Abstract
Background.
Indexes constructed from components may identify individuals who age well across systems. We studied the associations of a Modified Physiologic Index (systolic blood pressure, forced vital capacity, Digit Symbol Substitution Test score, serum cystatin-C, serum fasting glucose) with mortality and incident disability.
Methods.
Data are from the Health, Aging, and Body Composition study on 2,737 persons (51.2% women, 40.3% black) aged 70–79 years at baseline and followed on average 9.3 (2.9) years. Components were graded 0 (healthiest), 1 (middle), or 2 (unhealthiest) by tertile or clinical cutpoints and summed to calculate a continuous index score (range 0–10). We used multivariate Cox proportional hazards regression to calculate risk of death or disability and determined accuracy predicting death using the area under the curve.
Results.
Mortality was 19% greater per index unit (p < .05). Those with highest index scores (scores 7–10) had 3.53-fold greater mortality than those with lowest scores (scores 0–2). The unadjusted index (c-statistic = 0.656, 95% CI 0.636–0.677, p < .0001) predicted death better than age (c-statistic = 0.591, 95% CI 0.568–0.613, p < .0001; for comparison, p < .0001). The index attenuated the age association with mortality by 33%. A model including age and the index did not predict death better than the index alone (c-statistic = 0.671). Prediction was improved with the addition of other markers of health (c-statistic = 0.710, 95% CI 0.689–0.730). The index was associated with incident disability (adjusted hazard ratio per index unit = 1.04, 95% CI 1.01–1.07).
Conclusions.
A simple index of available physiologic measurements was associated with mortality and incident disability and may prove useful for identifying persons who age well across systems.
Keywords: Aging, Index, Mortality, Disability, Longevity
The relationship between aging and disease is complex. One theory is that aging is independent of disease pathogenesis. This is based on the relatively consistent observation that a decline in function with age is seen between and within species with widely different physiology and pathology (1). This separation may be artificial, though, and belie our poor understanding of the aging–disease dichotomy (2). A second theory argues that aging and disease pathogenesis are inextricable and, in the extreme, are in fact the same process expressed in different terms (2). This theory may be less tenable because over time organisms exhibit facets of aging which do not appear like disease and which occur without concomitant disease pathogenesis (1). A third, more moderate theory contends that aging is partly divisible from disease pathogenesis, that the two have shared and independent causes and consequences, and that they comingle to transition a young, healthy, adaptable organism to an old, unhealthy organism that poorly responds to stress. Due to the strong tie between aging and many chronic diseases, and the imperfect ability to disentangle age-related changes from age-related disease, this third theory may be the most promising, though the debate continues.
Biomarkers of aging are measurable factors that ideally predict future events better than chronologic age (3,4). Preferably, a biomarker of aging would allow monitoring of a basic aging process independent of disease. As stated previously, because aging and age-related disease are likely linked, it is possible that strong biomarkers of aging may also straddle changes seen in age-related disease. Most advantageous may be validation of biomarkers that are composite indexes of measurements taken from several systems. By identifying individuals with exceptional values in several systems, these indexes may help uncover a rare group of older adults who have aged well systematically. This would be an ideal reference group in which to search for longevity-associated factors.
Newman and colleagues (5) created a 10-point physiologic index of comorbidity that tabulates severity of age-related chronic disease using tests involving the vasculature (carotid intima-media thickness), lungs (pulmonary vital capacity), kidneys (serum cystatin-C), brain (white matter grade), and glucose metabolism (serum fasting glucose). The index straddles changes seen in aging and age-related disease by using continuous measures of all components, a departure from traditional comorbidity counts based on the simple presence or absence of clinically diagnosed diseases. This physiologic index widely stratifies risk of death and incident disability (5). Such an index may advance the study of aging by serving as an outcome to discover risk factors for age-related pathology or serving as a predictor of adverse outcomes. The potential to tease out individuals who have aged well across systems makes it useful for identifying factors associated with healthy aging or longevity.
Nonetheless, some components of the physiologic index, such as carotid intima-media thickness obtained via ultrasound and white matter grade obtained via magnetic resonance imaging, are not widely available in epidemiologic studies, limiting its practicality for genome-wide association studies and other types of pooled analyses and hindering replication of results. Fortunately, other measures of key systems are commonly included in aging studies and could replace components in the original physiologic index that are not widely available. Blood pressure could substitute for carotid intima-media thickness and tests of cognitive function (e.g., the Digit Symbol Substitution Test [DSST]) could stand for white matter grade. This study tests whether a Modified Physiologic Index comprised of more widely available components predicts mortality and incident disability beyond chronologic age with the intent of producing a tool for broad application in aging research.
METHODS
Study Population
We used the Health, Aging, and Body Composition (Health ABC) study. All black and a randomly selected subset of Medicare enrollees aged 70–79 years in designated zip code areas in Memphis, Tennessee, and Pittsburgh, Pennsylvania, were eligible to participate in the Health ABC study, conducted in part to identify risk factors for mobility disability. Because the Health ABC study was originally designed to examine incident mobility disability, eligibility criteria were no reported difficulty in walking for 1/4 mile, walking up 10 steps, getting in and out of bed or chairs, bathing or showering, dressing, or eating; no need of using a cane, walker, crutches, or other special equipment to get around; not enrolled in a lifestyle intervention trial; free of life-threatening illness; and no plans to leave the geographic area for at least 3 years. These criteria assured a study population healthier at enrollment than an age-matched general population. The University of Pittsburgh and the University of Tennessee Institutional Review Boards approved all procedures.
Of the 3,075 Health ABC participants enrolled at baseline, 2,737 participants (89.0%) with complete data on all index components, mortality, and incident disability were included in the analysis (Supplementary Table 1). Systolic blood pressure (SBP) and incident disability were available for all participants, but some data were missing for forced vital capacity (FVC, N = 212, 6.9%), DSST score (N = 43, 1.4%), serum cystatin-C (N = 31, 1.0%), and serum fasting glucose (N = 98, 3.2%). Compared with excluded Health ABC study participants, included participants were healthier with respect to index components and some covariates (for characteristics of participants included and excluded from the analytic sample, see Supplementary Table 1).
Index Components
The choice of components in the original physiologic index was based on research that identified each as an important predictor of mortality and as a major indicator of a common age-related chronic disease (6,7). Replacement measurements (i.e., blood pressure replacing carotid thickness; DSST replacing white matter grade) were chosen as alternative indicators of organ/organ system health. Although the replacement measurements may not represent a phenotype identical to that represented by the original components, they are nonetheless indicators of health status for their respective physiologic systems. Blood pressure was measured with the participant seated for 5 minutes and then 1 minute after standing and averaged over 3 readings. Spirometry (FVC) was performed according to the standards of the American Thoracic Society (8). Cognitive function was evaluated with the DSST administered by trained interviewers (9). Serum fasting glucose was measured as previously described (coefficient of variance 1.2%, 10). Serum cystatin-C, a marker of glomerular filtration rate, was assessed using a BNII nephelometer that used a particle-enhanced immunonephelometric assay (coefficient of variance ∼2%, 11,12).
All-Cause Mortality and Incident Disability
Participants were followed from study inception in 1997/1998 through December 31, 2008. The mean (SD) length of follow-up was 9.34 (2.85) years, and the maximum length was 11.67 years. Death was documented by death certificate. Incident disability was defined as reporting during the follow-up period needing a cane or walker to get around, having severe mobility difficulty (severe difficulty or cannot walk 1/4 mile and/or climb 10 steps), or having any difficulty in activities of daily living. Disability was assessed at annual and semi-annual clinic visits and phone calls to participants or an identified proxy.
Confounders
Trained interviewers administered the baseline Health ABC questionnaire to assess demographic and socioeconomic characteristics, health behaviors, health status, and medical history. Within 2 weeks of the interview participants visited the University of Pittsburgh or University of Tennessee clinics for baseline anthropometric and functional measures and a blood draw. Participants were asked to bring all prescription and over the counter medications used in the previous 2 weeks. Medications were coded using the Iowa Drug Information System (13).
Covariates for this analysis included age, sex, race, study site, smoking (never/ever), body mass index (BMI), years of education, physical activity, and prevalent chronic conditions including cancer (except nonmelanoma skin cancer), cardiovascular disease, pulmonary disease, diabetes, hypertension, depression, kidney disease, osteoporosis, and osteoarthritis. BMI (kg/m2) was calculated using baseline height and weight and was categorized as underweight (BMI < 18.5, 1.3%), normal (18.5 ≤ BMI < 25, 30.7%), overweight (25 ≤ BMI < 30, 42.9%, reference category), or obese (BMI ≥ 30, 25.1%). Education was ascertained in the interview as highest grade completed. Total physical activity (kcal/kg/week) was determined by questionnaire with coding of reported physical activities into metabolic equivalents to derive caloric expenditure (14,15). The prevalence of physician-diagnosed cancer within the past 5 years, cardiovascular disease, pulmonary disease, diabetes, hypertension, kidney disease, and osteoporosis was determined using algorithms based on self-reporting and medication use. Depression was defined on the basis of a score >16 on a 20-item CES-D score (16,17). Osteoarthritis was determined by self-report of the presence of pain in the hip or knee in the last 12 months lasting at least 1 month or on most days.
Statistical Analysis
After visual and quantitative inspection of components categorized into tertiles, quartiles, and deciles, it appeared that associations of markers to mortality were linear, except for serum fasting glucose, which was J-shaped. Subsequently, the Modified Physiologic Index was constructed by scoring each marker by tertile (0 = healthiest tertile, 1 = middle tertile, 2 = unhealthiest tertile) according to the supposed direction of the association (e.g., higher pulmonary vital capacity = more healthy—see Table 1), except for glucose, which was categorized using clinical cutoffs. This was done for simplicity and also to allow comparison of the Modified Physiologic Index to the original physiologic index described by Newman and colleagues (5), which scored components by tertiles, except for glucose, which also used clinical cutoffs. SBP was scored as 0: <126 mmHg, 1: 126–142 mmHg, and 2: ≥142 mmHg. Because there was little overlap between men and women for FVC, tertile cut points for FVC were sex specific: men, 0: ≥3,700 mL, 1: 3,066–3,700 mL, 2: <3,066 mL; women, 0: ≥2,564 mL, 1: 2,127–2,564 mL, and 2: <2,127 mL. DSST was scored as: 0: ≥42 points, 1: 30–42 points, and 2: <30 points. Cystatin-C was scored as: 0: <0.90 mg/L, 1: 0.90–1.09 mg/L, and 2: ≥1.09 mg/L. In the original report of the physiologic index, fasting glucose was scored using clinical cutoffs suggested by the American Diabetes Association (0: <100 mg/dL, 1: 100–126 mg/dL, 2: ≥126 mg/dL). To account for the J-shaped association with mortality and to match construction of the original physiologic index, we scored fasting glucose using the same clinical cutoffs. Additional analyses categorizing glucose by tertile produced results that were not substantially different. Individual component scores were summed to create the index score ranging from 0 (healthiest) to 10 (unhealthiest).
Table 1.
Prediction of Death by Components of the Modified Physiologic Index
| Component Score | Events per 1,000 pyrs | HR (95% CI), Unadjusted | HR (95% CI), Age, Sex, Race | C-Statistic (95% CI), Unadjusted* |
| Systolic blood pressure | ||||
| 0 | 36 | 1.00 | 1.00 | |
| 1 | 37 | 1.05 (0.89–1.23) | 1.00 (0.85–1.17) | 0.530 (0.507–0.553) |
| 2 | 43 | 1.22 (1.05–1.42) | 1.13 (0.97–1.32) | |
| Forced vital capacity | ||||
| 0 | 26 | 1.00 | 1.00 | |
| 1 | 37 | 1.43 (1.21–1.69) | 1.28 (1.07–1.51) | 0.534 (0.511–0.557) |
| 2 | 55 | 2.19 (1.87–2.56) | 1.85 (1.56–2.19) | |
| DSST score | ||||
| 0 | 25 | 1.00 | 1.00 | |
| 1 | 39 | 1.61 (1.36–1.90) | 1.45 (1.23–1.72) | 0.634 (0.612–0.655) |
| 2 | 56 | 2.42 (2.06–2.85) | 1.97 (1.64–2.37) | |
| Serum cystatin-C | ||||
| 0 | 27 | 1.00 | 1.00 | |
| 1 | 35 | 1.32 (1.12–1.57) | 1.26 (1.06–1.49) | 0.620 (0.598–0.642) |
| 2 | 54 | 2.09 (1.78–2.50) | 1.90 (1.61–2.24) | |
| Serum fasting glucose | ||||
| 0 | 36 | 1.00 | 1.00 | |
| 1 | 37 | 1.05 (0.90–1.23) | 0.99 (0.85–1.16) | 0.538 (0.515–0.561) |
| 2 | 55 | 1.59 (1.34–1.89) | 1.42 (1.19–1.68) |
Notes: CI = confidence interval; DSST = Digit Symbol Substitution Test; HR = hazard ratio; and pyrs = person-years.
Unadjusted c-statistic for each component, modeled as a continuous variable, predicting death.
We assessed the association of the Modified Physiologic Index to potential confounders using a test of trend or χ2-test. We calculated the association of the Modified Physiologic Index to mortality and incident disability using univariate and multivariate Cox proportional hazards regression. The score cutpoints were taken from the original report of the physiologic index in Cardiovascular Health Study for comparability. In models of incident disability, individuals free of incident disability who died during the follow-up period were censored at date of death. A priori model building proceeded with adjustment for age, sex, and race, and then for additional covariates to guard against over-fitting. To assess the ability of the Modified Physiologic Index (modeled as a continuous variable) to predict mortality, we used the area under the receiver–operator curve method and calculated the concordance statistics (c-statistic). C-statistics from different models were formally compared using the method described by DeLong and colleagues (18). A significance level of p < .05 was used to determine statistical significance. SAS 9.2 (SAS Institute, Cary, NC) was used for all analyses.
RESULTS
The mean (SD) age of the population was 73.6 (2.9) years, 51.2% were women, and 40.3% were black (for additional characteristics, see Supplementary Table 1). Each component was associated with mortality independent of age, sex, and race (Table 1). Based on hazard ratios for death, of all components FVC, DSST, and serum cystatin-C were most strongly associated with mortality. Thus, the Modified Physiologic Index included all components (SBP, FVC, DSST score, serum fasting glucose, and serum cystatin-C).
The Modified Physiologic Index was relatively normally distributed, similar to the original physiologic index of comorbidity (Figure 1, reference 1). If the Modified Physiologic Index reflects an older adult’s health, it should track with other markers of health such as age, BMI, smoking, physical activity, and prevalence of diagnosed chronic health conditions, as was demonstrated for the original physiologic index of comorbidity. Higher scores on the Modified Physiologic Index were indeed associated with indicators of poorer health status (Table 2). This included older age, smoking, higher BMI, fewer years of education, lower physical activity, and higher prevalence of cardiovascular disease, diabetes, hypertension, and pulmonary disease. Cancer, depression, osteoarthritis, and kidney disease were not associated with the Modified Physiologic Index, though osteoporosis was associated with lower index scores.
Figure 1.
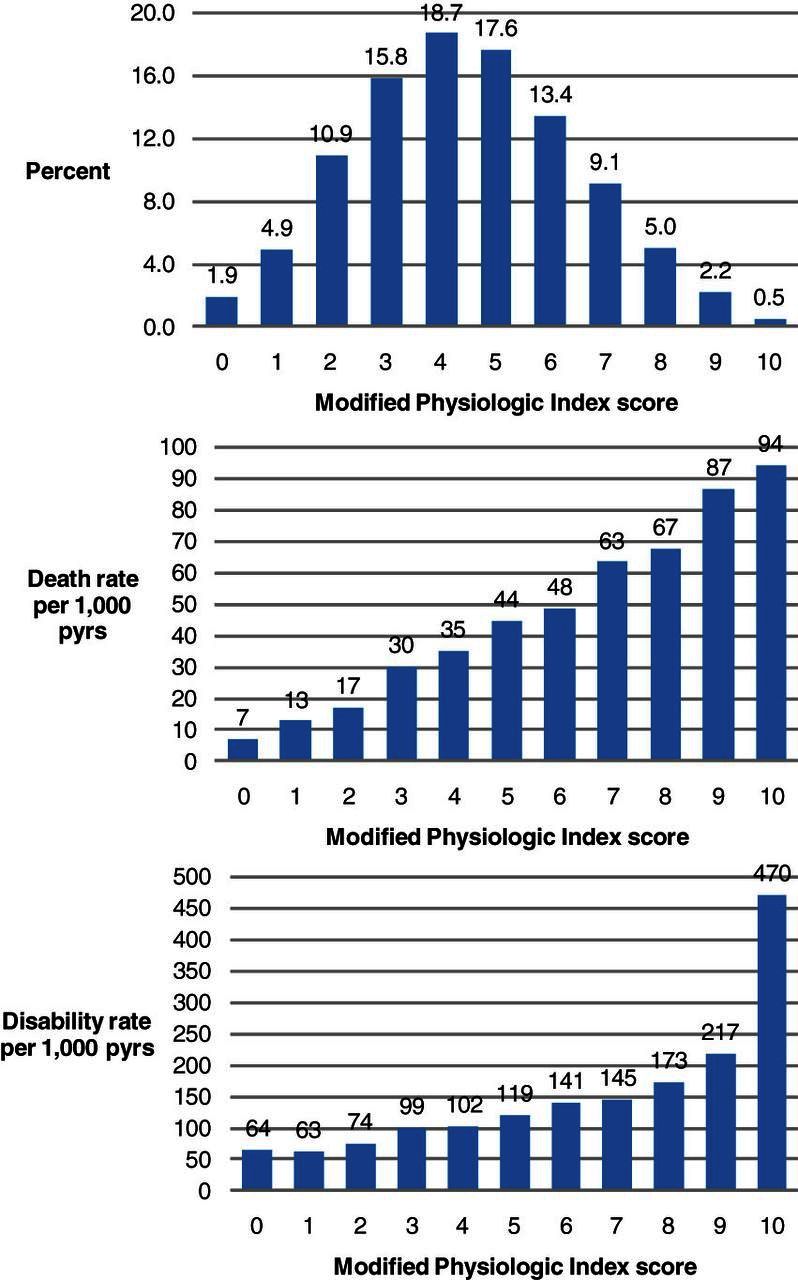
Distribution of the Modified Physiologic Index and death rate and disability rate by Modified Physiologic Index score.
Table 2.
Association of the Modified Physiologic Index with Baseline Demographics and Health Status
| Modified Physiologic Index | |||||
| Baseline covariate | Score 0–2 (N = 484) | Score 3–4 (N = 943) | Score 5–6 (N = 848) | Score 7–10 (N = 462) | p * |
| Age (years), mean (SD) | 72.7 (2.5) | 73.4 (2.8) | 73.9 (2.9) | 74.3 (2.9) | <.0001 |
| Women, N (%) | 304 (62.8) | 491 (52.1) | 406 (47.9) | 201 (43.5) | <.0001 |
| Black race, N (%) | 78 (16.1) | 292 (31.0) | 423 (49.9) | 311 (67.3) | <.0001 |
| Pittsburgh site, N (%) | 256 (52.9) | 454 (48.1) | 426 (50.2) | 249 (53.9) | .15 |
| Ever smoke, N (%) | 240 (49.6) | 533 (56.6) | 478 (56.4) | 281 (61.0) | <.01 |
| Body mass index (kg/m2), mean (SD) | 25.4 (3.7) | 26.9 (4.4) | 27.8 (4.9) | 29.5 (5.4) | <.0001 |
| Education (highest grade), mean (SD) | 14.6 (2.4) | 13.3 (3.0) | 12.5 (3.3) | 11.7 (3.3) | <.0001 |
| Physical activity (kcal/kg/week), mean (SD) | 93.8 (63.7) | 85.2 (67.7) | 82.8 (70.6) | 74.5 (77.7) | <.001 |
| Cancer, N (%) | 68 (14.1) | 162 (17.3) | 152 (18.0) | 83 (18.0) | .28 |
| Cardiovascular disease, N (%) | 76 (15.9) | 230 (24.9) | 248 (30.0) | 182 (40.2) | <.0001 |
| Depression, N (%) | 27 (5.6) | 47 (5.0) | 43 (5.1) | 35 (7.6) | .21 |
| Diabetes, N (%) | 13 (2.7) | 65 (6.9) | 139 (16.4) | 172 (37.2) | <.0001 |
| Hypertension, N (%) | 137 (28.3) | 418 (44.5) | 487 (58.0) | 330 (72.4) | <.0001 |
| Pulmonary disease, N (%) | 28 (5.8) | 99 (10.6) | 111 (13.2) | 69 (14.9) | <.0001 |
| Osteoporosis, N (%) | 61 (12.9) | 114 (12.3) | 71 (8.5) | 31 (6.8) | <.001 |
| Osteoarthritis, N (%) | 55 (11.5) | 105 (11.3) | 85 (10.2) | 44 (9.7) | .71 |
| Kidney disease, N (%) | 8 (1.7) | 9 (1.0) | 13 (1.5) | 10 (2.2) | .33 |
Note: * p value from test of trend or χ2-test.
There was a wide range in death rate across the span of the Modified Physiologic Index (Figure 1). Individuals with highest index scores had 10-fold greater crude mortality than individuals with lowest index scores. In adjusted models, mortality was 19% greater per unit of the Modified Physiologic Index (Table 3). Participants with the index scores 7–10 had 3.53-fold greater mortality than participants with index scores 0–2. Addition of the Modified Physiologic Index to a model of only age predicting death attenuated the association of age by 33%. Age alone predicted death better than chance (c-statistic = 0.591, 95% CI 0.568–0.613, p < .0001), but the Modified Physiologic Index (c-statistic = 0.656, 95% CI 0.636–0.677, p < .0001) predicted death better than age (for comparison, p < .0001). A model of age and the index had slightly better accuracy (c-statistic = 0.671, 95% CI 0.650–0.691), though it was not significantly different from the index alone. Accuracy increased with addition of other health indicators (noted in Table 3, c-statistic = 0.710, 95% CI 0.689–0.730).
Table 3.
Hazard Ratios for Mortality and Incident Disability by Modified Physiologic Index Scores
| Outcome | Events per 1,000 pyrs | HR (95% CI) Unadjusted | HR (95% CI) Age, Sex, Race | HR (95% CI) Multivariate* |
| Mortality (N = 983) | ||||
| HR per unit of index | 1.25 (1.22–1.30) | 1.21 (1.17–1.25) | 1.19 (1.14–1.24) | |
| 0–2 | 15 | 1.00 | 1.00 | 1.00 |
| 3–4 | 33 | 2.32 (1.80–2.99) | 2.09 (1.62–2.71) | 1.94 (1.48–2.54) |
| 5–6 | 46 | 3.34 (2.60–4.29) | 2.81 (2.17–3.64) | 2.46 (1.87–3.25) |
| 7–10 | 68 | 5.20 (4.01–6.74) | 4.07 (3.09–5.35) | 3.53 (2.61–4.79) |
| Incident disability (N = 1,384) | ||||
| HR per unit of index | 1.13 (1.10–1.16) | 1.13 (1.10–1.16) | 1.04 (1.01–1.07) | |
| 0–2 | 70 | 1.00 | 1.00 | 1.00 |
| 3–4 | 101 | 1.39 (1.18–1.65) | 1.40 (1.18–1.66) | 1.16 (0.97–1.39) |
| 5–6 | 128 | 1.73 (1.46–2.04) | 1.68 (1.41–2.00) | 1.26 (1.04–1.53) |
| 7–10 | 166 | 2.15 (1.79–2.57) | 2.07 (1.70–2.51) | 1.22 (0.98–1.53) |
Notes: CI = confidence interval; HR = hazard ratio; and pyrs, person-years.
Adjusted for age, sex, race, site, smoking, body mass index, years of education, physical activity, baseline cancer, hypertension, cardiovascular disease, diabetes, depression, kidney disease, pulmonary disease, osteoporosis, and osteoarthritis.
The Modified Physiologic Index stratified the population by rate of incident disability (Figure 1). In an unadjusted model, the index was associated with incident disability (hazard ratio per index unit = 1.13, 95% CI 1.10–1.16, Table 3). Participants with index scores 7–10 had a 2-fold greater risk of incident disability than participants with index scores 0–2. After adjustment, the index remained significantly though weakly associated with incident disability (hazard ratio per index unit = 1.04, 95% CI 1.01–1.07). Attenuation of the graded association with incident disability is somewhat artificial due to categorizing the index. The gradient is also difficult to visualize because the index is weakly associated with disability. Associations with mortality or incident disability were not modified by gender or race.
DISCUSSION
The Modified Physiologic Index stratified a population of older adults into a wide range of rates of death and disability. After adjustment for baseline confounders, the Modified Physiologic Index remained significantly associated with mortality and incident disability and predicted death better than age. Because the index includes physiologic changes with age that occur even in the absence of disease and can begin before age 50, the index may be a useful tool to study the biology of healthy aging, particularly as an intermediate phenotype of aging for genome-wide association studies. One might argue that the index is a marker of mortality and age-related ill health and not a marker of aging per se, but because aging is such a strong risk factor for these outcomes, the index could be a marker of mortality and age-related ill health because it is a marker of aging. Furthermore, it can be widely employed in nongenetic studies because the Modified Physiologic Index is constructed from components available in many studies of aging and cardiovascular disease.
In addition to predicting incident events better than chronologic age, attenuating the association of age with mortality strongly supports the validity of the Modified Physiologic Index as a marker of aging. This observation illustrates that the marker partly captures the effect of chronologic age—in essence, that the marker represents true biologic aging for which chronological age is only a surrogate—and is calculated using the percent change in the beta-coefficient of age before and after adding the index to the model. The Modified Physiologic Index attenuated the association of age with mortality by 33%. The original physiologic index of comorbidity attenuated the association of age with mortality by 40% in the Cardiovascular Health Study (5). It is important to note that the strength of age as a predictor is partly due to the age range of the study population (10 years in Health ABC, nearly 30 years in the Cardiovascular Health Study). A greater age range produces more variance and thus the ability to account for more variability in mortality and makes the effect of age more difficult to attenuate. Although Health ABC had a narrower age range, the substantial attenuation of the age association with mortality further supports the Modified Physiologic Index as a potential biomarker of aging.
The components of the Modified Physiologic Index that appeared most strongly associated with death were FVC, cystatin-C, and DSST. In another analysis using data from the Cardiovascular Health Study and the original physiologic index, these three components were also the ones most strongly associated with frailty (19). FVC, cystatin-C, and DSST might be stronger predictors of frailty and mortality because they may integrate many processes that contribute to aging rather than few processes. For example, FVC may register decreased lung volume, tissue elasticity, and muscle strength; cystatin-C may reflect kidney function, inflammation, and cardiovascular health. In contrast, SBP may chiefly reflect cardiovascular health alone. It is also possible that SBP and fasting glucose are weaker predictors because they are confounded by medical treatment for hypertension and diabetes; in contrast, few treatments affect FVC, cystatin-C, or DSST. Both low and high SBP and glucose may indicate increased risk for death, though it would be difficult to know if low values were true physiologic values or the result of treatment. Subsequently, FVC, cystatin-C, and DSST may be more useful than SBP and fasting glucose in the search for underlying mechanisms that contribute to healthy aging across systems.
Our results may depend in part on the construction of the Modified Physiologic Index, including the selected measures and cutpoints used to define the three-level categorization of each measure. Each component of the original index and Modified Physiologic Index is rooted in noninvasive measurement of organ structure and function without regard to clinical diagnosis of disease and as such permits quantification of a broad range of capacity from severely diseased or diminished to robust and healthy. Because aging and disease pathogenesis are difficult to disentangle (2) and may occur on a continuum (2,20), unlike yes/no classification of disease diagnosis, using noninvasive tests that provide continuous measurements of age and disease-related changes can identify individuals who are truly healthy.
In this analysis, we used the same reasoning and several of the same components (FVC, cystatin-C, fasting glucose,) used to construct the physiologic index of comorbidity. SBP and DSST score were purposefully substituted for carotid intima-media thickness and white matter grade because they are more widely available in epidemiologic studies. In Cardiovascular Health Study, linear correlations between SBP and carotid intima-media thickness (Spearman r = 0.11, p < .0001) and DSST score and white matter grade (Spearman r = −0.17, p < .0001) are significant but moderate. Nonetheless, SBP and DSST score are significant predictors of adverse outcomes in older adults independent of carotid thickness or white matter grade (21,22). The slightly weaker association of the Modified Physiologic Index to mortality (adjusted hazard ratio 3.53) compared with the physiologic index of comorbidity (adjusted hazard ratio 3.80) is likely because carotid intima-media thickness and white matter grade are more strongly associated with death than SBP and DSST score. In additional analyses not shown, we constructed alternative indexes using other components such as pulse pressure instead of SBP, serum creatinine instead of cystatin-C, and Modified Mini Mental Status Exam score instead of DSST score. These alternative indexes also stratified the study sample into a wide range of rates of death and incident disability, though the associations were attenuated more substantially with adjustment for other markers of health status. Because cystatin-C and DSST score are more strongly associated with mortality than creatinine and Modified Mini Mental Status Exam score, greater attenuation by other health indicators is expected. These additional analyses demonstrated that alternative indexes may be constructed using available organ-specific measurements and that these indexes can perform comparably with an understanding of the strengths and weaknesses of the components used to construct each index. Although some measurements, like cognitive testing, cannot be conducted retrospectively, blood-based biomarkers may be able to be measured years after a study has ceased, further expanding the availability of potential biomarkers to include in an index of healthy aging. Clearly, many physiologic and biologic measurements that have been examined with respect to aging were not included in the indexes presented here. Future research should investigate if inclusion of alternative factors adds predictive value.
Our analysis has several strengths and weaknesses. The Health ABC study afforded a large sample size for adequate power, the ability to conduct a prospective analysis, and the ability to adjust for potential confounders. Because the population was selected to be somewhat healthier and initially less functionally impaired than the general age-matched population, the results may not be generalizable to the most frail. Selection bias may have been introduced by use of an analytic subset of the Health ABC study, though 89.0% of participants were included. There are also other methods for constructing indexes that may influence results, such as choosing components in a more agnostic fashion using principal components analysis or factor analysis, or weighting components differently, such as with regression parameters (23). Finally, when scoring components, we may have misclassified participants with hypertension or diabetes who had low SBP and glucose due to medical treatment rather than having truly healthy physiologic values. In our sample, 35% of diabetics had fasting glucose <126 mg/dL and 56% of hypertensives had SBP <142 mmHg (the tertile cutoff for SBP). In a sensitivity analysis, we tested the effect of categorizing participants with diagnosed hypertension or diabetes into the worst group (component score = 2) regardless of their physiologic value for SBP and glucose. This had a small effect: the hazard ratio per unit of the continuous index increased from 1.25 to 1.26, and the unadjusted c-statistic increased from 0.656 to 0.663, with a similar 0.01 increase in the adjusted c-statistic. Subsequently, although misclassification may be moderate for SBP and glucose, misclassification has a small effect on prediction.
In conclusion, we found that a Modified Physiologic Index constructed from measurements available in many epidemiologic studies was associated with mortality and weakly with incident disability in an initially well-functioning cohort of older adults. The Modified Physiologic Index attenuated the association of age with mortality and was able to predict mortality with moderately high accuracy and better than chronologic age. Replication of these results in other cohorts may justify using the Modified Physiologic Index as a relevant endpoint for genetic studies searching for the biologic basis of healthy aging. As noted by Minne and colleagues (24), few predictive models of mortality in older adults have been externally validated and convincingly demonstrated to improve clinical decision making. Subsequently, given the ability of the Modified Physiologic Index to attenuate age and predict mortality in this population of older adults, it may be prudent to examine it as a tool to aid clinical decisions making—for example, as a preoperative screening tool to predict mortality and guide therapy via risk stratification.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/.
Acknowledgments
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. JLS is supported by a National Research Service Award from the National Institute on Aging (1F30-AG038093-01).
References
- 1.Miller RA. The biology of aging and longevity. In: Hazzard WR, Blass JP, Halter JB, Ouslander JG, Tinetti ME, editors. Principles of Geriatric Medicine and Gerontology. 5th ed. New York: McGraw-Hill; 2003. pp. 3–15. [Google Scholar]
- 2.Blumenthal HT. The aging-disease dichotomy: true or false? J Gerontol A Biol Sci Med Sci. 2003;58:138–145. doi: 10.1093/gerona/58.2.m138. [DOI] [PubMed] [Google Scholar]
- 3.Butler RN, Sprott R, Warner H, et al. Biomarkers of aging: from primitive organisms to humans. J Gerontol A Biol Sci Med Sci. 2004;59:B560, B–567. doi: 10.1093/gerona/59.6.b560. [DOI] [PubMed] [Google Scholar]
- 4.Sprott RL. Biomarkers of aging and disease: introduction and definitions. Exp Gerontol. 2010;45:2–4. doi: 10.1016/j.exger.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Boudreau RM, Naydeck BL, Fried LF, Harris TB. A physiologic index of comorbidity: relationship to mortality and disability. J Gerontol A Biol Sci Med Sci. 2008;63:603–609. doi: 10.1093/gerona/63.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 7.Kuller LH, Arnold AM, Longstreth WT, Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28:1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Standardization of spirometry, 1994 update. American Thoracic Society. Am J Resp Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 9.Wechsler D. Wechsler Adult Intelligence Scale—Revised. New York: Psychological Corp; 1988. [Google Scholar]
- 10.Resnick HE, Shorr RI, Kuller L, Franse L, Harris TB. Prevalence and clinical implications of American Diabetes Association-defined diabetes and other categories of glucose dysregulation in older adults: the Health, Aging and Body Composition Study. J Clin Epidemiol. 2001;54:869–876. doi: 10.1016/s0895-4356(01)00359-6. [DOI] [PubMed] [Google Scholar]
- 11.Shlipak MG, Wassel Fyr CL, Chertow GM, et al. Cystatin C and mortality risk in the elderly: the Health, Aging, and Body Composition Study. J Am Soc Nephrol. 2006;17:254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 12.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 13.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 14.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Colbert LH, Visser M, Simonsick EM, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 16.Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol. 1986;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000;160:1761–1768. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- 18.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 19.Sanders JL, Boudreau RM, Fried LP, Walston JD, Harris TB, Newman AB. Measurement of organ structure and function enhances understanding of the physiological basis of frailty: The Cardiovascular Health Study. J Am Geriatr Soc. 2011;59:1581–1588. doi: 10.1111/j.1532-5415.2011.03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taffet GA. Physiology of aging. In: Cassel CK, editor. Geriatric Medicine: An Evidence-Based Approach. 4th ed. New York: Springer-Verlag; 2003. pp. 27–35. [Google Scholar]
- 21.Psaty BM, Furberg CD, Kuller LH, et al. Isolated systolic hypertension and subclinical cardiovascular disease in the elderly. . Initial findings from the Cardiovascular Health Study. J Am Med Assoc. 1992;268:1287–1291. [PubMed] [Google Scholar]
- 22.Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56:1618–1625. doi: 10.1111/j.1532-5415.2008.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases—a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2011;66:301–311. doi: 10.1093/gerona/glq208. [DOI] [PubMed] [Google Scholar]
- 24.Minne L, Ludikhuize J, de Rooij SE, Abu-Hanna A. Characterizing predictive models of mortality for older adults and their validation for use in clinical practice. J Am Geriatr Soc. 2011;59:1110–1115. doi: 10.1111/j.1532-5415.2011.03411.x. [DOI] [PubMed] [Google Scholar]


