Abstract
Endothelial tip cells are leading cells at the tips of vascular sprouts coordinating multiple processes during angiogenesis. In the developing retina, tip cells play a tightly controlled, timely role in angiogenesis. In contrast, excessive numbers of tip cells are a characteristic of the chaotic pathological blood vessels in proliferative retinopathies. Tip cells control adjacent endothelial cells in a hierarchical manner to form the stalk of the sprouting vessel, using, among others, the VEGF-DLL-Notch signaling pathway, and recruit pericytes. Tip cells are guided toward avascular areas by signals from the local extracellular matrix that are released by cells from the neuroretina such as astrocytes. Recently, tip cells were identified in endothelial cell cultures, enabling identification of novel molecular markers and mechanisms involved in tip cell biology. These mechanisms are relevant for understanding proliferative retinopathies. Agents that primarily target tip cells can block pathological angiogenesis in the retina efficiently and safely without adverse effects. A striking example is platelet-derived growth factor, which was recently shown to be an efficacious additional target in the treatment of retinal neovascularization. Here we discuss these and other tip cell-based strategies with respect to their potential to treat patients with ocular diseases dominated by neovascularization.
Keywords: angiogenesis, endothelial tip cell, proliferative retinopathy, anti-angiogenesis therapy, retinal neovascularization, vascular sprouts, endothelial stalk cell, molecular mediators of angiogenesis, pericytes
Ocular diseases dominated by neovascularization, such as age-related macular degeneration (AMD), retinal vein occlusions, and diabetic retinopathy (DR), often culminate in severe visual loss and ultimately blindness. In most of these conditions, ischemic retinal areas produce abnormal amounts of angiogenic growth factors that induce excessive angiogenesis, overwhelming the effects of natural angiogenesis inhibitors (Witmer et al. 2003). The newly formed vessels occur as part of wound-healing responses and usually do not restore tissue integrity. Vision is disturbed when bleeding occurs by secondary macular edema, or when vessels finally grow into normally avascular transparent tissues such as the fovea and vitreous and develop into scar tissue (Schlingemann 2004). The most widely used therapeutic target in treating ocular neovascularization is vascular endothelial growth factor-A (VEGF-A) (Witmer et al. 2003). Currently, VEGF-A antagonists are standard care in the treatment of exudative AMD and have been found to be a valuable additional treatment strategy in several other vascular retinal diseases (Schlingemann and Witmer 2009).
VEGF and other endogenous factors that can drive angiogenesis have been studied in detail in the past decades, but our understanding of the complex molecular mechanisms that regulate endothelial cell differentiation during sprout formation has advanced only recently. Sprouting angiogenesis requires hierarchical organization and activation of endothelial cells from preexisting blood vessels to form new vessels, while at the same time the majority of endothelial cells need to remain quiescent. This hierarchical organization is mainly mediated by specialized motile endothelial cells located at the tips of growing vessels, termed tip cells. Simultaneously, complex interactions occur between endothelial cells and supporting cells in the microenvironment via angiogenic growth factors and inhibitors (Stahl et al. 2010). In the retina, supporting cells are mainly pericytes that envelope retinal capillaries to provide vessel stability.
Tip cells have a unique functional and molecular signature and are therefore an attractive target for therapeutic modulation of angiogenesis. The relative ease of accessibility for imaging and intervention makes the retina ideally suitable for research on tip cells (Stahl et al. 2010), when compared with models of tumor angiogenesis (reviewed in Chappell et al. 2012). This has spurred intensive research into the complex molecular mechanisms that regulate endothelial differentiation and has led to the screening of potential therapeutic agents. In fact, many studies on molecular targets of tip cells in the field of tumor angiogenesis also included mouse models of retinal angiogenesis. However, to date, no single antibody can serve as a unique marker of endothelial tip cells in vivo.
A recent study from our group demonstrated that cells with virtually all the known properties of tip cells are present in two-dimensional vascular endothelial cell cultures and that this subpopulation of cells can be isolated on the basis of CD34 expression (Siemerink et al. 2012). This in vitro tip cell model has opened alternative avenues for the study of molecular processes and functions in tip cells in angiogenesis and has allowed characterization of the full transcriptome of tip cells in vitro. This has identified a large number of tip cell-associated genes not previously published, in addition to the known genes previously associated with tip cells in vivo. The present review translates these findings to the context of angiogenesis in proliferative retinal diseases, as well as to the possible implications of these findings for development of therapeutic strategies.
Endothelial Cell Differentiation in Vascular Sprouts
Angiogenesis is tightly controlled by closely interacting angiogenic and angiostatic factors, and their balance ultimately determines if, where, and when the “angiogenic switch” is turned on, with angiogenesis as the result (Witmer et al. 2003). The angiogenic switch occurs only in a small subset of endothelial cells without disrupting the integrity of the preexisting vascular network. Subsequently, these endothelial cells differentiate to enable migration, proliferation, lumen formation, and the formation of vessel walls. These cellular processes are not taking place simultaneously in all cells involved. Instead, several highly specialized endothelial phenotypes with distinct functions form a vascular sprout in an orchestrated manner (reviewed in Herbert and Stainier 2011).
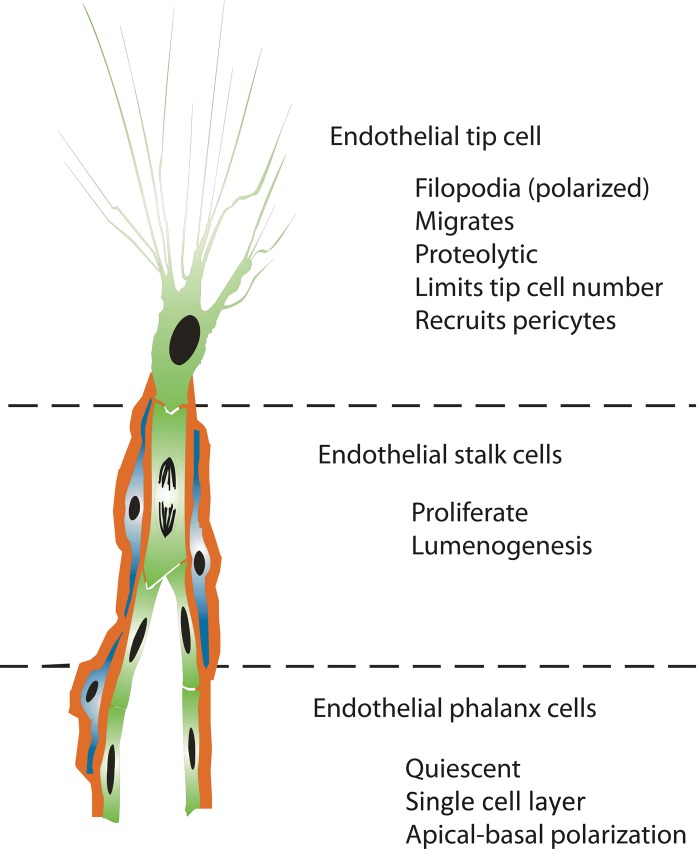
First, a single tip cell emerges from the existing blood vessel and becomes the leading cell of the sprouting vessel. Tip cells dynamically extend long filopodial protrusions in a polarized way, migrate into the extracellular matrix, and sense their environment for attractive and repulsive signals for guidance (Fig. 1) (Klosovskii and Zhukova 1963; Schoefl 1963; Marin-Padilla 1985; Schlingemann et al. 1990; Witmer et al. 2001, 2004; Gerhardt et al. 2003). Tip cells have a minimal proliferation rate or do not proliferate at all, are not involved in the formation of the vessel lumen, and actively recruit non-vascular cells, including pericytes.
Figure 1.
Concept of endothelial cell differentiation during angiogenesis. Angiogenic sprouts are formed by a subset of specialized endothelial cell phenotypes (green), each with a distinct cellular fate. Pericytes (blue) are instantly recruited to ensheathe the sprouting vessel and to produce a basal lamina (red).
Second, other endothelial cells, directly following the migrating tip cell, differentiate under the influence of the tip cell into stalk cells that proliferate and bridge the gap between the tip cell and the parent vasculature. Stalk cells generate the blood vessel lumen, a process called lumenogenesis (reviewed in Iruela-Arispe and Davis 2009). Together, the tip and stalk cell phenotypes form a vascular sprout, which grows toward an angiogenic stimulus, in response to chemical cues, mechanical factors, and some degree of random motility.
Third, endothelial cells behind the stalk cells differentiate into phalanx cells and align in a smooth cobblestone monolayer, becoming the most inner cell layer in the new blood vessel, where they no longer proliferate (reviewed in De Bock et al. 2009). Both stalk and phalanx cells express tight junctions and associate with supporting vascular smooth muscle cells or pericytes, depending on the type of vascular bed. The retinal vasculature appears to be particularly dependent on pericytes, and defective pericyte recruitment affects the retina more than other tissues (reviewed in Ejaz et al. 2008). Finally, endothelial tip cells of two sprouts come together and form new blood vessels, a process called anastomosis, mediated by tissue-resident macrophages (Fantin et al. 2010).
Angiogenic Tip Cells in the Retina
Embryo
Tip cells are distinguished predominantly on the basis of their location and specific morphology. Isolectin-B4 and anti-CD34, anti-VEGF receptor 3 (VEGFR3), and anti-laminin antibodies enable visualization of tip cells and their filopodia in angiogenic tissues, such as the developing retina. In these tissues, tip cells are found at the edge of the expanding vascular plexus, extending numerous filopodia that probe the environment (Fig. 2A) (Klosovskii and Zhukova 1963; Schoefl 1963; Marin-Padilla 1985; Schlingemann et al. 1990; Hughes et al. 2000; Gerhardt et al. 2003; Witmer et al. 2001, 2004). However, most of these antibodies also stain stalk cells and phalanx cells. To date, a single antibody that can be used as a specific marker of endothelial tip cells in vivo has not been identified.
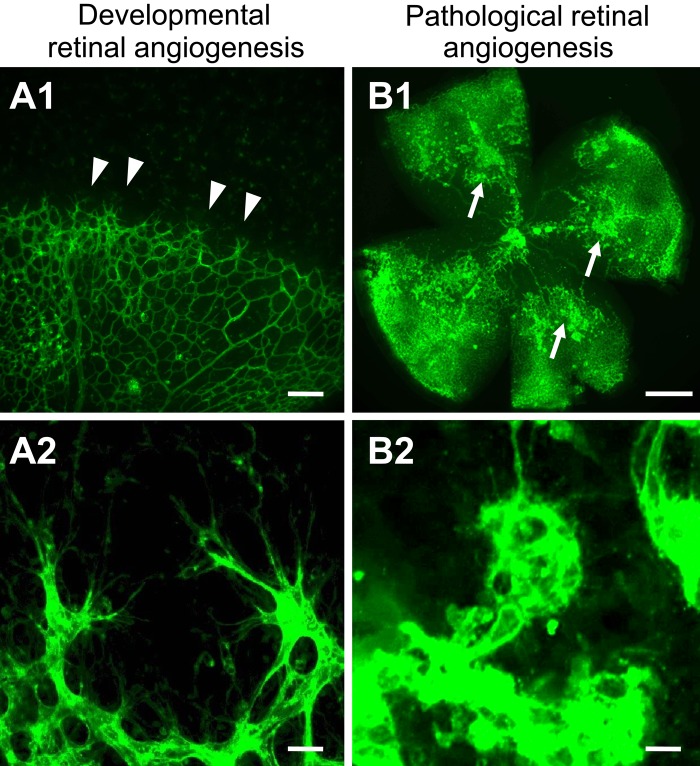
Figure 2.
Tip cells are actively generated in physiological and pathological conditions of the retina. Confocal images of blood vessels from mouse retinas stained with Alexa 488-conjugated isolectin-B4. (A1, A2) Retinal wholemount from postnatal day 5 shows that the superficial vascular plexus is formed by radial outgrowth of vessels from the optic nerve into the periphery. (A1) Tip cells are located at the angiogenic front (arrowheads). Scale bar = 50 µm. (A2) High magnification of tip cells with their typical morphology (highly polarized nature and numerous filopodia probing the environment). Scale bar = 20 µm. (B1, B2) Retinal wholemount from postnatal day 17 in the oxygen-induced retinopathy model when the maximum severity of the pathological neovascularization is reached during relative hypoxia. (B1) Pathological neovessels leave the retina and grow into the vitreous cavity to form unorganized, small-caliber vessels, termed preretinal tufts (arrows). Scale bar = 500 µm. (B2) High magnification of an epiretinal tuft that is formed by activated endothelial cells that extend numerous filopodia in all directions. Scale bar = 20 µm.
In contrast to humans, where development of the intraretinal vasculature is completed at the time of birth, retinal vascularization in mice occurs postnatally, which enables the study of various stages of vessel network formation in neonatal animals. The mouse retina has therefore contri-buted significantly to our understanding of mechanisms of endothelial cell differentiation during angiogenic sprouting (Hughes et al. 2000; Gerhardt et al. 2003; Chappell et al. 2012). In the first week after birth, retinal vessels immediately emerge from the optic nerve head, grow radially toward the retinal periphery, and form the laminar superficial vascular plexus. In the second postnatal week, branches of the superficial vessels sprout to generate the deep vascular plexus. A tertiary intermediate vascular plexus is formed in the third postnatal week. Tip cells have been found in all areas of this active retinal angiogenic network formation, indicating that tip cells are actively generated during physiological retinal neovascularization (Fantin et al. 2010; Caprara et al. 2011; Caprara and Grimm 2012).
During retinal development, the vascular and neuroretinal cell systems display a high degree of crosstalk and depend on each other functionally. Regulatory mechanisms respond to altered oxygen profiles during retinal development to induce a controlled and organized angiogenic response (reviewed in Caprara and Grimm 2012). The neuroretina acts primarily as an oxygen sensor, through the transcription factor hypoxia-inducible factor 1 alpha subunit (HIF-1α), which is required for proper vascular patterning in the retina (Caprara et al. 2011; Nakamura-Ishizu et al. 2012). In addition, an astrocytic network is established in the retina and serves as a template over which filopodia-mediated tip cell migration takes place (Dorrell et al. 2002).
Pathological Conditions
The typical morphological aspects of tip cells (highly polarized nature and numerous filopodia probing the environment) were also found in specimens of human pathological retinal neovascularization (Schlingemann et al. 1990; Schlingemann 2004) and in tumors (Schlingemann et al. 1990). Compared with physiological angiogenesis, both the number of tip cells as well as the number of filopodial protrusions per tip cell is highly increased in areas of pathological angiogenesis. Several in vivo models have been developed to study mechanisms of pathological neovascularization in retinal diseases (Grossniklaus et al. 2010). One of the major models is oxygen-induced retinopathy in mice: an acute biphasic model of preretinal neovascularization associated with ischemia in the retina that develops after a period of experimental hyperoxia (Smith et al. 1994; Stahl et al. 2010). These pathological newly formed vessels grow outside the highly organized layered structure of the retina into the normally avascular vitreous cavity to form disorganized, small-sized vascular structures, termed preretinal tufts. The tufts consist of activated endothelial cells and resemble pathological neovascularization observed in human proliferative retinopathies (Stahl et al. 2010). It has been shown that most endothelial cells from these tufts extend numerous filopodia (Budd et al. 2009; Fukushima et al. 2011; Hakansson et al. 2011; Zhang W et al. 2011). In contrast to tip cells in developmental angiogenesis, pathologically generated tip cells extend shorter filopodia, which grow in all directions (Fig. 2B). In addition to the formation of these “pathological” neovascular tufts, angiogenesis occurs also within the retina in this model, restoring the vasculature of the ischemic non-perfused areas.
What Controls the Differentiation of Tip Cells and Stalk Cells?
VEGF Induces the Tip Cell Phenotype
The VEGF family of growth factors are key molecules for initiation and direction of blood vessel sprouting. In mammals, the VEGF family includes VEGF-A, VEGF-B, placenta growth factor (PlGF), VEGF-C, and VEGF-D (reviewed in Witmer et al. 2003). It is widely accepted that VEGF-A is crucial for both vasculogenesis and angiogenesis: loss of only a single allele in mice or zebrafish is lethal due to severe vascular defects and abnormalities (Carmeliet et al. 1996). Tip cells, stalk cells, and phalanx cells are all dependent on the binding of VEGF to cell membrane VEGF receptors (VEGFRs). Interestingly, all three phenotypes react in distinct ways to VEGF-A signaling: Tip cells start to migrate and extend filopodia, stalk cells undergo proliferation, and phalanx cells need low levels of VEGF-A for survival. A first explanation for these multiple complex roles of VEGF-A in morphogenic events was based on observations showing that local concentrations of VEGF-A vary in areas of angiogenic tissues and that endothelial cells differentiate into tip cells and remain tip cells only when VEGF-A levels are above a certain threshold.9 Hence, endothelial cells exposed to the highest VEGF-A levels are the ones that likely become tip cells. Furthermore, the activation of cell membrane receptors has differential effects in tip cells, stalk cells, and phalanx cells, most likely due to combinations with other signals. VEGFs bind selectively to three distinct receptors with different affinities: VEGFR1, also known as FLT1; VEGFR2, also known as KDR or FLK1; and VEGFR3, also known as FLT4 (Witmer et al. 2003). VEGFR2 is considered to be the major receptor responsible for mediating the angiogenic effects of VEGF-A. Relative expression levels of VEGFR2 play a crucial role in endothelial cell differentiation: the highest levels of messenger RNA and protein of VEGFR2 were found in tip cells (Fig. 3) (Gerhardt et al. 2003).Furthermore, tip cells and their filopodia express VEGFR3 (Witmer et al. 2001; Siekmann and Lawson 2007; Tammela et al. 2008, 2011; Benedito et al. 2012). Activation of VEGFR3 by its ligand, VEGF-C, but also ligand-independent VEGFR3 activation allows angiogenic vessel growth (Galvagni et al. 2010; Zhang LQ et al. 2010; Tammela et al. 2011), even in the absence of VEGF-A/VEGFR2 signaling (Benedito et al. 2012). VEGFR1 is expressed in tip, stalk, and phalanx cells. A recent study in zebrafish showed that loss of VEGFR1 signaling increases the number of tip cells, suggesting that VEGFR1 acts as a negative regulator of tip cell differentiation and branching (Krueger et al. 2011).
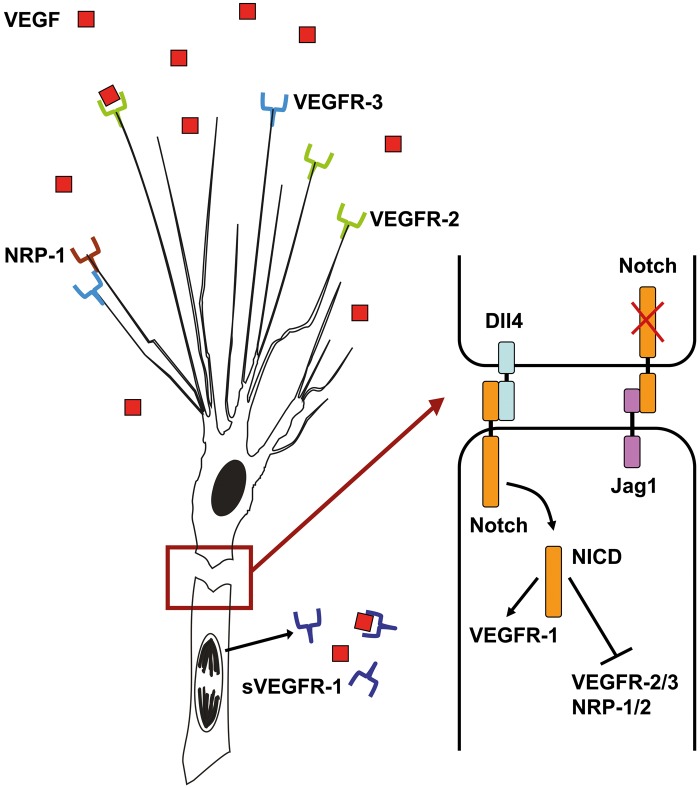
Figure 3.
Schematic representation of the VEGF-DLL4-Notch signaling pathway in angiogenic sprouts. Tip cells express vascular endothelial growth factor receptor 2 (VEGFR2) and vascular endothelial growth factor receptor 3 (VEGFR3) together with several co-receptors, including neuropilin-1 (NRP1), to sense for vascular endothelial growth factor (VEGF) signals. Extracellular gradients of VEGF provide a template for tip cell attraction. Upon activation, tip cells express DLL4, which binds to Notch receptors on follower stalk cells. In stalk cells, Notch is cleaved by gamma-secretase, releasing the Notch intracellular domain (NICD), which dampens the expression of VEGFR2 and VEGFR3, as well as increases VEGFR1 expression. sVEGFR1 is released by stalk cells and binds to VEGF molecules to block local VEGF signaling. Jagged1 is expressed by stalk cells and negatively regulates Notch activity in tip cells.
VEGF/VEGFR signaling complexes also include co-receptors such as neuropilin-1 (NRP1) and NRP2. A co-receptor is a cell surface receptor that binds a signaling molecule in addition to a primary receptor, facilitating ligand recognition, and inducing specific internal cellular signaling. The formation of VEGFR2/NRP complexes amplifies VEGF-induced angiogenesis (Gerhardt et al. 2004; Tammela et al. 2008). The filopodia of tip cells are positive for both NRP1 and NRP2 (Gerhardt et al. 2004). Another co-factor of VEGF signaling in tip cell induction is ephrin-B2 (EFNB2), a transmembrane ligand for EphB receptor tyrosine kinases (Bochenek et al. 2010). EFNB2 controls VEGFR2 internalization, which is necessary for activation and downstream signaling of the receptor, and is required for VEGF-induced tip cell filopodia extension (Sawamiphak et al. 2010; Wang et al. 2010). Finally, after activation by VEGF-A or VEGF-C, tip cells can form heterodimers of VEGFR2 and VEGFR3 that positively regulate angiogenic sprouting (Nilsson et al. 2010; Zhang LQ et al. 2010). Thus, once they have been induced by VEGF-A, tip cells become much more sensitive to VEGF than adjacent stalk cells. Endothelial cells determine the appropriate response to VEGF activation via crosstalk between different VEGF receptors in combination with the activity of co-receptors and other signaling molecules.
The hierarchical non-symmetric relations between endothelial cells in sprouts are dynamic, and differentiation of tip cells is reversible. Tip and stalk cells continuously compete for the tip cell position, controlled by differential VEGFR expression levels in individual endothelial cells (Jakobsson et al. 2010). A tip cell erroneously migrating toward an area containing lower levels of VEGF-A may lose sensitivity to VEGF-A, allowing the challenging neighbor to gain a competitive advantage (Jakobsson et al. 2010). Because the tip cell position seems to be controlled by survival and local adaptation, one can easily speculate that the tip cell phenotype is dominant over others, implicating that the tip cell controls adjacent endothelial cells and other cell types in terms of cell fate.
Lateral Cell-Cell Inhibition Limits Tip Cell Formation
Differentiation of endothelial tip cells from a population of quiescent endothelial cells has to be tightly regulated because excessive tip cell formation results in a poorly patterned, hyperdense vessel network that may not be functional. Therefore, tip cells signal to their adjacent endothelial cells to downregulate their tip cell phenotype and maintain the proliferative stalk cell phenotype. As stated above, relative expression levels of VEGFR2 and other receptors ultimately determine whether a cell becomes a tip cell or not. In tip cells, VEGFR2 signaling induces the expression of the Notch ligand delta-like 4 (DLL4), which is then transported to the cell membrane and binds to Notch receptors on adjacent endothelial cells (Fig. 3). Upon DLL4 binding, Notch is cleaved, generating the Notch intracellular domain, which acts as a transcriptional regulator and dampens VEGFR2, VEGFR3, and NRP1 expression in these future stalk cells, as well as induces the transcription of VEGFR1 and its soluble splice variant sVEGFR1 (Siekmann and Lawson 2007; Suchting et al. 2007; Harrington et al. 2008; Tammela et al. 2008). sVEGFR1 is secreted by stalk cells and binds to VEGF-A in the extracellular matrix, decreasing local levels of available VEGF-A (Harrington et al. 2008). Secretion of sVEGFR1 therefore decreases VEGF/VEGFR2 signaling in stalk cells, consolidating the stalk cell phenotype.
Cell-matrix interactions mediated by laminin/integrin-induced signaling are necessary to induce physiologically functional levels of DLL4 expression in tip cells (Estrach et al. 2011; Stenzel et al. 2011). VEGF treatment stimulates laminin production, leading to integrin signaling and induction of DLL4. Apparently, the extracellular matrix is important for promotion of DLL4 expression in endothelial tip cells. On the other hand, a recent study showed that VEGF-A/VEGFR2 signaling is not essential for DLL4 expression in tip cells (Benedito et al. 2012).
Another critical component in tip cell-stalk cell Notch signaling is Jagged1, which is abundantly expressed in stalk cells (Hofmann and Iruela-Arispe 2007; Benedito et al. 2009). Jagged1 negatively regulates Notch activity by antagonizing DLL4-induced Notch activation. Because Notch is expressed in both tip cells and stalk cells, it is likely that Jagged1 from stalk cells binds to Notch on adjacent tip cells, thereby inactivating this signaling pathway in tip cells (Benedito et al. 2009; Suchting and Eichmann 2009). Jagged1 also counteracts DLL4-Notch signaling interactions between adjacent stalk cells, which may help to sustain low VEGFR expression levels in stalk cells and keep them sensitive for VEGF-A to promote stalk cell proliferation (Fig. 3). However, the exact role of these DLL4/Jagged1 interactions in angiogenesis is still unclear.
A third Notch-ligand, DLL1, was also shown to be involved in endothelial cell differentiation but not via lateral inhibition. DLL1 is secreted by non-vascular cells in the retina during development and induces VEGFR3 and EFNB2 expression in endothelial tip cells (Napp et al. 2012). DLL1 is a less potent Notch activator than DLL4; induction of DLL4 in tip cells that binds to Notch on stalk cells therefore results in less sensitivity of the Notch receptor for DLL1 in stalk cells. Taken together, these data emphasize that specific Notch ligands, in crosstalk with VEGF, exert diverse effects in endothelial cell differentiation during angiogenesis and that both tip and stalk cells can use these different effects to control each other.
Tip Cells Control Sprout Behavior through Secretion of Specific Molecules
If and how tip cells signal to stalk cells that are not in direct cell-cell contact is less well understood. Several recent studies attempted to analyze the expression profiles of endothelial tip cells and identified a number of tip cell-associated genes that are specifically expressed by these cells (Harrington et al. 2008; del Toro et al. 2010; Strasser et al. 2010; Siemerink et al. 2012). These studies not only expand the tip cell gene expression profile but also contribute to our understanding of tip cell biology and tip cell-specific functions. Surprisingly, the largest cluster of upregulated genes in tip cells encodes for proteins that are secreted, including chemokines, proteases, adrenomedullin (ADM), platelet-derived growth factor-B (PDGF-B), angiopoietin-2 (ANG2), apelin (APLN), insulin-like growth factor binding proteins (IGFBPs), and endothelial specific molecule-1 (del Toro et al. 2010; Strasser et al. 2010; Siemerink et al. 2012). These molecules can potentially regulate many aspects of angiogenesis such as interactions with astrocytes, microglial cells, pericytes, and endothelial stalk cells. Secretion of molecules further indicates the dominant role of the tip cell over other endothelial cell phenotypes and other cell types. Some of these gene products have been studied for their functional role in angiogenesis. However, extensive further studies are necessary to elucidate their exact functions in tip cell biology. It may be assumed that tip cells secrete regulatory proteins while creating a path throughout the extracellular matrix to control behavior of trailing stalk cells and pericytes during angiogenesis.
Another mechanism of distant signaling by tip cells has been suggested on the basis of secretion of exosomes containing DLL4, indicating an additional long-range signaling mechanism involved in tip cell differentiation (Sheldon et al. 2010). These exosomes appeared to be able to confer a tip cell phenotype on recipient cells and may represent a mechanism by which stalk cell differentiation is restricted so that tip cell formation can recur. However, the exact role of these exosomes during angiogenesis in vivo is not clear.
Modulation of Tip Cells by MicroRNAs
MicroRNAs (miRNA) are a class of short non-coding RNA molecules that bind to complementary sequences of target messenger RNA transcripts (mRNAs) to regulate activity of transcripts at the posttranscriptional level. Because relative gene expression levels are crucial in the dynamic competition between tip cells and stalk cells, miRNAs may function as internal mechanisms to rapidly change the effects of gene expression levels in these cells. There is increasing evidence that miRNAs play important roles in vascular development as well as in vascular diseases, including DR and AMD (Shen et al. 2008; Kovacs et al. 2011). In addition, multiple miRNAs have been implicated in controlling the angiogenic response of endothelial cells to various growth factors (reviewed in Caporali and Emanueli 2011).
Two recent studies identified miR-221 and miR-27b to be important in regulation of tip cell functioning during angiogenesis in zebrafish models (Biyashev et al. 2012; Nicoli et al. 2012). miR-221 was found to repress two distinct pathways in sprouting endothelial cells: the phosphoinositide-3-kinase, regulatory subunit 1 alpha (PIK3R1) complex and cell cycle progression via cyclin-dependent kinase inhibitor 1b (CDKN1B). Downregulation of CDKNB1 and promotion of optimal PI3K output by reduction of PIK3R1 induced tip cell migration and proliferation (Nicoli et al. 2012). Furthermore, miR-221 levels were found to be negatively regulated downstream of DLL4/Notch signaling in stalk cells. Thus, miR-221 enables endothelial cells to dynamically respond to pro-angiogenic cues.
miR-27b was found to promote microvessel sprouting and branching by repressing DLL4 and therefore increasing the number of endothelial cells that have the tip cell phenotype (Biyashev et al. 2012). Blockade of miR-27b impaired sprouting and induced the generation of large open vessels in tumors (Biyashev et al. 2012).
miRNA-126 is regarded as one of the most important microRNAs governing vascular integrity and angiogenesis by regulating the signaling of angiogenic growth factors (Fish et al. 2008). Retinal levels of miR-126 were found to be decreased in the mouse model of oxygen-induced retinopathy, and intravitreal injection of miR-126 was found to reduce pathological neovascularization in this model (Bai et al. 2011). Both miR-27b and miR-126 share common target genes, suggesting that miR-126 regulates vascular sprouting and tip cell formation (Kuhnert et al. 2008). These findings suggest that miRNAs mediate a fine balance between inducers and suppressors of endothelial differentiation, as well as tip cell- and stalk cell-specific behavior in angiogenic sprouts.
Functional Characteristics of Endothelial Tip Cells
Migration and Searching for Guidance Cues
Tip cells are known to express receptors for guidance cues, such as the Netrin receptor uncoordinated-5B (UNC5B) and NRPs, by which they pick up attractive or repulsive signals from the tissue environment and translate them into a dynamic process of adhesion and detachment, leading to directed migration. In this process, the tip cell forms filopodia and lamellipodia (reviewed in Mattila and Lappalainen 2008). Filopodia are long, spiky plasma membrane protrusions containing tight parallel bundles of filamentous actin, and they function as antennae with which tip cells probe their environment. Lamellipodia are short protrusions that contain a highly branched actin network and are located at the front edge of the cell. In lamellipodia, the intracellular cytoskeleton is connected to the extracellular matrix via adhesion molecules, allowing stress fibers of actin/myosin filaments to pull the cell forward. The main regulators of filopodia and lamellipodia formation are members of the Rho small GTPases, which are induced by VEGF/VEGFR2 signaling in tip cells (Lamalice et al. 2007; Ispanovic et al. 2008). An extracellular VEGF-A gradient appears to be a strong attractant for migrating endothelial cells via binding to VEGFR2 and NRPs, which are prominently present on tip cell filopodia (Gerhardt et al. 2003, 2004; Lamalice et al. 2007). An important biological property of the different VEGF-A isoforms is their varying heparin- and heparansulfate-binding capacity (reviewed in Witmer et al. 2003). The larger VEGF-A isoforms bind very tightly to heparin and thus remain sequestered in the extracellular matrix, whereas the shorter VEGF-A isoforms diffuse freely. VEGF-A189 and VEGF-A165 promote polarized extension of tip cell filopodia, whereas VEGF-A121 induces endothelial cell proliferation but has little effect on tip cell guidance (reviewed in Carmeliet 2003).
Endothelial tip cells function in a remarkably similar way as axonal growth cones (reviewed in Carmeliet 2003; Dorrell and Friedlander 2006; Adams and Eichmann 2010). Blood vessels and nerve fibers course throughout the body alongside one another, and it has been reported that, during embryogenesis, their patterning is guided in large part by similar attractive and repulsive guidance cues. Thus far, four major families of receptors have been shown to regulate guidance events during axonal and vascular morphogenesis: plexin/NRP complexes with their ligands class 3 Semaphorins, UNC5 family and “deleted in colorectal cancer” (DCC) with their ligands Netrins, “roundabout” (ROBO) with their ligands Slits, and EPH and their ligands Ephrins (Table 1) (Carmeliet 2003).
Table 1.
Molecular Regulators of Tip Cell Behavior and Differentiation
| Genes Upregulated in Tip Cells | Description | Function(s) |
|---|---|---|
| VEGFR2 | Transmembrane receptor | Tip cell activation, filopodia formation, tip cell guidance (attractive), migration |
| VEGFR3 | Transmembrane receptor | Tip cell activation, tip cell guidance (attractive), modulates vascular endothelial growth factor receptor 2 (VEGFR2) signaling |
| NRP1 | Transmembrane receptor | Increased VEGF co-receptor, VEGFR2 and VEGFR3 signaling |
| PDGFB | Secreted molecule | Pericyte recruitment |
| DLL4 | Transmembrane protein | Tip cell-phenotype suppression in stalk cells |
| ANGPT2 | Secreted molecule | Vessel destabilization (only in the presence of VEGF), stalk cell proliferation |
| APLN | Secreted molecule | Stalk cell proliferation |
| UNC5B | Transmembrane receptor | Tip cell guidance (repulsive) |
| PLXND1 | Transmembrane receptor | Tip cell guidance (repulsive) |
| EFNB2 | Transmembrane protein | VEGFR2 internalization, filopodia formation, migration |
| MMP14 | Protease | Pericellular collagenase, extracellular matrix remodeling |
| CXCR4 | Chemokine receptor | Tip cell activation, mediation of tip cell morphology, tip cell guidance (attractive) |
Extracellular Matrix and Basal Lamina Interactions
To allow tip cells to penetrate avascular tissues, the basal lamina and extracellular matrix barriers that ensheathe the preexisting capillary have to be degraded. Therefore, tip cells acquire a proteolytic phenotype and secrete proteases, leading to remodeling of the extracellular matrix. First, the binding of tip cells to laminin in the basal lamina is disrupted, which activates signaling cascades that lead to reorganization of the cytoskeleton and a change in cell shape. The family of matrix metalloproteinases (MMPs) consist of 26 members, and the key players of the MMP family that participate in tip cell functioning and ocular angiogenesis are MMP2, MMP9, and MMP14 (Barnett et al. 2007; Karagiannis and Popel 2006). Furthermore, MMPs participate in the angiogenic switch because they increase the bioavailability of VEGF and other angiogenic factors by degradation of extracellular components, such as collagen type IV, that bind these factors (Bergers et al. 2000).
On the other hand, tip cells produce, in cooperation with pericytes, basal lamina components, which may serve as a template for follower stalk cells. To provide structural and organizational stability and to change the endothelial phenotype to a quiescent one, the endothelial tube is ensheathed by a new basal lamina (reviewed in Eble and Niland 2009). The basal lamina consists of a scaffold of laminins together with essential components, such as collagen IV, perlecan, nidogens, and collagen XVIII. Tip cells recruit and activate pericytes to upregulate expression of transforming growth factor-beta (TGF-β), which induces the production of basal lamina components (Van Geest et al. 2010).
Apart from its function as a scaffold and as a reservoir for growth factors, the extracellular matrix also provides positional information to tip cells and stalks cells. Cues from matrix proteins are transmitted to cells by integrins, which are transmembrane α and β heterodimers that convey signals regulating endothelial morphogenetic events (Estrach et al. 2011). There are 18 α integrin subunit family members and 8 β integrin subunit family members, and 24 αβ combinations are known to exist (Frijns et al. 2012). For angiogenesis, the α2β1 and α6β1 integrins are important. Ligation of these integrins induces DLL4/Notch signaling in endothelial cells, and a role of extracellular matrix proteins in the processes controlling tip cell versus stalk cell phenotype has also been suggested (Estrach et al. 2011).
Recruitment of Pericytes
Pericytes are indispensable as they provide pro-survival factors and anti-proliferative factors that stabilize newly formed vessels, and they prevent vessel regression. However, the hypothesis that pericyte loss initiates the first steps of angiogenesis whereas pericyte recruitment occurs only when angiogenesis is completed remains controversial, because large amounts of pericytes are found in endothelial sprouts in vivo (Ruiter et al. 1989; Schlingemann et al. 1990, 1991; Witmer et al. 2004).
Pericytes are present in early stages of angiogenesis, enabling stimulation of endothelial cell migration (Ruiter et al. 1989; Schlingemann et al. 1990, 1991; Witmer et al. 2004). The expression of proteases and proteoglycans by pericytes at the tip of vascular sprouts facilitates tip cell motility. Expression of MMPs, particularly MMP14, is coordinated by interactions between endothelial cells and pericytes (Yana et al. 2007). Once vessels have been established in the retina, pericytes are necessary for vessel stabilization. Pericyte-endothelial cell interactions result in the expression of tissue inhibitors of MMPs (TIMPs), reducing proteolysis, and subsequently stabilizing vessels (Saunders et al. 2006).
Recruitment of pericytes is controlled by PDGF-B signaling and angiopoietin-1 (ANGPT1) and ANGPT2 interactions with their receptor TIE2 (reviewed in Hammes et al. 2011). PDGFs are present as heterodimers (PDGF-AB) or homodimers composed of subunits A and B. Endothelial tip cells of growing vascular sprouts generate a PDGF-B concentration gradient that promotes the recruitment of pericytes expressing the PDGF-B receptor. ANGPT1 expressed by pericytes binds to and activates the TIE2 receptor on endothelial cells, thereby promoting pericyte attachment to capillaries. ANGPT2, a prominent marker of tip cells, was shown to have an antagonizing effect on TIE2, acting as an endogenous dominant negative ligand for ANGPT1. High levels of VEGF and elevated ANGPT2 levels destabilize vessels, causing endothelial cell proliferation and pericyte activation (del Toro et al. 2010; Strasser et al. 2010; Hammes et al. 2011). These two mechanisms together induce the timely detachment and attachment of pericytes in the process of vascular sprouting, allowing tip cells to migrate.
Therapeutic Strategies for Targeting Endothelial Tip Cells
Therapeutic targeting of angiogenesis is an attractive strategy in treating ocular diseases that are driven by pathological neovascularization. Anti-angiogenic agents currently in clinical development interfere with pro-angiogenic growth factors, their receptors, or the downstream signaling. Employing this strategy, the presently registered anti-VEGF drugs have made a significant advance in treating ocular neovascularization.
However, there are a number of reports arguing against anti-VEGF therapy. First, repeated monthly intravitreal anti-VEGF injections cause a tremendous burden on patients and national health care systems. Second, VEGF and its receptors have physiological functions in the normal retina, and blocking VEGF signals interferes with these functions (Blaauwgeers et al. 1999; Witmer et al. 2003; Saint-Geniez et al. 2008). Third, there is increasing evidence that angiogenesis can occur in the absence of VEGF signaling (Benedito et al. 2012). Fourth, adverse effects, such as promotion of fibrosis, scarring, and tractional retinal detachment, have been reported after intravitreal anti-VEGF injections due to the shift in the balance between VEGF and connective tissue growth factor (CTGF, also known as CCN2), which induces the angiofibrotic switch (Kuiper et al. 2007, 2008; Van Geest et al. 2012). Therefore, there is a great therapeutic demand and scope for identifying new determinants of angiogenesis besides anti-VEGF therapy that may ultimately translate into more effective and efficient therapies with less adverse effects.
Ideally, agents that primarily target tip cells and do not interfere with quiescent endothelium should block pathological angiogenesis in the retina efficiently and safely with less adverse effects. The tip cell gene expression pattern (Table 1) that is known now should facilitate the development of such therapeutic strategies. Promising agents specifically targeting tip cells are now in clinical trials or may be in the near future.
The VEGF-DLL4-Notch signaling axis in angiogenesis is a major focus of research for the development of novel therapies (reviewed in Oon and Harris 2011). Blockade of DLL4/Notch signaling enhances the chaotic, nonproductive vascular sprouting that is characteristic of tumor angiogenesis by promoting VEGF-driven tip cell formation and vascular leakage (Kalen et al. 2011). These events lead to disrupted neovascular perfusion, starvation of the tumor, and delay in tumor growth (Dong et al. 2011; Kalen et al. 2011). Therapeutic options in the DLL4-Notch-VEGF pathway were also studied in the mouse oxygen-induced retinopathy model. Interestingly, the intravitreal injection of a soluble DLL4 fusion protein, which attenuates DLL4/Notch signaling, stimulated extension of new vascular sprouts in the retina and limited ectopic growth of pathological neovascular tufts into the vitreous (Lobov et al. 2007).
A well-tolerated DNA vaccination strategy, targeting endothelial tip cells through the antigen DLL4, showed similar therapeutic efficacy in mouse tumor models (Haller et al. 2010). In this study, a DLL4-plasmid vaccine was generated and injected three times in mice to induce the production of antibodies recognizing the extracellular domain of DLL4, resulting in a retarded growth of implanted mammary carcinomas in these mice by induction of a non-productive angiogenic response. Blocking of the DLL4-Notch-VEGF pathway through injections of anti-DLL4 (together with anti-VEGF) antibodies or via the induction of immunity toward tip cells may provide protection against recurrent proliferative retinopathies or may reduce the necessity of monthly anti-VEGF injections.
PDGF is secreted by tip cells to recruit pericytes; blocking PDGF through injections of anti-PDGF antibodies results in immature blood vessels that lack pericytes. A recent clinical phase 2b trial of anti-PDGF therapy administered in combination with anti-VEGF therapy showed statistically significant superior efficacy over anti-VEGF monotherapy for the treatment of neovascular AMD (http://clinicaltrials.gov/ct2/show/record/NCT01089517).
VEGFR3 kinase activity inhibitors but not ligand-blocking antibodies suppressed the sprouting of endothelial cells that had low Notch signaling activity (Tammela et al. 2011; Zhang LQ et al. 2010). In settings of low Notch activity, VEGFR3 upregulation allows strong, ligand-independent and highly deregulated angiogenesis even in the absence of VEGF-VEGFR2 signaling (Benedito et al. 2012). Neutralizing antibodies against VEGFR3 ligands VEGF-C and VEGF-D therefore showed minimal or no effect on tumor angiogenesis. Recently, it has been proposed that VEGFR3 exerts its function in angiogenesis indirectly by regulating the level of VEGFR2 activation, providing an additional layer of control over VEGFR2-mediated signals (Zhang et al. 2010). Therefore, simultaneous blocking of VEGFR2 and VEGFR3 may be beneficial in the treatment of ocular neovascularization. Probing the status of vascular Notch or VEGFR3 activation may be relevant in patients who only partially respond to anti-VEGF treatment or do not respond at all (Benedito et al. 2012; Zhang et al. 2010).
EFNB2, the ligand for EphB4, was found to be upregulated in tip cells (Siemerink et al. 2012), where it controls the function of VEGFR2 and VEGFR3 (Sawamiphak et al. 2010; Wang et al. 2010). DLL4/Notch and EFNB2/EphB4 pathways play critical roles in tumor vessel development; combinational targeting of both pathways was shown to be highly effective in disrupting tumor angiogenesis (Djokovic et al. 2010). Endogenous EFNB2 and EphB4 are regulators of retinal neovascularization during oxygen-induced retinopathy and may be useful targets for therapeutic intervention, alone or in combination with other therapies (Zamora et al. 2005; Davies et al. 2009, 2010; Adams and Eichmann 2010).
APLN is an endogenous ligand for the angiotensin-1-like receptor APJ that induces proliferation of endothelial cells. APLN is an angiogenic factor in retinal endothelial cells, and loss of APLN-APJ signaling results in retardation of developmental retinal angiogenesis (Saint-Geniez et al. 2002; Kasai et al. 2008, 2010; Tao et al. 2010). Moreover, it has been shown that APLN acts as a potent activator of tumor angiogenesis (Sorli et al. 2007). Several recent studies identified APLN expression to be upregulated in tip cells, whereas its receptor APJ was found to be expressed in stalk cells (del Toro et al. 2010; Strasser et al. 2010; Siemerink et al. 2012). Based on these findings, APLN-APJ signaling has been suggested to stimulate stalk cell proliferation controlled by tip cells. Blocking the APLN-APJ pathway may prevent stalk cell proliferation and sprout elongation (Tao et al. 2010).
Production of ANGPT2 by endothelial cells stimulates angiogenesis in the presence of VEGF. ANGPT2 plays an important role in physiological and pathological retinal angiogenesis (Hackett et al. 2002; Takagi et al. 2003), and increased expression of ANGPT2 relative to ANGPT1 in tumors correlates with poor prognosis (reviewed in Huang et al. 2010). Several recent studies identified ANGPT2 expression to be specifically upregulated in tip cells (del Toro et al. 2010; Siemerink et al. 2012). Another recent study showed that a neutralizing antibody specific for ANGPT2 inhibits ocular angiogenesis (Rennel et al. 2011). Agents targeting ANGPT2 signaling in tumor angiogenesis are now under development, and some have reached the level of phase 2 clinical trials (Huang et al. 2010).
CXCR4, the receptor for stromal cell-derived factor-1 (SDF1; also known as CXCL12), was found to be abundantly expressed in tip cells (Strasser et al. 2010; Unoki et al. 2010; Siemerink et al. 2012). SDF1/CXCR4 signaling also contributes to the activation of resident macrophages (also called microglia) that promote neovascular sprouting in the retina through secretion of VEGF (Sengupta et al. 2010; Unoki et al. 2010). Inhibition of CXCR4 signaling by neutralizing antibodies against SDF1 or antagonists of the CXCR4 receptor in neonatal mice resulted in defects in retinal tip cell morphology (Sengupta et al. 2010; Strasser et al. 2010; Unoki et al. 2010). Moreover, SDF1 levels increased after chemotherapy and anti-VEGF therapy, favoring tumor recurrences (Xu et al. 2009). CXCR4 is also present in hematopoietic stem cells, and receptor activation stimulates homing of these cells in the bone marrow; in this context, CXCR4 antagonists are now approved for mobilization of stem cells for autologous and allogenic transplants in patients with leukemia (reviewed in Duda et al. 2011). Clinical trials combining anti-VEGF and CXCR4 antagonists are now under development to block tumor angiogenesis. Treatment using CXCR4 antagonists in combination with other therapies may be an interesting strategy to prevent recurrent neovascularization also in the context of proliferative retinopathies.
Conclusions and Future Directions
Understanding the complex interrelated directions of endothelial cell differentiation in the angiogenic processes and the molecular mediators involved has led to the development of strategies specifically targeting endothelial tip cells and to the screening of novel potential anti-angiogenic agents. Angiogenesis inhibitors targeting tip cells without affecting quiescent endothelial cells or their functions are likely to change the face of medicine in the next decade. The challenge for the future is to develop such novel anti-angiogenic strategies and to optimize combination treatment regimens to fully exploit the therapeutic potential of angiogenesis inhibition.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research and/or authorship of this article: This work was partially funded by the Algemene Nederlandse Vereniging ter Voorkoming van Blindheid, Stichting Blinden-Penning, Dr. F. P. Fisher-Stichting, Stichting Oogfonds Nederland, Landelijke Stichting voor Blinden en Slechtzienden. Retina Nederland Onderzoek Fonds, Stichting MD Fonds, combined in the Uitzicht grant 2009-18. The founding organizations had no participation in design or conduct of this study, data collection, management, analysis, interpretation, preparation, review, or approval of this manuscript.
References
- Adams RH, Eichmann A. 2010. Axon Guidance Molecules in Vascular Patterning. Cold Spring Harb Perspect Biol. 2:a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai YY, Bai X, Wang ZY, Zhang XF, Ruan CG, Miao JC. 2011. MicroRNA-126 inhibits ischemia-induced retinal neovascularization via regulating angiogenic growth factors. Exp Mol Pathol. 91:471–477 [DOI] [PubMed] [Google Scholar]
- Barnett JM, McCollum GW, Fowler JA, Duan JJW, Kay JD, Liu RQ, Bingaman DP, Penn JS. 2007. Pharmacologic and genetic manipulation of MMP-2 and -9 affects retinal neovascularization in rodent models of OIR. Invest Ophthalmol Vis Sci. 48:907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. 2009. The Notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 137:1124–1135 [DOI] [PubMed] [Google Scholar]
- Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. 2012. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 484:110–114 [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. 2000. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2:737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biyashev D, Veliceasa D, Topczewski J, Topczewska JM, Mizgirev I, Vinokour E, Reddi AL, Licht JD, Revskoy SY, Volpert OV. 2012. miR-27b controls venous specification and tip cell fate. Blood. 119:2679–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaauwgeers HGT, Hotkamp BW, Rutten H, Witmer AN, Koolwijk P, Partanen TA, Alitalo K, Kroon ME, Kijlstra A, van Hinsbergh VWM, et al. 1999. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris: evidence for a trophic paracrine relation. Am J Pathol. 155:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochenek ML, Dickinson S, Astin JW, Adams RH, Nobes CD. 2010. Ephrin-B2 regulates endothelial cell morphology and motility independently of Eph-receptor binding. J Cell Sci. 123:1235–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd S, Byfield G, Martiniuk D, Geisen P, Hartnett ME. 2009. Reduction in endothelial tip cell filopodia corresponds to reduced intravitreous but not intraretinal vascularization in a model of ROP. Exp Eye Res. 89:718–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporali A, Emanueli C. 2011. MicroRNA regulation in angiogenesis. Vasc Pharm. 55:79–86 [DOI] [PubMed] [Google Scholar]
- Caprara C, Grimm C. 2012. From oxygen to erythropoietin: relevance of hypoxia for retinal development, health and disease. Prog Retin Eye Res. 31:89–119 [DOI] [PubMed] [Google Scholar]
- Caprara C, Thiersch M, Lange C, Joly S, Samardzija M, Grimm C. 2011. HIF1A is essential for the development of the intermediate plexus of the retinal vasculature. Invest Ophthalmol Vis Sci. 52:2109–2117 [DOI] [PubMed] [Google Scholar]
- Carmeliet P. 2003. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Gen. 4:710–720 [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. 1996. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 380:435–439 [DOI] [PubMed] [Google Scholar]
- Chappell JC, Wiley DM, Bautch VL. 2012. How blood vessel networks are made and measured. Cells Tissues Organs. 195:94–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MH, Stempel AJ, Hubert KE, Powers MR. 2010. Altered vascular expression of EphrinB2 and EphB4 in a model of oxygen-induced retinopathy. Dev Dyn. 239:1695–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MH, Zamora DO, Smith JR, Powers MR. 2009. Soluble ephrin-B2 mediates apoptosis in retinal neovascularization and in endothelial cells. Microvasc Res. 77:382–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock K, De Smet F, Leite De Oliveira R, Anthonis K, Carmeliet P. 2009. Endothelial oxygen sensors regulate tumor vessel abnormalization by instructing phalanx endothelial cells. J Mol Med (Berl). 87:561–569 [DOI] [PubMed] [Google Scholar]
- Del Toro R, Prahst C, Mathivet T, Siegfried G, Kaminker JS, Larrivee B, Breant C, Duarte A, Takakura N, Fukamizu A, et al. 2010. Identification and functional analysis of endothelial tip cell-enriched genes. Blood. 116:4025–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djokovic D, Trindade A, Gigante J, Badenes M, Silva L, Liu R, Li XQ, Gong M, Krasnoperov V, Gill PS, et al. 2010. Combination of Dll4/Notch and Ephrin-B2/EphB4 targeted therapy is highly effective in disrupting tumor angiogenesis. BMC Cancer. 10:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Wang YS, Dou GR, Hou HY, Shi YY, Zhang R, Ma K, Wu L, Yao LB, Cai Y, et al. 2011. Influence of Dll4 via HIF-1α-VEGF signaling on the angiogenesis of choroidal neovascularization under hypoxic conditions. PloS ONE. 6:e18481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Friedlander M. 2002. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 43:3500–3510 [PubMed] [Google Scholar]
- Dorrell MI, Friedlander M. 2006. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res. 25:277–295 [DOI] [PubMed] [Google Scholar]
- Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. 2011. CXCL12 (SDF1 alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin Cancer Res. 17:2074–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble JA, Niland S. 2009. The extracellular matrix of blood vessels. Curr Pharm Des. 15:1385–1400 [DOI] [PubMed] [Google Scholar]
- Ejaz S, Chekarova I, Ejaz A, Sohail A, Lim CW. 2008. Importance of pericytes and mechanisms of pericyte loss during diabetic retinopathy. Diabetes Obes Metab. 10:53–63 [DOI] [PubMed] [Google Scholar]
- Estrach S, Cailleteau L, Franco CA, Gerhardt H, Stefani C, Lemichez E, Gagnoux-Palacios L, Meneguzzi G, Mettouchi A. 2011. Laminin-binding integrins induce Dll4 expression and Notch signaling in endothelial cells. Circ Res. 109:172–182 [DOI] [PubMed] [Google Scholar]
- Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. 2010. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 116:829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JE, Santoro MM, Morton SU, Yu SH, Yeh RF, Wythe JD, Lvey KN, Bruneau BG, Stainier DYR, Srivastava D. 2008. MiR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 15:272–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijns E, Kuikman I, Litjens S, Raspe M, Jalink K, Ports M, Wilhelmsen K, Sonnenberg A. 2012. Phosphorylation of threonine 1736 in the C-terminal tail of integrin beta 4 contributes to hemidesmosome disassembly. Mol Biol Cell. 23:1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S, Uemura A. 2011. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest. 121:1974–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvagni F, Pennacchini S, Salameh A, Rocchigiani M, Neri F, Orlandini M, Petraglia F, Gotta S, Sardone GL, Matteucci G, et al. 2010. Endothelial cell adhesion to the extracellular matrix induces c-Src-dependent VEGFR-3 phosphorylation without the activation of the receptor intrinsic kinase activity. Circ Res. 106:1839–1125 [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. 2003. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 161:1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. 2004. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dynam. 231:503–509 [DOI] [PubMed] [Google Scholar]
- Grossniklaus HE, Kang SJ, Berglin L. 2010. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res. 29:500–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA. 2002. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 192:182–187 [DOI] [PubMed] [Google Scholar]
- Hakansson J, Stahlberg A, Wolfhagen SF, Gerhardt H, Semb H. 2011. N-CAM exhibits a regulatory function in pathological angiogenesis in oxygen induced retinopathy. PLoS ONE. 6:e26026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller BK, Brave A, Wallgard E, Roswall P, Sunkari VG, Mattson U, Hallengard D, Catrina SB, Hellstrom M, Pietras K. 2010. Therapeutic efficacy of a DNA vaccine targeting the endothelial tip cell antigen delta-like ligand 4 in mammary carcinoma. Oncogene. 29:4276–4286 [DOI] [PubMed] [Google Scholar]
- Hammes HP, Feng YX, Pfister F, Brownlee M. 2011. Diabetic retinopathy: targeting vasoregression. Diabetes. 60:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LS, Sainson RCA, Williams CK, Taylor JM, Shi W, Li JL, Harris AL. 2008. Regulation of multiple angiogenic pathways by D114 and Notch in human umbilical vein endothelial cells. Microvasc Res. 75:144–154 [DOI] [PubMed] [Google Scholar]
- Herbert SP, Stainier DY. 2011. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 12:551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann JJ, Iruela-Arispe ML. 2007. Notch expression patterns in the retina: an eye on receptor-ligand distribution during angiogenesis. Gene Expr Patterns. 7:461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HH, Bhat A, Woodnutt G, Lappe R. 2010. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 10:575–585 [DOI] [PubMed] [Google Scholar]
- Hughes S, Yang HJ, Chan-Ling T. 2000. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 41:1217–1228 [PubMed] [Google Scholar]
- Iruela-Arispe ML, Davis GE. 2009. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 16:222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ispanovic E, Serio D, Haas TL. 2008. Cdc42 and RhoA have opposing roles in regulating membrane type 1-matrix metalloproteinase localization and matrix metalloproteinase-2 activation. Am J Physiol Cell Physiol. 295:600–610 [DOI] [PubMed] [Google Scholar]
- Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, et al. 2010. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 12:943–953 [DOI] [PubMed] [Google Scholar]
- Kalen M, Heikura T, Karvinen H, Nitzsche A, Weber H, Esser N, Yla-Herttuala S, Hellstrom M. 2011. Gamma-secretase inhibitor treatment promotes VEGF-A–driven blood vessel growth and vascular leakage but disrupts neovascular perfusion. PloS ONE. 6:e18709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis ED, Popel AS. 2006. Distinct modes of collagen type I proteolysis by matrix metalloproteinase (MMP) 2 and membrane type I MMP during the migration of a tip endothelial cell: insights from a computational model. J Theor Biol. 238:124–145 [DOI] [PubMed] [Google Scholar]
- Kasai A, Ishimaru Y, Kinjo T, Satooka T, Matsumoto N, Yoshioka Y, Yamamuro A, Gomi F, Shintani N, Baba A, et al. 2010. Apelin is a crucial factor for hypoxia-induced retinal angiogenesis. Arterioscler Thromb Vasc Biol. 30:2182–2187 [DOI] [PubMed] [Google Scholar]
- Kasai A, Shintani N, Kato H, Matsuda S, Gomi F, Haba R, Hashimoto H, Kakuda M, Tano Y, Baba A. 2008. Retardation of retinal vascular development in apelin-deficient mice. Arterioscler Thromb Vasc Biol. 28:1717–1722 [DOI] [PubMed] [Google Scholar]
- Klosovskii BN, Zhukova TP. 1963. Effect of colchicine on remote phases of growing capillaries in the brain [in Russian]. Arkh Patol. 35(3):38–44 [PubMed] [Google Scholar]
- Kovacs B, Lumayag S, Cowan C, Xu SB. 2011. microRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 52:4402–4409 [DOI] [PubMed] [Google Scholar]
- Krueger J, Liu D, Scholz K, Zimmer A, Shi Y, Klein C, Siekmann A, Schulte-Merker S, Cudmore M, Ahmed A, et al. 2011. Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development. 138:2111–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, Kuo CJ. 2008. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 135:3989–3993 [DOI] [PubMed] [Google Scholar]
- Kuiper EJ, Hughes JM, Van Geest RJ, Vogels IMC, Goldschmeding R, Van Noorden CJF, Schlingemann RO, Klaassen I. 2007. Effect of VEGF-A on expression of profibrotic growth factor and extracellular matrix genes in the retina. Invest Ophthalmol Vis Sci. 48:4267–4276 [DOI] [PubMed] [Google Scholar]
- Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, van Meurs JC, Tanck MW, Oliver N, Klaassen I, Van Noorden CJF, Goldschmeding R, Schlingemann RO. 2008. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PloS ONE 3:e2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamalice L, Le Boeuf F, Huot J. 2007. Endothelial cell migration during angiogenesis. Circ Res. 100:782–794 [DOI] [PubMed] [Google Scholar]
- Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. 2007. Delta-like ligand 4 (DII4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 104:3219–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M. 1985. Early vascularization of the embryonic cerebral cortex: Golgi and electron microscopic studies. J Comp Neurol. 241:237–249 [DOI] [PubMed] [Google Scholar]
- Mattila PK, Lappalainen P. 2008. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 9:446–454 [DOI] [PubMed] [Google Scholar]
- Nakamura-Ishizu A, Kurihara T, Okuno Y, Ozawa Y, Kishi K, Goda N, Tsubota K, Okano H, Suda T, Kubota Y. 2012. The formation of an angiogenic astrocyte template is regulated by the neuroretina in a HIF-1-dependent manner. Dev Biol. 363:106–114 [DOI] [PubMed] [Google Scholar]
- Napp LC, Augustynik M, Paesler F, Krishnasamy K, Woiterski J, Limbourg A, Bauersachs J, Drexler H, le Noble F, Limbourg FP. 2012. Extrinsic Notch ligand Delta-like 1 regulates tip cell selection and vascular branching morphogenesis. Circ Res. 110:530–550 [DOI] [PubMed] [Google Scholar]
- Nicoli S, Knyphausen CP, Zhu LHJ, Lakshmanan A, Lawson ND. 2012. miR-221 is required for endothelial tip cell behaviors during vascular development. Dev Cell. 22:418–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson I, Bahram F, Li XJ, Gualandi L, Koch S, Jarvius M, Soderberg O, Anisimov A, Kholova I, Pytowski B, et al. 2010. VEGF receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts. EMBO J. 29:1377–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oon CE, Harris AL. 2011. New pathways and mechanisms regulating and responding to Delta-like ligand 4-Notch signalling in tumour angiogenesis. Biochem Soc Trans. 39:1612–1618 [DOI] [PubMed] [Google Scholar]
- Rennel ES, Regula JT, Harper SJ, Thomas M, Klein C, Bates DO. 2011. A human neutralizing antibody specific to Ang-2 inhibits ocular angiogenesis. Microcirculation. 18:598–607 [DOI] [PubMed] [Google Scholar]
- Ruiter DJ, Schlingemann RO, Rietveld FJR, Dewaal RMW. 1989. Cellular and extracellular components of the tumor microvasculature visualized by immunohistochemistry. Ultramicroscopy. 31:480 [Google Scholar]
- Saint-Geniez M, Maharaj ASR, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ, D’Amore PA. 2008. Endogenous VEGF is required for visual function: evidence for a survival role on Muller cells and photoreceptors. PloS ONE. 3:e3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Geniez M, Masri B, Malecaze F, Knibiehler B, Audigier Y. 2002. Expression of the murine msr/apj receptor and its ligand apelin is upregulated during formation of the retinal vessels. Mech Dev. 110:183–186 [DOI] [PubMed] [Google Scholar]
- Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. 2006. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 175:179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. 2010. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 465:487–491 [DOI] [PubMed] [Google Scholar]
- Schlingemann RO. 2004. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 242:91–101 [DOI] [PubMed] [Google Scholar]
- Schlingemann RO, Rietveld FJ, de Waal RM, Bradley NJ, Skene AI, Davies AJ, Greaves MF, Denekamp J, Ruiter DJ. 1990. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest. 62:690–696 [PubMed] [Google Scholar]
- Schlingemann RO, Rietveld FJR, Kwaspen F, Vandekerkhof PCM, Dewaal RMW, Ruiter DJ. 1991. Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol. 138:1335–1347 [PMC free article] [PubMed] [Google Scholar]
- Schlingemann RO, Witmer AN. 2009. Treatment of retinal diseases with VEGF antagonists. Prog Brain Res. 175:253–267 [DOI] [PubMed] [Google Scholar]
- Schoefl GI. 1963. Studies on inflammation, III: growing capillaries: their structure and permeability. Virchows Arch Pathol Anat Physiol Klin Med. 337:97–141 [PubMed] [Google Scholar]
- Sengupta N, Afzal A, Caballero S, Chang KH, Shaw LC, Pang JJ, Bond VC, Bhutto I, Baba T, Lutty GA, et al. 2010. Paracrine modulation of CXCR4 by IGF-1 and VEGF: implications for choroidal neovascularization. Invest Ophthalmol Vis Sci. 51:2697–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, Leek R, Edelmann M, Kessler B, Sainson RCA, et al. 2010. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 116:2385–2394 [DOI] [PubMed] [Google Scholar]
- Shen JK, Yang XR, Xie B, Chen YJ, Swaim M, Hackett SF, Campochiaro PA. 2008. MicroRNAs regulate ocular neovascularization. Mol Ther. 16:1208–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND. 2007. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 445:781–784 [DOI] [PubMed] [Google Scholar]
- Siemerink MJ, Klaassen I, Vogels IMC, Griffioen AW, Van Noorden CJF, Schlingemann RO. 2012. CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis. 15:151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. 1994. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 35:101–111 [PubMed] [Google Scholar]
- Sorli SC, Le Gonidec S, Knibiehler B, Audigier Y. 2007. Apelin is a potent activator of tumour neoangiogenesis. Oncogene. 26:7692–7699 [DOI] [PubMed] [Google Scholar]
- Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, et al. 2010. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 51:2813–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel D, Franco CA, Estrach S, Mettouchi A, Sauvaget D, Rosewell I, Schertel A, Armer H, Domogatskaya A, Rodin S, et al. 2011. Endothelial basement membrane limits tip cell formation by inducing Dll4/Notch signalling in vivo. EMBO Rep. 12:1135–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser GA, Kaminker JS, Tessier-Lavigne M. 2010. Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood. 115:5102–5110 [DOI] [PubMed] [Google Scholar]
- Suchting S, Eichmann A. 2009. Jagged gives endothelial tip cells an edge. Cell. 137:988–990 [DOI] [PubMed] [Google Scholar]
- Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. 2007. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 104:3225–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H, Koyama S, Seike H, Oh H, Otani A, Matsumura M, Honda Y. 2003. Potential role of the angiopoietin/Tie2 system in ischemia-induced retinal neovascularization. Invest Ophthalmol Vis Sci. 44:393–402 [DOI] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, Nurmi H, Jakobsson L, Heinolainen K, Tvorogov D, Zheng W, Franco CA, Murtomaki A, Aranda E, et al. 2011. VEGFR-3 controls tip to stalk conversion at vessel fusion sites by reinforcing Notch signalling. Nat Cell Biol. 13:1202–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, et al. 2008. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 454:656–668 [DOI] [PubMed] [Google Scholar]
- Tao Y, Lu QA, Jiang YR, Qian J, Wang JY, Gao L, Jonas JB. 2010. Apelin in plasma and vitreous and in fibrovascular retinal membranes of patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 51:4237–4242 [DOI] [PubMed] [Google Scholar]
- Unoki N, Murakami T, Nishijima K, Ogino K, van Rooijen N, Yoshimura N. 2010. SDF-1/CXCR4 contributes to the activation of tip cells and microglia in retinal angiogenesis. Invest Ophthalmol Vis Sci. 51:3362–3371 [DOI] [PubMed] [Google Scholar]
- Van Geest RJ, Klaassen I, Vogels IMC, Van Noorden CJF, Schlingemann RO. 2010. Differential TGF-beta signaling in retinal vascular cells: a role in diabetic retinopathy? Invest Ophthalmol Vis Sci. 51:1857–1865 [DOI] [PubMed] [Google Scholar]
- Van Geest RJ, Lesnik-Oberstein SY, Tan HS, Mura M, Goldschmeding R, Van Noorden CJF, Klaassen I, Schlingemann RO. 2012. A shift in the balance of vascular endothelial growth factor and connective tissue growth factor by bevacizumab causes the angiofibrotic switch in proliferative diabetic retinopathy. Br J Ophthalmol. 96:587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YD, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, et al. 2010. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 465:483–486 [DOI] [PubMed] [Google Scholar]
- Witmer AN, van Blijswijk BC, Dai J, Hofman P, Partanen TA, Vrensen GF, Schlingemann RO. 2001. VEGFR-3 in adult angiogenesis. J Pathol. 195:490–497 [DOI] [PubMed] [Google Scholar]
- Witmer AN, van Blijswijk BC, Van Noorden CJ, Vrensen GF, Schlingemann RO. 2004. In vivo angiogenic phenotype of endothelial cells and pericytes induced by vascular endothelial growth factor-A. J Histochem Cytochem. 52:39–52 [DOI] [PubMed] [Google Scholar]
- Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. 2003. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 22:1–29 [DOI] [PubMed] [Google Scholar]
- Xu L, Duda DG, di Tomaso E, Ancukiewicz M, Chung DC, Lauwers GY, Samuel R, Shellito P, Czito BG, Lin PC, et al. 2009. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1 alpha, CXCR4, CXCL6, and Neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 69:7905–7910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yana I, Sagara H, Takaki S, Takatsu K, Nakamura K, Nakao K, Katsuki M, Taniguchi SI, Aoki T, Sato H, et al. 2007. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci. 120:1607–1614 [DOI] [PubMed] [Google Scholar]
- Zamora DO, Davies MH, Planck SR, Rosenbaum JT, Powers MR. 2005. Soluble forms of EphrinB2 and EphB4 reduce retinal neovascularization in a model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 46:2175–2182 [DOI] [PubMed] [Google Scholar]
- Zhang LQ, Zhou F, Han WC, Shen B, Luo JC, Shibuya M, He YL. 2010. VEGFR-3 ligand-binding and kinase activity are required for lymphangiogenesis but not for angiogenesis. Cell Res. 20:1319–1331 [DOI] [PubMed] [Google Scholar]
- Zhang W, Yokota H, Xu Z, Narayanan SP, Yancey L, Yoshida A, Marcus DM, Caldwell RW, Caldwell RB, Brooks SE. 2011. Hyperoxia therapy of pre-proliferative ischemic retinopathy in a mouse model. Invest Ophthalmol Vis Sci. 52:6384–6395 [DOI] [PMC free article] [PubMed] [Google Scholar]