Abstract
Islet-associated protein–2 (IA-2) and IA-2β (also known as phogrin) are unique neuroendocrine-specific protein tyrosine phosphatases (PTPs). The IA-2 family of PTPs was originally identified from insulinoma cells and discovered to be major autoantigens in type 1 diabetes. Despite its expression in the neural and canonical endocrine tissues, data on expression of the IA-2 family of PTPs in gastrointestinal endocrine cells (GECs) are limited. Therefore, we immunohistochemically investigated the expression of the IA-2 family of PTPs in the rat gastrointestinal tract. In the stomach, IA-2 and IA-2β were expressed in GECs that secrete serotonin, somatostatin, and cholecystokinin/gastrin-1. In addition to these hormones, secretin, gastric inhibitory polypeptide (also known as the glucose-dependent insulinotropic peptide), glucagon-like peptide-1, and glucagon, but not ghrelin were coexpressed with IA-2 or IA-2β in duodenal GECs. Pancreatic islet cells that secrete gut hormones expressed the IA-2 family of PTPs. The expression patterns of IA-2 and IA-2β were comparable. These results reveal that the IA-2 family of PTPs is expressed in a cell type–specific manner in rat GECs. The extensive expression of the IA-2 family of PTPs in pancreo-gastrointestinal endocrine cells and in the enteric plexus suggests their systemic contribution to nutritional control through a neuroendocrine signaling network.
Keywords: IA-2, islet cell antigen ICA512, IA-2β, phogrin, protein tyrosine phosphatases, gastrointestinal endocrine cell, secretory granules, gut hormones
The gastrointestinal tract is the largest endocrine organ in the body, and hormones produced within it exert profound physiological effects. In contrast to endocrine glands, the gastrointestinal endocrine system is diffuse; single hormone-secreting gastrointestinal endocrine cells (GECs) are scattered among other types of epithelial cells in the mucosa of the stomach and intestine. Thus far, more than a dozen subsets of distinct endocrine cells have been identified in the gastrointestinal endocrine system. Gut hormones have a wide range of functions, such as control of gastrointestinal motility, sensing of nutrients in the diet, and regulation of food intake and glucose homeostasis (Moran-Ramos et al. 2012). Altered gut hormone homeostasis has been found in humans with eating disorders and has been implicated in its pathophysiology (Prince et al. 2009).
Islet-associated protein–2 (IA-2; PTPRN, also known as ICA-512) and IA-2β (PTPRN2, also known as phogrin) are type I transmembrane proteins that possess one inactive protein tyrosine phosphatase (PTP) domain in the cytoplasmic region (reviewed by Torii 2009). The IA-2 family of PTPs is expressed in neuroendocrine-specific organs, such as pancreatic islets, pituitary and adrenal glands, and the brain, and primarily localized to dense-core secretory granules (SGs) (Solimena et al. 1996; Wasmeier and Hutton 1996; Shimizu et al. 2001; Takeyama et al. 2009; Torii et al. 2009). Studies using genetic knockout mouse models have shown the involvement of IA-2 PTPs in secretory functions of pancreatic β-cells, the anterior pituitary, and learning-related or circadian rhythm-related neuroendocrine cells (Kubosaki et al. 2006; Mziaut et al. 2008; Kim SM et al. 2009; Nishimura et al. 2009; Cai et al. 2011). Recent molecular and cellular analyses have suggested that the IA-2 family of PTPs specifically contributes to exocytosis of SGs, SG stability, hormone sorting into SGs, and pancreatic β-cell growth (Mziaut et al. 2008; Trajkovski et al. 2008; Torii et al. 2009; Saito et al. 2011; Cai et al. 2011).
The IA-2 family of PTPs is known to act as autoantigens in type 1 diabetes, and previous studies have focused on determining which pancreatic islet cell types express the IA-2 family of PTPs and on exploring their roles in insulin-producing β-cells. In the pancreo-gastrointestinal endocrine system, which develops from a common endodermal origin and is important for systemic regulation of digestive function, the IA-2 family of PTPs is also expressed in α-cells and δ-cells in the pancreatic islets and in somatostatin-containing D-cells in the stomach (Solimena et al. 1996; Takeyama et al. 2009; Nakajima et al. 2011). Recent reports used immunohistochemical and immunoblot analyses with specific monoclonal antibodies to demonstrate that IA-2 and IA-2β are expressed in the mouse stomach and intestine (Takeyama et al. 2009; Nakajima et al. 2011). Immunoblot analysis, particularly of IA-2 expression, has shown that the major immunoreactive signals originate from a neural component (the enteric plexus) of the gastrointestinal tract, despite the apparent immunohistochemical signals from GECs, such as gastric somatostatin-producing cells. However, little information is available about the type of cells that express the IA-2 family of PTPs. Moreover, the comparative expression patterns of IA-2 and IA-2β in pancreo-gastrointestinal endocrine cells are unclear. In the present study, to gain insight into the potential role of the IA-2 family of PTPs in digestive function, we immunohistochemically examined the cell types expressing the IA-2 family of PTPs in the gastrointestinal tract and pancreas of rats.
Materials and Methods
Animals and Tissue Preparation
Two Long-Evans male rats, aged 8 weeks, were purchased from Japan SLC, Inc. (Hamamatsu, Japan). The rats were deeply anesthetized by intraperitoneal injection of sodium pentobarbital (0.1 mg/g body weight) and were then processed for sample preparation. For paraffin sections (4 µm thick), rats were first perfused intracardially with 50 ml of saline and then with 250 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3) at room temperature. Pancreata and guts were recovered from each rat and were then dissected into small pieces and postfixed in the same fixative for 3 days. Tissue blocks were then dehydrated and embedded in paraffin. Cross and longitudinal sections were mounted on 0.2% gelatin-coated glass slides and processed for immunohistochemistry. All animal experiments were conducted in accordance with the Guidelines for Animal Care, Regulation of Infectious Agents and Experimental Committee, Gunma University.
Primary Antibodies
The rabbit polyclonal antibodies against IA-2 and IA-2β used in this study had been prepared previously (Torii et al. 2009). These antibodies were diluted (1:200 for anti–IA-2 antibody or 1:500 for anti–IA-2β antibody) for use in the immunohistochemical analyses. Other antibodies against gut hormones secreted by GECs used in the present study are listed in Table 1.
Table 1.
Antibodies Used against Gut Hormones
| Specificity | Developed in | Dilution | Productsa (No.) |
|---|---|---|---|
| Serotonin | Mouse | 1:500 | Abcam (ab16007) |
| Ghrelin | Mouse | 1:200 | Enzo (BPD-ABS-052-121) |
| Somatostatin | Rat | 1:400 | Chemicon (MAB354) |
| GIP | Mouse | 1:200 | Enzo (BPD-ABS-021-04) |
| GLP-1 | Mouse | 1:500 | BioPorto (HYB147-06) |
| Secretin | Rabbit | 1:500 | Phoenix (H-067-04) |
| Glucagon | Guinea Pig | 1:400 | Linco (4031-01) |
| CCK/gastrin-1b | Rabbit | 1:500 | Phoenix (H-069-04) |
GIP, Gastric inhibitory polypeptide (alias of glucose-dependent insulinotropic peptide); GLP-1, glucagon-like peptide-1.
Products from Abcam Plc (Tokyo, Japan); Enzo Life Sciences International, Inc. (Plymouth Meeting, PA); Chemicon/Millipore (Temecula, CA); BioPorto Diagnostics (Gentofte, Denmark); Phoenix Pharmaceuticals, Inc. (Burlingame, CA); and Linco Research, Inc. (St. Charles, MO).
The anti-cholecystokinin (CCK) antibody cross-reacts with gastric gastrin-1.
Antigen-Antibody Specificity Experiments
To assess antibody specificity, the rat pancreatic β-cell line INS-1 cells were infected with adenovirus integrating or expressing shVector (shVec), shIA2 (shRNA for IA-2), or shPh3 (shRNA for IA-2β), as described previously (Torii et al. 2009). After 48 hr, cell extracts were prepared and each extract was normalized for total protein content (20 µg) before being subjected to SDS-PAGE. Tissue lysates prepared from rat brain, pituitary, pancreatic islets, stomach, and small intestine were also subjected to immunoblotting with anti–IA-2 or anti–IA-2β antibody. INS-1 cells infected with adenovirus shIA-2 or shPh3 were fixed with 4% paraformaldehyde, and immunofluorescence staining was performed with antibodies against IA-2 or IA-2β, as described previously (Torii et al. 2009).
Immunohistochemistry
Paraffin sections were deparaffinized in xylene and rehydrated in a series of graded ethanol and water solutions. For double-immunofluorescence staining, deparaffinized sections were immersed in 0.01 M sodium citrate buffer (pH 6.0) and processed with microwave antigen retrieval in boiling buffer (500 W, 6 min), according to the antigen retrieval method previously applied in immunohistochemistry of the IA-2 family of PTPs (Takeyama et al. 2009; Nakajima et al. 2011), as described for the recovery of protein immunoreactivity on formalin-fixed paraffin-embedded tissue sections (Fowler et al. 2011). The slides were washed with phosphate-buffered saline (PBS; 137 mM NaCl, 2.68 mM KCl, 8.10 mM Na2HPO4, 1.47 mM KH2PO4, pH 7.3), and the background was blocked by incubation with 0.1 M NH4Cl/PBS solution and 5% normal goat or donkey serum (Vector Laboratories, Burlingame, CA) for 30 min before incubation with primary antibodies. Incubation with primary antibodies was performed overnight at 4C. Single immunohistochemical labeling was performed by the avidin-biotin-peroxidase technique using the Vectastain Elite ABC kit (Vector Laboratories). Deparaffinized sections were treated with 0.3% hydrogen peroxide in methanol for 20 min and blocked with 5% normal goat serum. After incubation with the primary antibody, sections were rinsed in PBS and incubated with biotinylated goat anti-rabbit IgG (Vector Laboratories; 1:200 dilution) for 1 hr. After washing with PBS, the sections were visualized with a peroxidase substrate kit (SK-4100; Vector Laboratories) and then counterstained with Mayer’s hematoxylin. For fluorescent double-immunohistochemical labeling of the IA-2 family of PTPs and gut hormones, the sections were incubated first with a mixture of primary antibodies and then with a secondary antibody combination (1:2000 dilution): Alexa 555–conjugated anti-mouse IgG and donkey Alexa 488– conjugated anti-rabbit IgG antibodies, goat Alexa 564–conjugated anti-rabbit IgG and donkey Alexa 488–conjugated anti-mouse IgG antibodies, goat Alexa 564–conjugated anti-rabbit IgG and donkey Alexa 488–conjugated anti-rat IgG antibodies, or goat Alexa 564–conjugated anti-rabbit IgG and goat Alexa 488–conjugated anti–guinea pig IgG antibodies (Invitrogen, Carlsbad, CA). After washing in PBS, the sections were counterstained with Hoechst 33258 (Sigma-Aldrich, St. Louis, MO). Images were acquired with a Zeiss Axio imager A1 microscope equipped with an AxioCam HRc charge-coupled device (CCD) camera or with an Axio imager M1 equipped with an AxioCam MRm CCD camera (Carl Zeiss Meditec, Inc., Dublin, CA). Images were digitized using Zeiss Axiovision software. For the negative controls, sections were incubated with 5% blocking serum in place of primary antibodies.
Immunohistochemical Restaining Method
To identify the type of endocrine cells, we used a restaining method that employed two rabbit polyclonal antibodies against the IA-2 family of PTPs and gut hormones (Miyazaki et al. 2007). After immunohistochemical visualization of the IA-2 family of PTPs with the avidin-biotin-peroxidase method, as described previously, the slides were treated by microwave boiling (500 W, 6 min) in 0.01 M sodium citrate buffer (pH 7.4) to elute the anti–IA-2 family PTP antibody. Then, the sections were reacted with an anti-gut hormone antibody overnight at 4C. After washing with PBS, the sections were incubated with a donkey Alexa 488–conjugated anti-rabbit IgG secondary antibody. To ensure removal of the first labeling, the boiled slides were labeled with a secondary antibody. Finally, the sections were counterstained with Hoechst 33258.
Results
Antigen-Antibody Specificity in the Rat IA-2 Family of PTPs
We first assessed the specificity of anti–IA-2 and anti–IA-2β antibodies to the rat IA-2 family of PTP antigens. INS-1 cells were extracted 48 hr after infection with adenoviruses expressing a short hairpin RNA for IA-2 (shIA2) or IA-2β (shPh3), which specifically silence IA-2 or IA-2β expression, respectively (Torii et al. 2009). Immunoblot analysis demonstrated that both antibodies detected the endogenous proteins in INS-1 cells and that they did not cross-react (Fig. 1A). Immunofluorescence staining of INS-1 cells expressing shIA-2 or shPh3 confirmed their specific detection of endogenous proteins (Fig. 1B). Thus, the antibodies prepared against the mouse IA-2 family of PTPs showed target-specific signals for IA-2 or IA-2β of rat origin. We further analyzed IA-2 or IA-2β expression in rat tissue lysates. In the protein extracts from the brain, pituitary, and pancreatic islets (4 µg of each protein), single or double main bands were reacted with anti–IA-2 or anti-IA–2β antibodies (Fig. 1C). However, even with a large amount of protein loading (100 µg of protein) for the stomach and small intestine protein extracts, no signal was detected for IA-2, and only a weak signal in the stomach extracts and a very weak signal in the small intestine extracts were detected for IA-2β. Therefore, the expression level of the endogenous IA-2 family of PTPs in the stomach and small intestine was very low.
Figure 1.
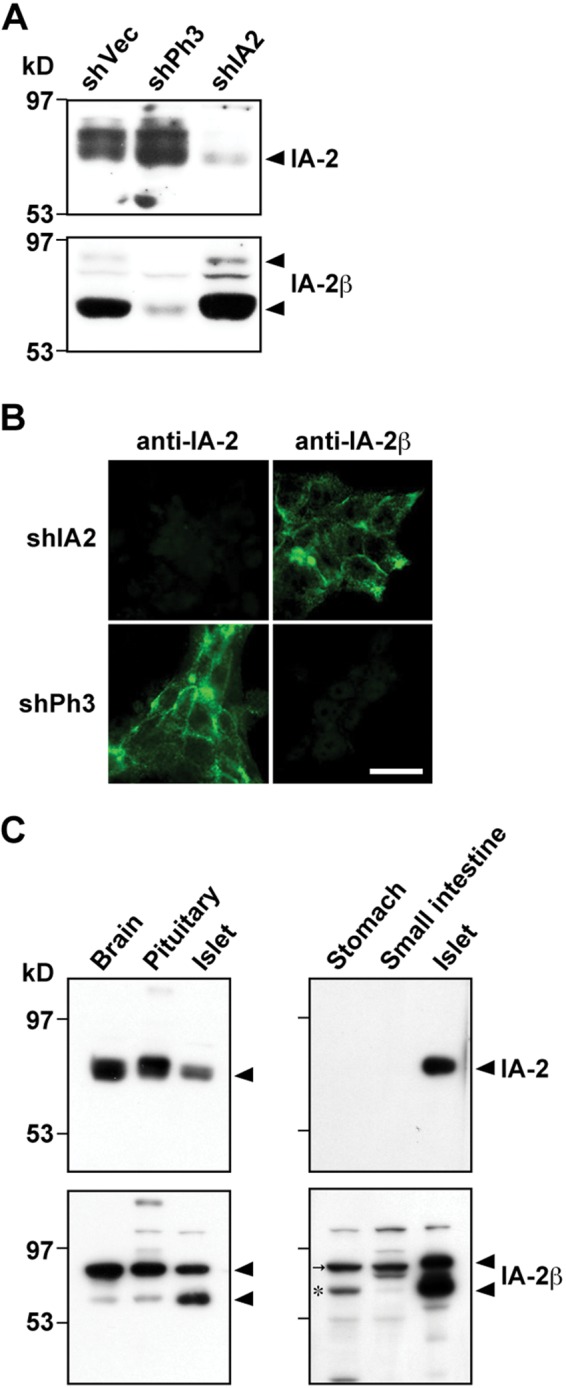
Immunoblot and immunofluorescence analyses with antibodies against islet-associated protein–2 (IA-2) and IA-2β in INS-1 rat pancreatic β-cell line and rat tissues. (A) INS-1 cells were infected with the adenovirus integrating or expressing shVector (shVec), shIA2 (shRNA for IA-2), or shPh3 (shRNA for IA-2β). After 48 hr, cell extracts were prepared, and each extract normalized for total protein content (20 µg) was subjected to immunoblotting with anti–IA-2 (upper panels) or anti–IA-2β (lower panels). (B) The INS-1 cells expressing shIA-2 or shPh3 were fixed and immunostained with antibodies against IA-2 or IA-2β. Bar, 10 µm. (C) Rat tissue lysates (4 µg of protein content extracted from the whole brain, pituitary, and pancreatic islets [left panels]; 100 µg of protein content extracted from stomach and small intestine; and 1 µg of islet protein [right panels]) were subjected to SDS-PAGE/immunoblot analysis. An asterisk indicates specific bands labeled with anti–IA-2β antibody in the stomach and small intestine (very weak), and an arrow indicates nonspecific bands in the same tissues.
Localization of the IA-2 Family of PTPs in Rat Pituitary and Pancreatic Islets
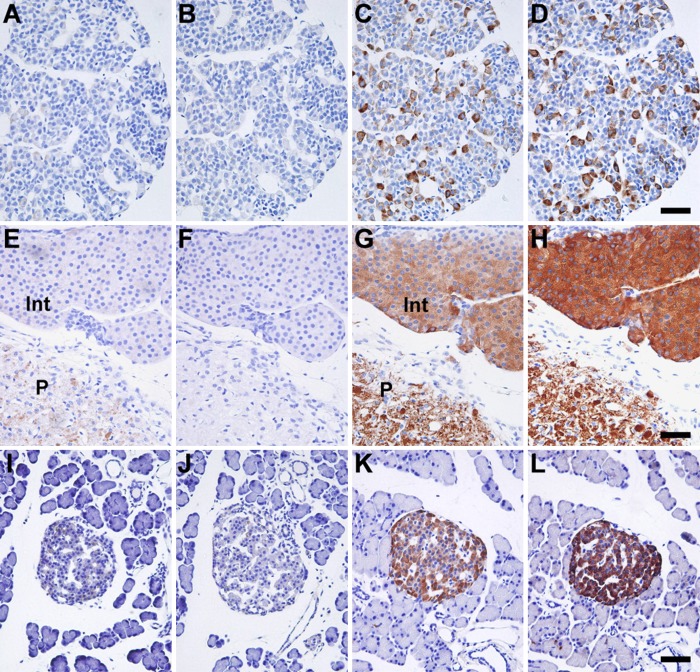
We used paraffin-embedded tissue sections and applied antigen retrieval techniques for immunohistochemical analysis of the IA-2 family of PTPs. Localization of IA-2– or IA-2β–positive cells was examined in canonical endocrine tissues (pituitary and pancreatic islets) of rat. Without antigen retrieval processing, very weak immunoreactivity was detected in the sections (Fig. 2A, B, E, F, I, J). However, after antigen retrieval was performed, the two proteins showed significant immunoreactivity with similar distributions (Fig. 2C, D, G, H, K, L). Thus, antigen retrieval was essential for detecting the immunohistochemical signal of the IA-2 family of PTPs. In the anterior pituitary lobe and pancreatic islets, endocrine cells with varying intensity of immunoreactivity were observed in both IA-2– and IA-2β–stained sections (Fig. 2C, D, K, L), whereas relatively uniform immunoreactivity was observed in the intermediate and posterior pituitary lobes (Fig. 2G, H). In the posterior lobes, the neuronal axons showed IA-2 and IA-2β immunoreactivities. The overall staining pattern of the IA-2 family of PTPs in rat endocrine tissues was consistent with the results of previous studies that used immunohistochemical staining with rabbit polyclonal antibodies in rat tissues (Solimena et al. 1996) and monoclonal antibodies in mice tissues (Takeyama et al. 2009; Nakajima et al. 2011).
Figure 2.
Localization of the islet-associated protein (IA-2) family of protein tyrosine phosphatases (PTPs) in rat pituitary and pancreatic islets. Paraffin sections of anterior pituitary lobes (A–D), intermediate and posterior pituitary lobes (E–H), and pancreatic islets (I–L) were immunostained with anti–IA-2 (A, C, E, G, I, K) or anti–IA-2β (B, D, F, H, J, L) antibodies. Control sections without processing of antigen retrieval are shown in the left two rows of panels (A, B, E, F, I, J). Sections were counterstained with hematoxylin. Int, intermediate pituitary lobe; P, posterior pituitary lobe. Bars, 50 µm.
Localization of the IA-2 Family of PTPs in Rat Stomach, Duodenum, and Pancreatic Duct
The localization of the IA-2 family of PTPs was examined in rat gastrointestinal tissues. In the stomach, IA-2– and IA-2β–positive cells were distributed throughout the gastric mucosa and were abundant in the fundic (Suppl. Fig. S1A, B) and pyloric glands (Suppl. Fig. S1C, D), which are located near the muscularis mucosae. Several to a few IA-2– and IA-2β–positive cells were observed in the surface epithelium. In the duodenum, IA-2– and IA-2β–positive cells were distributed sparsely in the intestinal crypts and villi (Suppl. Fig. S1E, F). Immunoreactivity against the IA-2 family of PTPs was also demonstrated in the myenteric and submucosal plexuses and in the tunica muscularis (Suppl. Fig. S1G, H). In addition, a small subpopulation of duct cells of the pancreatic exocrine gland showed IA-2 and IA-2β immunoreactivities (Suppl. Fig. S1I, J).
Identification of Cell Types Expressing the IA-2 Family of PTPs in Rat Stomach and Duodenum
To determine the type of epithelial cells displaying IA-2 or IA-2β immunoreactivity, we performed double-immunofluorescence staining with six antibodies against gut hormones (serotonin, ghrelin, somatostatin, gastric inhibitory polypeptide [GIP], glucagon-like peptide-1 [GLP-1], and glucagon) prepared by immunization of mice or guinea pigs and immunohistochemical restaining with two antibodies against gut hormones (secretin and cholecystokinin [CCK]/gastrin-1) prepared by immunization of rabbits (Table 1).
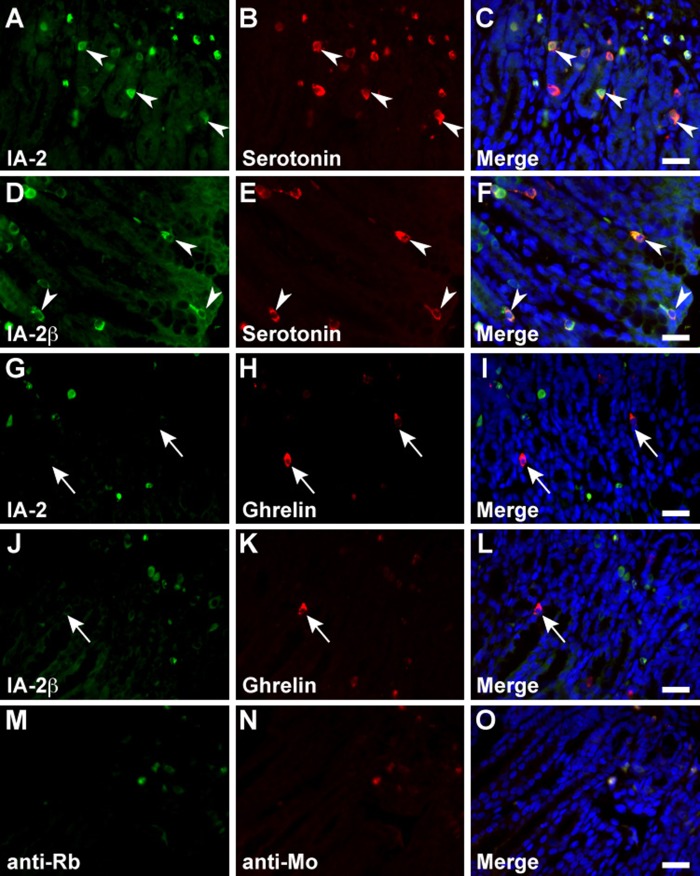
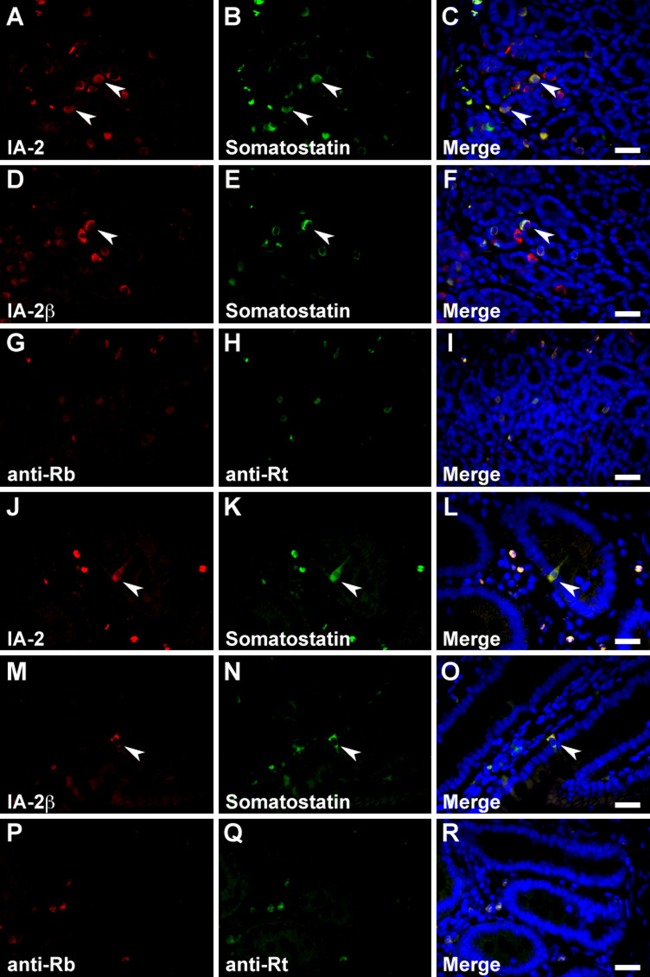
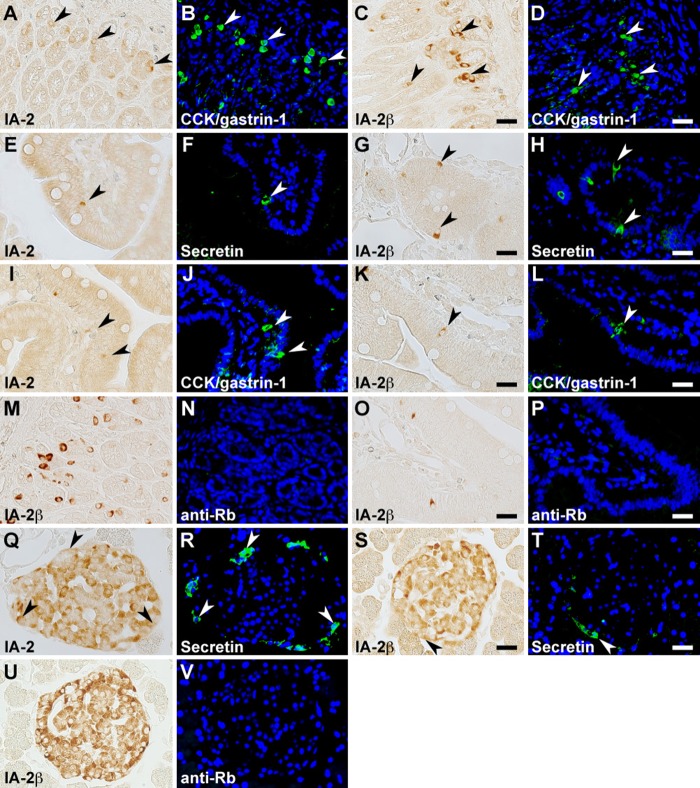
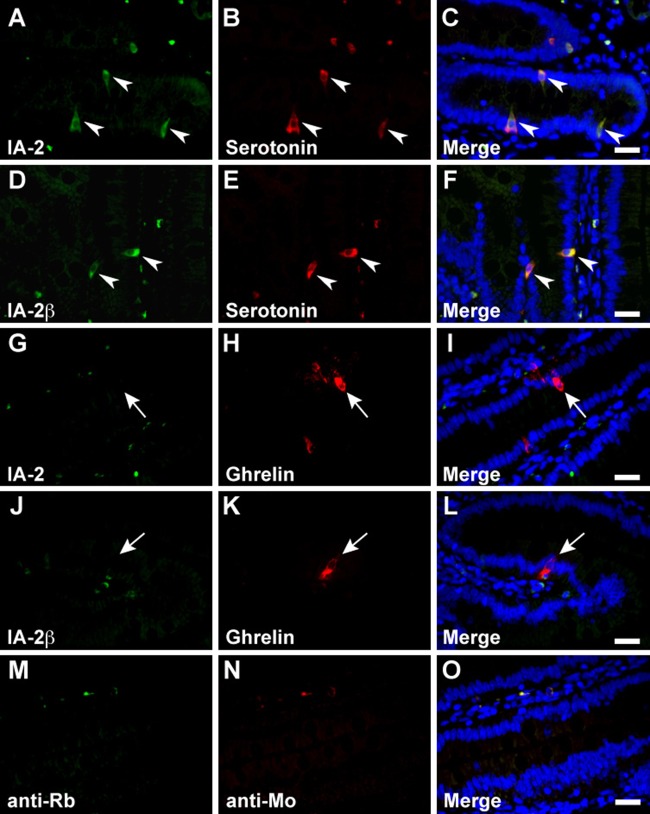
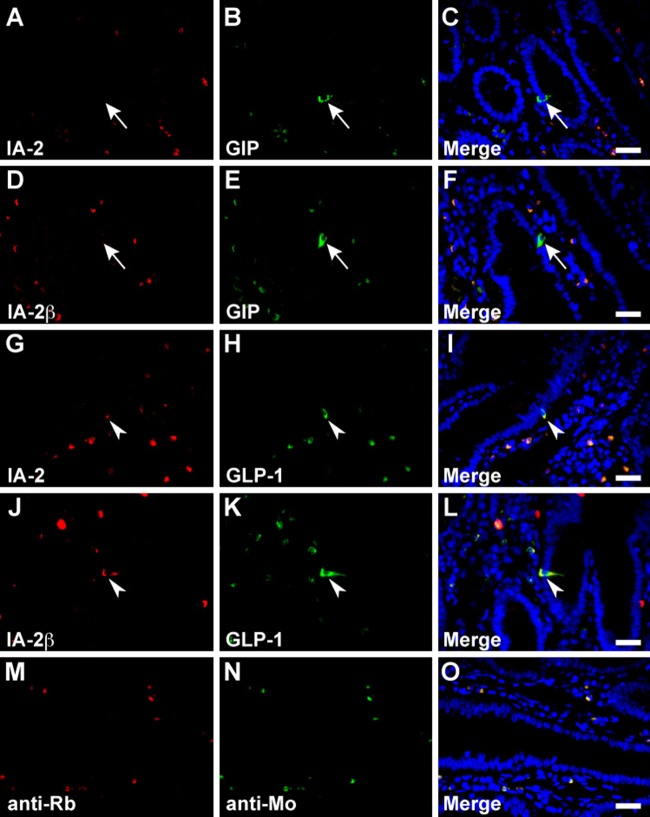
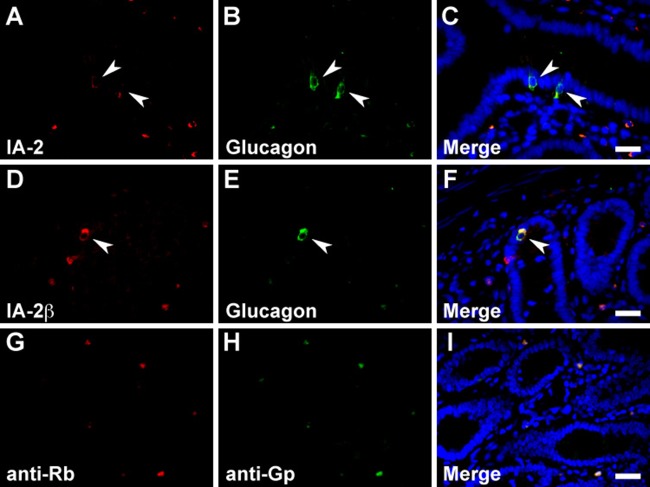
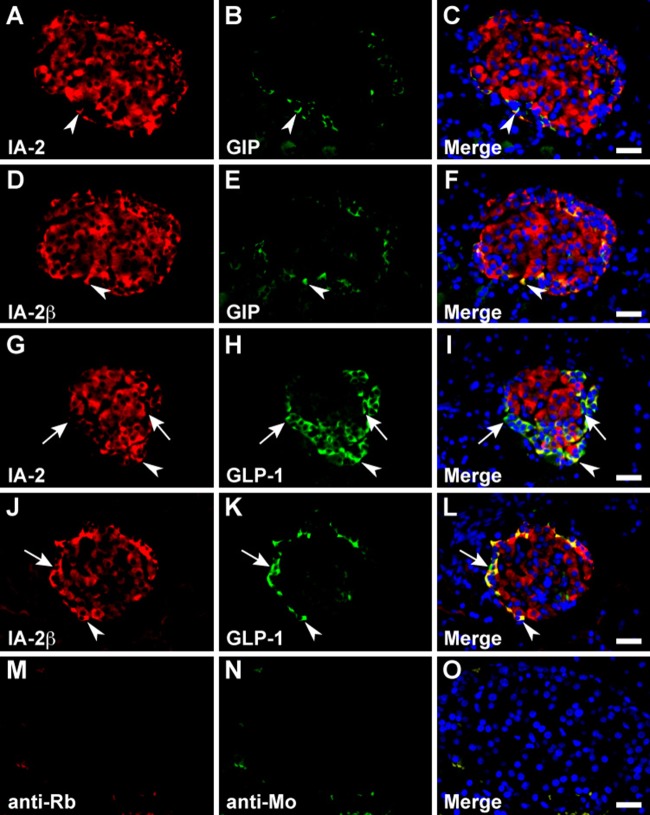
In the stomach, serotonin, somatostatin, and CCK/gastrin-1 were expressed along with IA-2 in the epithelial cells (Figs. 3A–C, 4A–C, and 6A, B), but GECs that expressed ghrelin did not coexpress IA2 (Fig. 3G–I). No stomach GECs with a significant expression of GIP, GLP-1, secretin, or glucagon were observed (data not shown). The same results were obtained with an analysis of IA-2β–immunolabeled epithelial cells (Figs. 3D–F, J–L; 4D–F; and 6C, D). Somatostatin, serotonin, secretin, CCK/gastrin-1, GIP, GLP-1, and glucagon were expressed in IA-2–immunolabeled epithelial cells in the duodenum (Figs. 4J–L; 5A–C; 6E, F,I,J; 7G–I; and 8A–C). However, in the majority of GIP-immunolabeled GECs, IA-2 was not coexpressed (Fig. 7A–C). Similar to the stomach, the duodenal ghrelin-immunolabeled GECs did not show coexpression with IA-2 (Fig. 5G–I). Comparable distribution patterns of IA-2β–immunolabeled GECs were observed in the duodenum (Figs. 4M–O; 5D–F, J–L; 6G, H,K,L; 7D–F, J–L; and 8D–F). However, the proportions of each cell type that were positive for IA-2 or IA-2β differed. A rough estimate of the coexpression of IA-2 or IA-2β with gut hormones revealed that the proportion of IA-2–positive cells was lower than that of IA-2β–positive cells (e.g., 70% IA-2 positivity [n=10] vs. 100% IA-2β positivity [n=6] in somatostatin-positive cells, 11% IA-2 positivity [n=19] vs. 17% IA-2β positivity [n=18] in GIP-positive cells, 39% IA-2 positivity [n=18] vs. 63% IA-2β positivity [n=27] in GLP-1-positive cells, and 57% IA-2 positivity [n=28] vs. 100% IA-2β positivity [n=21] in glucagon-positive cells, respectively). The number of cells (n) is the total number of hormone-positive cells counted in six different duodenal sections from two animals (three sections per animal). Basal background signaling in double-immunofluorescence staining was observed in parietal cell-like cells in the proper gastric gland and in plasma cell-like cells distributed in the lamina propria, in the central lymphoduct, and in the submucosa of the gastric and intestinal mucosa in the control sections (Figs. 3M–O; 4G–I, P–R; 5M–O; 7M–O; and 8G–I).
Figure 3.
Double immunofluorescence staining of the islet-associated protein (IA-2) family of protein tyrosine phosphatases (PTPs) with serotonin and ghrelin in rat stomach. Sections reacted with anti–IA-2 (A–C and G–I) or anti–IA-2β (D–F and J–L) antibodies in combination with anti-serotonin (A–F) and anti-ghrelin (G–L) antibodies. Arrowheads indicate double-immunolabeled gastrointestinal endocrine cells (GECs), and arrows indicate GECs that do not express the IA-2-family of PTPs. Negative control sections were stained with a mixture of anti-rabbit/anti-mouse secondary antibodies without primary antibodies (M–O). Blue fluorescence indicates nuclear counterstaining. Bars, 20 µm.
Figure 4.
Double immunofluorescence staining of the islet-associated protein (IA-2) family of protein tyrosine phosphatases (PTPs) with somatostatin in rat stomach and duodenum. Sections reacted with anti–IA-2 (A–C and J–L) or anti–IA-2β (D–F and M–O) antibodies in combination with anti-somatostatin antibody in the stomach (A–I) and duodenum (J–R). Arrowheads indicate double-immunolabeled gastrointestinal endocrine cells (GECs). Negative control sections were stained with a mixture of anti-rabbit/anti-rat secondary antibodies without primary antibodies in the stomach (G–I) or duodenum (P–R). Blue fluorescence indicates nuclear counterstaining. Bars, 20 µm.
Figure 6.
Dual immunostaining for the islet-associated protein (IA-2) family of protein tyrosine phosphatases (PTPs) with cholecystokinin (CCK)/gastrin-1 and secretin in rat stomach, duodenum, and pancreatic islets. Immunohistochemical restaining was performed on the sections using anti–IA-2 (A, B, E, F, I, J, Q, R) or anti–IA-2β (C, D, G, H, K, L, S, T) antibodies in combination with anti-CCK/gastrin-1 (A–D and I–L) and anti-secretin (E–H and Q–T) antibodies. Pairs of the same section are arranged in the two left and two right panels in each row. Gastrointestinal endocrine cells (GECs) and islet cells showing co-localization of the IA-2 family of PTPs with gut hormones in the stomach (A–D), duodenum (E–L), and pancreatic islets (Q–T) are indicated by arrowheads. Negative control sections were reacted with Alexa 488–conjugated anti-rabbit secondary antibody after boiling anti–IA-2β–labeled sections of the stomach (M and N), duodenum (O and P), and pancreatic islets (U and V). Bars, 20 µm.
Figure 5.
Double immunofluorescence staining of the islet-associated protein (IA-2) family of protein tyrosine phosphatases (PTPs) with serotonin and ghrelin in rat duodenum. Rat duodenal sections were concurrently incubated with anti–IA-2 (A–C and G–I) or anti–IA-2β (D–F and J–L) and anti-serotonin (A–F) or anti-ghrelin (G–L) antibodies. Arrowheads indicate double-immunolabeled gastrointestinal endocrine cells (GECs) and arrows indicate GECs that do not express the IA-2 family of PTPs. Negative control sections were stained with a mixture of anti-rabbit/anti-mouse secondary antibodies without primary antibodies (M–O). Blue fluorescence indicates nuclear counterstaining. Bars, 20 µm.
Figure 7.
Double immunofluorescence staining of the islet-associated protein (IA-2) family of protein tyrosine phosphatases (PTPs) with gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) in rat duodenum. Sections were reacted with anti–IA-2 (A–C and G–I) or anti–IA-2β (D–F and J–L) antibodies in combination with anti-GIP (A–F) or anti–GLP-1 (G–L) antibodies. Arrowheads indicate doubly immunolabeled gastrointestinal endocrine cells (GECs) and arrows indicate GECs that do not express the IA-2 family of PTPs. Negative control sections were stained with a mixture of anti-rabbit/anti-mouse secondary antibodies without primary antibodies (M–O). Blue fluorescence indicates nuclear counterstaining. Bars, 20 µm.
Figure 8.
Double immunofluorescence staining of the islet-associated protein (IA-2) family of protein tyrosine phosphatases (PTPs) with glucagon in rat duodenum. Sections were reacted with anti–IA-2 (A–C) or anti–IA-2β (D–F) antibodies in combination with anti-glucagon antibody. Arrowheads indicate doubly immunolabeled gastrointestinal endocrine cells (GECs). Negative control sections were stained with a mixture of anti-rabbit/anti–guinea pig secondary antibodies without primary antibodies (G–I). Blue fluorescence indicates nuclear counterstaining. Bars, 20 µm.
Identification of Cell Types Expressing the IA-2 Family of PTPs in Rat Pancreatic Islets
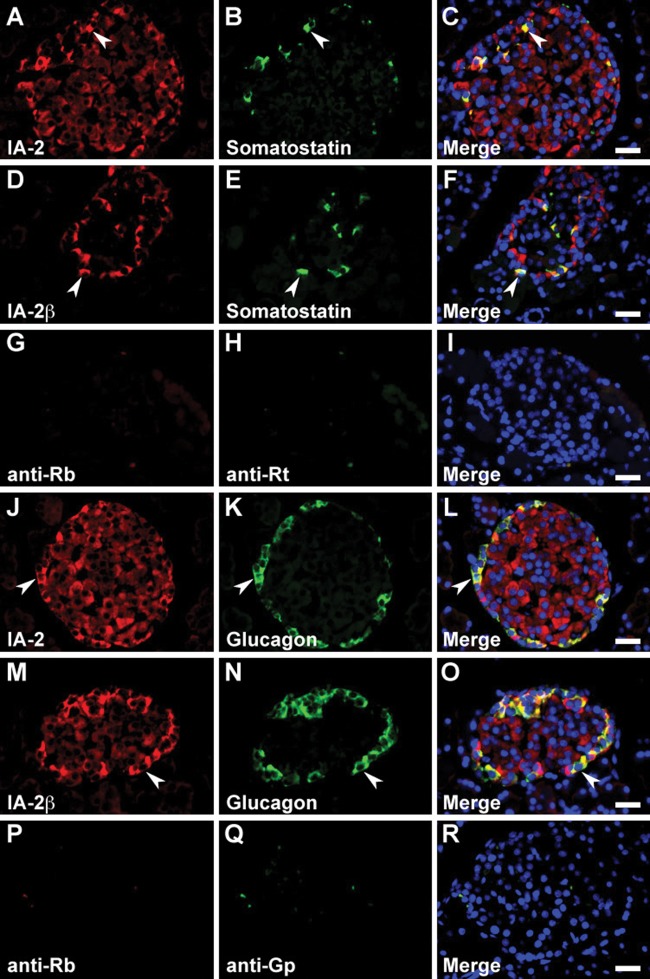
In the pancreo-gastrointestinal endocrine system, certain endocrine cells express the same hormones in the pancreatic islets and gastrointestinal tract. Therefore, we determined the cell types that express gut hormones in the pancreatic islets. Islet cells stained positive for secretin, somatostatin, GIP, GLP-1, and glucagon, but no significant immunoreactive signal was observed for serotonin, ghrelin, and CCK/gastrin-1 (data not shown). Although the above-mentioned difference in localization of the IA-2– or IA-2β–immunolabeled cells in each islet was generally reproducible using double-immunofluorescence staining, IA-2 or IA-2β was essentially expressed in gut hormone–positive cells (Figs. 6Q–T; 9A–F, J–O; and 10A–L). However, we observed a lower proportion of GLP-1/IA-2 double-positive cells than GLP-1/IA-2β double-positive cells (Fig. 10G–L). In the control pancreatic islets, a relatively low level of background signal was observed in double-immunofluorescence staining (Figs. 9G–I, P–R and 10M–O).
Figure 9.
Double immunofluorescence staining of the islet-associated protein (IA-2) family of protein tyrosine phosphatases (PTPs) with somatostatin and glucagon in rat pancreatic islets. Sections were reacted with anti–IA-2 (A–C and J–L) or anti–IA-2β (D–F and M–O) antibodies in combination with anti-somatostatin (A–F) and anti-glucagon (J–O) antibodies. Arrowheads indicate double-immunolabeled gastrointestinal endocrine cells (GECs). Negative control sections were stained with a mixture of anti-rabbit/anti-rat (G–I) or anti-rabbit/anti–guinea pig (P–R) secondary antibodies without primary antibodies. Blue fluorescence indicates nuclear counterstaining. Bars, 20 µm.
Figure 10.
Double immunofluorescence staining of the islet-associated protein (IA-2) family of protein tyrosine phosphatases (PTPs) with gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) in rat pancreatic islets. Sections were reacted with anti–IA-2 (A–C and G–I) or anti–IA-2β (D–F and J–L) antibodies with a combination of anti-GIP or anti–GLP-1 antibodies. Arrowheads indicate double-immunolabeled gastrointestinal endocrine cells (GECs) and arrows indicate GECs that do not express the IA-2 family of PTPs. Negative control sections were stained with a mixture of anti-rabbit/anti-mouse secondary antibodies without primary antibodies (M–O). Blue fluorescence indicates nuclear counterstaining. Bars, 20 µm.
Discussion
In the present study, we examined the distribution of the IA-2 family of PTPs and their coexpression with eight representative gut hormones in rat stomach, duodenum, and pancreas. Our antibodies against IA-2 and IA-2β could detect specific proteins in rat endocrine tissues. In the gastrointestinal tract, the IA-2 family of PTP-positive cells was distributed in the gastric mucosa, intestinal crypts and villi, and the myenteric and submucosal plexuses. In the pancreas, all islet endocrine cells and a few cell subpopulations in the exocrine duct expressed the IA-2 family of PTPs.
In the gastrointestinal tract, GECs secrete various peptide hormones. These hormone-producing cells are widely distributed with regional specificity, and some are also expressed in the pancreatic islet cells. Secreted gut hormones not only transmit signals to target digestive organs (endocrine function) but also act within the local gastrointestinal epithelium (paracrine function). Recent studies suggest that they participate in mutual interactions in a pancreo-gastrointestinal hormone network (Drucker 2006). The main functions of the gut hormones analyzed in the present study include modulation of (1) gastric secretion and motility (gastrin-1, secretin, GIP, and ghrelin), (2) intestinal motility (serotonin), (3) exocrine pancreatic secretion and choleresis (secretin and CCK), and (4) endocrine functions of GECs and pancreatic islet cells (somatostatin, CCK, GIP, and GLP-1). A previous study by Takeyama et al. (2009) described the expression of IA-2 in the somatostatin-containing D-cells in the mouse stomach and suggested the possibility of expression of the IA-2 family of PTPs in various types of GECs. However, no detailed analysis of GECs that express IA-2 and IA-2β has been conducted yet. In the present study, we confirmed that various gut hormone-producing cells expressed the IA-2 family of PTPs (Table 2). However, interestingly, we found that only GECs that expressed ghrelin, an appetite-stimulating peptide hormone, did not coexpress the IA-2 family of PTPs. These results suggest that the IA-2 family of PTPs plays a role in regulating the secretory pathway of specific gut hormones in GECs. Expression of the IA-2 family of PTPs in the myenteric and submucosal plexuses of the gastrointestinal tract also suggests their involvement in the neuronal secretory pathways of norepinephrine, acetylcholine, or other neuromodulatory substances from autonomic nerve endings to support homeostasis in the pancreo-gastrointestinal tract.
Table 2.
Summary of Coexpression of Gut Hormones and the Islet-Associated Protein–2 (IA-2) Family of Protein Tyrosine Phosphatases
| Hormone/Tissue | Stomach | Duodenum | Pancreatic Islet |
|---|---|---|---|
| Serotonin | + | + | NS |
| Ghrelin | − | − | NS |
| Somatostatin | + | + | + |
| GIP | NS | + | + |
| GLP-1 | NS | + | + |
| Secretin | NS | + | + |
| Glucagon | NS | + | + |
| CCK/gastrin-1 | + | + | NS |
CCK, cholecystokinin; GIP, gastric inhibitory polypeptide; GLP-1, glucagon-like peptide-1; NS, no significant immunolabeling of gut hormones.
The basal expression patterns of IA-2 and IA-2β in GECs were very similar, but the degree of co-localization of various gut hormones with IA-2 or IA-2β differed slightly in each cell type. For instance, a higher proportion of co-localization was observed in GECs double-positive for somatostatin, GIP, GLP-1, or glucagon and IA-2β in the duodenum and for GLP-1 and IA-2β in pancreatic islets. Although it is difficult to explain the association between the differential expression of the IA-2 family of PTPs and physiological function, these results reinforce the notion that the roles of IA-2 and IA-2β are similar in general but may differ in detail. Indeed, differential control of the expression of IA-2 and IA-2β has been reported. In developmental processes, IA-2 expression is increased in the pancreatic islets and the brain, whereas IA-2β shows a more consistent pattern of expression (Chiang and Flanagan 1996; Roberts et al. 2001; Shimizu et al. 2001; Löbner et al. 2002). Furthermore, the expression of IA-2, but not of IA-2β, is induced by glucose stimulation in pancreatic β-cells (Seissler et al. 2000; Löbner et al. 2002). In contrast, the transcriptional level of IA-2β, but not of IA-2, is increased in the brain and pancreas by the administration of ghrelin (Doi et al. 2006). More interestingly, Nakajima et al. (2011) have reported that three IA-2β isoforms—IA-2β60, IA-2β64, and IA-2β71—are expressed in the brain, stomach, large intestine, and pancreatic α- and δ-cells, whereas pancreatic β-cells exclusively expressed IA-2β60 in a mouse model. An analysis of sucrose gradient fractions of brain homogenates in a mouse model suggests that IA-2β64 and IA-2β71 are co-localized with IA-2 and involved in secretion from secretory granules, whereas IA-2β60 plays a role in secretion from synaptic vesicles. These data would clarify the roles of each peptide, which may have differentiated over the course of the functional evolution of endocrine cells.
A recent report by Cai et al. (2011) demonstrated that the decrease in insulin content and secretion in pancreatic β-cells from mice deficient in IA-2, IA-2β, or both genes is due to a decrease in the number of insulin granules. The reduction in insulin granules may have derived from defects in gene expression, including genes for insulin (mediated by autocrine insulin signaling or STAT5 activity), insulin transport and sorting to secretory granules, or the stability of insulin granules (Mziaut et al. 2008; Trajkovski et al. 2008; Torii et al. 2009; Saito et al. 2011; Cai et al. 2011). Moreover, several abnormalities have been detected in double-knockout mice, including female infertility (caused by a deficit in the luteinizing hormone surge) and disturbances in learning and in circadian rhythm (because of an impaired release of neurotransmitters such as norepinephrine, dopamine, and serotonin) (Kubosaki et al. 2006; Kim SM et al. 2009; Nishimura et al. 2009). Although the impairment in each tissue in knockout mice cannot rule out secondary influences from other IA-2 (IA-2β)–deficient tissues, the IA-2 family of PTPs presumably influences the modes of action of various physiologically active substances in the body. In the present study, we confirmed that numerous GECs express the IA-2 family of PTPs in the rat gastrointestinal tract. However, to date, no evidence has been presented to support the altered function of GECs in gene-deficient model mice. However, disruption of gut-specific ia2 expression in Drosophila revealed the function of IA-2 in modulating the expression of insulin-like peptide ilp-6 and hexokinase (Kim J et al. 2008). Because hormone-producing cells in the stomach, duodenum, and pancreas are associated with the regulation of homeostasis, further studies using conditional knockout mice will be required to demonstrate whether the IA-2 family of PTPs function in GECs.
In conclusion, the IA-2 family of PTPs is expressed in gut hormone-producing GECs in the rat gastrointestinal tract and pancreatic islet cells, with cell-type specificity. Our findings provide a basis for an understanding of the systemic contribution of these proteins to the nutritional control by the pancreo-gastrointestinal hormone network.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Grants-in-Aid from the Japan Society for the Promotion of Science (JSPS) #22500367 (HG) and #21689007 (ST). It was also supported in part by the joint research program of the Institute for Molecular and Cellular Regulation, Gunma University.
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
References
- Cai T, Hirai H, Zhang G, Zhang M, Takahashi N, Kasai H, Satin LS, Leapman RD, Notkins AL. 2011. Deletion of Ia-2 and/or Ia-2β in mice decreases insulin secretion by reducing the number of dense core vesicles. Diabetologia. 54:2347–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MK, Flanagan JG. 1996. PTP-NP, a new member of the receptor protein tyrosine phosphatase family, implicated in development of nervous system and pancreatic endocrine cells. Development. 122:2239–2250 [DOI] [PubMed] [Google Scholar]
- Doi A, Shono T, Nishi M, Furuta H, Sasaki H, Nanjo K. 2006. IA-2β, but not IA-2, is induced by ghrelin and inhibits glucose-stimulated insulin secretion. Proc Natl Acad Sci U S A. 103:885–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ. 2006. The biology of incretin hormones. Cell Metab. 3:153–165 [DOI] [PubMed] [Google Scholar]
- Fowler CB, Evers DL, O’Leary TJ, Mason JT. 2011. Antigen retrieval causes protein unfolding: evidence for a linear epitope model of recovered immunoreactivity. J Histochem Cytochem. 59:366–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Bang H, Ko S, Jung I, Hong H, Kim-Ha J. 2008. Drosophila ia2 modulates secretion of insulin-like peptide. Comp Biochem Physiol A Mol Integr Physiol. 151:180–184 [DOI] [PubMed] [Google Scholar]
- Kim SM, Power A, Brown TM, Constance CM, Coon SL, Nishimura T, Hirai H, Cai T, Eisner C, Weaver DR, et al. 2009. Deletion of the secretory vesicle proteins IA-2 and IA-2β disrupts circadian rhythms of cardiovascular and physical activity. FASEB J. 23:3226–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubosaki A, Nakamura S, Clark A, Morris JF, Notkins AL. 2006. Disruption of the transmembrane dense core vesicle proteins IA-2 and IA-2β causes female infertility. Endocrinology. 147:811–815 [DOI] [PubMed] [Google Scholar]
- Löbner K, Steinbrenner H, Roberts GA, Ling Z, Huang GC, Piquer S, Pipeleers DG, Seissler J, Christie MR. 2002. Different regulated expression of the tyrosine phosphatase-like proteins IA-2 and phogrin by glucose and insulin in pancreatic islets: relationship to development of insulin secretory responses in early life. Diabetes. 51:2982–2988 [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Kanaya T, Tanaka S, Takakura I, Watanabe K, Ohwada S, Kitazawa H, Rose MT, Sakaguchi S, Katamine S, et al. 2007. Immunohistochemical characterization of the cell types expressing the cellular prion protein in the small intestine of cattle and mice. Histochem Cell Biol. 127:291–301 [DOI] [PubMed] [Google Scholar]
- Moran-Ramos S, Tovar AR, Torres N. 2012. Diet: friend or foe of enteroendocrine cells—how it interacts with enteroendocrine cells. Adv Nutr. 3:8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mziaut H, Kersting S, Knoch KP, Fan WH, Trajkovski M, Erdmann K, Bergert H, Ehehalt F, Saeger HD, Solimena M. 2008. ICA512 signaling enhances pancreatic β-cell proliferation by regulating cyclins D through STATs. Proc Natl Acad Sci U S A. 105:674–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Wu G, Sakudo A, Onodera T, Takeyama N. 2011. Distinct subcellular localization of three isoforms of insulinoma-associated protein 2β in neuroendocrine tissues. Life Sci. 88:798–802 [DOI] [PubMed] [Google Scholar]
- Nishimura T, Kubosaki A, Ito Y, Notkins AL. 2009. Disturbances in the secretion of neurotransmitters in IA-2/IA-2beta null mice: changes in behavior, learning and lifespan. Neuroscience. 159:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince AC, Brooks SJ, Stahl D, Treasure J. 2009. Systematic review and meta-analysis of the baseline concentrations and physiologic responses of gut hormones to food in eating disorders. Am J Clin Nutr. 89:755–765 [DOI] [PubMed] [Google Scholar]
- Roberts C, Roberts GA, Löbner K, Bearzatto M, Clark A, Bonifacio E, Christie MR. 2001. Expression of the protein tyrosine phosphatase-like protein IA-2 during pancreatic islet development. J Histochem Cytochem. 49:767–776 [DOI] [PubMed] [Google Scholar]
- Saito N, Takeuchi T, Kawano A, Hosaka M, Hou N, Torii S. 2011. Luminal interaction of phogrin with carboxypeptidase E for effective targeting to secretory granules. Traffic. 12:499–506 [DOI] [PubMed] [Google Scholar]
- Seissler J, Nguyen TB, Aust G, Steinbrenner H, Scherbaum WA. 2000. Regulation of the diabetes-associated autoantigen IA-2 in INS-1 pancreatic β-cells. Diabetes. 49:1137–1141 [DOI] [PubMed] [Google Scholar]
- Shimizu S, Saito N, Kubosaki A, SungWook S, Takeyama N, Sakamoto T, Matsumoto Y, Saeki K, Matsumoto Y, Onodera T. 2001. Developmental expression and localization of IA-2 mRNA in mouse neuroendocrine tissues. Biochem Biophys Res Commun. 288:165–171 [DOI] [PubMed] [Google Scholar]
- Solimena M, Dirkx R, Hermel JM, Pleasic-Williams S, Shapiro JA, Caron L, Rabin DU. 1996. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J. 15:2102–2114 [PMC free article] [PubMed] [Google Scholar]
- Takeyama N, Ano Y, Wu G, Kubota N, Saeki K, Sakudo A, Momotani E, Sugiura K, Yukawa M, Onodera T. 2009. Localization of insulinoma associated protein 2, IA-2 in mouse neuroendocrine tissues using two novel monoclonal antibodies. Life Sci. 84:678–687 [DOI] [PubMed] [Google Scholar]
- Torii S. 2009. Expression and function of IA-2 family proteins, unique neuroendocrine-specific protein-tyrosine phosphatases. Endocr J. 56:639–648 [DOI] [PubMed] [Google Scholar]
- Torii S, Saito N, Kawano A, Hou N, Ueki K, Kulkarni RN, Takeuchi T. 2009. Gene silencing of phogrin unveils its essential role in glucose-responsive pancreatic β-cell growth. Diabetes. 58:682–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovski M, Mziaut H, Schubert S, Kalaidzidis Y, Altkrüger A, Solimena M. 2008. Regulation of insulin granule turnover in pancreatic β-cells by cleaved ICA512. J Biol Chem. 283:33719–33729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmeier C, Hutton JC. 1996. Molecular cloning of phogrin, a protein-tyrosine phosphatase homologue localized to insulin secretory granule membranes. J Biol Chem. 271:18161–18170 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.