Abstract
Endogenous soluble vascular endothelial growth factor receptor-2 (esVEGFR-2), a new splicing variant of VEGFR-2, was shown to be the first endogenous specific inhibitor of lymphatic vessel growth. The expression of esVEGFR-2 and its clinicopathological roles in esophageal squamous cell carcinoma (ESCC) are unclear. In this article, quantitative RT-PCR was employed to detect the mRNA levels of esVEGFR-2 and VEGF-C in 90 paired primary ESCC tissues, along with immunohistochemical staining to measure esVEGFR-2 protein in 182 ESCC primary tissues. Correlations between esVEGFR-2 expression and clinicopathological features were also analyzed. Compared with the corresponding non-neoplastic esophageal mucosa tissues, the mRNA level of esVEGFR-2 was decreased, whereas the mRNA level of VEGF-C was increased in ESCCs. Downregulation of esVEGFR-2 mRNA level was significantly correlated with pTNM stages (χ2 = 7.790, p=0.02). Immunohistochemical staining of esVEGFR-2 was inclined to be reduced in ESCC tissues; lower esVEGFR-2 protein expression was related to better prognosis (χ2 = 6.366, p=0.012), whereas higher esVEGFR-2 protein accumulation in ESCC tissues was an independent prognostic factor for poor survival of patients (hazard ratio, 1.606; 95% confidence interval, 1.042–2.476; p=0.032). Taken together, altered expression of esVEGFR-2 is correlated with progression of ESCC. esVEGFR-2 might serve as a new independent prognostic marker for ESCC patients.
Keywords: esVEGFR-2, ESCC, biomarker, prognosis
Esophageal cancer is the eighth most common cancer and the sixth most common cause of cancer deaths worldwide. New cases of esophageal carcinoma in China make up more than half of all annual global incidences (Parkin et al. 2005). Adenocarcinoma is the most rapidly increasing cancer in Western countries, whereas esophageal squamous cell carcinoma (ESCC) is still the dominant histological type in East Asia (Matsushima K et al. 2010). Although surgery and radiation therapy effectively control primary lesions, prognosis remains poor due to development of metastatic disease (Steeg 2006).
It is known that tumor cells must invade the tissue surrounding the primary tumor, enter either the lymphatic system or the bloodstream, survive and eventually arrest in the circulation, extravasate into a tissue, and proliferate at new sites (Steeg 2006). Tumor cells enter the lymphatic vasculature by invading preexisting lymphatic vessels in the tumor periphery or by eliciting lymphangiogenesis via growth factor production in regional lymph node metastasis, which represents the first step of tumor dissemination for a variety of human common cancers (Sundar and Ganesan 2007; Tammela and Alitalo 2010). Studies over the past decade have revealed a signal-transduction system for lymphatic endothelial cell proliferation, migration, and survival. This system consists of vascular endothelial growth factors (VEGF)–C and –D and their receptor vascular endothelial growth factor receptor (VEGFR)–3 (Shibuya et al. 2006; Sundar and Ganesan 2007). Recently, a natural endogenous soluble form of VEGFR-2 (esVEGFR-2) has been described in mouse and human (Albuquerque et al. 2009). esVEGFR-2, the product of alternative mRNA splicing, is composed of only six of the seven extracellular immunoglobulin-like domains, and it is distinguished from the soluble VEGFR-2 (sVEGFR-2), which is produced by proteolytic shedding from the cell surface and contains the seven extracellular immunoglobulin-like domains (Pavlakovic et al. 2010; Shibata et al. 2010). Both esVEGFR-2 and sVEGFR-2 can inhibit developmental and reparative lymphangiogenesis by trapping VEGF-C at a circulating level, indicating that modulation of esVEGFR-2/sVEGFR-2 might have therapeutic effects in the treatment of lymphatic vascular disorders, including tumor lymphangiogenesis (Albuquerque et al. 2009; Shibata et al. 2010). Furthermore, circulating esVEGFR-2/sVEGFR-2 and their correlation with clinicopathological features have been described in several cancers, including gastric cancer, cervical cancer, and neuroblastoma (Kuemmel et al. 2009; Becker et al. 2010; Kikuchi et al. 2011). However, the expression and clinical significance of esVEGFR-2 in ESCCs have not been elucidated.
In this study, quantitative RT-PCR (qRT-PCR) and immunohistochemical staining were performed to determine the expression of esVEGFR-2 in ESCC tissues. Furthermore, the clinical significance of esVEGFR-2 was also explored.
Materials and Methods
Patients and Clinical Samples
The primary tumor tissues from patients diagnosed with ESCC at Shantou Central Hospital were collected before treatment/chemotherapy. Informed consent was obtained from all subjects. The tissues used for qRT-PCR were collected between December 2007 and October 2010, and the paraffin-embedded specimens used for immunohistochemistry (IHC) were collected between February 2000 and December 2006. The clinical information of all patients is listed in Table 1. Staging was defined according to the seventh edition of the TNM Classification of Malignant Tumors of the International Union against Cancer (UICC). This project was approved by the Ethics Committee of Shantou Central Hospital. All work was done in accordance with the Ethics Declaration of Shantou Central Hospital.
Table 1.
Patient Information for Samples Used in Quantitative RT-PCR (qRT-PCR) and Immunohistochemistry (IHC) Assays
| Parameters | qRT-PCR (90 Pairs) | IHC (182 Cases) |
||
|---|---|---|---|---|
| Cases | Survival Rate of 5 Years, % | p Valuea | ||
| Age, y | ||||
| <58 | 49 | 85 | 52.1 | 0.088 |
| ≥58 | 41 | 97 | 37.5 | |
| Sex | ||||
| Male | 67 | 135 | 46.3 | 0.629 |
| Female | 23 | 47 | 37.0 | |
| Grade of differentiation | ||||
| Well | 22 | 54 | 50.3 | 0.570 |
| Moderate | 56 | 107 | 45.3 | |
| Poor | 12 | 21 | 27.3 | |
| Depth of tumor invasion | ||||
| T1 and T2 | — | 10 | 47.3 | 0.661 |
| T3 | 24 | 171 | 43.8 | |
| T4 | 66 | 1 | — | |
| Lymph node metastases | ||||
| N0 | 56 | 99 | 60.8 | 0.00002 |
| N1 | 23 | 67 | 24.8 | |
| N2 and N3 | 11 | 16 | 0.00 | |
| pTNM stages | ||||
| I and IIa | 31 | 42 | 60.7 | 0.001a |
| IIb | 30 | 62 | 56.8 | |
| III | 29 | 78 | 22.2 | |
The long dashes in the table indicate that no statistics were computed because only one case was involved.
Log-rank test of Kaplan-Meier method; p<0.05 was considered significant.
qRT-PCR
Total RNA was isolated from 90 paired primary tissues by TRIzol Reagent (Invitrogen; Carlsbad, CA), and cDNA was synthesized according to the Reverse Transcription System instructions (Promega; Madison, WI). Amplification primers were as follows:
esVEGFR-2: 5′-GCCTTGCTCAAGACAGGAA G-3′ (forward) and 5′-CAACTGCCTCTGCAC AATGA-3′ (reverse)
VEGF-C: 5′-TGAACACCAGCACGAGCTAC-3′ (forward) and 5′-GCCTTGAGAGAGAGGCACT G-3′ (reverse)
β-actin: 5′-CAACTGGGACGACATGGAGAAA-3′ (forward) and 5′-GATAGCAACGTACATGGCT GGG-3′ (reverse)
All primers were designed to produce fragments that spanned exon-intron boundaries and excluded amplification of genomic DNA. The primers were also designed to detect all known splice variants of the respective gene of interest. For esVEGFR-2, the reverse primer recognized the intron 13 motif, which was specific for the truncated transcript variant of this secreted form of VEGFR-2 (Albuquerque et al. 2009). For the normalization of the results, β-actin was used as the internal reference. qRT-PCR was performed with a Rotor-Gene 6000 (Corbett Life Science; Sydney, Australia), using SYBR PreMix Ex Taq (TaKaRa, Otsu, Japan). The amplification protocol was as follows: 30 sec preincubation at 95C followed by 45 cycles of 10 sec at 95C, 5 sec at 58C, and 15 sec at 72C. The ΔΔCt method was employed to analyze the data. Ct values were calculated following the manufacturer’s instructions (Corbett Life Science).
Immunohistochemical Staining for esVEGFR-2
A total of 182 formalin-fixed, paraffin-embedded ESCC specimens were placed in a tissue microarray, the protocol of which was described in our previous article (Zhang et al. 2008). An antibody against human esVEGFR-2 was produced by Capital Bio-sciences (Beijing, China), as reported by Albuquerque et al. (2009). To ensure the specificity of the primary antibody (Suppl. Fig. S1), we replaced it with PBS, preimmune rabbit serum, and blocking peptide as a negative control, as described in the protocol by Albuquerque et al. In addition, normal skin tissue was examined as a positive control. After deparaffinization and rehydration, all sections were incubated in 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity. Antigen retrieval was performed by microwave oven heating (10 min) in 0.01 M sodium citrate buffer (pH 6.0). Sections were incubated with 10% normal goat serum in PBS for 15 min at room temperature to block nonspecific binding. Then, all sections were incubated at 4C overnight with rabbit polyclonal antibody against esVEGFR-2 (1:50 dilution). Next, slide immunostaining was detected by the application of the SuperPic Ture Polymer Detection kit and Liquid DAB Substrate kit (Invitrogen), counterstained with hematoxylin, and then dehydrated and mounted.
Evaluation of Immunohistochemical Staining
Evaluation of immunohistochemical staining was described in detail in our previous article (Cui et al. 2008). Positive reactions were defined as those showing brown signals in the cell cytoplasm. Each separate tissue core was scored on the basis of the intensity and area of the positive staining. The intensity of positive staining was scored as follows: 0, negative; 1, weak staining; 2, moderate staining; and 3, strong staining. The rate of positive cells was scored on a 0 to 4 scale: 0, 0–5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; and 4, >75%. If the positive staining was homogeneous, a final score was achieved by multiplication of the two scores above, producing a total range of 0 to 12. When the staining was heterogeneous, we scored it as follows: each component scored independently and summed for the results. For example, a specimen containing 25% tumor cells with moderate intensity (1 × 2 = 2), 25% tumor cells with weak intensity (1× 1 = 1), and 50% tumor cells without immunoreactivity received a final score of 2 + 1 + 0 = 3. Strong immunoreactivity of esVEGFR-2 in squamous epithelial cells of mouse and human was observed by an immunohistochemical method in a previous study (Albuquerque et al. 2009). In our study, predominant expression of esVEGFR-2 protein was also observed in adjacent normal esophageal squamous epithelial cells. The scores of adjacent normal esophageal epithelial tissues were 9 to 12. For statistical analysis, we divided all the samples of ESCC into two groups according to positive expression as follows: scores of 0 to 8 as low expression and scores of 9 to 12 as high expression.
Statistical Methods
Statistical analyses were performed using the statistical package SPSS version 13.0 (SPSS, Inc, an IBM Company, Chicago, IL). Using the Chi-square test, we compared high expression with low expression of esVEGFR-2 and VEGF-C mRNA in ESCC samples on the basis of clinicopathological factors, including age at diagnosis, sex, histologic type, pathological stage, and pTNM. When we obtained expected numbers of less than 5, Fisher’s exact test was applied. Survival analysis of ESCC patients was performed using the log-rank test for comparison of low-expression with high-expression esVEGFR-2 immunohistochemical staining. Kaplan-Meier curves for the two groups were plotted based on overall survival. We accounted for clinical factors by univariate analysis and Cox proportional hazards models; p values of less than 0.05 were considered significant.
Results
RNA Levels of esVEGFR-2 and VEGF-C in ESCCs
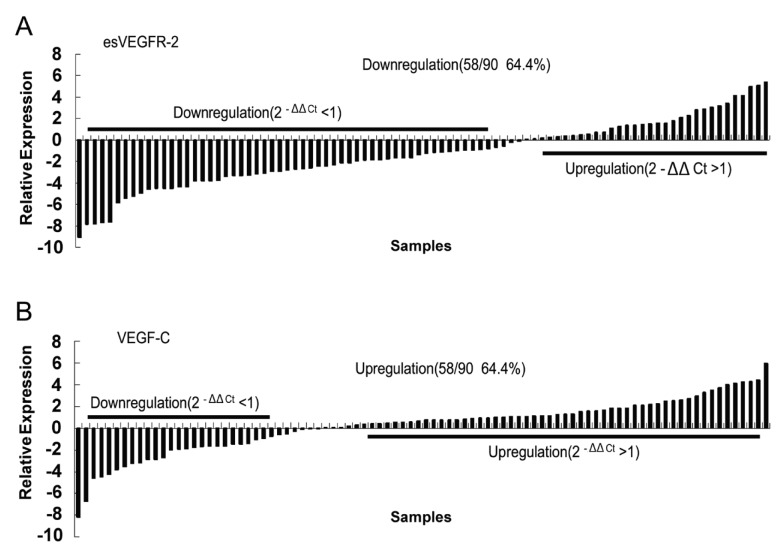
Levels of esVEGFR-2 and VEGF-C mRNA were addressed by qRT-PCR. Results showed that esVEGFR-2 mRNA levels in 64.4% of ESCC tissues (58/90) were significantly lower (2–ΔΔCt <1) than in corresponding non-neoplastic esophageal mucosa tissues (Fig. 1A). Furthermore, we observed that esVEGFR-2 mRNA downregulation was correlated with the pTNM stages of ESCC patients. In stage I/IIa, 45.4% cases (14/31) displayed downregulation (2−ΔΔCt <1), whereas 76.7% (23/30) of stage IIb and 72.4% (21/29) of stage III cases were downregulated (χ2 = 7.790, p=0.020; Table 2). In contrast, the level of VEGF-C mRNA was obviously increased in 64.4% (58/90) of cases (Fig. 2B). No significant differences between VEGF-C mRNA level and ESCC clinicopathological features were found (Table 2).
Figure 1.
Endogenous soluble vascular endothelial growth factor receptor-2 (esVEGFR-2) and vascular endothelial growth factor C (VEGF-C) mRNA relative expression in 90 patients with esophageal squamous cell carcinoma (ESCC) as measured by quantitative RT-PCR. β-Actin was applied as an internal reference. ΔΔCt method and Chi-square test were employed to analyze the results. Each bar is the log2−ΔΔCt value of esVEGFR-2 or VEGFC expression level. (A) Statistically significant downregulation of esVEGFR-2. (B) Upregulation of VEGF-C. Bar indicates relative expression level.
Table 2.
Correlations between esVEGFR2 and VEGF-C mRNA Levels and Clinicopathological Features in 90 ESCC Cases
| Clinical Parameter | esVEGFR-2 Expression, No. (%) |
VEGF-C Expression, No. (%) |
|||||
|---|---|---|---|---|---|---|---|
| 2−ΔΔCt <1 | 2−ΔΔCt >1 | p Valuea | 2−ΔΔCt <1 | 2−ΔΔCt >1 | p Valuea | ||
| Total | 58 (64.4) | 32 (35.6) | 32 (35.6) | 58 (64.4) | |||
| Age, y | <58 | 32 (65.3) | 17 (34.7) | 0.852 | 17 (34.7) | 32 (65.3) | 0.852 |
| ≥58 | 26 (63.4) | 15 (36.6) | 15 (36.6) | 26 (63.4) | |||
| Sex | Female | 15 (65.2) | 8 (34.8) | 0.928 | 9 (39.1) | 14 (60.9) | 0.678 |
| Male | 43 (64.2) | 24 (35.8) | 23 (34.3) | 44 (65.7) | |||
| Grade of differentiation | Well | 13 (59.1) | 9 (40.9) | 0.833 | 8 (36.4) | 14 (63.6) | 0.711 |
| Moderate | 37 (66.1) | 19 (33.9) | 21 (37.5) | 35 (62.5) | |||
| Poor | 8 (66.7) | 4 (33.3) | 3 (25.0) | 9 (75.0) | |||
| Depth of tumor invasion | T1 and T2 | 17 (70.8) | 7 (29.2) | 0.445 | 11 (45.8) | 13 (54.2) | 0.219 |
| T3 | 41 (62.1) | 25 (37.9) | 21 (31.8) | 45 (68.2) | |||
| Lymph node metastases | N0 | 34 (60.7) | 22 (39.3) | 0.627 | 18 (32.1) | 38 (67.6) | 0.364 |
| N1 | 16 (69.6) | 7 (30.4) | 8 (34.8) | 15 (58.2) | |||
| N2 + N3 | 8 (72.7) | 3 (27.3) | 6 (54.5) | 5 (45.5) | |||
| pTNM stages | I + IIa | 14 (45.4) | 17 (54.6) | 0.020a | 9 (29.0) | 22 (71.0) | 0.600 |
| IIb | 23 (76.7) | 7 (23.3) | 11 (36.7) | 19 (63.3) | |||
| III | 21 (72.4) | 8 (27.6) | 12 (41.4) | 17 (58.6) | |||
ESCC, esophageal squamous cell carcinoma; esVEGFR-2, endogenous soluble vascular endothelial growth factor receptor-2; VEGF-C, vascular endothelial growth factor C.
Pearson’s Chi-square test; p<0.05 was considered significant.
Figure 2.
Immunohistochemical staining of endogenous soluble vascular endothelial growth factor receptor-2. Normal mucous membrane of esophagus (A), intense expression (B, score: 12), moderate expression in (C, score: 8), and mild expression in esophageal squamous cell carcinoma (D, score: 4). Scale bars: A, 100 µm; B–D, 50 µm.
Protein Expression of esVEGFR-2 and Its Effect on Overall Survival
Immunohistochemical staining showed that esVEGFR-2 was distributed in the cytoplasm of cells. In normal esophagus, esVEGFR-2 protein was detected in epithelial cells from basal to superficial layers, and stronger immunoreactivity was observed in prickle and basal cell layers (Fig. 2A). In ESCCs, diffuse cytoplasmic staining to different degrees was observed (Fig. 2B –D). In total, 65.9% (120/182) of ESCC cases showed esVEGFR-2 low expression (scores of 0–8), and 34.1% (62/182) exhibited high expression (scores of 9–12; Table 3), indicating that esVEGFR-2 protein level was inclined to be reduced. The correlations between esVEGFR-2 protein level and clinicopathological parameters were also analyzed. A significant positive association between esVEGFR-2 protein level in the cytoplasm and lymph node metastases was identified (χ2 = 7.325, p=0.026; Table 3).
Table 3.
Association between esVEGFR-2 Protein Staining and Clinicopathological Parameters in 182 ESCC Cases
| Clinical Parameter | esVEGFR-2 Protein Status, No. (%) |
p Valuea | ||
|---|---|---|---|---|
| Low (0–8) | High (9–12) | |||
| Total | 120 (65.9) | 62 (34.1) | ||
| Age, y | <58 | 54 (63.5) | 31 (36.5) | 0.522 |
| ≥58 | 66 (68.0) | 31 (32.0) | ||
| Sex | Female | 32 (68.1) | 15 (31.9) | 0.718 |
| Male | 88 (65.2) | 47 (34.8) | ||
| Grade of differentiation | Well | 35 (64.8) | 19 (35.2) | 0.978 |
| Moderate | 71 (66.4) | 36 (33.6) | ||
| Poor | 14 (66.7) | 7 (33.3) | ||
| Depth of tumor invasion | T1 and T2 | 6 (60.0) | 4 (40.0) | 0.737 |
| T3 and T4 | 114 (66.3) | 58 (33.7) | ||
| Lymph node metastases | N0 | 71 (71.7) | 28 (28.3) | 0.026 |
| N1 | 43 (64.2) | 24 (35.8) | ||
| N2 and N3 | 6 (37.5) | 10 (62.5) | ||
| pTNM stage | I and IIa | 27 (64.3) | 15 (35.7) | 0.379 |
| IIb | 45 (72.6) | 17 (27.4) | ||
| III | 48 (61.5) | 30 (38.5) | ||
ESCC, esophageal squamous cell carcinoma; esVEGFR-2, endogenous soluble vascular endothelial growth factor receptor-2.
Pearson’s Chi-square test; p<0.05 was considered significant.
Kaplan-Meier survival analysis was used to address the impact of esVEGFR-2 expression on the overall survival of ESCC patients. Results showed that high esVEGFR-2 expression was associated with decreased overall survival (χ2 = 6.366, p=0.012; Fig. 3).
Figure 3.

Kaplan-Meier survival curves for endogenous soluble vascular endothelial growth factor receptor-2 (esVEGFR-2) in patients with esophageal squamous cell carcinoma (ESCC). Patients were divided into two subgroups: low expression (bold line, score <9) and high expression (dotted line, score ≥9). Kaplan-Meier analysis of esVEGFR-2 expression in 182 patients with ESCC illustrated that survival was lower in 62 cases where esVEGFR-2 expression was high, as compared with 120 cases where esVEGFR-2 expression was low.
Next, we included esVEGFR-2 expression in further prognostic value analysis. In the univariate analysis, esVEGFR-2 protein accumulation in the cytoplasm was a significant prognostic indicator for ESCC patients (F = 4.955, p=0.027; Table 4). Multivariate analysis showed that high esVEGFR-2 expression (hazard ratio, 1.606; 95% confidence interval [CI], 1.042–2.476; p=0.032), as well as lymph node metastasis (hazard ratio, 2.651; 95% CI, 1.701–4.132; p<0.001), was a significant independent prognostic factor for poor overall survival of ESCC patients (Table 4).
Table 4.
Univariate and Multivariate Cox Regression Analysis of esVEGFR-2 Protein Status
| esVEGFR-2 Status | Mean Survival Time, mo | % of 5-Year Survival (95% CI) | p Value a |
|---|---|---|---|
| Univariate | |||
| Low (scores of 0–8) | 70.1 | 51.2 (1.3–112.8) | 0.012 |
| High (scores of 9–12) | 48.9 | 30.2 (1.6–106.3) | |
|
|
|||
| esVEGFR-2 Status | Hazard Ratio | 95% CI | p Valuea |
|
| |||
| Multivariate | |||
| Lymph node metastases (N1) | 2.651 | 1.701–4.132 | 0.000 |
| esVEGFR-2 expression (high) | 1.606 | 1.042–2.476 | 0.032 |
CI, confidence interval; esVEGFR-2, endogenous soluble vascular endothelial growth factor receptor-2.
p<0.05 was considered significant; Cox proportional hazards model.
Discussion
To date, although there have been prior studies of esVEGFR-2 in diverse cancers, these studies have not been wholly focused on the relationship between esVEGFR-2 expression and prognosis of cancer patients. In the current study, we explored the expression pattern of esVEGFR-2 in ESCC tissue samples and showed that esVEGFR-2 was downregulated in ESCC tissues, and also showed that high expression of esVEGFR-2 protein in ESCC tissues was a significant independent prognostic factor for poor survival of patients.
esVEGFR-2, a new splicing variant of VEGFR-2, could inhibit developmental and reparative lymphangiogenesis by blocking VEGF-C function. Here, we revealed that the mRNA level of esVEGFR-2 was decreased in ESCCs, whereas the mRNA level of VEGF-C was increased. Moreover, a positive correlation was found in the subsequent analysis between downregulated esVEGFR-2 mRNA expression and the pTNM stages of ESCC patients, which was characterized by lymphatic metastasis in progressed stages. This result was in accordance with the results of a previous study in neuroblastoma (Becker et al. 2010). It was reported that variable promoter hypermethylation of VEGFR-2, from which mRNA alternative splicing generates the esVEGFR-2 isoform (Albuquerque et al. 2009), influenced its expression in stomach cancer, colon cancer, and hepatocellular carcinoma (Kim et al. 2009). We presumed that in ESCC, methylated VEGFR-2/KDR might lead to the downregulation of esVEGFR-2 mRNA expression.
As a lymphangiogenesis inhibitor, higher esVEGFR-2 protein should inhibit tumor lymphangiogenesis and adversely affect tumor metastasis via lymphatic vessels, resulting in better survival of patients with ESCC. However, it was both interesting and puzzling that our immunohistochemical data for esVEGFR-2 protein expression contradicted this presumption. There was a positive correlation between lymph node metastasis and esVEGFR-2 protein expression detected by the immunohistochemical method. Furthermore, we also found that esVEGFR-2 protein level was inclined to be reduced, and that a lower esVEGFR-2 protein level in the cytoplasm was significantly related with a better prognosis. Multivariate analysis revealed that high esVEGFR-2 protein expression was a significant independent prognostic factor for poor cancer-specific survival of patients with ESCC. These data indicated that esVEGFR-2 might have some new functions in the progression of ESCC. The data from Lorquet et al. (2010) supported that, in addition to trapping VEGF at a circulating level, sVEGFRs could form a signaling-inactive membrane-associated complex consisting of sVEGFR/VEGF/VEGFR-2 that prevents VEGF-driven angiogenesis and/or lymphangiogenesis, and that can also bind endothelial cells through interaction with a coreceptor, which could subsequently generate a trans-signaling to induce the migration of neighbor cells. Therefore, we hypothesized that, at the molecular level, a secretary dysfunction of mature esVEGFR-2 in cancer cells and/or endocytosis of mature esVEGFR-2 in the microenvironment by cancer cells could accompany the downregulation of esVEGFR-2 expression in the cells and aggravate a reduction of esVEGFR-2 in blood circulation, resulting in more free VEGF-C to activate the pathways responsible for lymphatic vessel growth. The accumulation of esVEGFR-2 in cancer cells could subsequently induce their migration and invasion by activating some signaling pathways. Further studies are under way.
VEGF-C can specifically bind to VEGFR-3 and induce tyrosine autophosphorylation of VEGFR-3, indicating its role in lymphangiogenesis (Joukov et al. 1996). VEGF-C binding to VEGFR-2 is also involved in hemangiogenesis, but its affinity is about an order of magnitude weaker than that of VEGF-A for VEGFR-2 (Kiec-Wilk et al. 2009). Furthermore, the VEGF-C/VEGFR-3 pathway can be blocked by esVEGFR-2 (Albuquerque et al. 2009). Thus, we detected VEGF-C expression in ESCC patients. This study showed that VEGF-C mRNA expression in ESCC lesions was upregulated, but we did not observe any statistically significant differences between VEGF-C mRNA level and clinicopathological features of ESCC patients. Therefore, the clinical significance of VEGF-C and the relationship between VEGF-C and esVEGFR-2 needs further explanation in ESCC patients.
In conclusion, we showed that expression of esVEGFR-2 was significantly downregulated at the mRNA and protein level in ESCC lesions in comparison with non-neoplastic esophageal mucosa. The altered expression of esVEGFR-2 was correlated with tumor progression and shorter survival of ESCC patients. To conclude, esVEGFR-2 could be used as a new independent prognostic marker for ESCC patients.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (No. 81071737), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20104402110003), the National High Technology Research and Development Program of China (No. 2012AA02A403), and the Natural Science Foundation of China-Guangdong Joint Fund (No. U0932001).
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
References
- Albuquerque RJ, Hayashi T, Cho WG, Kleinman ME, Dridi S, Takeda A, Baffi JZ, Yamada K, Kaneko H, Green MG, et al. 2009. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat Med. 15:1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Pavlakovic H, Ludewig F, Wilting F, Weich HA, Albuquerque R, Ambati J, Wilting J. 2010. Neuroblastoma progression correlates with downregulation of the lymphangiogenesis inhibitor sVEGFR-2. Clin Cancer Res. 16:1431–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Xu LY, Shen ZY, Tao Q, Gao SY, Lv Z, Du ZP, Fang WK, Li EM. 2008. NGALR is overexpressed and regulated by hypomethylation in esophageal squamous cell carcinoma. Clin Cancer Res. 14:7674–7681 [DOI] [PubMed] [Google Scholar]
- Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. 1996. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 15:290–298 [PMC free article] [PubMed] [Google Scholar]
- Kiec-Wilk B, Razny U, Mathers JC, Dembinska-Kiec A. 2009. DNA methylation, induced by beta-carotene and arachidonic acid, plays a regulatory role in the pro-angiogenic VEGF-receptor (KDR) gene expression in endothelial cells. J Physiol Pharmacol. 60:49–53 [PubMed] [Google Scholar]
- Kikuchi S, Obata Y, Yagyu K, Lin Y, Nakajima T, Kobayashi O, Kikuichi M, Ushijima R, Kurosawa M, Ueda J. 2011. Reduced serum vascular endothelial growth factor receptor-2 (sVEGFR-2) and sVEGFR-1 levels in gastric cancer patients. Cancer Sci. 102:866–869 [DOI] [PubMed] [Google Scholar]
- Kim JY, Hwang JH, Zhou W, Shin J, Noh SM, Song IS, Kim JY, Lee SH, Kim J. 2009. The expression of VEGF receptor genes is concurrently influenced by epigenetic gene silencing of the genes and VEGF activation. Epigenetics. 4:313–321 [PubMed] [Google Scholar]
- Kuemmel S, Thomas A, Landt S, Fuger A, Schmid P, Kriner M, Blohmer JU, Sehouli J, Schaller G, Lichtenegger W, et al. 2009. Circulating vascular endothelial growth factors and their soluble receptors in pre-invasive, invasive and recurrent cervical cancer. Anticancer Res. 29:641–645 [PubMed] [Google Scholar]
- Lorquet S, Berndt S, Blacher S, Gengoux E, Peulen O, Maquoi E, Noël A, Foidart JM, Munaut C, Péqueux C. 2010. Soluble forms of VEGF receptor-1 and -2 promote vascular maturation via mural cell recruitment. FASEB J. 24:3782–3795 [DOI] [PubMed] [Google Scholar]
- Matsushima K, Isomoto H, Kohno S, Nakao K. 2010. MicroRNAs and esophageal squamouscell carcinoma. Digestion. 82:138–144 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. 2005. Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108 [DOI] [PubMed] [Google Scholar]
- Pavlakovic H, Becker J, Albuquerque R, Wilting J, Ambati J. 2010. Soluble VEGFR-2: an antilymphangiogenic variant of VEGF receptors. Ann N Y Acad Sci. 1207:E7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata MA, Ambati J, Shibata E, Albuquerque RJ, Morimoto J, Ito Y, Otsuki Y. 2010. The endogenous soluble VEGF Receptor-2 isoform suppresses lymph node metastasis in a mouse immunocompetent mammary cancer model. BMC Med. 8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M, Claesson-Welsh L. 2006. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 312:549–560 [DOI] [PubMed] [Google Scholar]
- Steeg PS. 2006. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 12:895–904 [DOI] [PubMed] [Google Scholar]
- Sundar SS, Ganesan TS. 2007. Role of lymphangiogenesis in cancer. J Clin Oncol. 25:4298–4307 [DOI] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. 2010. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 140:460–476 [DOI] [PubMed] [Google Scholar]
- Zhang FR, Tao LH, Shen ZY, Lv Z, Xu LY, Li EM. 2008. Fascin expression in human embryonic, fetal, and normal adult tissue. J Histochem Cytochem. 56:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.