Abstract
Background and aims: The goal of this study was to evaluate the efficacy of platelet dressing in the treatment of burn wounds and compare its results with silver sulfadiazine dressing. Material and methods: Between 21 march 2011 to 21 September, 50 patients with burn injuries were selected by a randomized double-blind controlled trial. In order to eliminate the biological and personal variables among the various treated burn wounds, in the same patient, distal or proximal, lateral or medial part of burn wound were selected for dressing with platelet or silver sulfadiazine. All patients were designated for homologous component use. The dressing was repeated every day up to complete healing. Results: The results indicated that treatment with platelet enhanced epithelialization and accelerate epithelialization and granulation tissue formation. Platelet dressing to be most significant in this respect compared with silver sulfadiazine dressing. Conclusion: It is concluded that topical application of platelet enhanced the wound healing process in burn patients.
Keywords: Platelet, burn wound, clinical study
Introduction
Humans, unlike (for instance) salamanders, lack the ability to regenerate specialized structures; instead, they heal by forming a scar that lacks the complex and important skin structures seen in unwounded skin [1].
The treatment and healing of wounds are some of the oldest subjects discussed in the medical literature. The same events, in the same order, occur in every healing process regardless of the tissue type or the inciting injury. Knowledge of the steps involved allows physicians to manipulate wounds to achieve optimal results in a short period. With recent basic science discoveries of the 1980s and 1990s, physicians can now manipulate the wound with cellular and molecular biology techniques and thus improve outcomes. Even with recent advances, the exact mechanisms underlying wound healing are not completely understood. Normal wound healing follows a predictable pattern that can be divide into over-lapping phases defined by the cellular populations and biochemical activities: 1: hemostasis and inflammation; 2: proliferation, and 3: maturation and remodeling. This sequence of events in fluid and overlapping, and in most circumstances spans the time from injury to resolution of acute wounds. All wounds needs to progress through this series of cellular and biological events that characterizes the phases of healing in order to successfully re-establish tissue injury. The wounds that the burn team deals, will do not follow the simplified healing of an incision. With large wounds as burns, all three phases tend to blend together.
Studies on the restoration of tissue integrity have shown the role of the platelets in the wound healing process: during coagulation and the inflammation phase, the formation of a blood clot induces adhesion, aggregation and degranulation of circulating platelets. Platelet x-granules release numerous GF: platelet derived growth factor (PDGF) [2], transforming growth factor beta (TGF-B) [3], epidermal growth factor (EGF) [4], insulin -like growth factor-1 e 2 (IGF 1-2) [5], and vascular endothelial growth factor (VEGF) [6]. These factors play an important role in the tissue remodeling phase (re-epithelialization and neovascularization) by mesenchymal cell recruitment and extra-cellular matrix synthesis [7,8].
Platelet derived growth factor (PDGF) stimulate collagen and proteoglycans synthesis. PDGF exists as three isomers: PDGF-AA, PDGF-AB, AND PDGF-BB; PDGF-BB is currently the only growth factor licensed for topical use by the US Food and Drug Administration and is the most studied clinically. The efficacy of recombinant PDGF-BB (becaplermin gel) in diabetic foot ulcers has been proven in a number of randomized trials [9,10]. In a meta-analysis of 4 randomized controlled trials, patients with a median ulcer area of 1.5 cm2 treated with becaplermin gel at a concentration of 100 µg/g, achieved a 39% higher healing rate when compared to placebo gel [11]. Topical application of recombinant PDGF improves wound healing-breaking strength and healing time in both human and murine of acute wounding. Administration of PDGF-BB improved wound closure in chronic and diabetic nonhealing ulcers in both humans and rodents but did not have the same effect in steroid- treated animals.
Most of cytokines and growth factors have the potential to improve wound healing through several mechanisms [1,7,8,12]: 1. They have chemotactic activities that attract inflammatory cells, fibroblasts and keratinocytes into the wound; 2. They act as mitogens to stimulate cellular proliferation; 3. Cytokines and growth factors can stimulate angiogenesis, the in growth of new blood vessels into the wound; 4. They have a profound effect on the production and degradation of the extracellular matrix; 5. They influence the synthesis of other cytokines and growth factors by neighboring cells.
TGF-1 isolated from blood platelets has major effect on tissues of mesenchymal origin. It has been shown to induce wound repair following application in rats and in humans. Addition of TGF-3 prior to experimentally induced excision wounds enhanced wound repair [12]. Growth factors are good candidates for the treatment of wound healing because they are naturally produced by the cells and enhance tissue repair. Clinical studies have shown that FGF applied to chronic and diabetic ulcers significantly enhanced their repair [13].
The use of cytokines to enhance wound healing is crucial in cases of burns, chronic pressure wounds, diabetic wounds, and chronic ulcers. The possibility of enhancing wound healing by cytokines is beneficial in long-term hospitalization, in the elderly, in accident wounds, and burns sustained on the battle field [13-15].
The purpose of the present research was to study possible ways of enhancing burn wound injury repaired by using platelet dressing and to compare with the effect of silver-sulfadiazine dressing.
Materials and methods
A randomized double-blind controlled trial was used to study the efficacy platelet dressing in promoting healing of burn wounds. Between 21 March 2011 and 21 September 2011, 50 patients from our burn center were screened. Predetermined criteria used for patient selection were grade II and III burn wounds.
In order to eliminate the biological and personal variables among the various treated burn wounds, in the same burn patient, distal or proximal, lateral or medial part of burn wound were selected for dressing with platelet or silver sulfadiazine. The burned area uniform in each patient for dressing with platelet or silver sulfadiazine (e.g. grade II or III in all area studied in each patient). Patients in two groups were matched by age, sex, TBSA%, and depth of burns. Patients are excluded if there is uncontrolled infection or cellulitis at the site of target ulcer or if there is vascular insufficiency in the wound area. Inclusion criteria are: grade II or III burns with TBSA%<20%. The study protocol and consent form were approved by the ethics committee of the Tabriz University Medical Sciences and the Sina hospital. The blood bank department of our hospital prepared the platelet concentrate, and both patients and physicians were blind to the type and location of dressing. All patients had been recommended which did not tell anything about dressing for blind visiting physicians. The dressing is removed by nursing and the wound cleaned thoroughly before the physician making the assessment is allowed to look the wound. All patients are designated for homologous component use. The traceability and suitability of the hemocomponents is assured according to the hemovigilance criteria: ABO compatibility, Rh compatibility, donor/patient cross match, and biological qualification.
Informed written consent from the patient is always acquired. Platelet is applied to the burn bed and then covered by an occlusive dressing. Antiseptics did not used with the platelet dressing and platelet dressing area was cleaned with normal saline. The dressing was repeated every day up to complete healing. Photo-documentation of the target ulcer is performed at recruitment time and also periodically during the therapeutic period. The response to topical hemotherapy with platelet concentrate is evaluated according to the following criteria: wound area re-epithelialization, granulation tissue formation. All wounds were cultured at first day and 5th day of admission in all patients. After granulation tissue formations, wounds were grafted in deep second and third degree burns.
Results
A total of 50 patients were screened between 21 March2006 and 21 September 2006. The end of point of treatment was defined as complete re-epithelialization or granulation of the burn wound. The base line profile of the two wound group was similar in regard to wound size and burn grade (Table 2). 45 of patients (90%) in the platelet group achieved complete healing, which is a significantly higher value than those obtained for the silver sulfadiazine group [19 patients (38%), (P<0.0001)]. Patients in the platelet group also healed more quickly than those in the silver sulfadiazine group (Figures 1, 2, 3, 4 and Table 1). The mean time to complete healing in the platelet group was 9.5-/+4.6days versus 12.2+/-5.4 in the silver sulfadiazine group (P<0.0001). Healing rates were 90% and 86% for the silver sulfadiazine group and platelet group, respectively. There was significant difference in healing time between the plate and silver sulfadiazine groups (Table 1, P<0.0001).
Table 2.
Demographic data of patients
| Demographic data | Platelet group | Silver sulfadiazine group |
|---|---|---|
| Mean age | 27.4 (range; 2-56) | 27.4 (range 2-56) |
| mean TBSA% | 14.5 (range 10-19) | 14.5 (10-19) |
| Depth of burns | 45 grade II, 5 grade III | 43 grade II, 7 grade III |
| Blood culture | Negative | Negative |
| Wound culture | Mixed: 4, cocci: 27, bacilli: 10 | Mixed: 3, cocci: 24, bacilli: 7 |
| Mortality | None | None |
Figure 1.
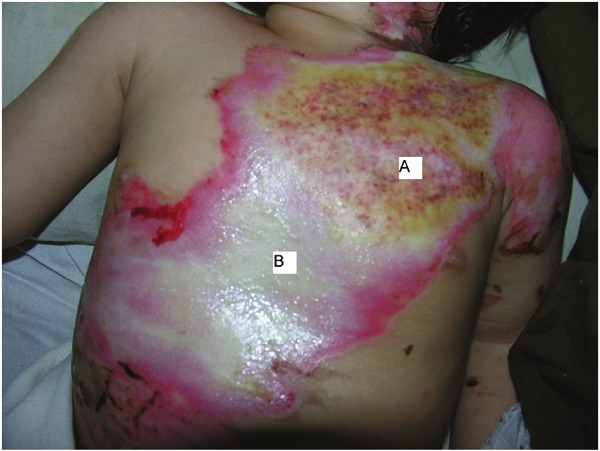
Morphologic evaluation of paired grade II burn wounds treated with platelet or silver sulfadiazine dressing on 4th days after burn injury; A: silver sulfadiazine, B: platelet.
Figure 2.
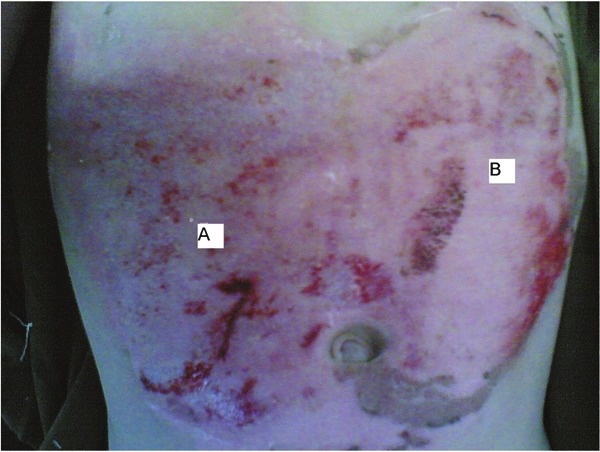
Morphologic evaluation of paired grade II burn wounds treated with platelet or silver sulfadiazine dressing on 6th days after burn injury; A: silver sulfadiazine, B: platelet.
Figure 3.

Morphologic evaluation of paired grade II burn wounds treated with platelet or silver sulfadiazine dressing on 5th days after burn injury; A: silver sulfadiazine, B: platelet.
Figure 4.
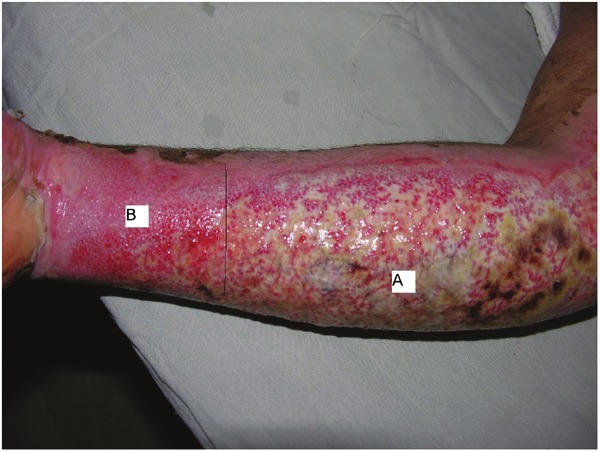
Morphologic evaluation of paired grade III burn wounds treated with platelet or silver sulfadiazine dressing on 7th days after burn injury; A: silver sulfadiazine, B: platelet.
Table 1.
Days until reepithelialization and granulation of burn wounds in platelet and silver sulfadiazine dressing
| Number of patients (wounds) | Minimum (days) | Maximum (days) | Mean (days) | S.T Deviation | P Value | |
|---|---|---|---|---|---|---|
| Date of first epithelial tissue occur (platelet) | 45 | 3 | 10 | 5.7 | 1.9 | 0.0001 |
| Date of first epithelial tissue occur (silver sulfadiazine) | 43 | 3 | 15 | 7.4 | 2.4 | <0.0001 |
| Date of epithelialization completed (platelet) | 45 | 4 | 22 | 9.5 | 4.6 | <0.0001 |
| Date of epithelialization completed (silver sulfadiazine) | 43 | 5 | 26 | 12.2 | 5.4 | <0.0001 |
| Date of granulation tissue completed (platelet) | 5 | 5 | 16 | 9.8 | 4.3 | <0.007 |
| Date of granulation tissue completed (silver sulfadiazine) | 7 | 7 | 18 | 11.7 | 3.9 | <0.007 |
The mean follow-up time was 16 week (range; 12-18). The fact that all of the burn wounds from the platelet group healed with a mean healing time of 9.5 days supports the efficacy of platelet in enhancing burn wound healing. No adverse effects have been occurred so far. Patients’ compliance was optimum and all the patients agreed that the pain decreased during platelet dressing.
Discussion
Major burn is a particularly severe form of trauma characterized by a hypermetabolic state. This vulnerable state compromises the immune system and attenuates wound healing. Moreover, it causes tissue damage by membrane destabilization and energy depletion at the cellular level, resulting in tissue necrosis [16,17]. A logical therapeutic approach to promote recovery after burn would therefore, be to block the immediate triggering of the inflammatory cascades that result in prolonged metabolic imbalances. A second component of the therapy would be to enhance wound healing, several molecular elements of which are regulated in part by components of the inflammatory cascade [16]. The prospects that the effect of “positive” growth hormones and cytokines may be enhanced and that of “negative” factors suppressed through molecular or genetic manipulation open new therapeutic venues [16]. Findings suggest that an endogenous growth factor mediated pathway during wound repair may be amenable to exogenous manipulation [16,17]. As promising as it may be, research into this domain has yet to overcome numerous obstacles the least of which is still our incomplete understanding of the intricate mechanisms involved. Local application of cytokines as proteins has been shown to be ineffective and of little clinical value due to enzymes and proteases locally present in the wound and because of lack of adequate receptors [16-18]. Large amounts of systemic cytokines needed for the desired therapeutic effects may result, however, in serious side-effects limiting their potential therapeutic utility in burn treatment [16]. Although gene therapy is emerging as an effective therapeutic approach to improve clinical outcomes after thermal injury [16,17], numerous hurdles still need to be overcome before these new technologically advanced modalities become practical for routine clinical usage [16]. Cytokine modulation by local application of therapeutic agents is an extremely appealing modality. Interpretation of these effects and determination of the exact significance of specific cellular and cytokine modulation on wound healing, in isolation or in combination with other cells and cytokines, remain to be clearly made.
The main conclusion of this study is that all burn wounds completely healed or granulation tissue occurred with daily application in less time under the management of a multidisciplinary team. The difference in healing rate compared with that seen without the platelet dressing was statistically significant. The healing with platelet was accompanied by a significant reduction in mean healing time. A Medline search using the key phrases ‘platelet dressing’, ‘clinical study’ and ‘burn wound’ did not yield a randomized control study with platelet dressing for the management of burn wounds. To our knowledge, this is the first study to demonstrate significant and positive effects of platelet in the healing of burn wounds in a randomized double-blind control trial.
Progressive skin necrosis after trauma such as burn wounds and local trauma is a frequent occurrence. During this process, tissue initially appears to be viable, with clinical evidence of perfusion. This tissue eventually dies, which has a profound clinical significance as the ultimate tissue loss in much greater than that estimated at the time of initial injury. The injured skin directly initiates an inflammatory response that includes the release of neutrophil chemoattractants [14,15]. It is possible that during the process of certain cytokines and growth factors levels are low or missing, which could slow down or inhibit the healing process [1,15].
Our innovation will contribute to the improvement of burn wound healing. We proved that platelet significantly reduced the total healing time more than the silver sulfadiazine. This was to be expected as we are aware of platelet role in the tissue remodeling phase.
In conclusion, our data support the contention that platelet dressing enhances burn wound healing and significantly reduces the healing time. Further study is required to define the optimal platelet dressing dose, the optimal frequency of application, and potential interaction of platelet factors with other factors in promoting burn wound healing.
In the light of the promising and encouraging results of our study, we are optimistic as regards the power of our innovation to contribute to the improvement and enhancement of burn wounds. To our best knowledge there are no recognized limitations to this treatment. However, further large-scale experiments are needed before clinical trials can be performed. In addition, more studies are needed in order to understand the mechanisms of this enhancement of wound healing by platelet dressing. A great deal more needs to be discovered about the concentration, temporal release and receptor cell population before growth therapy is to make a consistent impact on wound healing.
Topical agents may have profound effects on wound healing kinetics and should not be used indiscriminately without understanding the basic mechanisms involved. Silver sulfadiazine, undoubtedly is a very efficient antibacterial agent, however, its effect on wound healing is rather negative. Its use, therefore, should be tailored to each particular situation. It must be stressed also that the various available silver sulfadiazine preparations may not have the same effects on the wound bed and on the healing mechanisms. Other agents or base vehicles present in the preparation being used may have an effect of their own that should not be attributed to silver sulfadiazine as such. The ultimate effective topical burn therapy remains in the choice of a product with a superior profile of antimicrobial activity over cellular toxicity. Topical intervention should be modified as well as the wound status is changing opting for the treatment modality with optimal modulation potential of wound healing kinetics.
References
- 1.Greenhalah G. The role of growth factors in wound healing. J Trauma. 1996;41:159–67. doi: 10.1097/00005373-199607000-00029. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Platelet-drived growth factor. Ann Rev Med. 1987;38:71–9. doi: 10.1146/annurev.me.38.020187.000443. [DOI] [PubMed] [Google Scholar]
- 3.O’Kane S, Ferguson MW. Trasforming growth factor betas and wound healing. Int J Biochem Cell Biol. 1997;29:63–78. doi: 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- 4.Cohen S, Carpenter G. Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci U S A. 1975;72:1317–21. doi: 10.1073/pnas.72.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhora FY, Dunkin BJ, Batzri S, Aly HM, Bass BL, Sidawy AN, Harmon JW. Effect of growth factors on cell proliferation and epitheliazation in human skin. J Surg Res. 1995;59:236–244. doi: 10.1006/jsre.1995.1160. [DOI] [PubMed] [Google Scholar]
- 6.Dvorak HF, Brown LF, Detmor M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability and angiogenesis. Am J Pathol. 1995;146:1029–39. [PMC free article] [PubMed] [Google Scholar]
- 7.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 8.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 9.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers: a phase III, randomised, placebo-controlled, double-blind study. Diabetes care. 1998;21:822–7. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 10.dÕHemecourt PA, Smiell JM, Karim MR. Sodium carboxymethyl cellulose aqueous based gel versus beclapermin in patients with nonhealing, lower extremity diabetic ulcers. Wounds. 1998;10:69–73. [Google Scholar]
- 11.Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor- BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomised studies. Wound Repair Regen. 1999;7:335–46. doi: 10.1046/j.1524-475x.1999.00335.x. [DOI] [PubMed] [Google Scholar]
- 12.Bennett NT, Schultz GS. Growth factors and wound healing: Biochemical properties of growth factors and their receptors. Am J Surg. 1993;165:728–37. doi: 10.1016/s0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- 13.Mustoe TA, Pierce GF, Morishima C, Deuel TF. Growth factor induced acceleration of tissue repair through direct and inductive activities. J Clin Invest. 1991;87:694–703. doi: 10.1172/JCI115048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman T, Lusthaus SN, Sagher U, Wexler MR. Deep partial skin thickness burns: a reproducible animal model to study burn wound healing. Burns. 1990;16:13–6. doi: 10.1016/0305-4179(90)90199-7. [DOI] [PubMed] [Google Scholar]
- 15.Pierce GF, Mustoe TA. Phatnlacoloeical enhancement of wound healing. Ann Rev Med. 1996;16:467–81. doi: 10.1146/annurev.med.46.1.467. [DOI] [PubMed] [Google Scholar]
- 16.Jurjus A, Atiyeh BS, Abdallah IM, Jurjus RA, Hayek SN, Jaoude MA, Gerges A, Tohme RA. Pharmacological Modulation Of Wound Healing In Experimental Burns. Burns. 2007 Nov;33:892–907. doi: 10.1016/j.burns.2006.10.406. [DOI] [PubMed] [Google Scholar]
- 17.Atiyeh BS, Hayek SN, Gunn SW. New Technologies For Burn Wound Closure And Healing - Review Of The Literature. Burns. 2005 Dec;31:944–56. doi: 10.1016/j.burns.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 18.Atiyeh BS, Dham R, Costagliola M, Al-Amm CA, Belhaouari L. Moist Exposed Therapy: An Effective and Valid Alternative to Occlusive Therapy for Postlaser Resurfacing Wound Care. Dermatol Surg. 2004 Jan;30:18–25. doi: 10.1111/j.1524-4725.2004.30006.x. discussion 25. [DOI] [PubMed] [Google Scholar]


