Abstract
Background.
Physical function declines, and markers of inflammation increase with advancing age, even in healthy persons. Microbial translocation (MT) is the systemic exposure to mucosal surface microbes/microbial products without overt bacteremia and has been described in a number of pathologic conditions. We hypothesized that markers of MT, soluble CD14 (sCD14) and lipopolysaccharide (LPS) binding protein (LBP), may be a source of chronic inflammation in older persons and be associated with poorer physical function.
Methods.
We assessed cross-sectional relationships among two plasma biomarkers of MT (sCD14 and LBP), physical function (hand grip strength, short physical performance battery [SPPB], gait speed, walking distance, and disability questionnaire), and biomarkers of inflammation (C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), TNF-α soluble receptor 1 [TNFsR1]) in 59 older (60–89 years), healthy (no evidence of acute or chronic illness) men and women.
Results.
LBP was inversely correlated with SPPB score and grip strength (p = .02 and p < .01, respectively) and positively correlated with CRP (p = 0.04) after adjusting for age, gender, and body mass index. sCD14 correlated with IL-6 (p = .01), TNF-α (p = .05), and TNFsR1 (p < .0001). Furthermore, the correlations between LBP and SPPB and grip strength remained significant after adjusting for each inflammatory biomarker.
Conclusions.
In healthy older individuals, LBP, a surrogate marker of MT, is associated with worse physical function and inflammation. Additional study is needed to determine whether MT is a marker for or a cause of inflammation and the associated functional impairments.
Key Words: Microbial translocation, Inflammation, Physical function, Microbiome
Age-associated increases in inflammatory biomarkers are associated with poor physical function (1–4). However, the mechanisms that initiate this increased inflammatory burden are not well understood. A previously unconsidered source of inflammatory initiation is mucosal surface microbes, primarily the gastrointestinal (GI) microbiome, through a process of microbial translocation (MT), the movement of microbes and/or microbial products across mucosal barriers without overt bacteremia. MT occurs in inflammatory bowel disease (IBD (5)), graft versus host disease (6), invasive GI surgery (7), sepsis (8), HIV infection (9,10), and obesity (11–13). Direct evidence of microbes include bacterial 16s rRNA and lipopolysaccharide (LPS) from gram-negative bacterial cell walls. Two endogenously produced reactive biomarkers formed in response to MT are soluble CD14 (sCD14), secreted by the liver and intestinal monocytes in response to LPS and other bacterial substances, and LPS-binding protein (LBP), a soluble acute-phase protein produced by hepatocytes (14,15) and intestinal epithelial cells (16,17), which binds LPS and promotes immune responses by presenting the LPS to CD14 (18). It is plausible that aging could increase MT due to age-related changes in GI function (19), immune responses (20), or both.
Associations among MT and inflammatory biomarkers have not been previously explored in older persons, nor has there been any prior investigation linking MT to physical function. Therefore, we evaluated the relationship of the MT markers sCD14 and LBP and biomarkers of inflammation and physical function in 60- to 89-year-old participants of the HEALTHY study, a population selected to be free from any overt clinical disease and not using any prescription medications.
Methods
Participants
The HEALTHY study was a cross-sectional study that enrolled nonsmoking older persons who reported no acute or chronic medical disorders, including hypertension or diabetes; who were taking no medications except aspirin for primary prevention; and who could discontinue aspirin at least 10 days prior to testing. The purpose of the study was to provide a comparison group for studies of age-related health conditions in order to understand the relative contributions of age and disease. Participants were recruited primarily by direct mail from community-dwelling persons aged ≥60 years. Eligibility was based on a medical history, physical examination by a board-certified internal medicine physician, medical record review, and multiple screening examinations including normal rest and exercise electrocardiogram (ECG) and echocardiogram; pulmonary function test; and chemistry and electrolyte panel. Participants who regularly participated in vigorous exercise >120 minutes per week or competitive sports were excluded. All participants provided written informed consent, and the protocol was approved by the Wake Forest University School of Medicine Institutional Review Board.
Protocol
Participants reported in the morning following an overnight fast, and any medications were held for 10 days. Blood samples were drawn in the supine, resting position and specimens were spun in a refrigerated centrifuge and plasma and serum were aliquoted and stored in 0.5mL samples at −80°C. Plasma samples were available from 59 participants for analysis.
Body Mass Index
Body mass (kg) was measured on a standard calibrated scale, and height (cm) was measured using a stadiometer.
Physical Function
Short physical performance battery.—Physical function was assessed using the Short physical performance battery (SPPB) (21–23). A score (0-12) is calculated based on (a) the time (s) needed to walk 4 m at a normal pace; (b) the time needed to stand up and sit down 5 times as quickly as possible from a chair with the arms folded across the chest; and (c) a timed balance test that measures the ability to maintain balance with feet together in three positions (side by side, semitandem, and tandem) with 12 being the best score.
Gait speed.—Participants were asked to walk at their usual pace over a 4-m course. Two trials were performed, and the faster of the two walks was used to compute gait speed.
Six-minute walk distance.—Walking distance (ft) was determined by measuring how far the participants walked in 6 minutes at normal pace.
Hand grip strength.—Grip strength in both hands was measured using an adjustable, hydraulic grip strength dynamometer (Jamar Hydraulic Hand Dynamometer, Model No. BK-7498, Fred Sammons, Inc., Burr Ridge, IL). If a participant reported wrist or hand pain, or had undergone surgery of the upper extremity in the past 3 months, the affected hand was not tested, and results of the other hand were used. The two trials for each hand were averaged with the larger score being between the two hands used in the analysis.
Self-reported disability questionnaire.—The Pepper Assessment Tool for Disability (PAT-D) is a 19-item self-report disability questionnaire to assess functional difficulty (24–26).
Maximal oxygen uptake (VO2).—Exercise capacity was assessed by measuring maximal VO2 (VO2max). VO2max was measured with a modified Bruce protocol during a progressive exercise test on a motorized treadmill according to standards established by the American Heart Association and as previously described in detail (27,28). Expired gases were measured with a Med Graphics (Minneapolis, MN) CardiO2 system following calibration for VO2 and VCO2. Patients were encouraged to give a maximal, exhaustive effort as judged by respiratory exchange ratio >1.05, rate of perceived exertion >15, and >90% age-predicted maximal heart rate. Blood pressure, heart rate, and ECG were continuously monitored. VO2 was assessed by averaging the final three 15-second averages, ventilatory threshold by the Wasserman method, and VE/VCO2 slope as previously described in detail in older patients in our laboratory (27,28).
Inflammatory Cytokines and MT Markers
Inflammatory biomarker concentrations were measured from plasma collected after an overnight fast. Fasting plasma concentrations of IL-6 (Intra-assay CV: 8.6%; Inter-assay CV, low: 0.98%; high: 0.70%), TNF-α (Intra-assay CV: 6.5%; Inter-assay CV, low: 0.17%; high: 2.2%), TNF-α soluble receptor 1 (TNFsR1; Intra-assay CV: 8.8%; Inter-assay CV, low: 3.0%; high: 4.4%), and sCD14 (Intra-assay CV: 5.7%; Inter-assay CV, low: 2.5%; high: 8.4%) were determined by high-sensitivity Quantikine immunoassay kits (R&D Systems, Minneapolis, MN). CRP was measured using an automated immunoanalyzer (Immulite; Diagnostics Products Corp.). Finally, plasma LBP (Intra-assay CV: 4.2%; Inter-assay CV, low: 0.22%; high: 5.0%) was determined with an ELISA kit (Cell Sciences, Canton, MA).
Statistics
Means and standard deviations were calculated for the continuous variables: age, BMI, SPPB, grip strength, gait speed, 6-minute walk distance, PAT-D MT biomarkers (LBP and sCD14), and inflammatory biomarkers (IL-6, CRP, TNFsR1, and TNF-α). Proportions were calculated for categorical variables such as gender and race. Distributions of all the biomarkers were skewed; so, logarithm transformations were applied before further analyses. Unadjusted and partial (adjusted for age, gender, and BMI) Pearson correlations were obtained among all the biomarkers. Multiple regression models were used to examine associations between physical performance or disability measures and MT biomarkers (individual and combined, respectively) in a stepwise fashion: first an unadjusted model was fitted (Model 1); then age, gender, BMI, and relative VO2max were adjusted in a second model (Model 2); finally, a series of models were fitted by adding other individual inflammatory biomarkers to Model 2. Because there were only three African American participants, race was not included for all the adjusted analyses, but the conclusions did not change even when adjusting for race in addition to other covariates. All analyses were performed using SAS 9.2 (Cary, NC). A p-value of .05 was considered as statistical significant.
Results
Table 1 provides descriptive statistics for participant characteristics, body composition, physical function, inflammatory biomarkers, and MT markers. The participants were predominantly women, white, and normal to overweight. The mean (±standard deviation) age was 69.2±7.4 years. Given the selection criteria, the sample performed well across all functional assessments. The inflammatory biomarkers are comparable to prior reports for these markers in older populations (1,4), and the level of the MT markers (LBP, sCD14) in this population was comparable to levels previously reported in healthy controls (9,29). However, the LBP levels previously reported in young, healthy controls appear to be a mean of approximately 10 µg/mL, whereas the mean LBP levels in this healthy, older population are 22.4±9.4 µg/mL.
Table 1.
Descriptive Statistics of Participants
| Characteristic | n | Mean or Percent | SD | Median | Range | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 59 | 69.2 | 7.4 | 68.0 | 60–89 | |||||
| Women | 37 | 63.0 | ||||||||
| Caucasian (%) | 56 | 95 | ||||||||
| African American (%) | 3 | 5.0 | ||||||||
| BMI (kg/m2) | 59 | 25.9 | 5.0 | 25.2 | 17.1–42.4 | |||||
| SPPB score (0–12) | 58 | 11.2 | 0.80 | 11.0 | 9–12 | |||||
| Grip strength (kg) | 56 | 34.2 | 11.8 | 31.8 | 15.5–62.5 | |||||
| Gait speed, 4 m (s) | 58 | 1.22 | 0.17 | 1.25 | 0.89–1.60 | |||||
| PAT-D (1–5) | 58 | 1.09 | 0.20 | 1.05 | 1.00–2.33 | |||||
| Walk distance, 6-min (ft) | 58 | 1,855.2 | 227.8 | 1861.0 | 1,363.0–2,478.0 | |||||
| VO2 max (mL/kg/min) | 58 | 25.1 | 7.1 | 23.9 | 14.0–50.4 | |||||
| IL-6 (pg/mL) | 59 | 1.42 | 0.92 | 1.11 | 0.42–4.73 | |||||
| TNF-α (pg/mL) | 59 | 1.90 | 5.6 | 0.79 | 0.52–41.9 | |||||
| CRP (mg/L) | 59 | 2.29 | 2.74 | 1.10 | 0.30–15.0 | |||||
| TNFsR1 (pg/mL) | 59 | 1,604.7 | 571.3 | 1,458.6 | 884.5–3,180.1 | |||||
| sCD14 (ng/mL) | 59 | 2,114.6 | 1,316.5 | 1,524.8 | 816.6–8,570.0 | |||||
| LBP (ng/mL) | 59 | 22,394.8 | 9,356.8 | 20,316.0 | 4,400.0–63,104.0 | |||||
Figure 1 shows the distribution of the MT markers, sCD14 and LBP, in this healthy, older population. Similar to inflammatory biomarkers, MT markers were not normally distributed. Age was not associated with either inflammatory or MT biomarkers (data not shown). After adjusting for gender, race, and BMI, the markers with the strongest association with age are LBP (r = 0.18, p = .18) and IL-6 (r = 0.21, p = .13).
Figure 1.
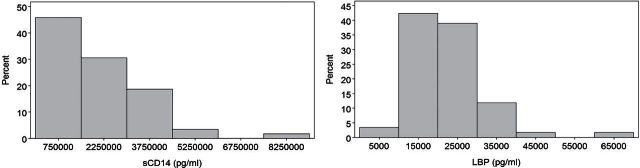
Histograms of the distribution of the microbial translocation markers sCD14 and lipopolysaccharide-binding protein in the healthy, older population.
The relationships among inflammatory and MT biomarkers are shown in Table 2. LBP was most strongly correlated with CRP (0.27, p = .04). SCD14 was directly associated with IL-6 (0.33, p = .01) and TNF-α (0.26, p = 0.05) and inversely associated with sTNFR1 (−0.52, p < .0001). Partial correlations adjusting for age, gender, race, and BMI did not materially differ from unadjusted associations (data not shown).
Table 2.
Correlation (Pearson’s) Matrix Showing the Relationships Among Inflammatory Biomarkers and MT Markers
| Log sCD14 | Log IL-6 | Log CRP | Log TNF-α | Log TNFsR1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log LBP | 0.19 | 0.04 | 0.27 | 0.05 | −0.11 | |||||
| p-Value | 0.15 | 0.77 | 0.04 | 0.69 | 0.41 | |||||
| Log sCD14 | 0.33 | 0.21 | 0.26 | −0.52 | ||||||
| p-Value | 0.01 | 0.10 | 0.05 | <0.0001 | ||||||
| Log IL-6 | 0.31 | 0.24 | −0.03 | |||||||
| p-Value | 0.02 | 0.06 | 0.81 | |||||||
| Log CRP | 0.13 | 0.09 | ||||||||
| p-Value | 0.33 | 0.52 | ||||||||
| Log TNF-α | −0.21 | |||||||||
| p-Value | 0.11 | |||||||||
Bold values represent significant correlations at a p value of .05 or less.
We assessed associations between physical function and MT markers using multiple linear regression modeling (Table 3). The unadjusted associations are shown along with adjustments for age, gender, and BMI (Model 1), or age, gender, BMI, and relative VO2max (Model 2). Because inflammatory biomarkers may mediate the association between MT markers and function, further adjustment for each biomarker was done in a separate step. LBP showed a strong association with both SPPB and grip strength, even after adjusting for each inflammatory biomarker. Furthermore, in the unadjusted models, LBP showed significant associations across all measures of physical function (data not shown) including gait speed (−0.10, p = .05), 6-minute walk distance (−170.4, p = .02), and PAT-D (0.13, p = .05). Although sCD14 associated with several inflammatory biomarkers, it did not associate with any of the physical function measurements in the unadjusted or adjusted models. When both MT markers were combined (data not shown), the pattern of associations changed little, suggesting independent contributions to function.
Table 3.
Association Between MT Markers and Physical Function Measures with Adjustment for Different Covariates
| SPPB | Grip Strength | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regression coefficient | SE | Change in 1 SD | p-Value | Regression coefficient | SE | Change in 1 SD | p-Value | |||||||||
| Log LBP | ||||||||||||||||
| Unadjusted | −0.76 | 0.24 | −0.39 | <0.01 | −3.89 | 3.77 | −0.14 | 0.31 | ||||||||
| Model 1 | −0.59 | 0.24 | −0.31 | 0.02 | −6.46 | 1.96 | −0.23 | <0.01 | ||||||||
| Model 2 | −0.58 | 0.24 | −0.33 | 0.02 | −6.39 | 2.01 | −0.23 | <0.01 | ||||||||
| Model 2 + TNFsR1 | −0.56 | 0.25 | −0.29 | 0.03 | −6.54 | 2.04 | −0.24 | <0.01 | ||||||||
| Model 2 + TNF-α | −0.57 | 0.24 | −0.29 | 0.02 | −6.45 | 2.02 | −0.23 | <0.01 | ||||||||
| Model 2 + IL-6 | −0.64 | 0.24 | −0.33 | 0.01 | −6.16 | 2.05 | −0.22 | <0.01 | ||||||||
| Model 2 + CRP | −0.55 | 0.25 | −0.28 | 0.04 | −5.56 | 2.06 | −0.20 | 0.01 | ||||||||
| Log sCD14 | ||||||||||||||||
| Unadjusted | −0.35 | 0.22 | −0.21 | 0.11 | −4.13 | 3.77 | −0.15 | 0.28 | ||||||||
| Model 1 | −0.30 | 0.21 | −0.18 | 0.16 | 0.08 | 2.14 | 0.003 | 0.97 | ||||||||
| Model 2 | −0.31 | 0.23 | −0.18 | 0.19 | 0.02 | 2.38 | 0.02 | 0.81 | ||||||||
| Model 2 + TNFsR1 | −0.30 | 0.27 | −0.17 | 0.27 | 0.49 | 2.81 | 0.02 | 0.86 | ||||||||
| Model 2 + TNF-α | −0.28 | 0.24 | −0.16 | 0.25 | 0.20 | 2.54 | 0.01 | 0.94 | ||||||||
| Model 2 + IL-6 | −0.24 | 0.24 | −0.14 | 0.34 | −0.09 | 2.46 | 0.00 | 0.97 | ||||||||
| Model 2 + CRP | −0.27 | 0.24 | −0.16 | 0.26 | 2.05 | 2.36 | 0.07 | 0.39 | ||||||||
Bold values represent significant associations at a p value of .05 or less.
Discussion
We believe that this is the first report of an association between markers of MT and physical function in older adults. MT markers were associated with physical function independently of age, gender, BMI, aerobic fitness level (VO2), and inflammatory biomarkers. MT (increased plasma LBP and sCD14) was associated with poorer physical function across several measures (SPPB and grip strength). This association is particularly notable given the very truncated distribution of physical function in this sample (all SPPB scores >8). Moreover, the population was free of clinically manifest disease; so, the associations are unlikely to be due to confounding medical comorbidities. As expected, after adjusting for age, gender, and BMI, MT markers were correlated with several inflammatory biomarkers (IL-6, TNF-α, CRP, and TNFsR1). An inverse correlation of sCD14 with TNFsR1 appears to be contrary to the other results (−0.53, p < .0001) and requires further exploration. Finally, there were no significant age-associated differences perhaps due to the restricted age range and modest sample size.
The mechanisms linking LBP to physical function also require further elucidation. There are limited data suggesting both direct and indirect mechanisms. MT could be responsible for physical function changes indirectly through an inflammatory intermediary (1). Because increased inflammation is strongly correlated with decreased physical function, it could be a likely explanation for the observed results. However, because LBP maintains its significant association with physical function after adjusting for inflammatory biomarkers, it suggests that alternative pathways may play a role. For example, LPS can directly induce muscle catabolism through TLR4 by the activation of ubiquitin–proteasome and autophagy-lysosome pathways (30). Furthermore, work by Frost and Lang (31–33) support several other ways in which MT products, including LPS and peptidoglycan, can directly impact myocytes. In their work, they show that endotoxin can affect muscle/myocytes directly by inhibiting translation through the stimulation of nitric oxide synthase activity (31). In addition, the myocyte can directly recognize and respond to LPS by producing cytokines (TNF-α) which work in an autocrine loop on the myocyte by altering nuclear factor kappaB and mTor signaling ultimately resulting in altered muscle growth (32,33). Directly measuring LPS in populations may aid in confirming this as a possible mechanism, but difficulties with the assay, the need to collect samples under LPS-free conditions, and high variability of LPS throughout the day (eg, similar to blood glucose) may mean LBP is a better marker of MT burden (eg, analogous to blood glucose and glycated hemoglobin [HgbA1C]).
Cross-talk that occurs between the microbiome and its host could occur in a variety of scenarios. For example, the microbiome’s role in food digestion will ultimately cause downstream effects to the host stemming from the harvested nutrients and vitamins as well as from caloric extraction (34,35). On the other hand, as previously described (9–13), microbial components can be directly responsible for the microbiome–host cross-talk. Such direct cross-talk could stem from direct mucosal damage or permeability changes or mucosal immunology changes that occur in response to a variety of factors including diet and the microbiome. Examples of such changes have been reported in aging humans and animal models (36,37). Another potential mechanism of MT involves the active transport of microbial products, such as LPS, through lipid transport pathways (38,39), which is supported by recent reports suggesting an endotoxemia–obesity link (11–13). However, regardless of the translocation mechanism, the GI tract is likely one of the principal sources of microbial products because of its massive bacterial load compared with other anatomical sites. At the same time, the GI microbiome is also under the most constant selective pressure stemming from diet changes, disease states, and medications, all important influences in the older population.
Limitations
The primary limitation of this study is the use of endogenously produced markers of MT. Endogenous biomarkers of MT (LBP and sCD14) have the advantage that they are easily measured and reflect the host’s response to microbial products. However, their level should be interpreted with caution because they are also acute phase reactant proteins which can be directly influenced by various inflammatory factors such as IL-1, IL-6, IL-22, and TNF-α (14,15). We attempted to account for any such influences in this study by adjusting for measured inflammatory biomarkers in our linear regression models. In addition, levels of these biomarkers depend on the ability of the individual to produce responsive proteins which may vary among individuals or change with age as liver function changes (40). This healthy, older population is ideal to assess an association with age because there is little interference from chronic conditions making it less likely that associations will be confounded by illness or treatment. At the same time, this limits the applicability of these findings to the majority of older adults. The study’s sample size was modest, and a number of statistical comparisons were made. This means that the Type I and Type II error rates are relatively high; thus, these results should be interpreted cautiously. The 95% confidence intervals from the models provide information regarding the range of probable effect sizes that are consistent with the presented data. Finally, because this was a cross-sectional study with no interventions, only a correlation can be stated and therefore the association may or may not be causally related. For these reasons, it will be important to measure MT markers (sCD14, LBP, LPS, 16s rRNA, etc.) in more diverse populations.
In conclusion, this study has shown for the first time, increased concentrations of endogenous markers of MT (sCD14 and LBP) to be associated with impaired physical function in an older population. This suggests that MT is a mechanism by which the microbiome can affect the host and may play a previously unrecognized role, resulting in adverse consequences including impaired physical function.
Funding
This work was partially supported by the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332). JS is supported by a NIA T32 Training grant (PI: Kritchevsky). The HEALTHY study was also supported partially by National Institute of Health grant R37-AG018915 (PI: Kitzman).
Acknowledgments
The authors thank Joel Eggebeen, MS, Karin Murphy, John Stone, and Heather Gregory, for technical assistance, and the HEALTHY study participants.
References
- 1. Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci 2009. 64 455––461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci 1997. 52 M201––M208 [DOI] [PubMed] [Google Scholar]
- 3. Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol 2004. 39 687––699 [DOI] [PubMed] [Google Scholar]
- 4. Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc 2004. 52 1105––1113 [DOI] [PubMed] [Google Scholar]
- 5. Caradonna L, Amati L, Magrone T, Pellegrino NM, Jirillo E, Caccavo D. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res 2000. 6 205––214 [PubMed] [Google Scholar]
- 6. Cooke KR, Olkiewicz K, Erickson N, Ferrara JL. The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J Endotoxin Res 2002. 8 441––448 [DOI] [PubMed] [Google Scholar]
- 7. Schietroma M, Carlei F, Cappelli S, Amicucci G. Intestinal permeability and systemic endotoxemia after laparotomic or laparoscopic cholecystectomy. Ann Surg 2006. 243 359––363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fink MP. Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Crit Care Med 1991. 19 627––641 [DOI] [PubMed] [Google Scholar]
- 9.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006. 12 1365––1371 [DOI] [PubMed] [Google Scholar]
- 11. Amar J, Burcelin R, Ruidavets JB, et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 2008. 87 1219––1223 [DOI] [PubMed] [Google Scholar]
- 12. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007. 56 1761––1772 [DOI] [PubMed] [Google Scholar]
- 13. Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008. 57 1470––1481 [DOI] [PubMed] [Google Scholar]
- 14. Wolk K, Witte E, Hoffmann U, et al. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J Immunol 2007. 178 5973––5981 [DOI] [PubMed] [Google Scholar]
- 15. Wan Y, Freeswick PD, Khemlani LS, et al. Role of lipopolysaccharide (LPS), interleukin-1, interleukin-6, tumor necrosis factor, and dexamethasone in regulation of LPS-binding protein expression in normal hepatocytes and hepatocytes from LPS-treated rats. Infect Immun 1995. 63 2435––2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dentener MA, Vreugdenhil AC, Hoet PH, et al. Production of the acute-phase protein lipopolysaccharide-binding protein by respiratory type II epithelial cells: implications for local defense to bacterial endotoxins. Am J Respir Cell Mol Biol 2000. 23 146––153 [DOI] [PubMed] [Google Scholar]
- 17. Su GL, Freeswick PD, Geller DA, et al. Molecular cloning, characterization, and tissue distribution of rat lipopolysaccharide binding protein. Evidence for extrahepatic expression. J Immunol 1994. 153 743––752 [PubMed] [Google Scholar]
- 18. Muta T, Takeshige K. Essential roles of CD14 and lipopolysaccharide-binding protein for activation of toll-like receptor (TLR)2 as well as TLR4 Reconstitution of TLR2- and TLR4-activation by distinguishable ligands in LPS preparations. Eur J Biochem 2001. 268 4580––4589 [DOI] [PubMed] [Google Scholar]
- 19. Thomson AB. Small intestinal disorders in the elderly. Best Pract Res Clin Gastroenterol 2009. 23 861––874 [DOI] [PubMed] [Google Scholar]
- 20. Boren E, Gershwin ME. Inflamm-aging: autoimmunity, and the immune-risk phenotype. Autoimmun Rev 2004. 3 401––406 [DOI] [PubMed] [Google Scholar]
- 21. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000. 55 M221––M231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995. 332 556––561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994. 49 M85––M94 [DOI] [PubMed] [Google Scholar]
- 24. Berry MJ, Rejeski WJ, Adair NE, Ettinger WH, Jr, Zaccaro DJ, Sevick MA. A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2003. 23 60––68 [DOI] [PubMed] [Google Scholar]
- 25. Mangani I, Cesari M, Kritchevsky SB, et al. Physical exercise and comorbidity. Results from the Fitness and Arthritis in Seniors Trial (FAST). Aging Clin Exp Res 2006. 18 374––380 [DOI] [PubMed] [Google Scholar]
- 26. Rejeski WJ, Ettinger WH, Jr, Schumaker S, James P, Burns R, Elam JT. Assessing performance-related disability in patients with knee osteoarthritis. Osteoarthr Cartil 1995. 3 157––167 [DOI] [PubMed] [Google Scholar]
- 27. Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil 2006. 26 86––89 [DOI] [PubMed] [Google Scholar]
- 28. Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 2002. 288 2144––2150 [DOI] [PubMed] [Google Scholar]
- 29. Redd AD, Dabitao D, Bream JH, et al. Microbial translocation, the innate cytokine response, and HIV-1 disease progression in Africa. Proc Natl Acad Sci USA 2009. 106 6718––6723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doyle A, Zhang G, Abdel Fattah EA, Eissa NT, Li YP. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J 2011. 25 99––110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frost RA, Nystrom GJ, Lang CH. Endotoxin and interferon-gamma inhibit translation in skeletal muscle cells by stimulating nitric oxide synthase activity. Shock 2009. 32 416––426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frost RA, Lang CH. mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiology (Bethesda) 2011. 26 83––96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frost RA, Lang CH. Regulation of muscle growth by pathogen-associated molecules. J Anim Sci 2008. 86(14 Suppl):E84––E93 [DOI] [PubMed] [Google Scholar]
- 34. Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004. 101 15718––15723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schwiertz A, Taras D, Schäfer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010. 18 190––195 [DOI] [PubMed] [Google Scholar]
- 36. Schmucker DL, Thoreux K, Owen RL. Aging impairs intestinal immunity. Mech Ageing Dev 2001. 122 1397––1411 [DOI] [PubMed] [Google Scholar]
- 37. Wade PR. Aging and neural control of the GI tract. I. Age-related changes in the enteric nervous system. Am J Physiol Gastrointest Liver Physiol 2002. 283 G489––G495 [DOI] [PubMed] [Google Scholar]
- 38. Grunfeld C, Feingold KR. Endotoxin in the gut and chylomicrons: translocation or transportation? J Lipid Res 2009. 50 1––2 [DOI] [PubMed] [Google Scholar]
- 39. Sharma R, von Haehling S, Rauchhaus M, et al. Whole blood endotoxin responsiveness in patients with chronic heart failure: the importance of serum lipoproteins. Eur J Heart Fail 2005. 7 479––484 [DOI] [PubMed] [Google Scholar]
- 40. Schmucker DL. Age-related changes in liver structure and function: Implications for disease ? Exp Gerontol 2005. 40 650––659 [DOI] [PubMed] [Google Scholar]


