Abstract
Specialized protein domains bind to posttranslational modifications (PTMs) of proteins, such as phosphorylation or glycosylation. When such PTM-binding protein domains are used as analytical tools, the functional states of cells and tissues can be determined with high precision. Here, we describe the use of recombinant CLEC10A (CD301), a human glycoreceptor of the C-type lectin family, for the detection of ligands in sections from formalin-fixed, paraffin-embedded normal and cancerous mammary tissues. A construct, in which part of the carbohydrate recognition domain (CRD) was deleted, was used as a negative control. In comparison to normal mammary glands, a pronounced staining of tumor tissues was observed. Because the construct with the truncated CRD did not show any tissue staining, the binding of the wild-type glycoreceptor can be attributed to its carbohydrate recognition domain. To distinguish our novel approach from immunohistochemistry, we propose the designation “protein domain histochemistry” (PDH).
Keywords: breast cancer, CD301, CLEC10A, C-type lectin, glycoreceptor, histochemistry, protein binding modules
Posttranslational modifications (PTMs) such as phosphorylation and glycosylation are important regulators of functional states of cells and tissues. The human genome encodes special protein modules, which recognize PTMs. For example, phosphotyrosine-containing amino acid motifs are bound by SH2 domains, and cell surface glycans interact with carbohydrate recognition domains (CRDs) of glycoreceptors of the C-type lectin family. If expressed as recombinant proteins, PTM-binding domains can be used as analytical tools. This approach has been applied to tyrosine phosphorylated proteins by recombinant SH2 domains (Dierck et al. 2009) and to glycoproteins by recombinant human glycoreceptors (Samsen et al. 2010). When different PTM-binding protein modules are applied in parallel, functional states of cells and tissues can be monitored with high precision.
In comparison with antibodies, the recognition of PTMs by cellular proteins reflects the physiological situation and, for this reason, the respective protein modules carry biological information. However, in general, the binding affinity of monomeric protein modules is relatively low. For this reason, monomeric protein domains have to be multimerized to achieve efficient binding in analytical settings (Powlesland et al. 2008). Furthermore, binding is favored if PTM-modified proteins are present at high concentrations. This condition is fulfilled in far Western blots because the transfer of focused protein bands from SDS-PAGE to membranes results in high concentrations of immobilized modified proteins. It is more challenging to use protein domains for the detection of interacting partners in tissue sections, particularly if formalin-fixed, paraffin-embedded (FFPE) tissues are used. In this setting, the concentration of PTMs may be limiting, and the penetration of bulky oligomers into the section may pose problems.
Here, we describe the binding of the human glycoreceptor C-type lectin domain 10, member A (CLEC10A), also known as CD301, to sections from FFPE tissues of mammary carcinomas and normal mammary glands. CLEC10A recognizes GalNAc-containing epitopes frequently expressed on the surface of cancer cells, is involved in the regulation of the adaptive and innate immune response, and was chosen as a probe for glycoprofiling to gain deeper insights in tumor-specific alterations of the glycostructures of breast cancer (Dwek et al. 2001; Welinder et al. 2011). Because, in negative controls, the CRD has been mutated, binding can be assigned to the CRD. The technology differs from immunohistochemistry insofar as antibodies are replaced by protein domains. To distinguish the technique from the use of antibodies in immunohistochemistry, we propose the designation “protein domain histochemistry” (PDH).
Materials and Methods
Cloning and Expression of Recombinant CD301
The extracellular domain (aa 67-292) of CD301 (CLEC10A; NCBI ref seq: NP 006335.2) was amplified by PCR from a human cDNA pool library (Clontech; Mountain View, CA) and cloned into the eukaryotic EBB expression vector as previously described (Bogoevska et al. 2006). For secretion, a human IgGk-leader was attached at the N-terminus followed by a myc-tag for CD301 complexing and detection. The CD301 binding mutant was generated by truncation of 57 aa of the C-terminus, removing approximately 60% of the C-terminal part of the CRD (aa 164–281). Plasmid-DNA was transiently transfected into HEK293T cells applying Lipofectamin 2000 (Invitrogen; Karlsruhe, Germany). Tissue culture supernatants were harvested after 48 hr and cleared by centrifugation. Recombinant CD301 was further characterized by Western blot analysis in comparison with an internal standard for quantification as previously described (Bogoevska et al. 2006). For determination of the exact molecular weight, glycans were removed from CD301 applying the protein deglycosylation mix (NEB; Frankfurt, Germany). Supernatants were either directly used or stored at 4C in the presence of 0.01% sodium azide for further analysis.
Glycan-ELISA
Glycoconjugates were purchased from Lectinity (Moscow, Russia) composed of a poly[N-(2-hydroxyethyl)acrylamide] backbone (PAA) to which different carbohydrates were linked via aminoglucitol (HOCH2(HOCH)4CH2NH-X). PAA-conjugated glycans (1 µg/well) were immobilized to flat-bottom MaxiSorp 96-well plates (Thermo Fisher Scientific/Nunc; Rockford, IL) in 100 µl PBS (pH 7.4) at 4C overnight under constant agitation. Plates were blocked using 1× carbo-free blocking solution (Biozol; Eching, Germany) for 1 hr at room temperature. CD301 complexes were prepared as described below, and 100 µl of complex was added per well and incubated for 2 hr at room temperature. After washing (3×) with Tris saline magnesium (TSM) buffer (20 mM Tris/HCl [pH 7.4], 150 mM NaCl, 2 mM MgCl2, 1 mM CaCl2) in the presence of 0.1% Tween-20, ABTS (2,2′-azino-di-[3-ethylbenzthiazoline sulfonate (6)] diammonium salt) was added as a substrate according to the instructions of the manufacturer (Roche; Mannheim, Germany). Absorbance was measured at 405 nm on a microplate reader (Tecan; Männedorf, Switzerland) at 37C.
Tissue Microarrays and CD301 Staining
Paraffin-embedded tissue microarrays (TMAs) of formalin-fixed human breast cancer and corresponding normal tissue were purchased from US Biomax (Rockville, MD). As assured by US Biomax, written informed consent was given by all donors, and tissues were collected under Health Insurance Portability and Accountability Act (HIPPA)–approved protocols. Studies on human tissue samples were approved by the local ethical review board (Ärztekammer Hamburg, Germany, PV3548). Tissue sections were deparaffinized in xylol (2× for 5 min each) and rehydrated in PBS. Antigen retrieval was achieved by boiling (5× for 2 min each) in a microwave (900 W) in 0.1 M sodium citrate buffer (pH 5.0). Slides were washed in PBS, endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 5 min at room temperature, and slides were washed once in TSM buffer. Subsequently, nonspecific binding sites were blocked at 4C for 2 hr in TSM buffer in the presence of 0.2% BSA, 10% fetal calf serum, and 0.3% Triton X-100. During the blocking step, a complex of myc-tagged CD301, streptavidin–horseradish peroxidase (HRP) conjugate (Thermo Fisher Scientific/Pierce; Rockford, IL), and the biotinylated anti-myc antibody 9E10 (Santa Cruz Biotechnology; Santa Cruz, CA) was generated. In brief, 100 to 200 ng/ml of recombinant CD301, as estimated from Western blot analysis in comparison to an internal standard (data not shown), was incubated with 2 µg/ml of 9E10 antibody for 1 hr at 4C. Streptavidin-HRP conjugate was added to a final concentration of 2.5 µg/ml and incubated for 1 hr at 4C. Tissue sections were covered with 200 µl of complex and incubated at 4C overnight in a wet chamber. After washing (3× for 5 min each) in TSM buffer, staining was performed with 3,3′-diaminobenzidine chromogen solution (Dako; Hamburg, Germany) for 10 min at room temperature, slides were washed in H2O once, and nuclei were counterstained by hematoxylin. Stained tissue sections were coverslipped, applying Glycergel Mounting Medium (Dako). Antibodies directed against HER2/neu and the blood group A1/A2 antigens were purchased from Dako and ViroGen (Watertown, MA), respectively, and were applied in a dilution of 1:500; as secondary antibody, an HRP-labeled, rabbit anti-mouse polyclonal antiserum (Dianova; Hamburg, Germany) was used (dilution 1:1000).
Microscopic Evaluation and Statistics
Microscopic examination was performed with an Axiphot microscope (Zeiss; Jena, Germany) and pictures were taken with an AxioCam (MRc5; Zeiss) camera. Tissue sections were independently reviewed and scored by two pathologists. Fisher’s exact test was applied for statistical analysis.
Results
To generate functionally active glycoreceptors, the extracellular part of CD301 was amplified from human cDNA, cloned in a eukaryotic expression system, and expressed after transient transfection of HEK293T cells. As a negative control, a CD301 binding mutant was generated in parallel lacking approximately 60% of the C-terminus of the carbohydrate recognition domain.
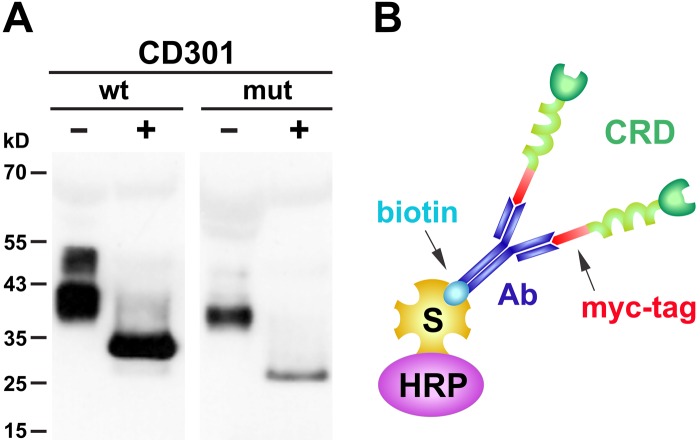
To enforce secretion into the tissue culture supernatant, both constructs carried a human IgGκ-leader at the N-terminus followed by a myc-tag, allowing subsequent complexing and detection. Soluble, recombinant CD301 domains were harvested from tissue culture supernatants and analyzed by Western blot analysis via the myc-tag (Fig. 1A). The CD301 wild-type domain migrated as two broad bands with a molecular weight of approximately 38 kD and 50 kD, respectively, whereas the mutant domain was detectable with an apparent molecular weight of approximately 35 kD. The migratory behavior of the recombinant proteins differs from the calculated theoretical molecular weights with 30 kD for the CD301 wild-type domain and 24 kD for the mutant domain, respectively, indicating that both proteins are posttranslationally modified, most likely by glycosylation during the expression in HEK293T cells. Deglycosylation of wild-type and mutant CD301 resulted in a shift in electrophoretic mobility to the expected molecular weights, confirming the identity and size of our recombinant probes.
Figure 1.
(A) Western blot analysis of CD301 wild-type (wt) and mutant (mut) extracellular domains expressed in tissue culture supernatants of HEK293T cells. Soluble domains were detected applying the anti-myc antibody 9E10 and a horseradish peroxidase (HRP)–conjugated polyclonal rabbit anti-mouse serum followed by enhanced chemiluminescence. For validation of the probes, native preparations (–) were compared with CD301 preparations after deglycosylation (+). (B) Multimeric complex formed between the extracellular domain of CD301, the biotinylated anti-myc antibody 9E10 recognizing the myc-tag attached at the N-terminus of CD301, and HRP-conjugated streptavidin (S). The extracellular part of CD301 is composed of the carbohydrate recognition domain (CRD) followed by a coiled-coiled domain.
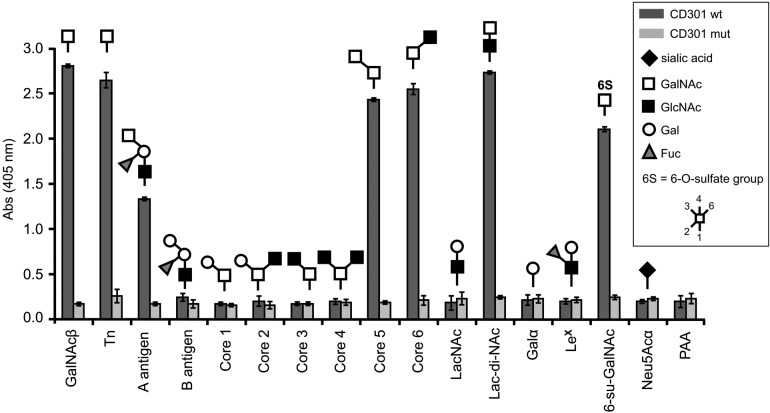
Interactions between carbohydrate recognition domains and their ligands are generally weak in nature, with affinities in the nano- to micromolar range. For the analysis of native tissues, where glycosylated ligands are expected to be expressed at relatively low levels, low affinities may substantially limit the applicability of glycoreceptors as probes for glycoprofiling. To overcome this limitation and to improve the binding strength of our glycoreceptor probes, we increased the avidity by multimerization of CD301 domains. For this purpose, recombinant CD301 was bound to a biotinylated anti-myc antibody via the myc-tag. Multimerization was achieved by complexing the CD301-antibody complex via biotin to streptavidin conjugated to HRP for detection (Fig. 1B). To test the functionality and binding specificity of our multimerized probes, a glycan-based ELISA was applied. Sixteen different PAA-conjugated glycans were immobilized on microtiter plates and incubated with wild-type and mutant CD301 multimers in the presence of calcium and magnesium, and binding was photometerically determined using ABTS as a substrate (Fig. 2). The wild-type CD301 carbohydrate recognition domain strongly bound terminal GalNAc glycostructures present in the Tn-antigen, blood group A antigen, Lac-di-NAc sequence (GalNAcβ1-4GlcNAc-R), 6-su-GalNAc, as well as core 5 and core 6 structures. No binding was observed for the CD301 mutant, confirming that partial truncation of the CRD fully abrogates the binding of the mutant. Our binding results are in good accordance with published data on human CD301 (MGL) (van Vliet et al. 2005).
Figure 2.
Functional characterization and determination of binding specificity of wild-type (wt) and mutant CD301 (mut) determined by glycan ELISA with 16 immobilized glycoconjugates. The composition of glycans conjugated to polyacrylamide (PAA) is given by the pictograms. Measurements were performed in triplicate; error bars indicate the standard error of the mean. PAA served as background control.
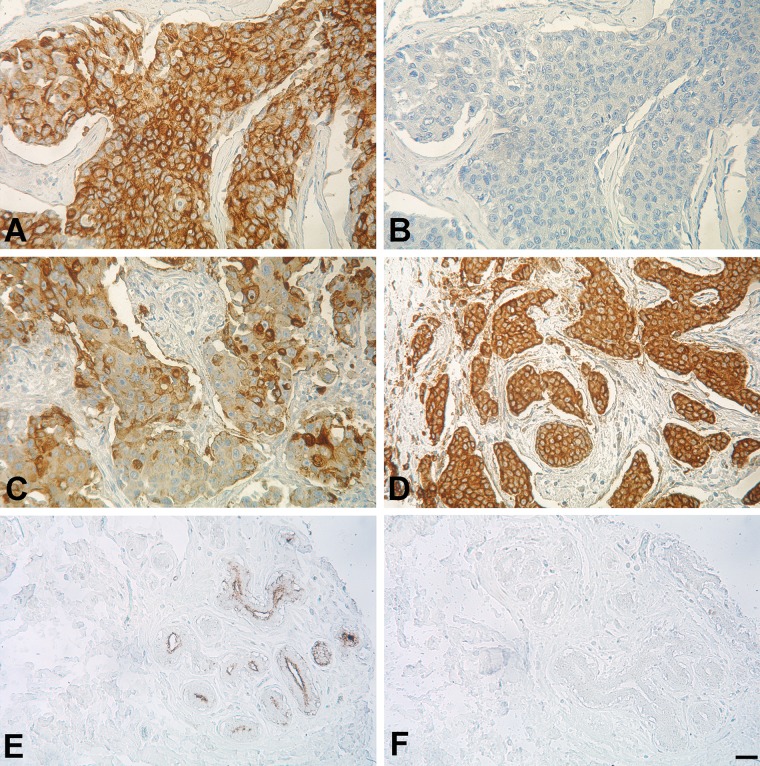
Having confirmed the functionality and specificity of our CD301 probes, the wild-type and mutant multimers were applied for staining of paraffin-embedded breast cancer specimen (n=97) and normal corresponding tissue (n=10) arranged on a tissue microarray (Fig. 3; Suppl. Fig. S1). CD301 staining in carcinoma and corresponding normal tissue was evaluated with regard to intensity and distribution (Suppl. Table S1). We observed moderate or strong staining intensity in approximately 80% of the carcinoma samples (77/97). In contrast, CD301 staining was weak or moderate in normal breast epithelia. Staining patterns in the parenchyma of tumors were focal in approximately two-thirds of samples (62/97); approximately one-third (35/97) of specimens showed diffuse patterns of staining. Tumor stroma stained negative in 40 cases or was weakly positive in 57 of 97 specimens with faint and sporadic staining of single fibroblasts. Occasionally, inflammatory cells present in the stroma of tumors were found to stain positive. For normal epithelial cells that scored weakly or moderately positive, staining was focal, primarily localized to lobular epithelial cells, and was accentuated in the luminal part of the acinus (Fig. 3E). Connective tissues of the normal parenchyma and fat cells stained completely negative. No staining was observed for the mutant CD301 domain, demonstrating the binding specificity of our CD301 wild-type domain (Fig. 3; Suppl. Fig. S1).
Figure 3.
CD301 protein domain histochemistry on paraffin-embedded human breast cancer and normal tissue. Representative areas and staining patterns are given for the different breast cancer specimens of the tissue microarray. (A) Strong and diffuse staining (3+) within the tumor parenchyma with most of the tumor stroma being completely negative. (B) No staining was observed on a consecutive tissue section corresponding to the tissue area shown in A, when the mutant CD301 probe was applied. (C) Focal, heterogeneous staining (3+) with minimum density of the tumor parenchyma. Stromal fibroblast and fibers are negative. (D) Strong, diffuse staining (3+) of tumor parenchyma. (E) Heterogeneous, moderate (2+) staining of normal breast epithelium with pronounced staining within the luminal part of the acinus. (F) Negative staining of the CD301 mutant in normal breast tissue corresponding to the tissue area given in E. Panels A and B were taken from the tissue spot C6, panel C from spot C3, panel D from F2, and panels E and F from the tissue spot K5 (see Suppl. Fig. S1 and Suppl. Table S1 for details). Scale bar: 50 µm.
As shown by our glycan ELISA, CD301 recognizes the blood group A antigen among other terminal GalNAc glycostructures. To exclude that positive staining for CD301 is simply reflecting neo-expression of the blood group A antigen on breast cancer cells of carriers of blood group A, we investigated the expression of the blood group A antigen in our breast cancer specimens. For this purpose, a consecutive section of the breast cancer TMA was probed with an antibody specific for the blood group antigens A1 and A2, respectively (Suppl. Fig. S2 and Suppl. Table S1). Positive staining for the blood group A antigen was limited to erythrocytes and blood vessels and was observed in approximately one-third of samples (27/97). In these cases, staining patterns of blood group A were completely different from CD301 patterns of staining, demonstrating that our CD301 probe recognizes glycostructures distinct from the blood group A antigen (Suppl. Fig. S2). The discrepancy between binding of CD301 to terminal glycostructures of the A antigen observed by ELISA and the lack of staining of CD301 toward erythrocytes and blood vessels on the TMA is currently unclear and is probably due to differences in the density and spacing of the A antigen present on the surface of cells and our ELISA platform.
Although the number of carcinoma specimens investigated in this study is limited, we tested for associations between staining intensity and histopathological as well as clinical parameters. No obvious association was observed between staining intensity and tumor grade, size of the tumor, and lymph node status. As no information was available on the HER2/neu status, we also stained our breast cancer samples for HER2/neu expression (Suppl. Fig. S3). Moderate or strong expression of HER2/neu was observed in 22.7% (22/97) of cases (Supp. Table S1). Interestingly, staining intensity of CD301 significantly correlated (p<0.001) with HER2/neu receptor expression. Among the breast cancer specimens stained strongly positive for CD301, approximately 75% (36/46) of the samples were positive for HER2/neu expression (Supp. Table S1). Because we have demonstrated here that staining of formalin-fixed, paraffin-embedded tissues by the recombinant CD301 glycoreceptor is feasible, larger numbers of breast cancer samples will be analyzed to establish potential correlations among molecular, histopathological, and clinical parameters.
Discussion
In the present communication, we describe the use of recombinant CLEC10A (CD301), a human glycoreceptor of the C-type lectin family, for the detection of ligands in sections from formalin-fixed, paraffin-embedded normal and cancerous mammary tissues. Because recombinant CLEC10A with a non-functional CRD was applied as a negative control, ligand binding is mediated by the CRD of the non-mutated receptor. By using polyvalent constructs, binding avidity was increased. The negative control, in which part of the CRD was deleted, did not show any background staining.
The binding specificity of recombinant CLEC10A was analyzed by defined glycans conjugated to polyacrylamide. The observed binding preferences for CD301 to terminal GalNAc structures present in Tn antigen, Lac-di-NAc, A antigen, 6-su-GalNAc, core 5, and core 6 are in accordance with the literature (van Vliet et al. 2005). In the past, a number of lectins have been used to detect the Tn structure in tissues and cells. In breast cancer, most clinical information was obtained by the use of the Helix pomatia agglutinin (HPA) (Dwek et al. 2001). However, this lectin binds to additional structures such as NeuAcα2-3(GalNacβ1-4)Galβ1-R, Fucα1-2(GalNAcα1-3)Galβ1-R, and Forssman glycolipid (Ju et al. 2011). As an alternative to lectins, monoclonal antibodies have been used to detect the Tn antigen in tissue sections. However, monoclonal antibodies may crossreact with other glycan structures (Ju et al. 2011). The main difference between lectins from plants and mollusks as well as monoclonal antibodies, on one hand, and human recombinant glycoreceptors, on the other hand, is the fact that the binding specificity of human receptors corresponds to the in vivo situation in humans.
CLEC10A is expressed by intermediate monocytes (Ziegler-Heitbrock et al. 2010; Wong et al. 2011) and macrophages (van Vliet et al. 2008). It becomes increasingly evident that tumor-associated macrophages (TAMs) play an important role in tumor progression. Although many different macrophage populations exist, there appears to be consensus that TAMs, which foster tumor progression, exhibit a status of alternative activation (M2 macrophages) (Mantovani et al. 2008; Qian and Pollard 2010). Because M2 macrophages express CLEC10A (Solinas et al. 2010; Prokop et al. 2011), the CLEC10A glycoreceptor of M2 macrophages may make contact with glycans binding to its CRD. The use of recombinant human CLEC10A and other glycan-binding receptors is most appropriate to identify those glycans in human tumors, which would be able to interact with TAMs expressing the respective glycoreceptors.
It has been argued that tumor progression may be mitigated by the inhibition of TAMs (Mantovani and Sica 2010; Qian and Pollard 2010). The identification of those tumor-associated glycans, which potentially interact with the CRDs of TAMs, may become a tool to identify those patients who will profit from such novel therapeutic approaches.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported within the collaborative project GlycoBioM, Grant Agreement Number 259869, in Framework 7 of the European Commission and by the German Federal Ministry of Education and Research, BMBF research grant 0315500B.
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
References
- Bogoevska V, Horst A, Klampe B, Lucka L, Wagener C, Nollau P. 2006. CEACAM1, an adhesion molecule of human granulocytes, is fucosylated by fucosyltransferase IX and interacts with DC-SIGN of dendritic cells via Lewis x residues. Glycobiology. 16:197–209 [DOI] [PubMed] [Google Scholar]
- Dierck K, Machida K, Mayer BJ, Nollau P. 2009. Profiling the tyrosine phosphorylation state using SH2 domains. Methods Mol Biol. 527:131–155 [DOI] [PubMed] [Google Scholar]
- Dwek MV, Ross HA, Streets AJ, Brooks SA, Adam E, Titcomb A, Woodside JV, Schumacher U, Leathem AJ. 2001. Helix pomatia agglutinin lectin-binding oligosaccharides of aggressive breast cancer. Int J Cancer. 95:79–85 [DOI] [PubMed] [Google Scholar]
- Ju T, Otto VI, Cummings RD. 2011. The Tn antigen-structural simplicity and biological complexity. Angew Chem Int Ed Engl. 50:1770–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. 2008. Cancer-related inflammation. Nature. 454:436–444 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A. 2010. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 22:231–237 [DOI] [PubMed] [Google Scholar]
- Powlesland AS, Fisch T, Taylor ME, Smith DF, Tissot B, Dell A, Pohlmann S, Drickamer K. 2008. A novel mechanism for LSECtin binding to Ebola virus surface glycoprotein through truncated glycans. J Biol Chem. 283:593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop S, Heppner FL, Goebel HH, Stenzel W. 2011. M2 olarized macrophages and giant cells contribute to myofibrosis in neuromuscular sarcoidosis. Am J Pathol. 178:1279–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. 2010. Macrophage diversity enhances tumor progression and metastasis. Cell. 141:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsen A, Bogoevska V, Klampe B, Bamberger AM, Lucka L, Horst AK, Nollau P, Wagener C. 2010. DC-SIGN and SRCL bind glycans of carcinoembryonic antigen (CEA) and CEA-related cell adhesion molecule 1 (CEACAM1): recombinant human glycan-binding receptors as analytical tools. Eur J Cell Biol. 89:87–94 [DOI] [PubMed] [Google Scholar]
- Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, Pasqualini F, Nebuloni M, Chiabrando C, Mantovani A, et al. 2010. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 185:642–652 [DOI] [PubMed] [Google Scholar]
- van Vliet SJ, Saeland E, van Kooyk Y. 2008. Sweet preferences of MGL: carbohydrate specificity and function. Trends Immunol. 29:83–90 [DOI] [PubMed] [Google Scholar]
- van Vliet SJ, van Liempt E, Saeland E, Aarnoudse CA, Appelmelk B, Irimura T, Geijtenbeek TB, Blixt O, Alvarez R, van Die I, et al. 2005. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int Immunol. 17:661–669 [DOI] [PubMed] [Google Scholar]
- Welinder C, Baldetorp B, Borrebaeck C, Fredlund BM, Jansson B. 2011. A new murine IgG1 anti-Tn monoclonal antibody with in vivo anti-tumor activity. Glycobiology. 21:1097–1107 [DOI] [PubMed] [Google Scholar]
- Wong KL, Tai JJ, Wong WC, Han H, Sem X, Yeap WH, Kourilsky P, Wong SC. 2011. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 118:e16–e31 [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, et al. 2010. Nomenclature of monocytes and dendritic cells in blood. Blood. 116:e74–80 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.