by diazoxide, but the sensitivity to the drug progressively diminished (22), perhaps because of the high activity of the mutated enzyme in a number of β-cells that increased with aging. Patients with an inherited glucokinase mutation have only rarely undergone subtotal pancreatectomy. No islet abnormalities were detected in two patients (40,41). In a third patient, islets were increased in size and contained β-cells with relatively large nuclei (42). These two features also were observed in pathological regions of case 6 with a glucokinase mutation and in pathological regions of the five cases with HK-I expression, which displayed similar features.
Functionally, also, the cases with HK-I expression closely resembled the case with a glucokinase mutation. However, we do not know what caused expression of HK-I in a subset of β-cells segregated from the others. We can only suggest that a somatic genetic event occurred during development of the pancreas, which may have affected the HK-1 gene itself or another gene that normally represses expression of the latter. Increased expression of HK-I and excessive insulin secretion at low glucose levels have been observed in islets from mice lacking PKC-λ in their β-cells, and both defects were corrected by re-expression of the simultaneously decreased Foxa2 (43). INS-1 cells expressing a dominant-negative Foxa2 also showed upregulation of HK-I, which explained the left shift of the glucose dependency of insulin secretion (44). Deletion of Foxa2 in all β-cells caused severe neonatal hypoglycemia with relative hyperinsulinism (45) that was at least partly attributable to defects in KATP channels (46). KATP channels are functional in pathological islets of our patients, and islets positive for HK-I also stain for Foxa2 (Supplementary Fig. 1C and D). Inactivating mutations in Foxa2 cannot be excluded, however. In summary, the primary genetic cause may be different in the five cases, but the common functional abnormality appears to be inappropriate expression of HK-I in a subset of β-cells.
Dominant mutations in ABCC8 cause a mild form of CHI that can often (47,48), although not always (49,50), be controlled by diazoxide. Expression studies under simulated heterozygous conditions produced channels with variably impaired sensitivity to physiological and pharmacological regulators. In our cases, in vitro sensitivity of pathological islets to diazoxide and tolbutamide thus is theoretically compatible with a somatic dominant mutation in ABCC8 or KCNJ11. Formal exclusion of this possibility faces the major difficulty of sequencing the two genes in tiny amounts of fixed material, and several arguments speak against that explanation. Impairing the function of β-cell KATP channels might increase insulin secretion rate in low glucose levels but is not expected to cause a large response to 1 mmol/L glucose without intervention of an abnormal phosphorylation of the sugar. It is not plausible that mutations in KATP channel subunits and glucokinase occurred simultaneously, and there is no published evidence that HK-I expression occurs secondarily to KATP channel dysfunction. We reinvestigated several cases from our recently studied series of CHI caused by ABCC8 mutations (14) and found that immunostaining for HK-I was negative in islets from patients lacking KATP channels in all their β-cells (diffuse form attributable to homozygous recessive mutations) and in focal lesions (paternal recessive mutation with loss of heterozygosity). Importantly, no staining for HK-I was detected in islets outside these KATP channel–related focal lesions, where β-cells are heterozygous for the mutation (Supplementary Fig. 2). Functional data also are inconsistent with this explanation. Thus, in some of these KATP channel–related focal or diffuse cases (14), the experimental protocol to test insulin secretion in vitro involved a change from 0 to 1 mmol/L glucose and no increase was observed (Supplementary Fig. 3).
Because of the islet mosaicism that characterizes the pancreas of studied patients, complete separation of normal and pathological pancreatic fragments for subsequent functional studies is virtually impossible. The presence of some hyperactive islets within “normal” fragments is the most plausible explanation for the small but indisputable response to 1 mmol/L glucose in some cases. However, the possible presence of a minority of normal islets within “pathological” fragments would not invalidate our main conclusions because it could not explain why 1 mmol/L glucose evokes a large peak of insulin secretion and how diazoxide completely inhibits insulin secretion if abnormal β-cells were not also sensitive to the drug. We acknowledge that our study suffers from certain limitations, but we emphasize that the amounts of available tissue are limited, which restricts the number of possible investigations.
In conclusion, we have identified undue presence of HK-I for an unknown cause (five cases) and an activating mutation in GCK (one case) in subsets of β-cells from CHI patients. These molecular abnormalities, which lead to inappropriate secretion of insulin at low glucose levels in vitro, may explain the hyperinsulinemic hypoglycemia in vivo. The pathogeny appears unrelated to KATP channel dysfunction but typical of a metabolic dysfunction that is sensitive to diazoxide treatment. The peculiarity in this group of subjects is that the genetic events are somatic and therefore affect only a fraction of islets concentrated in a focal lesion, the surgical resection of which can cure or markedly improve the patients.
Supplementary Material
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
J.-C.H. and J.R. designed the study. J.-C.H., C.S., M.N., and J.R. analyzed data. J.-C.H. wrote the manuscript. C.S., J.M., S.G., Y.G., and M.N. performed research. C.S., J.M., S.G., Y.G., M.N., and J.R. edited the manuscript. J.-C.H. is the guarantor of this work and, as such, had full access to all the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are grateful to their colleagues who provided medical and surgical treatment of the patients in Hospital Necker, Paris, France (C. Bellané-Chantelot, P. de Lonlay, and C. Fekete), Hospital St. Luc, Brussels, Belgium (M. Maes, M.C. Nassogne, and R. Reding), and Hospital Charité, Berlin, Germany (O. Blankenstein).
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1414/-/DC1.
See accompanying commentary, p. 1373.
REFERENCES
- 1.De León DD, Stanley CA. Mechanisms of Disease: advances in diagnosis and treatment of hyperinsulinism in neonates. Nat Clin Pract Endocrinol Metab 2007;3:57–68 [DOI] [PubMed] [Google Scholar]
- 2.Arnoux JB, de Lonlay P, Ribeiro MJ, et al. Congenital hyperinsulinism. Early Hum Dev 2010;86:287–294 [DOI] [PubMed] [Google Scholar]
- 3.Senniappan S, Shanti B, James C, Hussain K. Hyperinsulinaemic hypoglycaemia: genetic mechanisms, diagnosis and management. J Inherit Metab Dis 2012;35:589–601 [DOI] [PubMed] [Google Scholar]
- 4.Thomas PM, Cote GJ, Wohllk N, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science 1995;268:426–429 [DOI] [PubMed] [Google Scholar]
- 5.Thomas P, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet 1996;5:1809–1812 [DOI] [PubMed] [Google Scholar]
- 6.Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 1999;20:101–135 [DOI] [PubMed] [Google Scholar]
- 7.Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest 2005;115:2047–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahier J, Fält K, Müntefering H, Becker K, Gepts W, Falkmer S. The basic structural lesion of persistent neonatal hypoglycaemia with hyperinsulinism: deficiency of pancreatic D cells or hyperactivity of B cells? Diabetologia 1984;26:282–289 [DOI] [PubMed] [Google Scholar]
- 9.Goossens A, Gepts W, Saudubray JM, et al. Diffuse and focal nesidioblastosis. A clinicopathological study of 24 patients with persistent neonatal hyperinsulinemic hypoglycemia. Am J Surg Pathol 1989;13:766–775 [PubMed] [Google Scholar]
- 10.de Lonlay P, Fournet JC, Rahier J, et al. Somatic deletion of the imprinted 11p15 region in sporadic persistent hyperinsulinemic hypoglycemia of infancy is specific of focal adenomatous hyperplasia and endorses partial pancreatectomy. J Clin Invest 1997;100:802–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahier J, Sempoux C, Fournet JC, et al. Partial or near-total pancreatectomy for persistent neonatal hyperinsulinaemic hypoglycaemia: the pathologist’s role. Histopathology 1998;32:15–19 [DOI] [PubMed] [Google Scholar]
- 12.Straub SG, Cosgrove KE, Ammälä C, et al. Hyperinsulinism of infancy: the regulated release of insulin by KATP channel-independent pathways. Diabetes 2001;50:329–339 [DOI] [PubMed] [Google Scholar]
- 13.Dunne MJ, Cosgrove KE, Shepherd RM, Aynsley-Green A, Lindley KJ. Hyperinsulinism in infancy: from basic science to clinical disease. Physiol Rev 2004;84:239–275 [DOI] [PubMed] [Google Scholar]
- 14.Henquin JC, Nenquin M, Sempoux C, et al. In vitro insulin secretion by pancreatic tissue from infants with diazoxide-resistant congenital hyperinsulinism deviates from model predictions. J Clin Invest 2011;121:3932–3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaser B, Kesavan P, Heyman M, et al. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med 1998;338:226–230 [DOI] [PubMed] [Google Scholar]
- 16.Stanley CA, Lieu YK, Hsu BY, et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med 1998;338:1352–1357 [DOI] [PubMed] [Google Scholar]
- 17.Otonkoski T, Jiao H, Kaminen-Ahola N, et al. Physical exercise-induced hypoglycemia caused by failed silencing of monocarboxylate transporter 1 in pancreatic β cells. Am J Hum Genet 2007;81:467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson ER, Boj SF, Steele AM, et al. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med 2007;4:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clayton PT, Eaton S, Aynsley-Green A, et al. Hyperinsulinism in short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency reveals the importance of beta-oxidation in insulin secretion. J Clin Invest 2001;108:457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González-Barroso MM, Giurgea I, Bouillaud F, et al. Mutations in UCP2 in congenital hyperinsulinism reveal a role for regulation of insulin secretion. PLoS ONE 2008;3:e3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanescu DE, Hughes N, Kaplan B, Stanley CA, De León DD. Novel presentations of congenital hyperinsulinism due to mutations in the MODY genes: HNF1A and HNF4A. J Clin Endocrinol Metab 2012;97:E2026–E2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sempoux C, Capito C, Bellanné-Chantelot C, et al. Morphological mosaicism of the pancreatic islets: a novel anatomopathological form of persistent hyperinsulinemic hypoglycemia of infancy. J Clin Endocrinol Metab 2011;96:3785–3793 [DOI] [PubMed] [Google Scholar]
- 23.Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes 2006;55:3470–3477 [DOI] [PubMed] [Google Scholar]
- 24.Capito K, Hedeskov CJ. Inosine-stimulated insulin release and metabolism of inosine in isolated mouse pancreatic islets. Biochem J 1976;158:335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozem M, Garrino MG, Henquin JC. Inosine partially mimics the effects of glucose on ionic fluxes, electrical activity, and insulin release in mouse pancreatic B-cells. Pflugers Arch 1987;410:457–463 [DOI] [PubMed] [Google Scholar]
- 26.Ishihara H, Wang H, Drewes LR, Wollheim CB. Overexpression of monocarboxylate transporter and lactate dehydrogenase alters insulin secretory responses to pyruvate and lactate in β cells. J Clin Invest 1999;104:1621–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heissig H, Urban KA, Hastedt K, Zünkler BJ, Panten U. Mechanism of the insulin-releasing action of α-ketoisocaproate and related α-keto acid anions. Mol Pharmacol 2005;68:1097–1105 [DOI] [PubMed] [Google Scholar]
- 28.Verkarre V, Fournet JC, de Lonlay P, et al. Paternal mutation of the sulfonylurea receptor (SUR1) gene and maternal loss of 11p15 imprinted genes lead to persistent hyperinsulinism in focal adenomatous hyperplasia. J Clin Invest 1998;102:1286–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damaj L, le Lorch M, Verkarre V, et al. Chromosome 11p15 paternal isodisomy in focal forms of neonatal hyperinsulinism. J Clin Endocrinol Metab 2008;93:4941–4947 [DOI] [PubMed] [Google Scholar]
- 30.Kassem SA, Ariel I, Thornton PS, et al. p57(KIP2) expression in normal islet cells and in hyperinsulinism of infancy. Diabetes 2001;50:2763–2769 [DOI] [PubMed] [Google Scholar]
- 31.Sempoux C, Guiot Y, Dahan K, et al. The focal form of persistent hyperinsulinemic hypoglycemia of infancy: morphological and molecular studies show structural and functional differences with insulinoma. Diabetes 2003;52:784–794 [DOI] [PubMed] [Google Scholar]
- 32.Bedoya FJ, Wilson JM, Ghosh AK, Finegold D, Matschinsky FM. The glucokinase glucose sensor in human pancreatic islet tissue. Diabetes 1986;35:61–67 [DOI] [PubMed] [Google Scholar]
- 33.De Vos A, Heimberg H, Quartier E, et al. Human and rat β cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest 1995;96:2489–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doliba NM, Qin W, Najafi H, et al. Glucokinase activation repairs defective bioenergetics of islets of Langerhans isolated from type 2 diabetics. Am J Physiol Endocrinol Metab 2012;302:E87–E102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuit F, Moens K, Heimberg H, Pipeleers D. Cellular origin of hexokinase in pancreatic islets. J Biol Chem 1999;274:32803–32809 [DOI] [PubMed] [Google Scholar]
- 36.Epstein PN, Boschero AC, Atwater I, Cai X, Overbeek PA. Expression of yeast hexokinase in pancreatic β cells of transgenic mice reduces blood glucose, enhances insulin secretion, and decreases diabetes. Proc Natl Acad Sci USA 1992;89:12038–12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishihara H, Asano T, Tsukuda K, et al. Overexpression of hexokinase I but not GLUT1 glucose transporter alters concentration dependence of glucose-stimulated insulin secretion in pancreatic β-cell line MIN6. J Biol Chem 1994;269:3081–3087 [PubMed] [Google Scholar]
- 38.Becker TC, BeltrandelRio H, Noel RJ, Johnson JH, Newgard CB. Overexpression of hexokinase I in isolated islets of Langerhans via recombinant adenovirus. Enhancement of glucose metabolism and insulin secretion at basal but not stimulatory glucose levels. J Biol Chem 1994;269:21234–21238 [PubMed] [Google Scholar]
- 39.Pal P, Miller BG. Activating mutations in the human glucokinase gene revealed by genetic selection. Biochemistry 2009;48:814–816 [DOI] [PubMed] [Google Scholar]
- 40.Sayed S, Langdon DR, Odili S, et al. Extremes of clinical and enzymatic phenotypes in children with hyperinsulinism caused by glucokinase activating mutations. Diabetes 2009;58:1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gloyn AL, Noordam K, Willemsen MA, et al. Insights into the biochemical and genetic basis of glucokinase activation from naturally occurring hypoglycemia mutations. Diabetes 2003;52:2433–2440 [DOI] [PubMed] [Google Scholar]
- 42.Cuesta-Muñoz AL, Huopio H, Otonkoski T, et al. Severe persistent hyperinsulinemic hypoglycemia due to a de novo glucokinase mutation. Diabetes 2004;53:2164–2168 [DOI] [PubMed] [Google Scholar]
- 43.Hashimoto N, Kido Y, Uchida T, et al. PKClambda regulates glucose-induced insulin secretion through modulation of gene expression in pancreatic β cells. J Clin Invest 2005;115:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Gauthier BR, Hagenfeldt-Johansson KA, Iezzi M, Wollheim CB. Foxa2 (HNF3β ) controls multiple genes implicated in metabolism-secretion coupling of glucose-induced insulin release. J Biol Chem 2002;277:17564–17570 [DOI] [PubMed] [Google Scholar]
- 45.Sund NJ, Vatamaniuk MZ, Casey M, et al. Tissue-specific deletion of Foxa2 in pancreatic β cells results in hyperinsulinemic hypoglycemia. Genes Dev 2001;15:1706–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lantz KA, Vatamaniuk MZ, Brestelli JE, Friedman JR, Matschinsky FM, Kaestner KH. Foxa2 regulates multiple pathways of insulin secretion. J Clin Invest 2004;114:512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huopio H, Reimann F, Ashfield R, et al. Dominantly inherited hyperinsulinism caused by a mutation in the sulfonylurea receptor type 1. J Clin Invest 2000;106:897–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinney SE, MacMullen C, Becker S, et al. Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest 2008;118:2877–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flanagan SE, Kapoor RR, Banerjee I, et al. Dominantly acting ABCC8 mutations in patients with medically unresponsive hyperinsulinaemic hypoglycaemia. Clin Genet 2011;79:582–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macmullen CM, Zhou Q, Snider KE, et al. Diazoxide-unresponsive congenital hyperinsulinism in children with dominant mutations of the β-cell sulfonylurea receptor SUR1. Diabetes 2011;60:1797–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.