Abstract
The galectin-4 protein is mostly expressed in the digestive tract and is associated with lipid raft stabilization, protein apical trafficking, wound healing, and inflammation. While most mammalian species, including humans, have a single Lgals4 gene, some mice have two paralogues: Lgals4 and Lgals6. So far, their significant similarities have hindered the analysis of their respective expression and function. We took advantage of two antibodies that discriminate between the galectin-4 and galectin-6 proteins to document their patterns of expression in the normal and the dextran sodium sulfate (DSS)-damaged digestive tract in the mouse. In the normal digestive tract, their pattern of expression from tongue to colon is quite similar, which suggests functional redundancy. However, the presence of galectin-4, but not galectin-6, in the lamina propria of the DSS-damaged colon, its association with luminal colonic bacteria, and differences in subcellular localization of these proteins suggest that they also have distinct roles in the normal and the damaged mouse digestive tract. Our results provide a rare example of ancestral and derived functions evolving after tandem gene duplication.
Keywords: digestive tract, experimental colitis, galectin, lectin, mice, paralogues
The term lectin commonly refers to a range of carbohydrate-binding proteins that have been implicated in a large variety of biological functions. Galectins are soluble lectins characterized by a conserved carbohydrate recognition domain (CRD) and, for most of its members, an affinity for β-galactosides in vitro (Barondes et al. 1994; Liu and Rabinovich 2010). Galectins have been identified, and their function investigated, in a number of phyla including fungi (Butschi et al. 2010), cnidarians (Hwang et al. 2010), ecdysozoans [insects (Kamhawi et al. 2004), nematodes (Ideo et al. 2009)], lophotrochozoans [Molluscs (Tasumi and Vasta 2007)], and deuterostomes [cephalochordates (Yu et al. 2007) and urochordates (Vizzini et al. 2012)], where they appear to be consistently associated with innate immunity and host/pathogen interplay. In vertebrates, the galectin family diversified through a series of duplications from an ancestral bi-CRD galectin. Ten to fifteen galectin-encoding genes have been identified in most mammalian species, where they participate in a large variety of biological processes. As gene duplications occurred throughout the whole vertebrate history, some galectin-encoding genes, such as Lgals4, 8, 9, 12 or Lgals3 can be tracked back to the origins of vertebrates, whereas others are present in only a subset of the species. For instance, Lgals2 is restricted to amniotes, Lgals7 to mammals, Lgals5 to rats, and Lgals6 to mice (Houzelstein et al. 2004).
The Lgals6 gene comes from a tandem duplication of Lgals4 in the mouse genome (Gitt, Colnot, et al. 1998; Gitt, Xia, et al. 1998; Houzelstein et al. 2008). Therefore, whereas most mammalian species, including humans, have a single Lgals4 gene, only mice have two paralogues. Like every tandem-repeat galectin in vertebrates, galectin-4 and -6 contain two CRDs joined by a linker region (Gitt, Xia, et al. 1998; Houzelstein et al. 2004). In addition, as they have as much as 83% identity at the amino-acid level (Gitt, Colnot, et al. 1998), these two proteins are likely to be, at least partially, functionally redundant. The Lgals6 gene evolution, however, has been affected by an episode of positive selection that prompted its divergence from Lgals4 and contributed to the accumulation of differences in the galectin-6 linker and its flanking regions as well as in its C-terminal CRD (Houzelstein et al. 2008). Galectin-6 might, therefore, have also developed a number of new properties (neofunctionalization). Positive selection facilitates the fixation of alleles under selection. The Lgals4-Lgals6 locus, however, is still polymorphic, both in wild-type populations and in laboratory mouse strains. For instance, whereas some mice, such as the 129/Sv laboratory strain, carry the Lgals4-Lgals6 gene duplication, others, such as the C57BL/6J laboratory strain, carry only the unduplicated Lgals4 gene (Houzelstein et al. 2008).
Nothing is known regarding the role galectin-6 might play, whereas galectin-4 (initially referred to as L36; Leffler et al. 1989; Oda et al. 1993) has been investigated in a number of studies. Galectin-4 is expressed almost exclusively in the digestive tract. It was initially identified as an adherens junction protein expressed in the tongue epithelium of the pig (Chiu et al. 1992; Chiu et al. 1994). It has also been shown to be a major component of lipid rafts in brush border membranes of the pig small intestine epithelial cells (reviewed in Danielsen and Hansen 2008). In cultures of human enterocyte-like HT-29 cells, galectin-4 binds to and recruits the apical glycoproteins in detergent-resistant membranes (Delacour et al. 2005; Morelle et al. 2009; Stechly et al. 2009). In cell cultures, galectin-4 is secreted both apically and, to a lesser extent, basolaterally (Stechly et al. 2009).
Galectin-4 has been associated with a number of disorders. Its expression is altered in several gastrointestinal cancers (Rechreche et al. 1997; Hippo et al. 2001; Nagy et al. 2003; Huflejt and Leffler 2004; van Baal et al. 2005; Rumilla et al. 2006; Duerr et al. 2008; Balan et al. 2010). Some authors suggested that it not only may have the properties of a tumor progression marker (Watanabe et al. 2011) but also may function as a tumor suppressor in human colorectal cancer (Satelli et al. 2011). Several studies have also implicated galectin-4 in the inflammatory response, though with conflicting conclusions. Some concluded that galectin-4 stimulates T-cells to produce interleukin-6 and contributes to the development of inflammatory bowel disease (Hokama et al. 2004). Others concluded that galectin-4 induces apoptosis of mucosal T-cells and promotes resolution of the inflammatory response (Paclik, Danese, et al. 2008; Paclik et al. 2011). The reason for these discrepancies is still unknown (reviewed in Liu and Rabinovich 2010). Galectin-4 has also been involved in intestinal epithelial wound healing (Paclik, Lohse, et al. 2008) and in the killing of human blood group antigen-expressing Escherichia coli present in the intestinal lumen (Stowell et al. 2010).
So far, the similarities between the Lgals4 and Lgals6 genes have hindered the analysis of their respective function (Gitt, Colnot, et al. 1998; Nio et al. 2005; Mathieu et al. 2008; Nio-Kobayashi et al. 2009). We have taken advantage of antibodies that discriminate between the two proteins to describe their patterns of expression in both normal and damaged mouse gastrointestinal tract.
We did this as a preliminary step to understand the reason why the Lgals4-Lgals6 locus remained polymorphic in wild mice for such an extended period of time; an intriguing question, with regard to the origin and maintenance of intraspecific genetic diversity. We also did it as a first step to document how the presence of the galectin-6 protein may alter the function of the galectin-4 protein, a question that cannot be ignored because of the key role of the mouse as a model organism in biomedical research.
Materials and Methods
The animal experiment procedures were approved by the Ethics Committee on animal experimentation, France (n° Ce5/2011/058).
Mouse Strains
The initial 129/Sv and C57BL/6J progenitors were purchased from Charles River (France). They were subsequently bred in our conventional animal facility with 50% humidity, 12:12 hr light:dark cycles, fed a standard pellet diet (SAFE, refA03), and tap water ad libitum. The experiments were performed on 7- to 9-week-old, 22- to 28-g males, unless otherwise specified.
Tissue Sampling
Mice were anesthetized by intraperitoneal co-injection of ketamine (100 mg/kg body weight) and xylazine (15 mg/kg body weight). However, because of variability between individuals within and between mouse strains, up to twice this dose had to be delivered in order to obtain a satisfactory level of anesthesia. Mice were then fixed by intracardiac perfusion of 100 ml to 120 ml 4% paraformaldehyde (Euromedex, France; 15714.S) in phosphate buffer saline (PBS) delivered for 45 min by a Minipuls peristaltic pump (Gilson, Middleton, WI), unless otherwise specified. The organs were then removed and immersed in the same fixative for an additional 18 to 24 hr at 4C. The tissues were then dehydrated in ethanol and embedded in paraffin by a conventional method.
For samples used in Figs 3a and b, mice were sacrificed by cervical dislocation, the organs dissected out immediately and fixed by immersion into cold 4% paraformaldehyde in PBS and incubated overnight at 4C.
Figure 3.
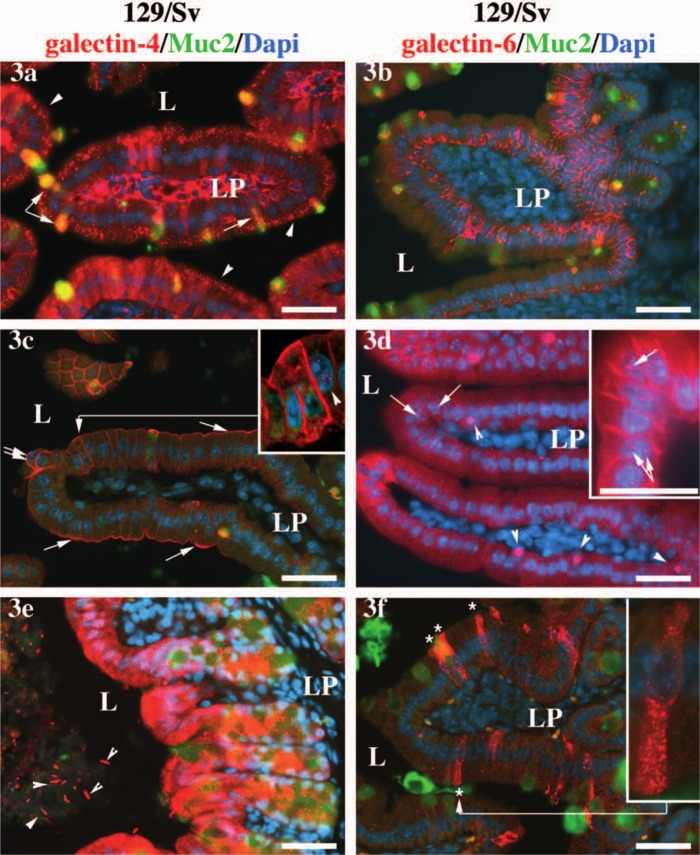
Differences in the subcellular localization of galectin-4 and galectin-6 in the 129/Sv background. Expression of galectin-4 (3a) and galectin-6 (3b) in samples of duodenum fixed by immersion into the fixative, instead of via intracardiac perfusion, as in the rest of the figures. In 3a, the arrowheads point toward endosomes in the enterocytes; the arrow points toward galectin-4 aggregates at the base of the secretion granule of a goblet cell, and the double arrow points toward galectin-4 and Muc-2 positive secretory granules also in goblet cells. In 3c, the arrows point toward an accumulation of galectin-4 protein in enterocyte apical membrane, and the double arrows point toward round shaped cells at the apex of the villus. In 3d, the arrows point toward galectin-6 positive nuclear granules in enterocytes, and the arrowheads point toward large nuclear granules in goblet cells. In 3e, the arrowheads point toward galectin-4-decorated bacteria present in the colonic lumen. In 3f, the asterisks label galectin-6-expressing enteroendocrine cells, and the inset shows an enlargement of one of such cells. The anti-galectin-4 or -6 staining is shown in red, the anti-Muc-2 in green, and the DAPI-stained nuclei is in blue. Scale bars = 40 µm. L = lumen; LP = lamina propria. The same settings and exposure times were used for all pictures. The levels were altered for the inset in 3c in order to enhance the enterocytic nuclear staining (arrowhead).
Induction of Acute Colitis in Mice
Acute colitis was induced on 22 g to 28 g, 6.5- to 8.5-week-old mice by replacing water with a 4% (w/v − 45 µ filtered) solution of dextran sulfate 40 sodium salt (VWR, A 3261) ad libitum for one to four days. The control mice received tap water ad libitum.
Double Fluorescent Labeling
The primary antibodies used in this study were: goat-anti-galectin-4 (G-20, sc-19288, Santa Cruz Biotechnology Inc., lot: C3103, dilution: 1/300); goat-anti-galectin-6: (F-16, sc-31798, Santa Cruz Biotechnology Inc., lot: A2006, dilution: 1/300); rabbit-anti-Muc2 (H-300, sc-15334, Santa Cruz Biotechnology Inc., lot: E3107, dilution: 1/100).
The secondary antibodies used in this study were: donkey-anti-rabbit-Alexa488-A21206 (Molecular Probes, Invitrogen, dilution: 1/600) and donkey-anti-goat-Alexa568-A11057 (Molecular Probes, Invitrogen, dilution: 1/600).
Sections, mounted on superfrost plus slides (05305190, Labonord, France), were deparaffinised in xylene, rehydrated through decreasing concentrations of ethanol, and rinsed twice in PBS (BD0435, Euromedex, France). Sections were unmasked (94C, 40 mins, Dako Target Retrieval Solution High pH, S3308), rinsed in PBS, and then blocked for 1 hr in 5% donkey serum (UP77719A, Interchim, France) in PBS in a humid chamber. Sections were then incubated overnight at 4C in a humid chamber in the primary antibody solutions (in PBS, 5% donkey serum). The sections were washed three times, for 5 min each time, in PBS, incubated for 1 hr at room temperature in the secondary antibody solutions (in PBS, 5% donkey serum), washed again three times in PBS, for 5 min each. Visualization of cell nuclei was achieved by incubating the sections for 10 min in a 1:4000 DAPI solution in a humid chamber and washing the sections three times in PBS for 5 min each. The sections were then mounted in Vectashield (Vector laboratories, Petersborough, England).
Western Blot
Portions of intestine were frozen and stored at -80C until processing. They were then crushed in a mortar using liquid nitrogen and homogenized in the extraction buffer (Hepes 50 mM pH7.5, NaCl 150 mM, Triton 1%, EGTA 2 mM, MgCl2 1.5 mM, glycerol 10%, antiproteases [Complete tablets, Roche, France]) on ice for 30 min and centrifuged at 15 000 g for 20 min. Supernatants were collected and a Bradford assay test used to evaluate protein concentration. Aliquots were then stored at -80C until use.
Proteins were denatured at 95C for 5 min in the loading buffer (Tris-HCl 60 mM pH6.8, SDS 2%, glycerol 10%, bromophenol blue 0.01%), separated on a 12% polyacrylamide gel, and transferred onto polyvinyl difluoride (PVDF) membranes (Millipore, France); transfer quality was assessed by Ponceau S red staining. The membrane was incubated in Tris-buffered saline (TBS) containing Tween 0.05% and 5% powered milk for 1 hr at room temperature to saturate nonspecific binding sites. Membranes were then incubated overnight at 4C in the primary antibody solution (anti-galectin-4 and anti-galectin-6, 1:200 in TBS-Tween 0.05%, powder milk 1%). The membrane was then rinsed thrice in TBS-Tween, incubated for 1 hr in HRP-conjugated anti-goat antibody solution and rinsed thrice in TBS-Tween. Staining was determined using the ECL+ kit (Amersham, Buckinghamshire, UK) according to the manufacturer’s instructions. A Fusion FX5 system (Vilber Lourmat, Marne-la Vallée, France) was used for the acquisition of the signal.
Results
Despite Their Sequence Identity, Galectin-4 and -6 Are Specifically Discriminated on Western Blots and by Immunofluorescence
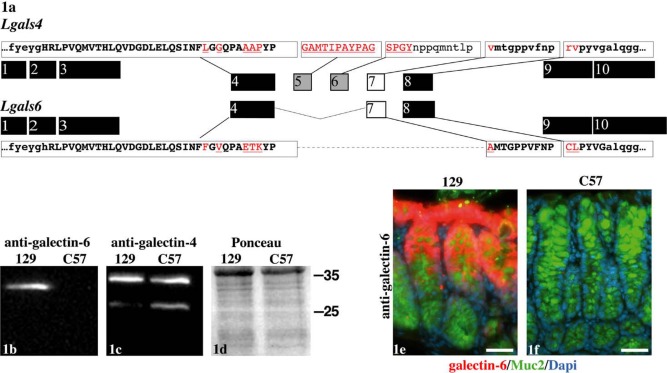
A difference between the Lgals4 and Lgals6 genes lies in the deletion of exons 5 and 6 in the Lgals6 compared to the Lgals4 gene (Fig. 1a). This in-frame deletion shortens the linker in galectin-6 without affecting the sequence of the CRDs. It interrupts the homologous region between galectin-4 and -6 and is associated with more residue substitutions in the regions flanking the deletion than in the remainder of the protein (Houzelstein et al. 2008). Santa Cruz Biotechnology Inc. (SCB; Dallas, TX) recently marketed antibodies designed to discriminate between the galectin-4 and galectin-6 proteins. The epitopes used to produce these antibodies overlap the region deleted in the galectin-6 compared to the galectin-4 protein as well as their linker N-terminal flanking region (see the epitope sequence shown in capitals in Fig. 1a). These antibodies should thus discriminate between galectin-4 and galectin-6. We first verified that these two antibodies were indeed specific on western blots and by immunofluorescence.
Figure 1.
Anti-galectin-4 and -6 specificity. Organization of the Lgals4 and Lgals6 exons in relation to the structure of the galectin-4 and galectin-6 proteins (1a). The exons encoding the carbohydrate recognition domains of galectin-4 and -6 are shown in black. The exons encoding the linker region of galectin-4 deleted in galectin-6 are shown in grey. The exon 7 that encodes the part of the galectin-4 linker region still present in galectin-6 is shown in white. The sequence of the epitopes used to produce the antibodies directed against galectin-4 and -6 are shown in capitals at the top (galectin-4) and bottom (galectin-6) of the figure. The boxes isolate the sequences from the corresponding exons. The epitope residues conserved between the galectin-4 and -6 proteins are in bold, and those not conserved are in red and underlined. Western blot on colon samples with the anti-galectin-6 (1b) and anti-galectin-4 (1c) antibodies, as well as a Ponceau S red staining to assess the quality of the transfer (1d). The mouse strain from which the samples were obtained is indicated over each lane (129 standing for 129/Sv, and C57 for C57BL/6J). The antibody or the staining procedure that has been used is indicated at the top. The position of the ladder bands is indicated on the right (in kD). By immunofluorescence, the anti-galectin-6 labels the colonic mucosa in the 129/Sv (1e) but not the C57BL/6J (1f) background. The anti-galectin-6 staining is shown in red, anti-Muc2 in green, and DAPI-stained nuclei in blue. Scale bars in 1e and 1f = 40 µm. The same settings and exposure times were used for both pictures.
We demonstrated earlier that the 129/Sv mouse strain contains the duplicated Lgals4-Lgals6 locus, whereas the C57BL/6J strain contains the unduplicated Lgals4 locus (Houzelstein et al. 2008). On western blots, the anti-galectin-6 antibody revealed a main band at about 30 kD in colon samples from the 129/Sv strain but not from the C57BL/6J strain (Fig. 1b), which demonstrates the specificity of this antibody. The anti-galectin-4 antibody revealed two bands in the colon samples from both the 129/Sv and C57BL/6J (Fig. 1c): a main band at about 35 kD (comparable to the 36 kD described by Gitt, Colnot, et al. 1998) and a fainter 28 kD band. These bands were clearly different in size from the 30 kD band revealed with the anti-galectin-6 antibody and were present in samples from the C57BL/6J strain that do not contain the Lgals6 gene (Fig. 1c). The presence of bands shorter than the full-length 36 kD have been described previously in tissue samples from mice and pigs and proposed to be proteolytic degradation products (Gitt, Colnot, et al. 1998; Wooters, Ropp, et al. 2005). Our results thus demonstrate the specificity of the anti-galectin-4 antibody in western blots.
By immunofluorescence, the anti-galectin-6 antibody stained the gastrointestinal mucosa from 129/Sv mice (Fig. 1e), but not that from C57BL/6J mice (Fig. 1f), a result that demonstrates the specificity of the galectin-6 antibody. In contrast, the anti-galectin-4 antibody stained the gastrointestinal mucosa from both 129/Sv and C57BL/6J mice (Figs 2d–r). The fact that the anti-galectin-4 antibody did not mark structures that were recognized by the anti-galectin-6 antibody such as the core of the filiform papillae in the tongue (compare Figs 2a, c), or the nucleus in the goblet cells (see arrowheads in Fig. 3d), shows that this antibody does not cross-react with the galectin-6 protein (see also the negative control without the primary antibody in Suppl. Fig. S3).
Figure 2.
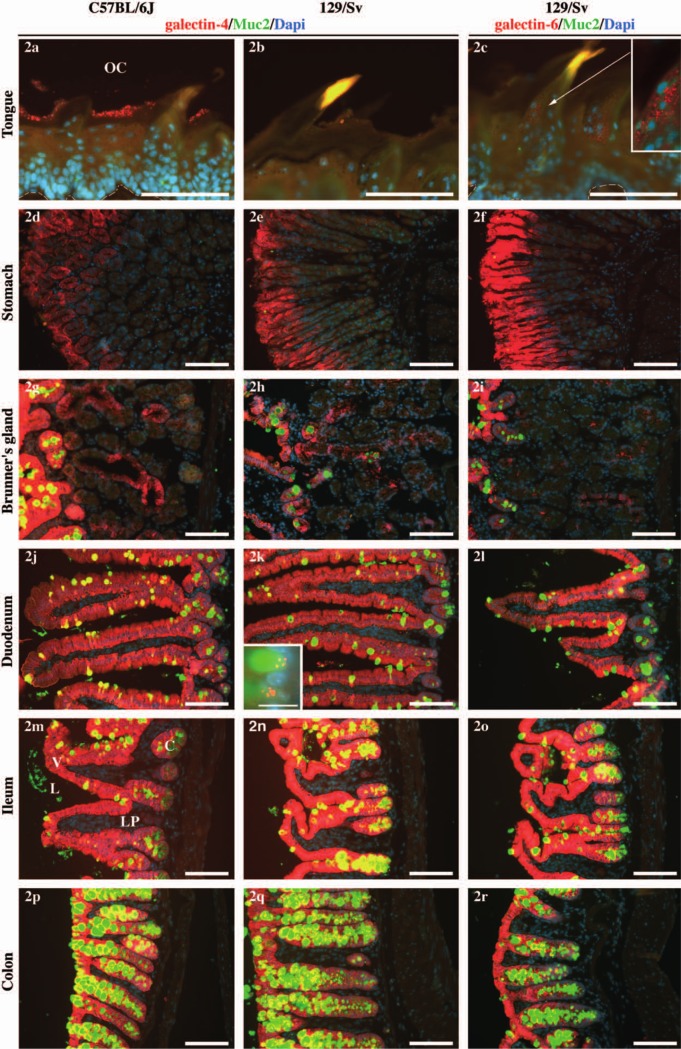
Expression of galectin-4 and -6 along the digestive tract. Expression of galectin-4 (two left columns) or galectin-6 (right column) in the C57BL/6J (left column) or 129/Sv (two right columns) background along the digestive axis (tongue, stomach, Brünner’s gland, duodenum, ileum, colon). In 2a and 2c, the white dashed line marks the limit between the lamina propria and the lingual epithelium. In 2d through 2r, the lumen is on the left. The anti-galectin-4 or -6 staining is shown in red, anti-Muc2, which specifically stains mucus in the goblet cells, in green, and the DAPI-stained nuclei in blue. The inset in 2c is an enlargement of the filiform papilla for which the contrast has been enhanced, as indicated by the arrow. The inset in 2k represents an enlargement of two other mucus vesicles from a different section of the same sample. The scale bars = 100 µm in all the pictures, except for the inset in 2k (scale bar = 15 µm). The same settings and exposure times were used for all pictures. The levels were altered for the inset in 2k in order to enhance the contrast. OC = oral cavity, L = lumen, V = villus, C = crypt, LP = lamina propria. Note that the colonic epithelium is recognized by its absence of villi.
Since both antibodies appeared specific, we investigated the patterns of expression of galectin-4 and galectin-6 along the gastrointestinal tract, from the tongue to the distal colon.
Galectin-4 and Galectin-6 Have Very Similar Patterns of Expression in the Digestive Tract
We first evaluated the influence of sex, age, and parental transmission on the galectin-4 and -6 pattern of expression. We could not detect any difference between males and females, which indicates that the galectin-4 and -6 patterns of expression are not likely to be influenced by sex-specific factors (data not shown). There was no obvious difference in expression pattern between 2- and 6-month-old mice, which suggests that although a change in the galectin-4 level of expression has been described at weaning in rats (Niepceron et al. 2004), no variation appears in adult mice (data not shown). Finally, we could not detect any difference between mice on a pure 129/Sv or on a mixed F1 129/Sv-C57BL/6J background. In the latter, the results were the same whether the Lgals6 gene was transmitted by the father or the mother (data not shown). The Lgals4-Lgals6 locus is, therefore, unlikely to be subject to maternal or paternal effects. Although we found no difference due to sex, age, or transmission, we chose to analyze 7- to 9-week-old inbred males only for homogeneity. The results are presented below in proximal to distal order along the digestive tract.
Tongue (Figs 2a–c). We detected a spatially restricted expression of galectin-4 in the interpapillary stratum corneum of the tongue in the C57BL/6J strain (Fig. 2a) whereas the protein was undetectable in 129/Sv (Fig. 2b). Only a faint expression of galectin-6 was detected in the filiform papillae (Fig. 2c and inset). This observation is congruent with the absence of the Lgals4/Lgals6 gene transcripts in tongue samples from the ddY mouse (Nio et al. 2005) and thus suggests that galectin-4 and -6 expression is very limited or absent in the tongue of mice. It contrasts strikingly, however, with the results of Markova et al. 2006, who describe the galectin-4 expression throughout the tongue epithelium in C57BL/6J mice, and in pigs, where the tongue is a major site of galectin-4 expression (Chiu et al. 1992).
Oesophagus (data not shown). We did not detect any expression of galectin-4 or -6 in the oesophagus. This result is in agreement with the results of Nio et al. 2005, who detected no Lgals4/6 RNA by in situ hybridization.
Stomach (Figs 2d–f). We detected galectin-4 in the glandular stomach mucosa (Figs 2d, e) as previously described (Nio et al. 2005; Nio-Kobayashi et al. 2009). The pattern of expression of galectin-6 was qualitatively the same as that of galectin-4 (Fig. 2f).
Brünner’s glands (Figs 2g–i). Galectin-4 and -6 were detected in these glands (described in Obuoforibo and Martin 1977; Treuting et al. 2012) with very similar patterns of expression. The presence of Lgals4 RNA in Brünner’s glands has already been reported (Nio et al. 2005).
Small intestine: duodenum (Figs 2j–l) and ileum (Figs 2m–o). The small and large intestines are the organs in which the level of expression of galectin-4 is highest in most mammalian species (apart from pigs, in which the tongue is the organ with the highest expression) and where its function has been investigated most thoroughly. We could not detect any difference between the duodenum and ileum regarding the patterns of expression of galectin-4 and -6. Both proteins were expressed strongly in the epithelium and both proteins were undetectable in the lamina propria, at least under our fixation conditions. A decreasing gradient of expression of the Lgals4 mRNA has been reported along the crypt to villus axis (Nio et al. 2005). In contrast, we observed that the protein expression appeared slightly weaker in the crypts than in the villi. These results suggest that, although the gene is strongly expressed in the intestinal crypts, the protein accumulates progressively as the cells differentiate and migrate from the crypt to the villus. The galectin-6 protein was expressed at a much lower level in the crypts than in the villi (Fig. 2l). The galectin-4 and -6 expression appeared regularly weaker at the tip of the villi compared with their core (Figs 2j, l), although some variability existed (e.g., compare with Fig. 3d).
In the large intestine, we observed identical patterns of expression in the cecum (data not shown) and distal colon (Figs 2p–r). As in the small intestine, we detected a strong expression of galectin-4 and -6 in the crypt epithelium but not in the lamina propria of either the cecum or the distal colon. Galectin-4 seemed expressed relatively homogenously along the crypt (Figs 2p, 2q, 3e; Nio-Kobayashi et al. 2009), whereas a base-apex gradient was evident in the case of galectin-6 (Figs 1e, 2r).
Galectin-4 and Galectin-6 Differ in Their Subcellular Localization
In this work, tissues were fixed by intracardiac perfusion of 4% paraformaldehyde in situ in deeply anesthetized mice. In this respect, note that all the figures shown in this article, apart from Figs 3a and 3b, come from mice fixed under such a protocol. In samples from individuals fixed by intracardiac perfusion, the galectin-4 and -6 proteins appeared abundant in the cytosol of the intestinal epithelial cells (Figs 2g–r) and only rarely in the lamina propria. For instance, we detected galectin-4 expression in the lamina propria of a very limited region of the colon in none of seven C57BL/6J mice and in only one of nine 129/Sv mice, and no aggregates of galectin-6 (data not shown).
In contrast, when tissues were fixed following the more usual two-step procedure (i.e., dissection followed by immersion into a fixative solution without any prior fixation) the proteins tended either to leak into the lamina propria (galectin-4, Fig. 3a) or to form cellular aggregates (galectin-6, Fig. 3b). However, even when tissues were fixed by intracardiac perfusion (e.g., Figs 3c–f), some variability in the retention of cytosolic galectin-4 and -6 was observed (compare galectin-4 in Fig. 3c and galectin-6 in Fig. 3d with Figs 2k and 2l). Such a loss of cytosolic galectin-4 at fixation had already been noted in human T84 cells (Huflejt et al. 1997) and could also be observed in studies by others in mice and pigs (Chiu et al. 1992; Nio-Kobayashi et al. 2009). This fainter cytosolic galectin-4 and -6 staining helped to reveal the presence of these proteins in otherwise unnoticed locations.
Endosomes in enterocytes (arrowheads in Fig. 3a and data not shown). A weaker galectin-4 cytosolic signal revealed subapical granules in the cytoplasm of enterocytes, the tall columnar cells that are responsible for the final digestion and absorption of nutrients, electrolytes, and water. In cell cultures, Stechly and colleagues (Stechly et al. 2009) have shown the granules to be early and apical recycling endosomes but not late endosomes or lysosomes. They also demonstrated that galectin-4 is endocytosed and then recycled back to the apical surface of the cells and how this endocytic-recycling pathway of galectin-4 is required for the apical trafficking of glycoproteins (Stechly et al. 2009). In contrast, galectin-6 was never seen in these endosomes and, therefore, in spite of the sequence similarities it shares with galectin-4, it is unlikely to be part of the endocytic–recycling pathway.
Cell membranes (Figs 3c, 3d, as well as inset in Fig. 3d showing an enlargement of another villus). Galectin-4 and -6 were bound to the cell membrane: staining was observed both apically and basolaterally for galectin-4 (Fig. 3c and inset) but only basolaterally for galectin-6 (Fig. 3d and inset). The expression of galectin-4 in the lateral membranes of the enterocytes is in agreement with the fact that galectin-4 was initially isolated biochemically in pigs as an adherens junction-associated protein (Chiu et al. 1992; Chiu et al. 1994), a property that might be preserved in galectin-6. In the distal part of the villus, a stronger galectin-4 staining was also consistently observed in the apical cellular membrane (Fig. 3c, arrows). This result is in agreement with the reported involvement of galectin-4 in apical trafficking and lipid raft stabilization in cell cultures (Danielsen and van Deurs 1997; Braccia et al. 2003; Delacour et al. 2005; Stechly et al. 2009). At the tip of the villus, galectin-4 was sometimes detected in association with the membrane of round-shaped cells (Fig. 3c, double arrow). This suggests that the galectin-4 protein organization might be altered in epithelial cells about to be shed at the top of the villus (Bullen et al. 2006).
Nuclei. Galectin-4 was consistently expressed in cell nuclei as numerous tiny dots (arrowhead in the inset in Fig. 3c and data not shown). Contrary to galectin-1 and galectin-3, for which a role in the spliceosome has been documented (reviewed in Haudek et al. 2010), a function for galectin-4 in the nucleus has never been investigated. Galectin-6 was detected as one or two larger dots in the nucleus in a number of enterocytes (Fig. 3d, arrows). A very large aggregate of galectin-6 was also often revealed in the nucleus of goblet cells, the specialized epithelial cells that secrete mucus into the intestinal lumen (Fig. 3d, arrowheads). The differences in this nuclear distribution of galectin-4 and -6 might provide a clue when investigating galectin-6 specific functions.
Enteroendocrine cells (Fig. 3f and inset). On a few occasions, weak cytosolic staining revealed galectin-6 labeling in some of the cells that we morphologically identified as enteroendocrine cells, the hormone-secreting cells present throughout the epithelium of the digestive tract (e.g., compare with Fig. 3L in Nio-Kobayashi et al. 2009). Expression of galectin-4 has been proposed in porcine intestinal crypt enteroendocrine cells (Wooters, Hildreth, et al. 2005) but has been ruled out in murine villi enteroendocrine cells (Nio-Kobayashi et al. 2009). The role played by galectin-6 in mouse enteroendocrine cell physiology may deserve further investigation.
Goblet cells (Figs 2k, 3a, 3c). The galectin-4 and -6 proteins were also detected in the cytoplasm of goblet cells, the mucus-secreting cells in the digestive tract. The signal appeared often stronger than in the neighboring enterocytes (e.g., Fig. 3a, arrow). The expression of galectin-4 and -6 in the goblet cells seemed even more sensitive to the stress induced by dissection than elsewhere in the epithelium, as their subcellular localization was more variable in these cells than in any other cell type. On several occasions, galectin-4 appeared concentrated at the base of the large mucus vesicle (inset in Fig. 2k, arrow in Fig. 3a). It also appeared associated with the mucus before and while the granule was secreted into the intestinal lumen (Fig. 3a, double arrow; see single and composite exposure images in Suppl. Fig. S2). Galectin-6 was also detected in the mucus vesicle of goblet cells, but to a much lesser extent than galectin-4 (Figs 2l, 2o, 2r, Suppl. Fig. S2).
Galectin-4, but Not Galectin-6, Is Detected in the Extracellular Space
Colonic lumen (Fig. 3e and data not shown). We have detected the expression of galectin-4 in the colonic lumen in association with resident bacteria (arrowheads in Fig. 3e and data not shown). In contrast, we were unable to detect any binding of galectin-6 to luminal bacteria.
Colonic lamina propria (Fig. 4). As galectin-4 passed rapidly from the intestinal epithelium to the lamina propria when tissues were dissected without prior in situ fixation, we wondered whether galectin-4 could also be secreted into the lamina under physiological conditions. Several authors linked galectin-4 to colitis in humans and mice and suggested that its secretion into the lamina propria and its binding to resident immune cells participate in the regulation of inflammation (Hokama et al. 2004; Mathieu et al. 2008; Paclik, Danese, et al. 2008; Paclik et al. 2011). However, although galectin-4 basolateral secretion was detected ex vivo in HT29 cells in culture (Stechly et al. 2009), the presence of galectin-4, and a fortiori galectin-6, in the inflamed lamina propria was never demonstrated in vivo.
Figure 4.
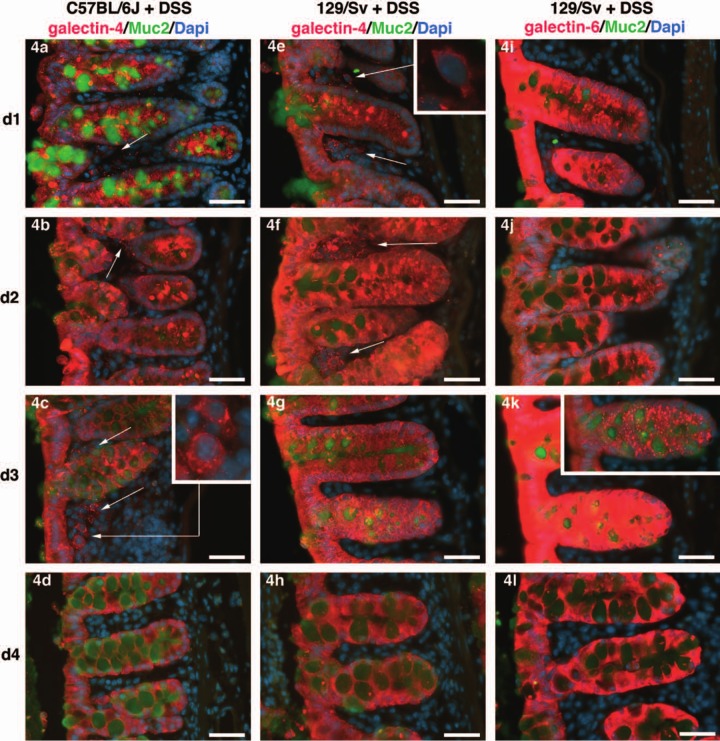
Galectin-4 and -6 expression in the colon of dextran sodium sulfate (DSS)-treated mice. Expression of galectin-4 (two left columns) or galectin-6 (right column) in the C57BL/6J (left column) or 129/Sv (two right columns) background. The arrows point toward galectin-4 decorated cells in the lamina propria. The insets in 4c and 4e are enlargements of the region to which the arrows point. The inset in 4k is a picture of the crypt shown in 4k, with a shorter exposure time in order to reveal the galectin-6-positive granules in the crypt. The labels d1 (day 1) to d4 (day 4) on the left represent the period during which DSS was in the drinking water. The anti-galectin-4 or -6 staining is shown in red, anti-Muc2 in green, and DAPI-stained nuclei in blue. Scale bars = 40 µm. The same settings and exposure times were used for all pictures, except for the inset in 4k.
Dextran sodium sulfate (DSS) is a polymer generally believed to be directly toxic to gut epithelial cells of the basal crypts and to affect the integrity of the mucosal barrier. Feeding mice for several days with DSS polymers added to the drinking water is sufficient to induce acute colitis. Hence, this protocol is widely used as a model to chemically induce intestinal inflammation in rodents (reviewed in Wirtz et al. 2007). In particular, DSS was chosen to induce acute colitis in the three studies that linked galectin-4 and inflammation in mice (Hokama et al. 2004; Mathieu et al. 2008; Paclik, Danese, et al. 2008). Ideo and colleagues (Ideo et al. 2007) showed, however, that DSS acts as an inhibitor of galectin-4 binding to cholesterol 3-sulfate, one of it ligands. It is thus possible that DSS also affects galectin-4 functions by inhibiting its interactions with its ligands.
We document here the effect on galectin-4 and galectin-6 patterns of expression in the mouse following consumption of 4% DSS in the drinking water for one to four days. Galectin-4 was not detected in the lamina propria of seven C57BL/6J control mice. It was detected in a very limited area in only one out of nine 129/Sv control individuals, possibly as part of spontaneous inflammation (Table 1). In contrast, at day 1 of DSS-treatment, galectin-4 was detected in the colonic lamina propria of 4/6 C57BL/6J DSS-treated mice (day1; arrows in Fig. 4a, numbers summarized in Table 1) and in every tested individual at day 2 (5/5, Fig. 4b) and day 3 (5/5, Fig. 4c), whereas it was undetectable at day 4 (0/5, Fig. 4d). In 129/Sv-treated individuals, galectin-4 was detected in the lamina propria of every treated individual at day 1 of DSS-treatment (4/4; Fig. 4e) and day 2 (5/5; Fig. 4f), whereas it was undetectable at day 3 (0/5; Fig. 4g) and detected in a limited colonic area in only one animal at day 4 (1/5; Fig. 4h). These results suggest temporal differences in the response to colonic epithelium damage between the C57BL/6J and 129/Sv mouse strains. For a given time-point and a given strain, the proportion of affected lamina propria varied widely between animals. Therefore, quantification of the surface of the lamina propria affected by the treatment was irrelevant.
Table 1.
Galectin-4 and galectin-6 expression in the colonic lamina propria cells in the C57BL/6J and 129/Sv mice following exposure to 4% dextran sodium sulfate
| Galectin-4 | C57BL/6J | 129/Sv |
|---|---|---|
| d0 | 0/7 | 1/9 |
| d1 | 4/6 | 4/4 |
| d2 | 5/5 | 5/5 |
| d3 | 5/5 | 0/5 |
| d4 | 0/5 | 1/5 |
|
| ||
| Galectin-6 | C57BL/6J | 129/Sv |
|
| ||
| d0 | - | 0/6 |
| d1 | - | 0/4 |
| d2 | - | 0/2 |
| d3 | - | 0/5 |
| d4 | - | 0/2 |
d0 (day 0): no treatment; d1 to d4: one to 4 days of treatment.
In contrast to galectin-4, galectin-6 was never detected in the intestinal lamina propria of 4% DSS-treated animals (Table 1; Figs 4i, j, k, l). Galectin-4 level increased in the colonic epithelium from day 1 to day 3 and, at day 3, it formed aggregates within the crypt (inset in Fig. 4k showing the same crypt as in Fig. 4k with a shorter exposition time). To determine whether these aggregates were detrimental or beneficial to the crypt cells was, however, beyond the scope of this work. As galectin-6 was not detected in the colonic lamina propria, our results show that, contrary to galectin-4, galectin-6 is unlikely to regulate inflammation through direct binding to lamina propria lymphocytes or macrophages, as proposed for galectin-4 (Hokama et al. 2008; Paclik, Danese, et al. 2008; Paclik et al. 2011).
Discussion
Galectin-4 and -6 Have Largely Overlapping Patterns of Expression that Suggest Functional Redundancy
In this work, our prime objective was to apprehend the reason why the Lgals4-Lgals6 locus remained polymorphic in wild and laboratory mice by carefully comparing the galectin-4 and -6 patterns of expression. The pattern of expression of galectin-4 generally confirms and extends published data (see Nio et al. 2005; Nio-Kobayashi et al. 2009, for the description of galectin-4 expression in mice). For instance, our results suggest strain-specific differences, not only in the expression of galectin-6 but also in the expression of galectin-4. We did not detect any expression of galectin-4 or -6 in the oesophagus, in agreement with the results of Nio et al. 2005. It is thus similar to that reported in humans, although galectin-4 expression increases dramatically in Barrett’s oesophagus (van Baal et al. 2005). However, it contrasts with results obtained in the pig (Chiu et al. 1992; Ideo et al. 2007) and the rat (Wasano and Hirakawa 1995), in which galectin-4 expression was detected in the oesophagus epithelium. Therefore, the results we obtained in the tongue and the oesophagus suggest species-specific diversity in the galectin-4 pattern of expression, at least in the proximal part of the digestive tract.
Galectin-6 is described here for the first time, and we show that its pattern of expression along the digestive tract is nearly identical to that of galectin-4, at least in healthy mice in the safe and controlled environment of the animal house. Our results, in conjunction with the noted 83% sequence identity for the two proteins, support the hypothesis of overall functional redundancy between the Lgals4 and Lgals6 genes in most organs. Our results indicate that duplication of the Lgals6 gene encompassed most, if not all, Lgals4 regulatory sequences. Hence, neofunctionalization of galectin-6 is unlikely to have been prompted by a change in its pattern of expression. Galectin-6 neofunctionalization would then rather be due to novelties in the protein structure leading to new ligand specificity and/or affinity.
Extracellular Galectin-4
For a long time, galectins were thought to bind only to endogenous “self” glycans in order to mediate a number of biological functions, including cell differentiation, tissue organization, and regulation of immune homeostasis. Even though galectins are synthesized and stored in the cytoplasm, following tissue damage or infection, cytosolic galectins isolated from a large number of phyla are either passively released or actively secreted from the cells. Host galectins would then function either as pattern recognition receptors (PRRs) that target “non-self” glycans on the surface of viruses, bacteria, and/or helminths, or as damage-associated molecular patterns (DAMPs) that emerge from dying host cells into the extracellular space upon damage. Their presence would then signal the invasion by pathogenic microorganisms or possible tissue damage (reviewed in Sato et al. 2009; Vasta 2009; Davicino et al. 2011). Reciprocally, some pathogens and parasites secrete their own galectins or subvert the roles of the host galectins to either attach to suitable epithelia in their insect vector or final host, or to enter the host cells to proliferate and disseminate systemically; therefore galectins are key players in the host versus pathogen everlasting war (see Ideo et al. 2009; Sato et al. 2009; Vasta 2009; Butschi et al. 2010, for some examples).
In 2009, Nio-Kobayashi and colleagues described the binding of galectin-3 to microorganisms in the mouse stomach (Nio-Kobayashi et al. 2009). In 2010, Stowell et al. described the binding of human galectin-3, -4 and -8 to human blood group antigen-expressing enteropathogenic Escherichia coli (EPECs) (Stowell et al. 2010). The binding of galectin-4 and -8, but not -3, to the pathogen led to a rapid loss of bacterial motility and viability. Murine galectin-4 also killed EPECs in vitro, although not as efficiently as human galectin-4 (Stowell et al. 2010, see also comment in Liu and Bevins 2010). We now show that galectin-4 is secreted into the lumen of the mouse intestine together with the content of mucus vesicles from goblet cells. There, galectin-4 would associate with the protective mucus layer and would bind to the surface of some of the luminal resident bacteria. This result supports the hypothesis that galectin-4 acts as a PRR and participates in the elimination of enteropathogenic bacteria in mice, as it does in humans.
The expression of galectin-4 in goblet cells, invites us to speculate whether galectin-4 and mucus might be stored in two distinct subcellular compartments in order to prevent any premature interaction (Ideo et al. 2002; Wu et al. 2002). Upon stimulation, these two compartments would merge and their content would be released into the lumen. Once there, the interaction between galectin-4 and these MUC proteins might contribute to the organization of the mucus-protecting coat, as described for galectin-3 in the eye (Argueso et al. 2009). Interestingly, galectin-4 and MUC proteins are also ectopically co-expressed in mucinous ovarian cancers (MOC; Heinzelmann-Schwarz et al. 2006) reinforcing the hypothesis of their functional association.
Galectin-6 is also secreted into the intestinal lumen, but to a much lesser extent than galectin-4, and was never found to bind to bacteria. As the effect of galectin-4 on E. coli motility and viability resides in its C-terminal domain (Stowell et al. 2010)—the part of galectin-6 most altered by positive selection (Houzelstein et al. 2008, and unpublished results)—galectin-6 might have lost its bactericide properties as part of the adaptive process, without losing its galactose-binding activity (Gitt, Colnot, et al. 1998).
Galectin-4 was only marginally detected in the lamina propria of healthy control mice. In contrast, it was present in the lamina propria of the large majority of the mice for which the colonic epithelium was damaged by the inflammatory agent DSS. Once in the lamina propria, galectin-4 is known to bind to activated neutrophils and to several leukocyte cell lines (Stowell et al. 2007), activated T lymphocytes (Hokama et al. 2004; Paclik, Danese, et al. 2008) as well as monocytes and macrophages (Paclik et al. 2011). In this work, the nuclear shape of the galectin-4 positive cells in the lamina propria suggests them to be mononuclear cells (plasma cells, lymphocytes, and macrophages) but clearly not polymorphonuclear cells (neutrophils, eosinophils, basophils). To determine their exact nature will require an in-depth analysis with a panel of immune cell-specific markers.
Galectin-4 and -6 Patterns of Expression Also Suggest Distinct Roles in Normal and Damaged Mouse Digestive Tract
Although traces of positive selection on the Lgals6 gene suggest a gain of new properties for the galectin-6 protein (Houzelstein et al. 2008), our results have indeed revealed more characteristics present in galectin-4 that galectin-6 may have lost than new characteristics for galectin-6 itself. Therefore, the galectin-6 properties that have been selected for remain elusive. Nevertheless, two new properties for galectin-6 might be noted: its expression in the enteroendocrine cells and its tendency to form aggregates. Actually, although both galectin-4 and -6 are detected in the nucleus, galectin-6 forms fewer and larger aggregates than does galectin-4. It also forms aggregates when the colonic mucosa is damaged by the inflammation-inducing agent DSS.
We thus show that galectin-4 and -6 differ in several aspects in their localization—within the cell (nucleus, apical membranes) and outside the cell (luminal colonic bacteria, lamina propria where only galectin-4 has been detected). This result, in conjunction with evidence of positive selection on the Lgals6 gene, supports the hypothesis of functional differences between the two proteins. Determining whether they favor or prevent fixation of the duplicated locus in wild and laboratory mice would require extensive functional analysis.
Concluding Remarks
The combination of redundant and galectin-4- and galectin-6-specific properties in mice illustrates how duplicated genes gradually acquire a new identity in the course of evolution. It also opens interesting possibilities for the analysis of galectin-4 with mice as an experimental organism. For instance, if a Lgals4 knockout mouse strain were to be produced, either embryonic stem (ES) cells from the 129/Sv strain, carrying the duplicated Lgals4-Lgals6 locus, or from the C57BL/6J strain, carrying the Lgals4 unduplicated locus, could be used. With ES cells carrying the duplicated Lgals4-Lgals6 locus, galectin-4-specific functions, such as in the lumen or the lamina propria, would be affected while galectin-6 would be able to fulfill most of the galectin-4/-6 redundant intracellular functions. On the contrary, with ES cells carrying the unduplicated Lgals4 locus, all the functions fulfilled by galectin-4 could be blocked at once. In this respect, the list of the major laboratory and wild-derived mouse strains carrying either the duplicated or unduplicated locus will also be useful (Houzelstein et al. 2008).
Supplementary Material
Acknowledgments
We wish to thank Léa Bidermann, Stephan Pinson, and Christine Lamouroux from the animal facility for their great help in the course of this project. We wish to thank Sarah Cormier and Aline Stedman for kindly sharing reagents. We thank Denise Busson and Marc Chodkiewicz for helpful discussions and comments on this article. Special thanks to Françoise Praz for her generous help at the beginning of this project.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Centre National pour la Recherche Scientifique (CNRS), the University Pierre et Marie Curie (UPMC), and the GEFLUC Paris.
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
References
- Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. 2009. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 284:23037–23045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan V, Nangia-Makker P, Raz A. 2010. Galectins as cancer biomarkers. Cancers. 2:592–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barondes SH, Cooper DN, Gitt MA, Leffler H. 1994. Galectins: structure and function of a large family of animal lectins. J Biol Chem. 269:20807–20810 [PubMed] [Google Scholar]
- Braccia A, Villani M, Immerdal L, Niels-Christiansen LL, Nystrom BT, Hansen GH, Danielsen EM. 2003. Microvillar membrane microdomains exist at physiological temperature: role of galectin-4 as lipid raft stabilizer revealed by “superrafts.” J Biol Chem. 278:15679–15684 [DOI] [PubMed] [Google Scholar]
- Bullen TF, Forrest S, Campbell F, Dodson AR, Hershman MJ, Pritchard DM, Turner JR, Montrose MH, Watson AJ. 2006. Characterization of epithelial cell shedding from human small intestine. Lab Invest. 86:1052–1063 [DOI] [PubMed] [Google Scholar]
- Butschi A, Titz A, Walti MA, Olieric V, Paschinger K, Nobauer K, Guo X, Seeberger PH, Wilson IB, Aebi M, et al. 2010. Caenorhabditis elegans N-glycan core beta-galactoside confers sensitivity towards nematotoxic fungal galectin CGL2. PLoS Pathog. 6:e1000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu ML, Jones JC, O’Keefe EJ. 1992. Restricted tissue distribution of a 37-kD possible adherens junction protein. J Cell Biol. 119:1689–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu ML, Parry DA, Feldman SR, Klapper DG, O’Keefe EJ. 1994. An adherens junction protein is a member of the family of lactose-binding lectins. J Biol Chem. 269:31770–31776 [PubMed] [Google Scholar]
- Danielsen EM, Hansen GH. 2008. Lipid raft organization and function in the small intestinal brush border. J Physiol Biochem. 64:377–382 [DOI] [PubMed] [Google Scholar]
- Danielsen EM, van Deurs B. 1997. Galectin-4 and small intestinal brush border enzymes form clusters. Mol Biol Cell. 8:2241–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davicino RC, Elicabe RJ, Di Genaro MS, Rabinovich GA. 2011. Coupling pathogen recognition to innate immunity through glycan-dependent mechanisms. Int Immunopharmacol. 11:1457–1463 [DOI] [PubMed] [Google Scholar]
- Delacour D, Gouyer V, Zanetta JP, Drobecq H, Leteurtre E, Grard G, Moreau-Hannedouche O, Maes E, Pons A, Andre S, et al. 2005. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol. 169:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr EM, Mizukami Y, Ng A, Xavier RJ, Kikuchi H, Deshpande V, Warshaw AL, Glickman J, Kulke MH, Chung DC. 2008. Defining molecular classifications and targets in gastroenteropancreatic neuroendocrine tumors through DNA microarray analysis. Endocr Relat Cancer. 15:243–256 [DOI] [PubMed] [Google Scholar]
- Gitt MA, Colnot C, Poirier F, Nani KJ, Barondes SH, Leffler H. 1998. Galectin-4 and galectin-6 are two closely related lectins expressed in mouse gastrointestinal tract. J Biol Chem. 273:2954–2960 [DOI] [PubMed] [Google Scholar]
- Gitt MA, Xia YR, Atchison RE, Lusis AJ, Barondes SH, Leffler H. 1998. Sequence, structure, and chromosomal mapping of the mouse Lgals6 gene, encoding galectin-6. J Biol Chem. 273:2961–2970 [DOI] [PubMed] [Google Scholar]
- Haudek KC, Patterson RJ, Wang JL. 2010. SR proteins and galectins: What’s in a name? Glycobiology. 20:1199–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry JP, Scolyer RA, Smith AN, Bali A, Vanden Bergh P, Baron-Hay S, Scott C, et al. 2006. A distinct molecular profile associated with mucinous epithelial ovarian cancer. Br J Cancer. 94:904–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippo Y, Yashiro M, Ishii M, Taniguchi H, Tsutsumi S, Hirakawa K, Kodama T, Aburatani H. 2001. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 61:889–895 [PubMed] [Google Scholar]
- Hokama A, Mizoguchi E, Mizoguchi A. 2008. Roles of galectins in inflammatory bowel disease. World J Gastroenterol. 14:5133–5137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokama A, Mizoguchi E, Sugimoto K, Shimomura Y, Tanaka Y, Yoshida M, Rietdijk ST, de Jong YP, Snapper SB, Terhorst C, et al. 2004. Induced reactivity of intestinal CD4(+) T cells with an epithelial cell lectin, galectin-4, contributes to exacerbation of intestinal inflammation. Immunity. 20:681–693 [DOI] [PubMed] [Google Scholar]
- Houzelstein D, Goncalves IR, Fadden AJ, Sidhu SS, Cooper DN, Drickamer K, Leffler H, Poirier F. 2004. Phylogenetic analysis of the vertebrate galectin family. Mol Biol Evol. 21:1177–1187 [DOI] [PubMed] [Google Scholar]
- Houzelstein D, Goncalves IR, Orth A, Bonhomme F, Netter P. 2008. Lgals6, a 2-million-year-old gene in mice: a case of positive Darwinian selection and presence/absence polymorphism. Genetics. 178:1533–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huflejt ME, Jordan ET, Gitt MA, Barondes SH, Leffler H. 1997. Strikingly different localization of galectin-3 and galectin-4 in human colon adenocarcinoma T84 cells: Galectin-4 is localized at sites of cell adhesion. J Biol Chem. 272:14294–14303 [DOI] [PubMed] [Google Scholar]
- Huflejt ME, Leffler H. 2004. Galectin-4 in normal tissues and cancer. Glycoconj J. 20:247–255 [DOI] [PubMed] [Google Scholar]
- Hwang JS, Takaku Y, Momose T, Adamczyk P, Ozbek S, Ikeo K, Khalturin K, Hemmrich G, Bosch TC, Holstein TW, et al. 2010. Nematogalectin, a nematocyst protein with GlyXY and galectin domains, demonstrates nematocyte-specific alternative splicing in Hydra. Proc Natl Acad Sci USA. 107:18539–18544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideo H, Fukushima K, Gengyo-Ando K, Mitani S, Dejima K, Nomura K, Yamashita K. 2009. A Caenorhabditis elegans glycolipid-binding galectin functions in host defense against bacterial infection. J Biol Chem. 284:26493–26501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideo H, Seko A, Ohkura T, Matta KL, Yamashita K. 2002. High-affinity binding of recombinant human galectin-4 to SO(3)(-)-->3Galbeta1-->3GalNAc pyranoside. Glycobiology. 12:199–208 [DOI] [PubMed] [Google Scholar]
- Ideo H, Seko A, Yamashita K. 2007. Recognition mechanism of galectin-4 for cholesterol 3-sulfate. J Biol Chem. 282:21081–21089 [DOI] [PubMed] [Google Scholar]
- Kamhawi S, Ramalho-Ortigao M, Pham VM, Kumar S, Lawyer PG, Turco SJ, Barillas-Mury C, Sacks DL, Valenzuela JG. 2004. A role for insect galectins in parasite survival. Cell. 119:329–341 [DOI] [PubMed] [Google Scholar]
- Leffler H, Masiarz FR, Barondes SH. 1989. Soluble lactose-binding vertebrate lectins: a growing family. Biochemistry. 28:9222–9229 [DOI] [PubMed] [Google Scholar]
- Liu FT, Bevins CL. 2010. A sweet target for innate immunity. Nat Med. 16:263–264 [DOI] [PubMed] [Google Scholar]
- Liu FT, Rabinovich GA. 2010. Galectins: regulators of acute and chronic inflammation. Ann N Y Acad Sci. 1183:158–182 [DOI] [PubMed] [Google Scholar]
- Markova V, Smetana K, Jr, Jenikova G, Lachova J, Krejcirikova V, Poplstein M, Fabry M, Brynda J, Alvarez RA, Cummings RD, et al. 2006. Role of the carbohydrate recognition domains of mouse galectin-4 in oligosaccharide binding and epitope recognition and expression of galectin-4 and galectin-6 in mouse cells and tissues. Int J Mol Med. 18:65–76 [PubMed] [Google Scholar]
- Mathieu A, Nagy N, Decaestecker C, Ferdinande L, Vandenbroucke K, Rottiers P, Cuvelier CA, Salmon I, Demetter P. 2008. Expression of galectins-1, -3 and -4 varies with strain and type of experimental colitis in mice. Int J Exp Pathol. 89:438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelle W, Stechly L, Andre S, Van Seuningen I, Porchet N, Gabius HJ, Michalski JC, Huet G. 2009. Glycosylation pattern of brush border-associated glycoproteins in enterocyte-like cells: involvement of complex-type N-glycans in apical trafficking. Biol Chem. 390: 529-544 [DOI] [PubMed] [Google Scholar]
- Nagy N, Legendre H, Engels O, Andre S, Kaltner H, Wasano K, Zick Y, Pector JC, Decaestecker C, Gabius HJ, et al. 2003. Refined prognostic evaluation in colon carcinoma using immunohistochemical galectin fingerprinting. Cancer. 97:1849–1858 [DOI] [PubMed] [Google Scholar]
- Niepceron E, Simian F, Louisot P, Biol-N’garagba MC. 2004. Expression of galectin 4 in the rat small intestine during postnatal development. Biochimie. 86:115–118 [DOI] [PubMed] [Google Scholar]
- Nio J, Kon Y, Iwanaga T. 2005. Differential cellular expression of galectin family mRNAs in the epithelial cells of the mouse digestive tract. J Histochem Cytochem. 53:1323–1334 [DOI] [PubMed] [Google Scholar]
- Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T. 2009. Immunohistochemical localization of six galectin subtypes in the mouse digestive tract. J Histochem Cytochem. 57:41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuoforibo AA, Martin BF. 1977. Postnatal growth of Brunner’s glands in the mouse. J Anat. 124:779–790 [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Herrmann J, Gitt MA, Turck CW, Burlingame AL, Barondes SH, Leffler H. 1993. Soluble lactose-binding lectin from rat intestine with two different carbohydrate-binding domains in the same peptide chain. J Biol Chem. 268:5929–5939 [PubMed] [Google Scholar]
- Paclik D, Danese S, Berndt U, Wiedenmann B, Dignass A, Sturm A. 2008. Galectin-4 controls intestinal inflammation by selective regulation of peripheral and mucosal T cell apoptosis and cell cycle. PLoS One. 3: e2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paclik D, Lohse K, Wiedenmann B, Dignass AU, Sturm A. 2008. Galectin-2 and -4, but not galectin-1, promote intestinal epithelial wound healing in vitro through a TGF-beta-independent mechanism. Inflamm Bowel Dis. 14: 1366-1372 [DOI] [PubMed] [Google Scholar]
- Paclik D, Werner L, Guckelberger O, Wiedenmann B, Sturm A. 2011. Galectins distinctively regulate central monocyte and macrophage function. Cell Immunol. 271:97–103 [DOI] [PubMed] [Google Scholar]
- Rechreche H, Mallo GV, Montalto G, Dagorn JC, Iovanna JL. 1997. Cloning and expression of the mRNA of human galectin-4, an S-type lectin down-regulated in colorectal cancer. Eur J Biochem. 248:225–230 [DOI] [PubMed] [Google Scholar]
- Rumilla KM, Erickson LA, Erickson AK, Lloyd RV. 2006. Galectin-4 expression in carcinoid tumors. Endocr Pathol. 17: 243-249 [DOI] [PubMed] [Google Scholar]
- Satelli A, Rao PS, Thirumala S, Rao US. 2011. Galectin-4 functions as a tumor suppressor of human colorectal cancer. Int J Cancer. 129:799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, St-Pierre C, Bhaumik P, Nieminen J. 2009. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol Rev. 230:172–187 [DOI] [PubMed] [Google Scholar]
- Stechly L, Morelle W, Dessein AF, Andre S, Grard G, Trinel D, Dejonghe MJ, Leteurtre E, Drobecq H, Trugnan G, et al. 2009. Galectin-4-regulated delivery of glycoproteins to the brush border membrane of enterocyte-like cells. Traffic. 10:438–450 [DOI] [PubMed] [Google Scholar]
- Stowell SR, Arthur CM, Dias-Baruffi M, Rodrigues LC, Gourdine JP, Heimburg-Molinaro J, Ju T, Molinaro RJ, Rivera-Marrero C, Xia B, et al. 2010. Innate immune lectins kill bacteria expressing blood group antigen. Nat Med. 16:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Karmakar S, Stowell CJ, Dias-Baruffi M, McEver RP, Cummings RD. 2007. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 109:219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasumi S, Vasta GR. 2007. A galectin of unique domain organization from hemocytes of the Eastern oyster (Crassostrea virginica) is a receptor for the protistan parasite Perkinsus marinus. J Immunol. 179:3086–3098 [DOI] [PubMed] [Google Scholar]
- Treuting PM, Valasek MA, Dintzis SM. Upper gastrointestinal tract. In Treuting PM, Dintzis SM, editors. Comparative anatomy and histology: a mouse and human atlas. Amsterdam (Netherlands: ): Elsevier; 2012. pp. 155-176 [Google Scholar]
- van Baal JW, Milano F, Rygiel AM, Bergman JJ, Rosmolen WD, van Deventer SJ, Wang KK, Peppelenbosch MP, Krishnadath KK. 2005. A comparative analysis by SAGE of gene expression profiles of Barrett’s esophagus, normal squamous esophagus, and gastric cardia. Gastroenterology. 129:1274–1281 [DOI] [PubMed] [Google Scholar]
- Vasta GR. 2009. Roles of galectins in infection. Nat Rev Microbiol. 7:424–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzini A, Parrinello D, Sanfratello MA, Salerno G, Cammarata M, Parrinello N. 2012. Inducible galectins are expressed in the inflamed pharynx of the ascidian Ciona intestinalis. Fish Shellfish Immunol. 32:101–109 [DOI] [PubMed] [Google Scholar]
- Wasano K, Hirakawa Y. 1995. Rat intestinal galactoside-binding lectin L-36 functions as a structural protein in the superficial squamous cells of the esophageal epithelium. Cell Tissue Res. 281:77–83 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Takemasa I, Kaneko N, Yokoyama Y, Matsuo E, Iwasa S, Mori M, Matsuura N, Monden M, Nishimura O. 2011. Clinical significance of circulating galectins as colorectal cancer markers. Oncol Rep. 25:1217–1226 [DOI] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF. 2007. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2:541–546 [DOI] [PubMed] [Google Scholar]
- Wooters MA, Hildreth MB, Nelson EA, Erickson AK. 2005. Immunohistochemical characterization of the distribution of galectin-4 in porcine small intestine. J Histochem Cytochem. 53:197–205 [DOI] [PubMed] [Google Scholar]
- Wooters MA, Ropp SL, Erickson AK. 2005. Identification of galectin-4 isoforms in porcine small intestine. Biochimie. 87:143–149 [DOI] [PubMed] [Google Scholar]
- Wu AM, Wu JH, Tsai MS, Liu JH, Andre S, Wasano K, Kaltner H, Gabius HJ. 2002. Fine specificity of domain-I of recombinant tandem-repeat-type galectin-4 from rat gastrointestinal tract (G4-N). Biochem J. 367:653–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yuan S, Yu Y, Huang H, Feng K, Pan M, Huang S, Dong M, Chen S, Xu A. 2007. Molecular and biochemical characterization of galectin from amphioxus: primitive galectin of chordates participated in the infection processes. Glycobiology. 17:774–783 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.