Glucose homeostasis reflects the integrated efficiencies of the islet β-cell to secrete insulin and of metabolically active tissues to transduce insulin signals. Conceptually, defects in either insulin secretion or insulin signaling can lead to glucose intolerance or frank diabetes. In the case of type 2 diabetes (T2D), the primary etiology of the disease is not only of academic significance but has important implications for the design of therapeutics. In recent years, there has been increasing evidence from rodent and human studies pointing to a primary defect in β-cell function in T2D. The results of genome-wide association studies provide genetic credence to the notion that defects in insulin secretion may be primarily causative of disease (1). Insulin secretion by the β-cell occurs in two distinct phases after an intravenous glucose load, with an acute phase occurring within minutes and a chronic phase persisting through the end of the glucose challenge. Among the earliest defects observed in humans and mice with T2D or at risk for T2D is the predominant loss of the acute phase of insulin secretion (2,3), and restoration of the acute phase can improve glycemic control in T2D subjects (4).
At present, only two major classes of drugs used clinically in T2D are capable of enhancing insulin release: sulfonylureas and incretins. Sulfonylureas cause closure of ATP-sensitive K+ channels, resulting in β-cell depolarization and calcium influx from voltage-dependent calcium channels, and leads to insulin granule exocytosis. The obvious advantage of sulfonylureas in T2D is enhanced insulin secretion, but a prominent drawback is that secretion occurs in a glucose-independent manner, increasing the risk of hypoglycemia (5,6). Whereas an increase in cytosolic calcium is required to trigger insulin exocytosis, additional metabolic and neurohormonal inputs play a vital role in the potentiation or amplification of insulin secretion. Chief among these inputs is signaling through the incretin hormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide. Notably, T2D is characterized by reduced secretion and action of GLP-1 (7), and extended infusions of GLP-1 are able to enhance the acute phase of insulin secretion to a greater extent than the chronic phase in both normal and T2D subjects with little risk of hypoglycemia (8).
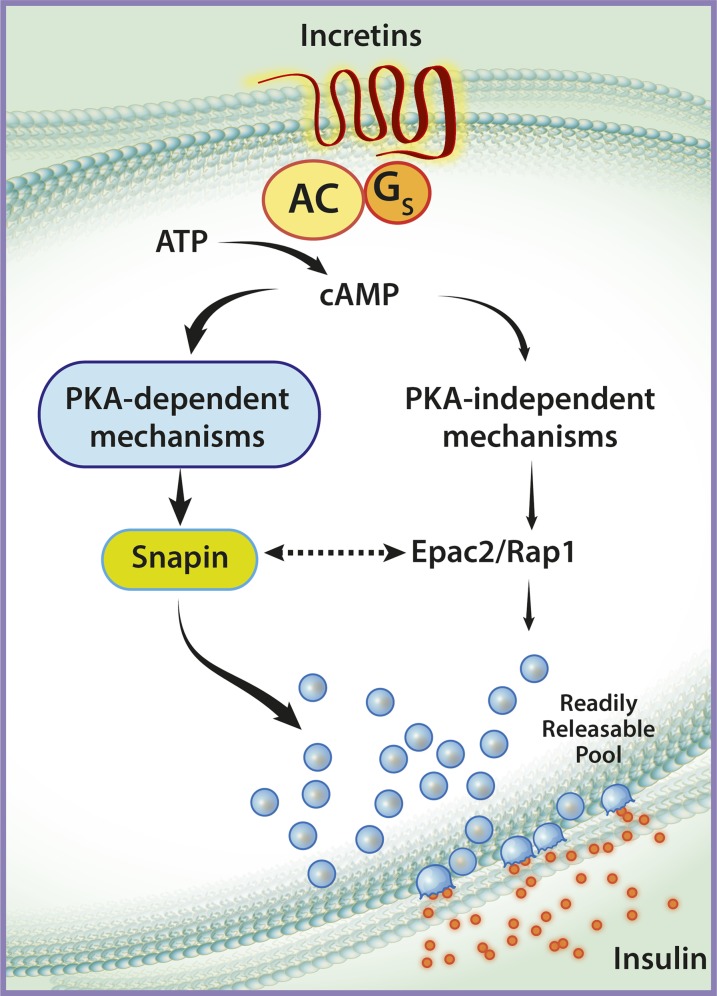
Incretins act through G-protein–coupled receptors on the β-cell plasma membrane to stimulate adenylyl cyclase and increase intracellular cyclic AMP (cAMP). The cAMP response in the β-cell is predominantly transmitted by two molecular pathways: a cAMP-dependent protein kinase A (PKA)-mediated pathway and a PKA-independent pathway (Fig. 1). PKA-dependent effects occur largely through phosphorylation of specific proteins such as Snapin, which are directly involved in the exocytosis process (9). PKA-independent effects are mediated through the cAMP-regulated guanine nucleotide exchange factor Epac2, which is thought to act in concert with the G-protein Rap1 to regulate the size of the readily releasable pool of insulin granules (10,11).
FIG. 1.
Incretin-induced cAMP signaling in the pancreatic β-cell. Incretins interact with G protein–coupled receptors at the cell membrane to stimulate production of cAMP from ATP. Subsequently, cAMP is thought to signal via PKA-dependent or PKA-independent pathways. The PKA-dependent pathway (left side of the figure) results in the phosphorylation of proteins such as Snapin, which activate the exocytosis machinery to cause fusion of insulin granules from a readily releasable pool. The PKA-independent pathway (right side of the figure) involves interactions between Epac2 and Rap1, which regulate the size of the readily releasable pool of insulin granules. The figure emphasizes the involvement of the PKA-dependent pathway in the stimulation of insulin release, as reflected by the studies of Kaihara et al. The figure also shows the potential interactions between the two major cAMP-signaling pathways (dotted arrow), wherein direct interactions between Snapin and Epac2 may be important for insulin release. AC, adenylyl cyclase; Gs, stimulative regulatory G protein.
Despite proposal of these two distinct cAMP-mediated pathways, the precise integration of PKA and Epac2 signals and their relative roles in the temporal regulation of insulin secretion remain incompletely understood. In this issue of Diabetes, Kaihara et al. (12) elegantly address these uncertainties by studying the phenotype of mice with constitutively active β-cell PKA (β-cell caPKA mice). In this novel model, basal β-cell PKA activity is increased approximately 10-fold compared with littermate controls, while maximal PKA activity in response to exogenous cAMP did not differ compared with control islets. During a hyperglycemic clamp, β-cell caPKA mice demonstrate a marked 2.5-fold enhancement of the acute phase of insulin secretion. Perifusion studies using isolated islets from β-cell caPKA mice confirmed the enhanced acute-phase insulin release, but additionally revealed a significant increase in the chronic phase of insulin release with sustained glucose stimulation.
The phenotype of the β-cell caPKA model is reminiscent of that observed by Song et al. (9) who recently showed that PKA disinhibition through conditional ablation of the gene encoding the inhibitory PKA regulatory subunit 1A (Prkar1a) also led to a remarkable increase in insulin secretion following both oral and intraperitoneal glucose administration. In that study, Song et al. used a knockout strategy wherein Prkar1a was deleted pancreas-wide from the inception of pancreas development, leaving open the possibility that effects of the knockout may have only secondarily affected the β-cell. By contrast, Kaihara et al. used an inducible Cre recombinase with demonstrated β-cell specificity (13), leaving little doubt of the intrinsic role for PKA in the β-cell.
Because incretins enhance acute-phase insulin secretion via cAMP, an important question arises whether this effect is via the PKA, Epac2, or both. The authors address this question in two ways: by oral glucose tolerance testing (whereby endogenous incretins are enhanced) and by exogenous administration of the GLP-1 mimetic exendin-4. Interestingly, no additional augmentation in acute-phase insulin release was observed when β-cell caPKA mice were challenged in either way. The authors suggest that increased PKA activity in this context may be sufficient to modulate the incretin effect on acute insulin secretion, de-emphasizing the need for Epac2. Although cAMP-induced vesicle fusion events are impaired in Epac2 knockout islets (10), insulin secretion and glucose tolerance appear to be unaffected by Epac2 deficiency (14). Interestingly, in the Prkar1a ablation model PKA-induced phosphorylation of Snapin and the interaction between Snapin and Epac2 were necessary for PKA-mediated insulin secretion. This finding shows significant overlap between PKA-dependent and -independent effects and suggests that these two arms of cAMP signaling are not completely independent of one another. Future studies that couple PKA activation in an Epac2-deficient model, or vice versa, may be able to definitively address which pathway plays the larger role in incretin augmentation of acute-phase insulin secretion.
Lastly, a notable finding in both the β-cell caPKA and Prkar1a ablation models was the absence of hypoglycemia at baseline or after glucose challenge—a finding also observed in humans with inactivating PRKAR1A mutations (9). This unique regulatory aspect of PKA physiology underscores the importance of continued interrogation of this pathway as a means to safely and effectively restore acute-phase insulin secretion in diabetes.
ACKNOWLEDGMENTS
Research in the laboratory of C.E.-M. is supported by National Institutes of Health (NIH) grants K08 DK080225, R03 DK 089147, and R01 DK093954 and by grants from the Juvenile Diabetes Research Foundation, the George and Frances Ball Foundation, and the Ball Bros. Foundation. Research in the laboratory of R.G.M. is supported by NIH grants R01 DK060581and R01 DK083583 and by grants from the Juvenile Diabetes Research Foundation, the George and Frances Ball Foundation, and the Ball Bros. Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 1527.
REFERENCES
- 1.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007;445:881–885 [DOI] [PubMed] [Google Scholar]
- 2.Nijpels G, Boorsma W, Dekker JM, et al. Absence of an acute insulin response predicts onset of type 2 diabetes in a Caucasian population with impaired glucose tolerance. J Clin Endocrinol Metab 2008;93:2633–2638 [DOI] [PubMed] [Google Scholar]
- 3.Nyholm B, Pørksen N, Juhl CB, et al. Assessment of insulin secretion in relatives of patients with type 2 (non-insulin-dependent) diabetes mellitus: evidence of early beta-cell dysfunction. Metabolism 2000;49:896–905 [DOI] [PubMed] [Google Scholar]
- 4.Basu A, Alzaid A, Dinneen S, Caumo A, Cobelli C, Rizza RA. Effects of a change in the pattern of insulin delivery on carbohydrate tolerance in diabetic and nondiabetic humans in the presence of differing degrees of insulin resistance. J Clin Invest 1996;97:2351–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu SC, Tu YK, Chien MN, Chien KL. Effect of antidiabetic agents added to metformin on glycaemic control, hypoglycaemia and weight change in patients with type 2 diabetes: a network meta-analysis. Diabetes Obes Metab 2012;14:810–820 [DOI] [PubMed] [Google Scholar]
- 6.Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 7.Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab 2001;86:3853–3860 [DOI] [PubMed] [Google Scholar]
- 8.Quddusi S, Vahl TP, Hanson K, Prigeon RL, D’Alessio DA. Differential effects of acute and extended infusions of glucagon-like peptide-1 on first- and second-phase insulin secretion in diabetic and nondiabetic humans. Diabetes Care 2003;26:791–798 [DOI] [PubMed] [Google Scholar]
- 9.Song WJ, Seshadri M, Ashraf U, et al. Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab 2011;13:308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibasaki T, Takahashi H, Miki T, et al. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA 2007;104:19333–19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seino S, Takahashi H, Fujimoto W, Shibasaki T. Roles of cAMP signalling in insulin granule exocytosis. Diabetes Obes Metab 2009;11(Suppl. 4):180–188 [DOI] [PubMed] [Google Scholar]
- 12.Kaihara KA, Dickson LM, Jacobson DA, et al. β-Cell–specific protein kinase A activation enhances the efficiency of glucose control by increasing acute-phase insulin secretion. Diabetes 2013;62:1527–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wicksteed B, Brissova M, Yan W, et al. Conditional gene targeting in mouse pancreatic ß-Cells: analysis of ectopic Cre transgene expression in the brain. Diabetes 2010;59:3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang CL, Katoh M, Shibasaki T, et al. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 2009;325:607–610 [DOI] [PubMed] [Google Scholar]