Dysregulated secretion of insulin, genetically determined and mediated by biochemical (e.g., phosphorylation) or biophysical (e.g., opening/closing of ion or voltage-gated channels) mechanisms is responsible for disorders of glucose homeostasis at both hyper- and hypo extremes of the glycemic spectrum (1–3). For hyperglycemia, the discoveries relating to genetic control of the development of the pancreas and the regulation of insulin secretion have revolutionized understanding of the etiology of rare and more common forms of diabetes. In addition, these insights enable rational treatments as best exemplified by the use of sulfonylureas to restore endogenous insulin secretion and ameliorate neurological impairment in neonatal diabetes caused by mutations in the ATP-sensitive K+ channel (KATP) channel (3).
For hypoglycemia, the current model recognizes nine genes involved in syndromes associated with dysregulated and excessive insulin secretion and hypoglycemia in infancy (HI), presenting, with some exceptions, mostly in the first year of life (Fig. 1) (1,2). An identical clinical (macrosomia) and biochemical (hypoglycemia, hypoketonemia, and hypo-fatty acidemia) phenotype, but with virtually undetectable insulin concentrations in serum, can result from activating mutations in AKT2, part of the insulin receptor signal transduction cascade (4). In this syndrome there is asymmetrical growth, as also occurs with activating mutations in AKT1 (5) (Proteus syndrome) and AKT3 (6), indicating that each isoform has a specific role in growth and metabolism, rather than representing evolutionary redundancy. The most common mutations involved in excessive insulin secretion are in the KATP genes, predominantly ABCC8 specifying the protein SUR-1 and occasionally the KCNJ11 gene, which specifies the inward rectifying potassium channel itself (KIR 6.2) (Fig. 1). Although autosomal recessive and some autosomal dominant forms of KATP channel defects result in diffuse involvement of the pancreatic islets, about 50% of neonatal HI caused by KATP mutations is due to a “double hit” mechanism, via inheritance of a paternal mutation and “extinction” of the normal and protective maternal allele in a patchy distribution resulting in focal lesions (1,2). In most but not all cases, positron emission tomography scanning with 18F-L-Dopa permits distinction of the diffuse from the focal form with high resolution and accuracy, providing preoperative guidance for surgical excision of the focal lesion resulting in cure, rather than the extensive resection of a diffusely involved pancreas which may not only fail to resolve the hypoglycemia in the short term but also evolves into diabetes later in life (7).
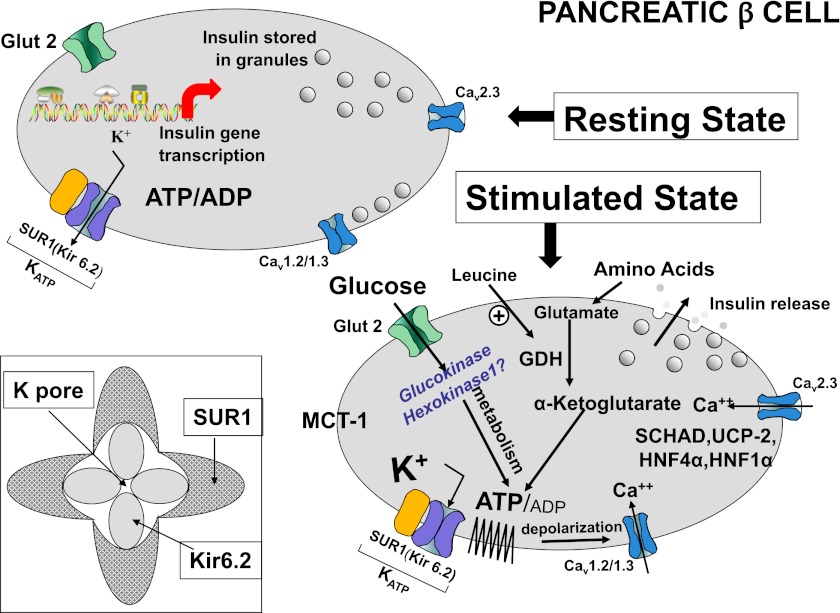
FIG. 1.
The question mark following hexokinase I indicates that the current article suggests but has not proven this mutation to be responsible for the syndrome described. Glucose and amino acids stimulate insulin release by generating ATP, which leads to closure of ATP-sensitive plasma membrane potassium channels, plasma membrane depolarization, activation of voltage-sensitive calcium channels, an increase of cytosolic calcium, and release of insulin from storage granules. Leucine is an allosteric activator of glutamate dehydrogenase that enables protein metabolism. Inactivating mutations in the KATP channel lead to closure and hence excessive unregulated insulin secretion causing hypoglycemia—these mutations may respond to diazoxide, an agent that promotes the opening of these channels. By contrast, activating mutations of the KATP keep the channel open, preventing insulin secretion and hence causing diabetes of varying degrees. These defects may be amenable to therapy with sulfonylureas that act on the sulfonylurea receptor 1 regulatory component to overcome the open state, induce closure, and hence restore insulin secretion. GDH, glutamate dehydrogenase; HK1, hexokinase I; HNF4α, hepatic nuclear factor 4α; HNF1α, hepatic nuclear factor 1α; Kir 6.2, inwardly rectifying potassium channel 6.2; MCT-1, monocarboxylic acid transporter 1; SCHAD, short-chain 3-OH acyl-CoA dehydrogenase; SUR1, sulfonylurea receptor 1; UCP2, uncoupling protein 2.
Activating mutations of glucokinase (GCK) (Fig. 1), the “glucose sensor” of the β-cell, are rare and, depending on the mutation, may cause fasting hypoglycemia in varying degrees at varying ages of life (8). GCK, also known as hexokinase IV or hexokinase D, has a lower affinity for glucose than other hexokinases and is most active in the physiological range of glucose of 4–10 mmol/L (72–180 mg/dL) with a Km of ∼8 mmol/L (144 mg/dL). There are other mammalian hexokinases, known as I, II, and III (A, B, C), designated as low-Km enzymes, displaying high affinity for glucose even at glucose concentrations as low as 1 mmol/L (18 mg/dL) or less. Hexokinase I/A, is found in all mammalian tissues and is considered a housekeeping enzyme, usually not regulated by hormonal or metabolic processes. Hexokinase II/B is the principal regulated isoform present in various cell types, whereas hexokinase III/C is normally substrate inhibited by glucose at physiological concentrations (9). Mutations in these isoforms have not, until recently, been implicated in any form of HI. Some inactivating mutations in the KATP channel, non- KATP focal lesions recently described, as well as glutamate dehydrogenase, short-chain 3-OH acyl-CoA dehydrogenase, and GCK (Fig. 1) are responsive to diazoxide, an agent used to keep the channel in an open and hence insulin-nonsecreting state. Despite the mounting and impressive body of data on genetic defects causing hyperinsulinism, the cause of ∼50% of syndromes of congenital hyperinsulinism in infants remains unknown (1,2).
In the current issue of Diabetes, Henquin et al. (10) describe the in vitro insulin secretory characteristics of fragments of pancreas from six patients with focal lesions not due to KATP mutations who underwent surgery and focal excision after sensitivity to diazoxide was lost; adjacent “normal” islet tissue removed at surgery served as controls. A brisk insulin response to 1 mmol/L glucose suggested the presence of low-Km hexokinase I, and this was confirmed by immunohistochemistry, which revealed its presence only in the β-cells of hyperfunctioning islets in five of six cases. In the sixth case, a known activating mutation in GCK was identified. Response to 15 mmol/L glucose was normal, as was the insulin secretory response to tolbutamide and suppression by diazoxide. The authors propose that somatic genetic events were responsible for both the confirmed mutation in the GCK and the aberrant responses in those β-cells retaining hexokinase I staining. These postzygotic events would explain the patchy, focal nature of the lesions along with normal secretory response to 15 mmol/L glucose or tolbutamide while retaining normal suppression by diazoxide. Although the reasoning is plausible, the case for hexokinase I mutation is not conclusive because genetic analysis of hexokinase I was not undertaken, as the authors themselves acknowledge. The authors indicate that “amounts of available tissue are limited, which restricts the number of possible investigations.” Still, this limitation was not apparent in the elegant mutational analysis for GCK, which proved positive in the sixth case. An additional quandary is the finding that in three out of four cases, in vitro insulin secretion was higher in the pathological than normal pancreatic tissue, even in the absence of glucose. Thus, the hypersecretion of insulin of these patients with the HI syndrome is more generalized and appears not to be solely restricted to an activating hexokinase mutation.
A more generalized defect in intrauterine insulin secretion is also suggested by the fact that birth weight was, on average, in the 64th percentile; the two subjects with neonatal presentation had birth weights in the 86th and 90th percentiles. Those with lower birth weights generally presented later, less severe manifestations, and in at least half of them positron emission tomography scanning was “inconclusive.” Furthermore, only three of the six patients were “cured” by the focal resection; recurrence occurred in the remaining three who then responded to diazoxide. All of these findings raise the possibility that the defect in insulin secretion was more generalized, at least in some subjects, with severity of presentation correlated to severity of the defect in insulin secretion.
Fetal glucose concentration is usually ∼80% that of the mother and hence would normally suffice to shut off hexokinase I activity unless fetal glucose fell to concentrations of 1 mmol/L or less. So, what possible purpose does its presence serve? It is now generally accepted that early in fetal development insulin acts more as a vital growth factor in utero rather than as the major regulator of carbohydrate metabolism. For example, insulin receptor number and tyrosine kinase phosphorylation are increased in a variety of fetal tissues including liver (11). However, in-vitro studies suggest that glucose concentration itself is the determinant of its incorporation into glycogen, without a demonstrable increase induced by insulin, whereas in adult tissue, over a wide range of glucose concentrations, insulin enhances the glucose incorporation into glycogen (11). Hence, it is tempting to propose that retention of an insulin secretory response to low glucose concentrations via hexokinase I may protect or facilitate in utero fetal growth, especially early in gestation when maternal glucose supply is diminished. Mutations in this enzyme might permit its expression at higher glucose concentrations and produce a syndrome of HI as recently proposed in a large kindred with HI, autosomal dominant transmission, and responsiveness to diazoxide first described by McQuarrie in 1954 (12). The current article suggests that hexokinase I mutation can also be postzygotic and localized in only some islets, but the findings are not yet conclusive, so a question mark remains for hexokinase I as indicated in the figure.
Why the lesions and/or mutations are focal and patchy is another mystery, though the phenotype of postzygotic mutation is commonly patchy, as noted in McCune-Albright syndrome, and even in acquired autoimmune entities such as pancreatic changes in type 1 diabetes or the cutaneous manifestations of vitiligo. These somatic mutations may be occurring in tissues other than pancreatic islets or skin, but remain undetected because their function is not as evident as that of abnormal insulin secretion that results in hypoglycemia or the cutaneous pigmentary changes seen in McCune-Albright syndrome.
The findings in the current report by Henquin et al. (10) are valid, though interpretation of some of the results may be open to debate. They remind us how much more remains to understand the normal regulation of insulin secretion and how, when, and where it is disturbed in such syndromes as HI.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
The author thanks Dr. Ram K. Menon for his valuable insights in reviewing this commentary and in the design of the figure.
Footnotes
See accompanying original article, p. 1689.
REFERENCES
- 1.Dekelbab BH, Sperling MA. Hypoglycemia in newborns and infants. Adv Pediatr 2006;53:5–22 [DOI] [PubMed] [Google Scholar]
- 2.De León DD, Stanley CA. Mechanisms of Disease: advances in diagnosis and treatment of hyperinsulinism in neonates. Nat Clin Pract Endocrinol Metab 2007;3:57–68 [DOI] [PubMed] [Google Scholar]
- 3.Murphy R, Ellard S, Hattersley AT. Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab 2008;4:200–213 [DOI] [PubMed] [Google Scholar]
- 4.Hussain K, Challis B, Rocha N, et al. An activating mutation of AKT2 and human hypoglycemia. Science 2011;334:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindhurst MJ, Sapp JC, Teer JK, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med 2011;365:611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindhurst MJ, Parker VE, Payne F, et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat Genet 2012;44:928–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardy OT, Hernandez-Pampaloni M, Saffer JR, et al. Accuracy of [18F]fluorodopa positron emission tomography for diagnosing and localizing focal congenital hyperinsulinism. J Clin Endocrinol Metab 2007;92:4706–4711 [DOI] [PubMed] [Google Scholar]
- 8.Marquard J, Palladino AA, Stanley CA, Mayatepek E, Meissner T. Rare forms of congenital hyperinsulinism. Semin Pediatr Surg 2011;20:38–44 [DOI] [PubMed] [Google Scholar]
- 9.Quintens R, Hendrickx N, Lemaire K, Schuit F. Why expression of some genes is disallowed in β-cells. Biochem Soc Trans 2008;36:300–305 [DOI] [PubMed] [Google Scholar]
- 10.Henquin J-C, Sempoux C, Marchandise J, et al. Congenital hyperinsulinism caused by hexokinase I expression or glucokinase-activating mutation in a subset of β-cells. Diabetes 2013;62:1689–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon RK, Sperling MA. Insulin as a growth factor. Endocrinol Metab Clin North Am 1996;25:633–647 [DOI] [PubMed] [Google Scholar]
- 12.Pinney SE, Ganapathy K, Bradfield J, et al. Novel form of autosomal dominant hyperinsulinism maps to chromosome 10q21 [abstract]. Monogenic Disorders of Insulin Secretion: Congenital Hyperinsulinism and Neonatal Diabetes, March 15–16, 2012 Faculty Synopsis. Pediatric Diabetes 2012;13:358 [Google Scholar]