Abstract
Tissue microarray (TMA) and cell microarray (CMA) are two powerful techniques that allow for the immunophenotypical characterization of hundreds of samples simultaneously. In particular, the CMA approach is particularly useful for immunophenotyping new stem cell lines (e.g., cardiac, neural, mesenchymal) using conventional markers, as well as for testing the specificity and the efficacy of newly developed antibodies. We propose the use of a tissue arrayer not only to perform protein expression profiling by immunohistochemistry but also to carry out molecular genetics studies. In fact, starting with several tissues or cell lines, it is possible to obtain the complete signature of each sample, describing the protein, mRNA and microRNA expression, and DNA mutations, or eventually to analyze the epigenetic processes that control protein regulation. Here we show the results obtained using the Galileo CK4500 TMA platform.
Keywords: tissue microarray, nucleic acids, IHC, cancer markers, cell type specific expression
Tissue microarray (TMA) is a widely accepted technology suitable for a large variety of possible applications (Kononen et al. 1998). Virtually all research involving in situ tissue studies can be carried out on a TMA format. The availability of a large collection of well-characterized specimens linkable to clinical data makes this technique a very powerful validation tool to complement the results obtained from different “omics” platforms. The tissue-arraying process itself is rather simple; more than 1000 different tissue samples can be combined on a single microscope slide to be simultaneously characterized by in situ analysis, but obviously, this depends on the needle diameter of the microarray device (Takikita et al. 2007). This technology not only reduces the laborious, time-consuming, and expensive conventional immunophenotypical characterizations on single slices but also diminishes the technical experimental variability in biomarker identification, allowing the analysis of a large number of samples at the same time. Exploiting this high-throughput tool, molecular pathology analysis could reach the capacity of “genome-scale” studies (Kallioniemi et al. 2001). TMAs have been constructed from paraffin-embedded tissue cell lines or cell blocks (cell microarray [CMA]; Waterworth et al. 2005; Wen et al. 2007), as well as from frozen tissues or cryoarrays (Schoenberg and Slamon 2001; Zhou et al. 2007).
A major concern in the routine diagnostic application of the TMA technology reflects the uncertainty of whether a small tissue core 0.6 mm in diameter could be representative of a heterogeneous tumor cell population. Several studies have shown a high concordance between immunohistochemical findings on TMAs and corresponding traditional large sections (Gillett et al. 2000), proving that two or three tissue cores could represent a single sample slice (Sauter and Mirlacher 2002; Kyndi et al. 2008). However, the lack of “perfect” concordance between the staining performed on multicores and the whole tissue sections makes this tool more suitable for new marker discovery rather than for diagnostic applications. Nonetheless, relevant data can be obtained from TMA studies, and improvements have been made to accommodate different necessities.
Overall, TMAs are categorized according to their applications:
Predictive TMAs, used to test markers that predict drug response (Andersson et al. 2006; Hewitt 2012)
Control TMAs, used to establish experimental protocols (Wan et al. 1987)
Validation TMAs, used to validate new markers discovered from DNA/RNA-based studies (Hewitt 2006)
Prognostic TMAs, used to correlate staining results with clinical end points (Lorente Garín et al. 2006)
Progression TMAs, used to follow tumor development or different tumor grades
Depending on the number of samples to be analyzed, it is possible to choose among different instruments, ranging from a completely manual arrayer to a fully automated one. In a manual system (e.g., Beecher Manual Tissue Arrayer I [Beecher, Sun Prairie, WI], Tissue Arrayer MiniCore, Alphelys, France, and others), tissue cores are extruded from a selected area of the donor block and inserted directly in the TMA recipient block. Obviously, the human-based operations of coring and subsequent deposition of samples are not only time-consuming but also subject to human errors. Semiautomated instruments (e.g., Galileo CK4500 Arrayer [Integrated System Engineering, Milan, Italy]) are associated with an X-Y-Z automated stage that allows one to directly place selected tissue cores in the recipient TMA block containing premade holes, ensuring not only a significant reduction in the array construction time but also an extreme alignment accuracy. Differently, a fully automated arrayer (e.g., Beecher Automated Tissue Arrayer ATA-27 [Beecher] and Quick-Ray Master Tissue Microarrayer, Sakura, Korea), once the coring sites in donor blocks are identified automatically, makes the recipient TMA block, without the intervention of the operator. This system is much less controllable by the operator, and errors cannot be corrected in real time; hence, sometimes a new TMA needs to be redesigned from the starting point or somehow completed manually. Ultimately, the use of fully automated instruments does not necessary imply saving time because it is often associated with complicated and time-consuming manipulations.
In this study, we have used the Galileo CK4500 Arrayer (www.isenet.it), a semiautomatic and computer-assisted TMA platform: It is a multimodular system with the characteristic to extract cores of interest, selected by the pathologist, from a tissue or cell paraffin block, to construct tissue/cell arrays or perform nucleic acid purification directly from the cores. The nucleic acid extraction from formalin-fixed paraffin-embedded tissues (FFPETs) is essentially problematic, as reported in several scientific studies. In particular, the use of formalin as a fixative agent is associated with the crosslinking of mRNA with proteins, negatively affecting the nucleic acid integrity (Ben-Ezra et al. 1991; Masuda et al. 1999). To minimize the negative effects on nucleic acid quality, the tissue biopsy should be fixed immediately (Gruber et al. 1994; Srinivasan et al. 2002; Abrahamsen et al. 2003), for no more than 2 days, preventing the deleterious consequences of overfixation (Gruber et al. 1994; Macabeo-Ong et al. 2002; Abrahamsen et al. 2003).
In this article, we show the purification of good-quality DNA, mRNA, and miRNA starting from extracted cores derived from archival tissues. On the same samples we also analyzed the expression pattern of SEL1L, a previously shown cancer-related gene (Orlandi et al. 2002; Cattaneo et al. 2003; Liu et al. 2012; Ashktorab et al. 2012). This gene encodes for several isoforms (Biunno et al. 2006; Cattaneo et al. 2009; Cattaneo et al. 2011), which, highly expressed by cancer cells, show different subcellular localizations that reflect the tumor subtype. Here we provide evidence that the TMA- and CMA-based staining method is compatible with the detection of nuclear (pH3), membrane (E-cadherin), and cytoplasmic markers (SEL1L).
Materials and Methods
Cell Lines and Culture Conditions
Human Burkitt lymphoma (Namalwa) and myeloma (KMS11) cell lines were maintained as suspension cultures in RPMI 1640 (Invitrogen; Monza, Italy), 10% FBS (Sigma; Milan, Italy), and 2 mM L-glutamine. The human glioblastoma-astrocytoma cell line U87 and embryonic kidney 293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen), supplemented with 10% FBS and 2 mM L-glutamine. The human pancreatic adenocarcinoma cell line CFPAC-1 was maintained in Iscove’s modified Dulbecco’s medium (Invitrogen), 10% FBS, and 2 mM L-glutamine, whereas mouse neural stem cells were cultured as neurospheres in Euromed-N (Euroclone; Pero, Italy) supplemented with 1% of N2 (Invitrogen) and 20 ng/ml of both epidermal growth factor (Peprotech; Rocky Hill, NJ) and fibroblast growth factor 2 (Peprotech). All cell lines were grown in a humidified incubator, at 37C with constant 5% CO2.
CMA and TMA Preparation
The CMA construction required the initial preparation of cell paraffin blocks (Andersson et al. 2006), which consisted of cell harvesting (about 1 × 106 cells) and incubation of the pellets for 20 min at 4C in 4% paraformaldehyde (PFA). The fixed cells were washed in PBS, resuspended in 100 µl of 2% low melting agarose, and finally applied in donut-shape agarose molds made up of 3% standard agarose. After solidification, the molds were incubated in PFA 1% for 24 hr and paraffin embedded. From this point onward, the cell blocks were treated exactly like the eight FFPETs analyzed. Three cores were extracted from five cancers and three normal tissues: One core was used for TMA construction, and two were stored for nucleic acid purification. Colon, spleen, prostatic normal tissues, and endometrial, liver, kidney, breast, and pancreatic cancer samples were obtained from the Anatomy Pathology Department of Multimedica Hospital (Milan, Italy). For endometrial, liver, kidney, breast, and pancreatic cancer samples, the tissues were cored after careful examination by the pathologist (FS). The criteria for core selection were the absence of necrotic tissue, the presence of more than 50% of cancer cells in the biopsy, the stromal reaction, and/or inflammatory cell presence less than 25%, irrespective of the cancer type.
Reference histological slides with the specific area marked by the pathologist were aligned with the respective donor block, and the Galileo TMA CK4500 hollow needle (diameter of 1 mm) was used to extract cells or tissue cores, which were then assembled in a recipient paraffin block; up to 200 consecutive sections of 4 µm thickness were cut from each TMA or CMA block, mounted on microscope slides, and assayed with different markers.
Immunophenotypical Analysis
Tissue slices were deparaffinized and rehydrated by incubating in solutions with decreasing alcohol content. Antigen retrieval was conducted by boiling the samples in citrate buffer (sodium citrate 10 mM, 0.05% Tween-20, pH 6) and blocking in 2% donkey serum, 1.5% BSA, and 0.5% fish gelatin for 45 min. Samples were immunostained at 4C in blocking solution with primary antibodies anti-SEL1L (5 µg/ml, kindly provided by Dr. Orlandi; Orlandi et al. 2002), anti–E-cadherin (1:100; Cell Signaling, Milan, Italy), and anti–phospho-histone 3 (pH3, 1:100; Cell Signaling). For immunohistochemical analysis, the endogenous peroxidase activity was blocked by treating with 3% H2O2 for 20 min, and, as well as for the immunofluorescence characterization, samples were incubated with appropriate secondary antibodies (rhodamine red anti-rabbit IgG [Jackson ImmunoResearch, West Grove, PA]; Alexa Fluor 488 anti-mouse IgG [Molecular Probes, Invitrogen]; anti-mouse horseradish peroxidase [HRP; Jackson ImmunoResearch]).
Immunohistochemistry signals were revealed by incubating the sections for 15 min with DAB (Pierce, Pero, Italy) and counterstaining with hematoxylin for 1 min, whereas for immunofluorescence, the nuclei were counterstained with Hoechst 33258. In both cases, the samples were mounted with GelMount aqueous mounting medium (Sigma).
The staining reaction was semiquantitatively evaluated; negative cases were those in which less than 5% of cells stained both at the nuclear or cytoplasmic level according to the different antibodies used. The positive samples were classified according to the following intensity scoring values, ranging from 1 to 3, where 1 represented weak staining; 2, moderate staining; and 3, intense staining. The percentage of positive cells was scored as 1 from 6% to 25% of stained cells, 2 from 26% to 50%, and 3 for more than 51% of stained cells. In particular, plasma cell reactivity of SEL1L was considered an index for score 3. The images were acquired using a Leica DMI4000B inverted microscope linked to DFC360FX or to DFC280 cameras (Leica Microsystems, Milan, Italy).
Nucleic Acid Extraction from Tissue Cores
Good-quality DNA was extracted following the previously described silica-based method (Malferrari et al. 2002); after deparaffinization and digestion of the tissue cores with proteinase K, 50 ng DNA was used as a template to amplify a region (137 base pairs) of SEL1L promoter with the following primers:
SEL1L F: 5′-tgagcctctttctcccagtc-3′
SEL1L R: 5′-ggccaatcattgtacgaagc-3′
Total RNA, also including small RNAs and microRNAs, was obtained using the Recover All Total Nucleic Acid Isolation Kit (Applied BioSystems, Monza, Italy), according to the manufacturer’s instructions. The RNA was retro-transcribed with the RevertAid H Minus Reverse Transcriptase (Fermentas, Cornaredo, Milan, Italy) and used to amplify a fragment (112 base pairs) of GAPDH mRNA with the following primers:
GAPDH F: 5′-gtggaagggctcatgacc-3′
GAPDH R: 5′-ggatgcagggatgatgttct-3′
To analyze the expression of let-7 miRNA, 100 ng total RNA was used as a template in a two-step TaqMan real-time PCR analysis, performed with a TaqMan microRNA reverse transcription kit (Applied BioSystems). Real-time PCRs were assembled on a Rotor-GeneQ (Qiagen, Milan, Italy) according to the TaqMan microRNA specific protocol, using 3 µl of the reverse transcribed product as a template.
Results
SEL1L Expression in CMA Slides
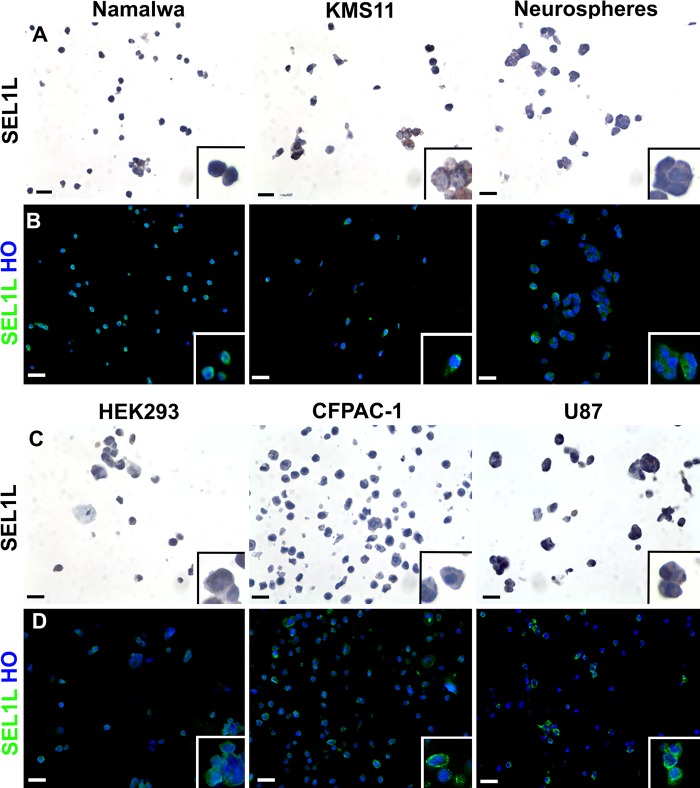
The immunophenotypical characterization of SEL1L by CMA (Fig. 1A–D) demonstrated the ubiquitous but differential expression of SEL1L in the different cell lines. The protein is highly present in human glioblastoma-astrocytoma (U87), pancreatic carcinomas (CFPAC-1), and undifferentiated murine neural stem cells (neurospheres). In contrast, the immunoreactivity was of intermediate levels in the Burkitt lymphoma (Namalwa) and myeloma (KMS11) cell lines and rather low in the embryonic kidney cells (HEK293). Moreover, in these cells, the protein localized in perinuclear and cytoplasmic areas, as previously reported (Cattaneo et al. 2009; Cardano et al. 2011).
Figure 1.
Immunocharacterization of six cell lines by cell microarray technology. After cell collection, pellets deriving from six different cell lines were resuspended in agarose and then embedded in paraffin. Two consecutive sections were analyzed for SEL1L expression by immunohistochemistry (A, C) and immunofluorescence (B, D) techniques (using the monoclonal antibody described by Orlandi et al. 2003), showing the typical perinuclear/cytoplasmic subcellular location of this protein. In the immunohistochemical images, the brown color reflects SEL1L expression and nuclei were counterstained with hematoxylin; differently, in immunofluorescence analysis, SEL1L is represented in green and nuclei in blue (counterstaining with Hoechst 33258, HO). Scale bars = 10 µm.
The CMA technique is very informative to immunophenotypically characterize any cell line, but it is easier for those cells that grow in suspension, such as Namalwa and KMS11, or those that create three-dimensional structures, such as neurospheres (neural stem cells aggregates) (Fig. 1C, D). More specifically, in neurospheres, the embedding process allows one to preserve the multicellular organization of the spheroid, which can efficiently be recognized after the staining (Fig. 1A, B, right panels), allowing the spatial evaluation of the differential expression of particular markers. Generally, neurospheres are intrinsically heterogeneous, because stemness markers are expressed by cells located toward the outside of the sphere, whereas glial and neuronal proteins are highly expressed in the center of the three-dimensional structure (Jensen and Parmar 2006). For these reasons, the CMA tool can be extremely useful to validate newly derived stemness or differentiation markers in neural cells using the neurosphere model. Using a wide panel of cell lines, newly derived antibodies can be tested quickly to assess their quality and set up the experimental conditions for their use.
Immunohistochemical and Immunofluorescence Characterization of TMA Slides
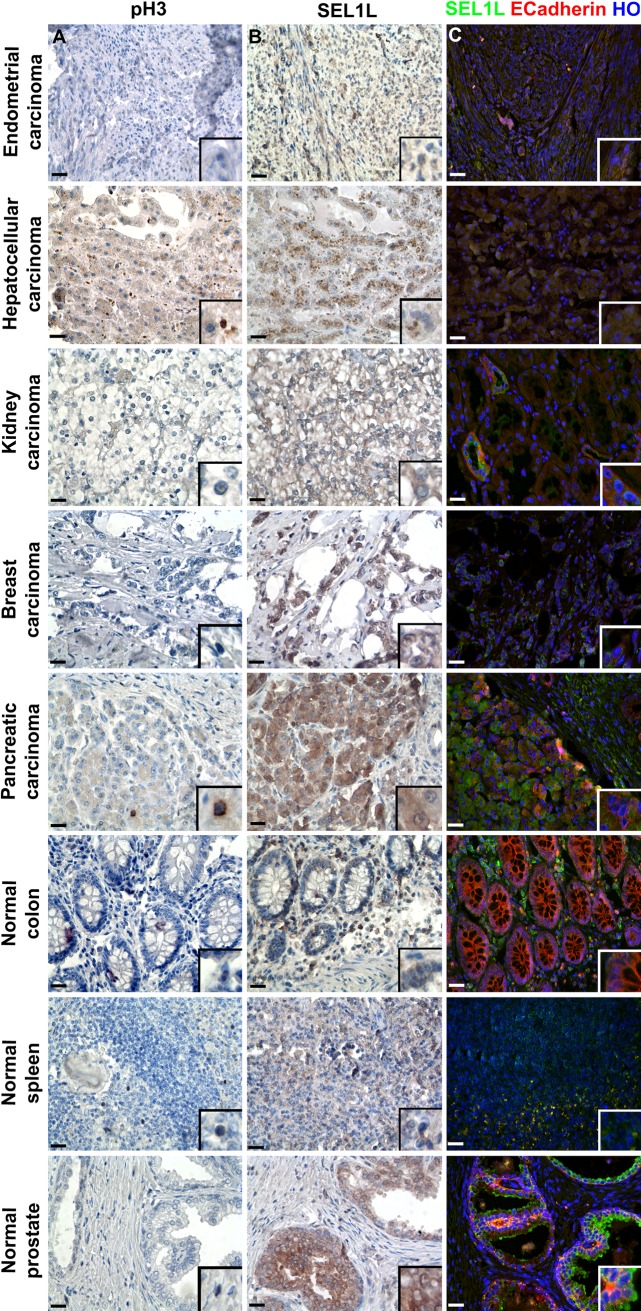
Currently, TMAs are predominantly used for research purposes but can be used profitably to rapidly screen different tissues to investigate new prognostic and molecular targets. In this study, we show that the same tissue area can be analyzed for the proliferative rate (pH3 immunoreactivity; Fig. 2A) and for prognostic expectations (SEL1L expression; Fig. 2B) or marked for normal epithelial cell presence (E-cadherin expression; Fig. 2C).
Figure 2.
Immunophenotypical analysis of tissue microarray (TMA) slices to identify nuclear, cytoplasmic, and membrane antigens. Five different tumoral and three normal tissues were used to construct a TMA, from which three sequential slices were analyzed for pH3 (A) and SEL1L (B; monoclonal antibody described by Orlandi et al. 2002) expression by immunohistochemistry and concurrently for SEL1L and E-cadherin (C) by immunofluorescence. The immunohistochemical figures show SEL1L and H3 proteins in brown, whereas nuclei were counterstained with hematoxylin. In the immunofluorescence study, SEL1L is depicted in green, E-cadherin in red, and nuclei in blue (counterstaining with Hoechst 33258, HO). Scale bars = 25 µm.
It was reported that SEL1L can reside in a different subcellular compartment, a feature that, at least in lung cancers, could differentiate the isotype (Ferrero et al. 2006). Here we show the simultaneous analysis (using the TMA platform) of SEL1L on a series of neoplastic tissue cores; the results confirm the cytoplasmic location of the protein in breast, kidney, pancreatic, endometrial, and liver cancers (Fig. 2B). As expected, SEL1L staining was more intense in pancreatic ductal cancer, medium in breast and liver, and fair in clear cells of renal cancer and in the endometrial carcinoma. Strong immunoreactivity was also detected in the normal prostatic gland and in plasma cells of the colon lamina propria; fair staining was observed in normal spleen (Fig. 2B). E-cadherin membrane reactivity was strongly observed in normal colon and pancreas, whereas it was less intense in pancreatic and kidney tumors (Fig. 2C), where loss of E-cadherin correlates with undifferentiated and anaplastic tumors (Onder et al. 2008).
Nucleic Acid Purification from Tissue Cores
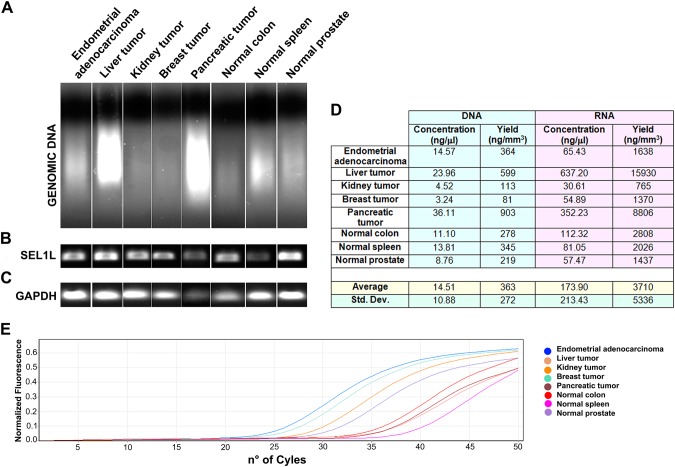
The eight tissues cored by the Galileo CK4500 Arrayer were processed for DNA purification (Fig. 3A), yielding an average of 14.5 ng/µl DNA with an expected variability (360 ± 272 ng/mm3; Fig. 3D), which depends on the nature and the quality of the original tissue. The extracted DNA is perfectly suitable for the necessary biomolecular applications, such as PCR amplification (Fig. 3B) or sequencing (data not shown).
Figure 3.
Nucleic acids extraction from tissues cored by the Galileo CK4500 Arrayer. Tissue cores were processed to extract both DNA (A) and RNA, showing good yields, but with an expected variability depending on the tissue quality and cellular content (D). The DNA can be used to amplify genes of interest (B), whereas the total RNA is suitable for RT-PCR (C) or to analyze the expression of specific microRNAs (E).
Total RNA extraction from the other eight tissue cores was characterized by an obvious variable yield of 3.7 ± 5.3 µg/mm3 (Fig. 3D); the obtained nucleic acid was reverse transcribed and the resulting cDNA was amplified to evaluate GAPDH expression (Fig. 3C). Moreover, this RNA was profitably used as a template in a two-step TaqMan real-time PCR that allows the amplification of specific microRNAs; here we show let-7 microRNA expression analysis (Fig. 3E).
Discussion
As new molecular targets are discovered, faster tools are necessary to rapidly validate the large amount of information deriving from genomics, proteomics, and other “omics” analysis on tissue samples. TMAs have been proven to be cost-effective, allowing the acquisition of a large amount of data from a single tissue. FFPETs represent an invaluable resource to obtain diagnostic and prognostic information connected to specific marker expression by immunophenotypical studies, but sometimes the molecular genetics is indispensable. Genomic DNA provides useful information to understand the molecular causes of specific diseases, and in some cases, such as in rare pathologies, it is the only source of investigation (Gilbert et al. 2007). In addition, modern mRNA profiling techniques, such as quantitative real-time PCR (qRT-PCR) and microarray platforms, are routinely used to elucidate the key molecular determinants of different diseases. Although paraffin embedding preserves the tissue architecture, the quality and the yield of nucleic acids extracted from FFPETs are not always satisfying, depending on several parameters connected to the tissue manipulation before nucleic acid isolation (Benavides et al. 2006; Gilbert et al. 2007). Many studies indicate that the use of formaldehyde (the principal constituent of the fixative formalin) and the length of the fixation process determine the crosslinking between nucleic acids and proteins, affecting nucleic acid integrity itself and subsequent downstream applications (Zsikla et al. 2004). Moreover, formalin fixation produces a covalent RNA modification that can interfere not only with the nucleic acid extraction but also with its quantification and reverse transcription (Feldman 1973; Auerbach et al. 1977). In addition, during DNA or RNA extraction from FFPETs, the most delicate process is deparaffinization that, if not correctly performed, could lead to poor quality of the nucleic acid, which on the other hand can determine the inhibition of successive enzymatic reactions (Santos et al. 2009).
In this study, we used the semiautomatic Galileo CK4500 TMA arrayer to validate the differential expression of SEL1L in a series of different cell lines and tissues. Several reasons encouraged us to use this platform, one being its multimodular characteristics able to accommodate various laboratory necessities. The modular sample holder allows the positioning of various items: standard histological cassettes, macrocassettes, microtiter plates, microfuge vials, custom-size paraffin blocks, and standard/macro glass slides. It is not a fully automated system, but the construction of TMA series is quite fast, requiring about 20 min to design the matrix and 3 min for tissue coring. CMAs are particularly useful in characterizing new molecular markers related to cancer or in selecting cell lines useful as models for drug discovery tests or to immunophenotype newly derived stem cell lines using specific markers. Moreover, this tool reduces the time to analyze molecular targets over conventional applications, particularly in testing the specificity and efficacy of newly developed antibodies. It is, however, important to prepare the cell-gel matrix in CMAs because it is necessary to maintain cell morphology to an acceptable level, preserving cell-cell interactions. Our results indicate that the SEL1L protein is highly expressed in tumor cells and tissues, particularly in pancreatic carcinomas, breast cancer, and human glioblastoma-astrocytoma, but also in undifferentiated neural stem cells, confirming previous reports (Orlandi et al. 2002; Cattaneo et al. 2003; Biunno et al. 2006; Cardano et al. 2011). High secreting cells, characterized by an endoplasmic reticulum biosynthetic machinery, such as myeloma cell lines or plasma cells infiltrating into the healthy colon tissue, showed the same elevated SEL1L expression, as previously verified in our laboratory (I. Biunno, personal communication 2009).
Here we favor the use of the TMA technology to screen cells and tissues to validate novel biomolecular markers. Several platforms are commercially available, including fully automated versions, connected to specific systems that are able to scan TMA slides and measure the staining intensity for each individual spot (Aperio, Olympus, Hamamatsu, and others). Truly, the concrete assembly of a TMA represents a small part of the entire process, in which the real time-consuming and error-prone step is the manual acquisition of immunohistochemical or immunofluorescence staining. Image scanning and automated scoring of the stained TMAs can facilitate the use of this technology for prognostic applications (Conway et al. 2008). One major concern in the use of TMAs is that small tissue samples of 0.6 to 1 mm in diameter might not be representative of the entire biopsy. By comparing staining results from TMAs with their corresponding tissue section, it has been shown that duplicate or triplicate tissue cores from each donor block are adequate to obtain matching results. In a TMA consisting of breast tumor material, even a single sample from each tumor was sufficient to identify relationships between molecular markers and clinical outcome. However, because the intratumoral heterogeneity of protein expression might differ between tumor types, the number of cores needed to ensure representivity should optimally be determined for every tumor type (Camp et al. 2000; Hoos and Cordon-Cardo, 2001; Torhorst et al. 2001).
Great effort is being made in trying to apply the TMA technology as a diagnostic tool and not only for research purposes. Sapino’s group evaluated hormone receptor expression in breast cancers (Sapino et al. 2006), but the question regarding the representation of the entire tissue in few cores remains an open issue. However, Thomson and coworkers (2010) demonstrated that two TMA cores from each sample could correctly account for the hormone receptor expression in breast cancers.
This report supports the use of the TMA and CMA technology as a high-throughput proteomics tool to validate the continuously increasing findings from the “omics”-based science.
Footnotes
Declaration of Conflicting Interests: The authors declared a potential conflict of interest (e.g. a financial relationship with the commercial organizations or products discussed in this article) as follows: Pasquale DeBlasio and Maurizio Falavigna work in the company that makes the TMA.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funds from Regione Lombardia “Metadistretto” (to IB and PD).
References
- Abrahamsen HN, Steiniche T, Nexo E, Hamilton-Dutoit SJ, Sorensen BS. 2003. Towards quantitative mRNA analysis in paraffin-embedded tissues using real-time reverse transcriptase polymerase chain reaction: a methodological study on lymphnodes from melanoma patients. J Mol Diagn. 5:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson AC, Strömberg S, Bäckvall H, Kampf C, Uhlen M, Wester K, Pontén F. 2006. Analysis of protein expression in cell microarrays: a tool for antibody-based proteomics. J Histochem Cytochem. 54(12):1413–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashktorab H, Green W, Finzi G, Sessa F, Nouraie M, Lee EL, Morgano A, Moschetta A, Cattaneo M, Mariani-Costantini R, et al. 2012. SEL1L, an UPR response protein, a potential marker of colonic cell transformation. Dig Dis Sci. 57(4):905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach C, Moutschen-Dahmen M, Moutschen J. 1977. Genetic and cytogenetical effects of formaldehyde and related compounds. Mutat Res. 39(3–4):317–361 [DOI] [PubMed] [Google Scholar]
- Benavides J, García-Pariente C, Gelmetti D, Fuertes M, Ferreras MC, García-Marín JF, Pérez V. 2006. Effects of fixative type and fixation time on the detection of Maedi Visna virus by PCR and immunohistochemistry in paraffin-embedded ovine lung samples. J Virol Methods. 137(2):317–324 [DOI] [PubMed] [Google Scholar]
- Ben-Ezra J, Johnson DA, Rossi J, Cook N, Wu A. 1991. Effect of fixation on the amplification of nucleic acids from paraffin-embedded material by the polymerase chain reaction. J Histochem Cytochem. 39:351–354 [DOI] [PubMed] [Google Scholar]
- Biunno I, Cattaneo M, Orlandi R, Canton C, Biagiotti L, Ferrero S, Barberis M, Pupa SM, Scarpa A, Ménard S. 2006. SEL1L a multifaceted protein playing a role in tumor progression. J Cell Physiol. 208(1):23–38 [DOI] [PubMed] [Google Scholar]
- Camp RL, Charette LA, Rimm DL. 2000. Validation of tissue microarray technology in breast carcinoma. Lab Invest. 80:1943–1949 [DOI] [PubMed] [Google Scholar]
- Cardano M, Diaferia GR, Cattaneo M, Dessì SS, Long Q, Conti L, Deblasio P, Cattaneo E, Biunno I. 2011. mSEL-1L (Suppressor/enhancer Lin12-like) protein levels influence murine neural stem cell self-renewal and lineage commitment. J Biol Chem. 27;286(21):18708–18719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo M, Lotti LV, Martino S, Alessio M, Conti A, Bachi A, Mariani-Costantini R, Biunno I. 2011. Secretion of novel SEL1L endogenous variants is promoted by ER stress/UPR via endosomes and shed vesicles in human cancer cells. PLoS ONE. 6(2):e17206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo M, Lotti LV, Martino S, Cardano M, Orlandi R, Mariani-Costantini R, Biunno I. 2009. Functional characterization of two secreted SEL1L isoforms capable of exporting unassembled substrate. J Biol Chem. 284(17):11405–11415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo M, Orlandini S, Beghelli S, Moore PS, Sorio C, Bonora A, Bassi C, Talamini G, Zamboni G, Orlandi R, et al. 2003. SEL1L expression in pancreatic adenocarcinoma parallels SMAD4 expression and delays tumor growth in vitro and in vivo. Oncogene. 22(41):6359–6368 [DOI] [PubMed] [Google Scholar]
- Conway C, Dobson L, O’Grady A, Kay E, Costello S, O’Shea D. 2008. Virtual microscopy as an enabler of automated/quantitative assessment of protein expression in TMAs. Histochem Cell Biol. 130:447–463 [DOI] [PubMed] [Google Scholar]
- Feldman M. 1973. Reactions of nucleic acids and nucleoproteins with formaldehyde. Prog Nucleic Acid Res Mol Biol. 13:1–49 [DOI] [PubMed] [Google Scholar]
- Ferrero S, Falleni M, Cattaneo M, Malferrari G, Canton C, Biagiotti L, Maggioni M, Nosotti M, Coggi G, Bosari S, et al. 2006. SEL1L expression in non-small cell lung cancer. Hum Pathol. 37(5):505–512 [DOI] [PubMed] [Google Scholar]
- Gilbert MT, Sanchez JJ, Haselkorn T, Jewell LD, Lucas SB, Van Marck E, Børsting C, Morling N, Worobey M. 2007. Multiplex PCR with minisequencing as an effective high-throughput SNP typing method for formalin-fixed tissue. Electrophoresis. 28(14):2361–2367 [DOI] [PubMed] [Google Scholar]
- Gillett CE, Springall RJ, Barnes DM, Hanby AM. 2000. Multiple tissue core arrays in histopathology research: a validation study. J Pathol. 192(4):549–553 [DOI] [PubMed] [Google Scholar]
- Gruber AD, Moennig V, Hewicker-Trautwein M, Trautwein G. 1994. Effect of formalin fixation and long-term storage on the detectability of bovine viral-diarrhoea-virus (BVDV) RNA in archival brain tissue using polymerase chain reaction. Zentralbl Veterinarmed B. 41:654–661 [DOI] [PubMed] [Google Scholar]
- Hewitt SM. 2006. The application of tissue microarrays in the validation of microarray results. Methods Enzymol. 410:400–415 [DOI] [PubMed] [Google Scholar]
- Hewitt SM. 2012. Tissue microarrays as a tool in the discovery and validation of predictive biomarkers. Methods Mol Biol. 823:201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoos A, Cordon-Cardo C. 2001. Tissue microarray profiling of cancer specimens and cell lines: opportunities and limitations. Lab Invest. 81:1331–1338 [DOI] [PubMed] [Google Scholar]
- Jensen JB, Parmar M. 2006. Strengths and limitations of the neurosphere culture system. Mol Neurobiol. 34(3):153–161 [DOI] [PubMed] [Google Scholar]
- Kallioniemi OP, Wagner U, Kononen J, Sauter G. 2001. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet. 10(7):657–662 [DOI] [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. 1998. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 4(7):844–847 [DOI] [PubMed] [Google Scholar]
- Kyndi M, Sørensen FB, Knudsen H, Overgaard M, Nielsen HM, Andersen J, Overgaard J. 2008. Tissue microarrays compared with whole sections and biochemical analyses: a subgroup analysis of DBCG 82 b&c. Acta Oncol. 47(4):591–599 [DOI] [PubMed] [Google Scholar]
- Liu Q, Chen J, Mai B, Amos C, Killary AM, Sen S, Wei C, Frazier ML. 2012. A single-nucleotide polymorphism in tumor suppressor gene SEL1L as a predictive and prognostic marker for pancreatic ductal adenocarcinoma in Caucasians. Mol Carcinog. 51(5):433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente Garín JA, Lloreta Trull J, Allepuz Losa C, Plaza Mas L, Rioja Sanz LA, Gelabert Mas A. 2006. Development of tissue microarray technology (TMA) for immunohistochemical study of molecular expression profiling in prostate cancer (part 1). Actas Urol Esp. 30(1):25–32 [DOI] [PubMed] [Google Scholar]
- Macabeo-Ong M, Ginzinger DG, Dekker N, McMillan A, Regezi JA, Wong DT, Jordan RC. 2002. Effect of duration of fixation on quantitative reverse transcription polymerase chain reaction analyses. Mod Pathol. 15:979–987 [DOI] [PubMed] [Google Scholar]
- Malferrari G, Monferini E, DeBlasio P, Diaferia G, Saltini G, Del Vecchio E, Rossi-Bernardi L, Biunno I. 2002. High-quality genomic DNA from human whole blood and mononuclear cells. Biotechniques. 33 (6):1228–1230 [DOI] [PubMed] [Google Scholar]
- Masuda N, Ohnishi T, Kawamoto S, Monden M, Okubo K. 1999. Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samples. Nucleic Acids Res. 27:4436–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. 2008. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 68(10):3645–3654 [DOI] [PubMed] [Google Scholar]
- Orlandi R, Cattaneo M, Troglio F, Casalini P, Ronchini C, Ménard S, Biunno I. 2002. SEL1L expression decreases breast tumor cell aggressiveness in vivo and in vitro. Cancer Res. 62(2):567–574 [PubMed] [Google Scholar]
- Santos S, Sà D, Bastos E, Guedes-Pinto H, Gut I, Gärtner F, Chaves R. 2009. An efficient protocol for genomic DNA extraction from formalin-fixed paraffin-embedded tissues. Res Vet Sci. 86(3):421–426 [DOI] [PubMed] [Google Scholar]
- Sapino A, Marchiò C, Senetta R, Castellano I, Macrì L, Cassoni P, Ghisolfi G, Cerrato M, D’Ambrosio E, Bussolati G. 2006. Routine assessment of prognostic factors in breast cancer using a multicore tissue microarray procedure. Virchows Arch. 449:288–296 [DOI] [PubMed] [Google Scholar]
- Sauter G, Mirlacher M. 2002. Tissue microarrays for predictive molecular pathology. J Clin Pathol. 55(8):575–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg Fejzo M, Slamon DJ. 2001. Frozen tumor tissue microarray technology for analysis of tumor RNA, DNA, and proteins. Am J Pathol. 159(5):1645–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M, Sedmak D, Jewell S. 2002. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 161:1961–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takikita M, Chumg JK, Hewitt SM. 2007. Tissue microarrays enabling high-throughput molecular pathology. Curr Opin Biotechnol. 18(4):318–325 [DOI] [PubMed] [Google Scholar]
- Thomson TA, Zhou C, Ceballos K, Knight B. 2010. Tissue microarray for routine clinical breast biomarker analysis. The British Columbia Cancer Agency 2008 experience. Am J Clin Pathol. 133:909–914 [DOI] [PubMed] [Google Scholar]
- Torhorst J, Bucher C, Kononen J, Haas P, Zuber M, Köchli OR, Mross F, Dieterich H, Moch H, Mihatsch M, et al. 2001. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol. 159:2249–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan WH, Fortuna MB, Furmanski P. 1987. A rapid and efficient method for testing immunohistochemical reactivity of monoclonal antibodies against multiple tissue samples simultaneously. J Immunol Methods. 103(1):121–129 [DOI] [PubMed] [Google Scholar]
- Waterworth A, Hanby A, Speirs V. 2005. A novel cell array technique for high-throughput, cell-based analysis. In Vitro Cell Dev Biol Anim. 41(7):185–187 [DOI] [PubMed] [Google Scholar]
- Wen CH, Su YC, Wang SL, Yang SF, Chai CY. 2007. Application of the microarray technique to cell blocks. Acta Cytol. 51(1):42–46 [DOI] [PubMed] [Google Scholar]
- Zhou L, Hodeib M, Abad JD, Mendoza L, Kore AR, Hu Z. 2007. New tissue microarray technology for analyses of gene expression in frozen pathological samples. Biotechniques. 43(1):101–105 [DOI] [PubMed] [Google Scholar]
- Zsikla V, Baumann M, Cathomas G. 2004. Effect of buffered formalin on amplification of DNA from paraffin wax embedded small biopsies using real-time PCR. J Clin Pathol. 57(6):654–656 [DOI] [PMC free article] [PubMed] [Google Scholar]