Abstract
Transferrin is an iron-binding protein that is expressed as a major product in liver and secreted into the plasma. To study the tissue-specific regulatory regions of this gene, the genomic mouse transferrin (mTf) gene was cloned and characterized by partial sequence analysis and S1 nuclease mapping of the transcriptional start site. Fusion genes containing the transferrin gene promoter and 5'-flanking sequences were ligated to the human growth hormone (hGH) gene and used to produce transgenic mice. A deletion construct containing the -581 to +50 region of the transferrin gene was sufficient to direct a high level of liver-specific expression resembling endogenous transferrin gene expression. Deletion to -139 base pairs of 5'-flanking sequence gave a construct which retained liver specificity, but the magnitude of expression decreased severalfold. These results demonstrate the presence of a liver-specific transcriptional element between -139 and +50 and suggest the presence of a distal element between -581 and -139 that can further increase expression. Surprisingly, fusion constructs containing -3 kilobase pairs (kb) of 5'-flanking sequence gave higher levels of mRNA in nonhepatic tissues than did either the -581 or -139 construct. Further studies indicated that the high levels of circulating hGH in these transgenic mice specifically induced the endogenous transferrin and albumin genes in liver and also stimulated the normally low levels of expression of the endogenous transferrin gene in brain, heart, kidney, and muscle. A mutated hGH gene that does not produce active growth hormone was fused to the -3- to +50-kb transferrin sequences to produce the -3-kb mTf-hGX construct. A liver-specific pattern of expression was observed in transgenic mice harboring the -3-kb mTf-hGX construct, and this mutated transgene was shown to be induced four- to sevenfold by either bovine or human growth hormone. These results demonstrate the presence of a growth hormone-responsive element between -3 and +50 kb in the 5'-flanking region of the mTf gene promoter.
Full text
PDF



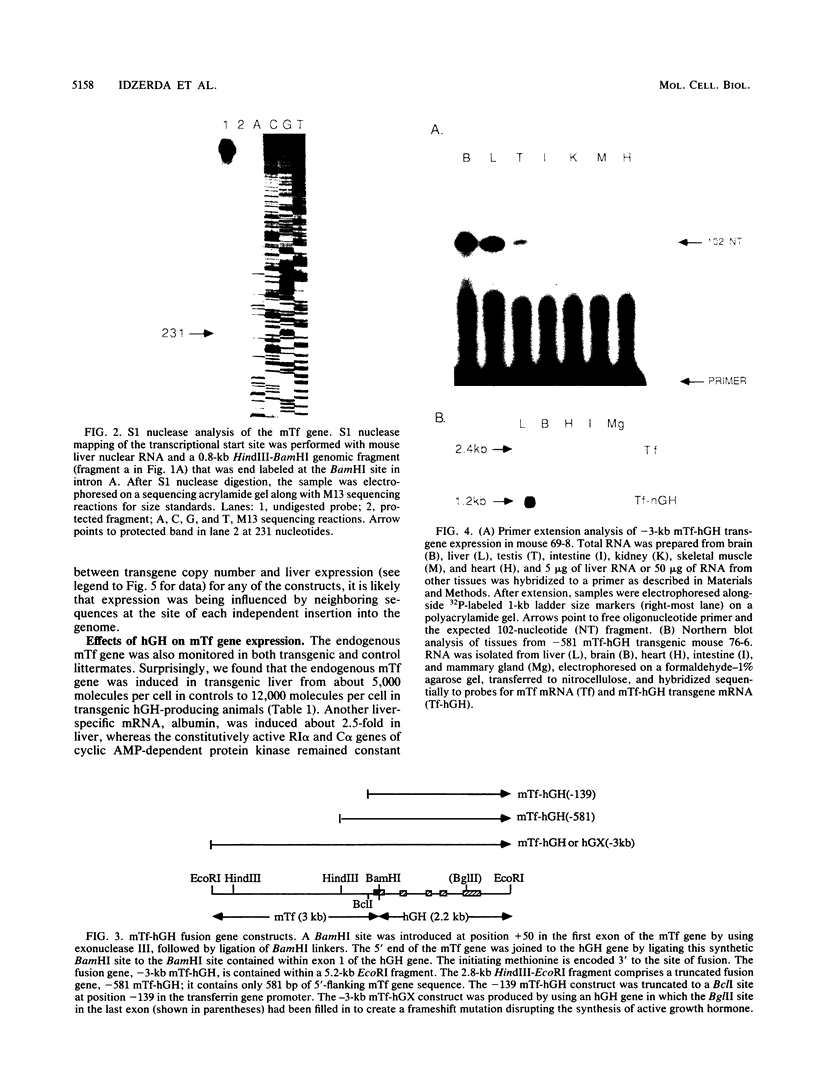
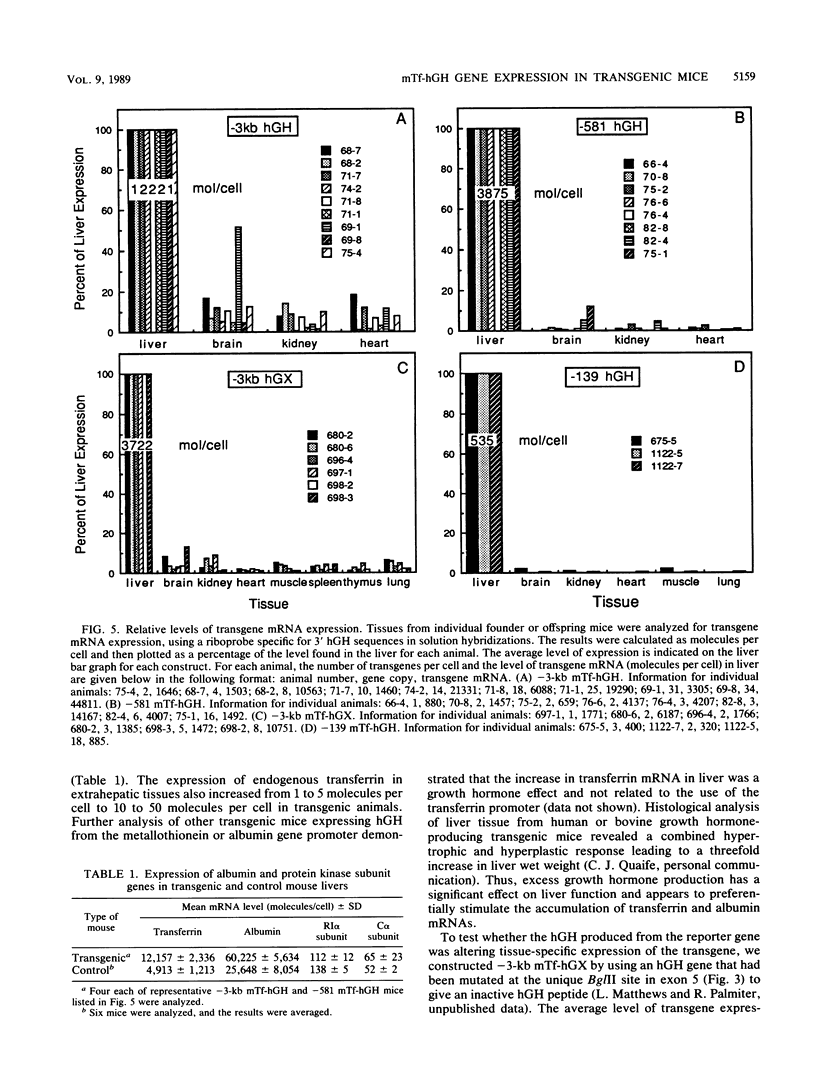



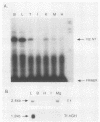
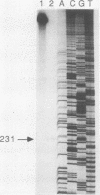
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldred A. R., Howlett G. J., Schreiber G. Synthesis of rat transferrin in Escherichia coli containing a recombinant bacteriophage. Biochem Biophys Res Commun. 1984 Aug 16;122(3):960–965. doi: 10.1016/0006-291x(84)91185-9. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M., Senear A. W., Warren R., Palmiter R. D. Somatic expression of herpes thymidine kinase in mice following injection of a fusion gene into eggs. Cell. 1981 Nov;27(1 Pt 2):223–231. doi: 10.1016/0092-8674(81)90376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Palmiter R. D. Introduction of genes into the germ line of animals. Harvey Lect. 1984 1985;80:1–38. [PMC free article] [PubMed] [Google Scholar]
- Brunel F., Ochoa A., Schaeffer E., Boissier F., Guillou Y., Cereghini S., Cohen G. N., Zakin M. M. Interactions of DNA-binding proteins with the 5' region of the human transferrin gene. J Biol Chem. 1988 Jul 25;263(21):10180–10185. [PubMed] [Google Scholar]
- Chen L. H., Bissell M. J. Transferrin mRNA level in the mouse mammary gland is regulated by pregnancy and extracellular matrix. J Biol Chem. 1987 Dec 25;262(36):17247–17250. [PubMed] [Google Scholar]
- Cochet M., Gannon F., Hen R., Maroteaux L., Perrin F., Chambon P. Organization and sequence studies of the 17-piece chicken conalbumin gene. Nature. 1979 Dec 6;282(5739):567–574. doi: 10.1038/282567a0. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Xanthopoulos K. G., Darnell J. E., Jr A liver-specific DNA-binding protein recognizes multiple nucleotide sites in regulatory regions of transthyretin, alpha 1-antitrypsin, albumin, and simian virus 40 genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3840–3844. doi: 10.1073/pnas.85.11.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler A., Cohen N., Maoz A. Human growth hormone but not ovine or bovine growth hormones exhibits galactopoietic prolactin-like activity in organ culture from bovine lactating mammary gland. Mol Cell Endocrinol. 1983 Dec;33(2-3):169–182. doi: 10.1016/0303-7207(83)90165-x. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Idzerda R. L., Brinster R. L., McKnight G. S. Estrogen regulation of the avian transferrin gene in transgenic mice. Mol Cell Biol. 1986 Apr;6(4):1010–1014. doi: 10.1128/mcb.6.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer R. E., Krumlauf R., Camper S. A., Brinster R. L., Tilghman S. M. Diversity of alpha-fetoprotein gene expression in mice is generated by a combination of separate enhancer elements. Science. 1987 Jan 2;235(4784):53–58. doi: 10.1126/science.2432657. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Swift G. H., Ornitz D. M., Quaife C. J., Palmiter R. D., Brinster R. L., MacDonald R. J. The rat elastase I regulatory element is an enhancer that directs correct cell specificity and developmental onset of expression in transgenic mice. Mol Cell Biol. 1987 Aug;7(8):2956–2967. doi: 10.1128/mcb.7.8.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard J. M., Herbomel P., Ott M. O., Mottura-Rollier A., Weiss M., Yaniv M. Determinants of rat albumin promoter tissue specificity analyzed by an improved transient expression system. Mol Cell Biol. 1987 Jul;7(7):2425–2434. doi: 10.1128/mcb.7.7.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hoffman L. M., Fritsch M. K., Gorski J. Probable nuclear precursors of preprolactin mRNA in rat pituitary cells. J Biol Chem. 1981 Mar 25;256(6):2597–2600. [PubMed] [Google Scholar]
- Huggenvik J. I., Idzerda R. L., Haywood L., Lee D. C., McKnight G. S., Griswold M. D. Transferrin messenger ribonucleic acid: molecular cloning and hormonal regulation in rat Sertoli cells. Endocrinology. 1987 Jan;120(1):332–340. doi: 10.1210/endo-120-1-332. [DOI] [PubMed] [Google Scholar]
- Idzerda R. L., Huebers H., Finch C. A., McKnight G. S. Rat transferrin gene expression: tissue-specific regulation by iron deficiency. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3723–3727. doi: 10.1073/pnas.83.11.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch J. M., Hen R., Maroteaux L., Garnier J. M., Chambon P. Sequence of the chicken ovotransferrin gene. Nucleic Acids Res. 1987 Sep 25;15(18):7643–7645. doi: 10.1093/nar/15.18.7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Barcellos-Hoff M. H., Chen L. H., Parry G., Bissell M. J. Transferrin is a major mouse milk protein and is synthesized by mammary epithelial cells. In Vitro Cell Dev Biol. 1987 Mar;23(3):221–226. doi: 10.1007/BF02623583. [DOI] [PubMed] [Google Scholar]
- Levin M. J., Tuil D., Uzan G., Dreyfus J. C., Kahn A. Expression of the transferrin gene during development of non-hepatic tissues: high level of transferrin mRNA in fetal muscle and adult brain. Biochem Biophys Res Commun. 1984 Jul 18;122(1):212–217. doi: 10.1016/0006-291x(84)90461-3. [DOI] [PubMed] [Google Scholar]
- Lucero M. A., Schaeffer E., Cohen G. N., Zakin M. M. The 5' region of the human transferrin gene: structure and potential regulatory sites. Nucleic Acids Res. 1986 Nov 11;14(21):8692–8692. doi: 10.1093/nar/14.21.8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Hammer R. E., Kuenzel E. A., Brinster R. L. Expression of the chicken transferrin gene in transgenic mice. Cell. 1983 Sep;34(2):335–341. doi: 10.1016/0092-8674(83)90368-9. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Lee D. C., Palmiter R. D. Transferrin gene expression. Regulation of mRNA transcription in chick liver by steroid hormones and iron deficiency. J Biol Chem. 1980 Jan 10;255(1):148–153. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller J., Bothwell A., Storb U. Physical linkage of the constant region genes for immunoglobulins lambda I and lambda III. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3829–3833. doi: 10.1073/pnas.78.6.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983 Jun 23;303(5919):674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Palmiter R. D., Hammer R. E., Brinster R. L., Swift G. H., MacDonald R. J. Specific expression of an elastase-human growth hormone fusion gene in pancreatic acinar cells of transgenic mice. Nature. 1985 Feb 14;313(6003):600–602. doi: 10.1038/313600a0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Chen H. Y., Brinster R. L. Differential regulation of metallothionein-thymidine kinase fusion genes in transgenic mice and their offspring. Cell. 1982 Jun;29(2):701–710. doi: 10.1016/0092-8674(82)90186-6. [DOI] [PubMed] [Google Scholar]
- Park I., Schaeffer E., Sidoli A., Baralle F. E., Cohen G. N., Zakin M. M. Organization of the human transferrin gene: direct evidence that it originated by gene duplication. Proc Natl Acad Sci U S A. 1985 May;82(10):3149–3153. doi: 10.1073/pnas.82.10.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkert C. A., Ornitz D. M., Brinster R. L., Palmiter R. D. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1987 May;1(3):268–276. doi: 10.1101/gad.1.3.268. [DOI] [PubMed] [Google Scholar]
- Roop D. R., Nordstrom J. L., Tsai S. Y., Tsai M. J., O'Malley B. W. Transcription of structural and intervening sequences in the ovalbumin gene and identification of potential ovalbumin mRNA precursors. Cell. 1978 Oct;15(2):671–685. doi: 10.1016/0092-8674(78)90035-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H. The human growth hormone gene family: nucleotide sequences show recent divergence and predict a new polypeptide hormone. DNA. 1982;1(3):239–249. doi: 10.1089/dna.1.1982.1.239. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Townes T. M., Lingrel J. B., Chen H. Y., Brinster R. L., Palmiter R. D. Erythroid-specific expression of human beta-globin genes in transgenic mice. EMBO J. 1985 Jul;4(7):1715–1723. doi: 10.1002/j.1460-2075.1985.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler M. D., Carmichael D. F., Lee D. C., Chrivia J. C., Krebs E. G., McKnight G. S. Isolation of cDNA clones coding for the catalytic subunit of mouse cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1300–1304. doi: 10.1073/pnas.83.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura K., Kudo A., Ebihara T., Kamino K., Araki K., Kumahara Y., Watanabe T. Cell-type-specific and regulated expression of a human gamma 1 heavy-chain immunoglobulin gene in transgenic mice. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2152–2156. doi: 10.1073/pnas.83.7.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]