Abstract
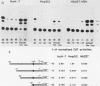
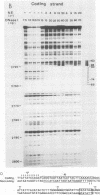
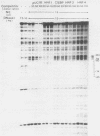
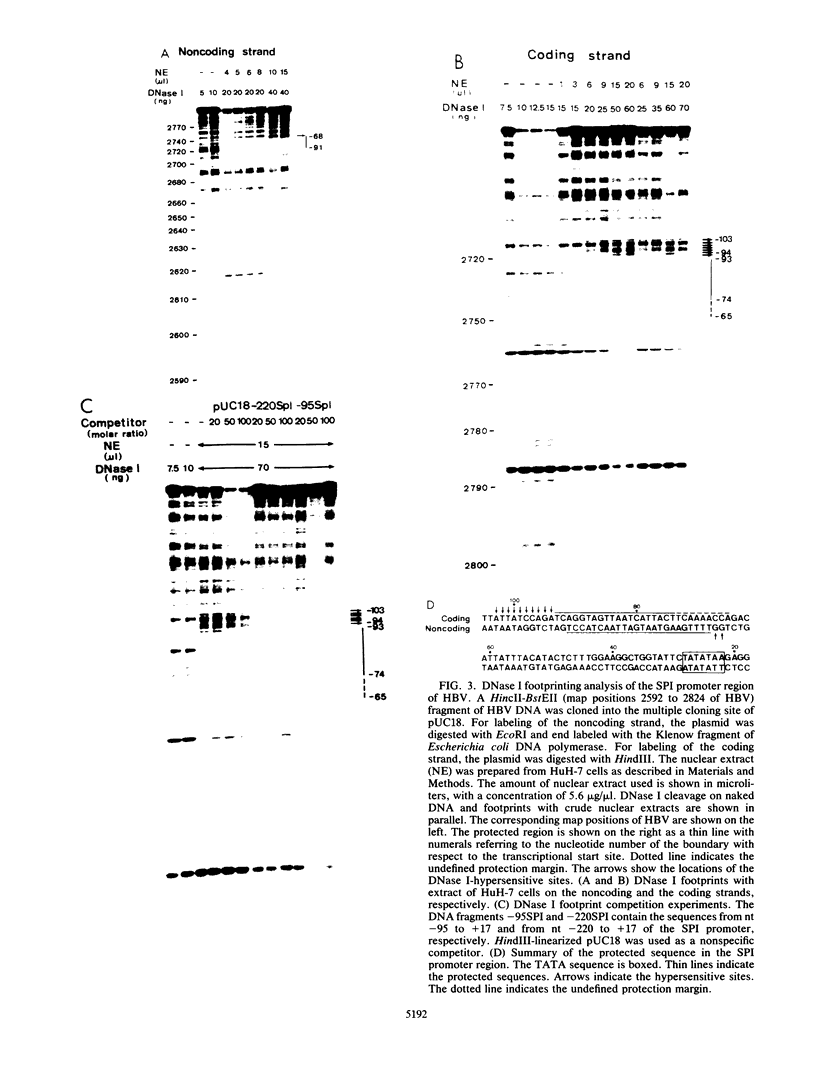
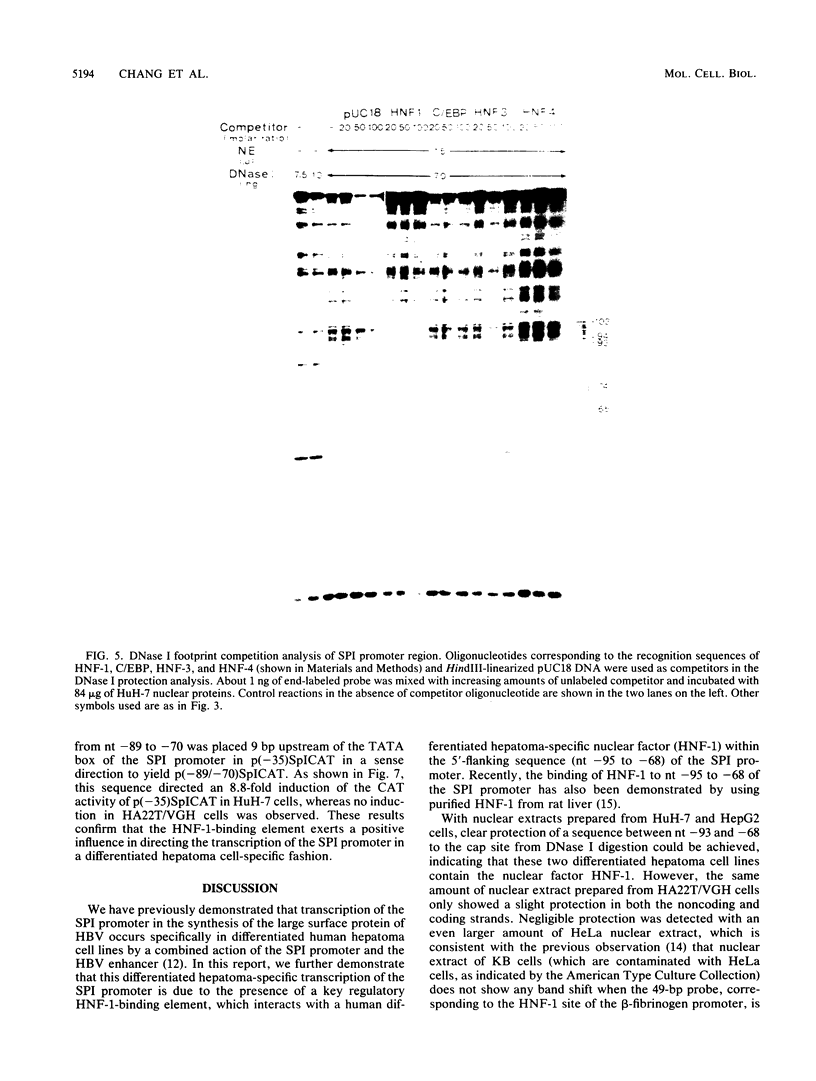
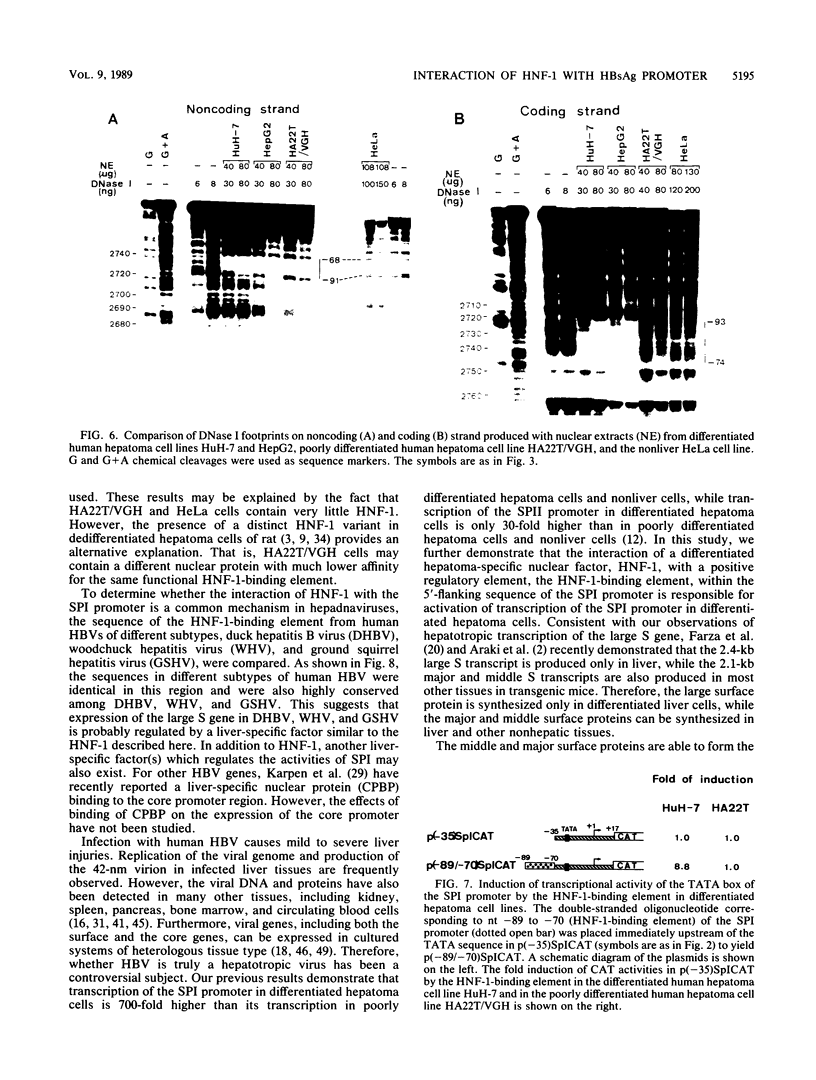
The outer envelope of the 42-nm virion of the human hepatitis B virus (HBV) is composed of the large, the middle, and the major surface proteins. Whereas the middle and the major surface proteins are transcribed from the SPII promoter of the pre-S/S gene, the large surface protein is transcribed from the SPI promoter located upstream of SPII. We have previously shown that transcription of SPI (comprising nucleotides [nt] -380 to +17) occurs preferentially in differentiated hepatoma cell lines (H.K. Chang and L.P. Ting, Virology 170:176-183, 1989). In this report, we further demonstrated that a sequence of 95 base pairs in the upstream region of SPI (nt -95 to +17) was necessary and sufficient for such preferential expression in differentiated hepatoma cells. By analysis of the expression of the chloramphenicol acetyltransferase gene in a series of mutants with deletions at the 5' end of SPI, we identified a positive transcriptional cis-acting element mapping at nt -95 to -72 which appears to play a key role in the regulation of the expression of the large surface protein. This region shared a high degree of sequence homology with regulatory sequences of several liver-specific genes from human, mouse, and rat, with a consensus sequence (G/A)GTTA(A/C)TNNT(C/T)NNC(A/C). We further identified a nuclear factor present in the nuclear extracts of differentiated human hepatoma cell lines which interacted specifically with this element of the SPI promoter. This nuclear factor was similar to the rat liver-specific factor HNF-1, since an oligonucleotide containing the recognition sequence of HNF-1 could efficiently compete for the human factor in a footprinting assay. The sequence at nt -93 to -68 which was bound by this factor in SPI was termed the HNF-1-binding element. Activation of the SPI promoter by human differentiated hepatocyte nuclear factor 1, described in this report, probably explains, first, the formation of the 42-nm virion specifically in liver but not in several other tissues despite the synthesis of the middle and the major surface proteins in those tissues, and second, why only differentiated hepatoma cell lines are able to produce 42-nm-like virion particles on transfection by HBV DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aden D. P., Fogel A., Plotkin S., Damjanov I., Knowles B. B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979 Dec 6;282(5739):615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- Araki K., Miyazaki J., Hino O., Tomita N., Chisaka O., Matsubara K., Yamamura K. Expression and replication of hepatitis B virus genome in transgenic mice. Proc Natl Acad Sci U S A. 1989 Jan;86(1):207–211. doi: 10.1073/pnas.86.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumhueter S., Courtois G., Crabtree G. R. A variant nuclear protein in dedifferentiated hepatoma cells binds to the same functional sequences in the beta fibrinogen gene promoter as HNF-1. EMBO J. 1988 Aug;7(8):2485–2493. doi: 10.1002/j.1460-2075.1988.tb03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Büscher M., Reiser W., Will H., Schaller H. Transcripts and the putative RNA pregenome of duck hepatitis B virus: implications for reverse transcription. Cell. 1985 Mar;40(3):717–724. doi: 10.1016/0092-8674(85)90220-x. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Will H., Hernandez N., Schaller H. Signals regulating hepatitis B surface antigen transcription. Nature. 1983 Sep 22;305(5932):336–338. doi: 10.1038/305336a0. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Will H., Schaller H. Hepatitis B virus transcription in the infected liver. EMBO J. 1984 Sep;3(9):2191–2196. doi: 10.1002/j.1460-2075.1984.tb02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghini S., Blumenfeld M., Yaniv M. A liver-specific factor essential for albumin transcription differs between differentiated and dedifferentiated rat hepatoma cells. Genes Dev. 1988 Aug;2(8):957–974. doi: 10.1101/gad.2.8.957. [DOI] [PubMed] [Google Scholar]
- Chang C., Lin Y., O-Lee T. W., Chou C. K., Lee T. S., Liu T. J., P'eng F. K., Chen T. Y., Hu C. P. Induction of plasma protein secretion in a newly established human hepatoma cell line. Mol Cell Biol. 1983 Jun;3(6):1133–1137. doi: 10.1128/mcb.3.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. K., Chou C. K., Chang C., Su T. S., Hu C., Yoshida M., Ting L. P. The enhancer sequence of human hepatitis B virus can enhance the activity of its surface gene promoter. Nucleic Acids Res. 1987 Mar 11;15(5):2261–2268. doi: 10.1093/nar/15.5.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. K., Ting L. P. The surface gene promoter of the human hepatitis B virus displays a preference for differentiated hepatocytes. Virology. 1989 May;170(1):176–183. doi: 10.1016/0042-6822(89)90364-4. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Darnell J. E., Jr Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol. 1989 Apr;9(4):1415–1425. doi: 10.1128/mcb.9.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G., Baumhueter S., Crabtree G. R. Purified hepatocyte nuclear factor 1 interacts with a family of hepatocyte-specific promoters. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7937–7941. doi: 10.1073/pnas.85.21.7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G., Morgan J. G., Campbell L. A., Fourel G., Crabtree G. R. Interaction of a liver-specific nuclear factor with the fibrinogen and alpha 1-antitrypsin promoters. Science. 1987 Oct 30;238(4827):688–692. doi: 10.1126/science.3499668. [DOI] [PubMed] [Google Scholar]
- De Simone V., Ciliberto G., Hardon E., Paonessa G., Palla F., Lundberg L., Cortese R. Cis- and trans-acting elements responsible for the cell-specific expression of the human alpha 1-antitrypsin gene. EMBO J. 1987 Sep;6(9):2759–2766. doi: 10.1002/j.1460-2075.1987.tb02570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejean A., Lugassy C., Zafrani S., Tiollais P., Brechot C. Detection of hepatitis B virus DNA in pancreas, kidney and skin of two human carriers of the virus. J Gen Virol. 1984 Mar;65(Pt 3):651–655. doi: 10.1099/0022-1317-65-3-651. [DOI] [PubMed] [Google Scholar]
- Dubois M. F., Pourcel C., Rousset S., Chany C., Tiollais P. Excretion of hepatitis B surface antigen particles from mouse cells transformed with cloned viral DNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4549–4553. doi: 10.1073/pnas.77.8.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders G. H., Ganem D., Varmus H. E. 5'-terminal sequences influence the segregation of ground squirrel hepatitis virus RNAs into polyribosomes and viral core particles. J Virol. 1987 Jan;61(1):35–41. doi: 10.1128/jvi.61.1.35-41.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farza H., Hadchouel M., Scotto J., Tiollais P., Babinet C., Pourcel C. Replication and gene expression of hepatitis B virus in a transgenic mouse that contains the complete viral genome. J Virol. 1988 Nov;62(11):4144–4152. doi: 10.1128/jvi.62.11.4144-4152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Chen T. N., Mandart E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J Virol. 1982 Jan;41(1):51–65. doi: 10.1128/jvi.41.1.51-65.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardon E. M., Frain M., Paonessa G., Cortese R. Two distinct factors interact with the promoter regions of several liver-specific genes. EMBO J. 1988 Jun;7(6):1711–1719. doi: 10.1002/j.1460-2075.1988.tb03000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L., Lubon H. Interaction of protein with DNA in vitro. Methods Enzymol. 1987;152:721–735. doi: 10.1016/0076-6879(87)52076-6. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Jose-Estanyol M., Danan J. L. A liver-specific factor and nuclear factor I bind to the rat alpha-fetoprotein promoter. J Biol Chem. 1988 Aug 5;263(22):10865–10871. [PubMed] [Google Scholar]
- Karpen S., Banerjee R., Zelent A., Price P., Acs G. Identification of protein-binding sites in the hepatitis B virus enhancer and core promoter domains. Mol Cell Biol. 1988 Dec;8(12):5159–5165. doi: 10.1128/mcb.8.12.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Hodnett J. L., Gray H. B., Jr Extracellular nucleases of pseudomonas BAL 31. III. Use of the double-strand deoxyriboexonuclease activity as the basis of a convenient method for the mapping of fragments of DNA produced by cleavage with restriction enzymes. Nucleic Acids Res. 1978 May;5(5):1445–1464. doi: 10.1093/nar/5.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie-Injo L. E., Balasegaram M., Lopez C. G., Herrera A. R. Hepatitis B virus DNA in liver and white blood cells of patients with hepatoma. DNA. 1983;2(4):301–308. doi: 10.1089/dna.1983.2.301. [DOI] [PubMed] [Google Scholar]
- Malpièce Y., Michel M. L., Carloni G., Revel M., Tiollais P., Weissenbach J. The gene S promoter of hepatitis B virus confers constitutive gene expression. Nucleic Acids Res. 1983 Jul 11;11(13):4645–4654. doi: 10.1093/nar/11.13.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaci P., Nicosia A., Cortese R. Two different liver-specific factors stimulate in vitro transcription from the human alpha 1-antitrypsin promoter. EMBO J. 1988 Jul;7(7):2075–2087. doi: 10.1002/j.1460-2075.1988.tb03047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982 Sep;42(9):3858–3863. [PubMed] [Google Scholar]
- Ou J. H., Rutter W. J. Regulation of secretion of the hepatitis B virus major surface antigen by the preS-1 protein. J Virol. 1987 Mar;61(3):782–786. doi: 10.1128/jvi.61.3.782-786.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. Inhibition of secretion of hepatitis B surface antigen by a related presurface polypeptide. Science. 1986 Dec 12;234(4782):1388–1391. doi: 10.1126/science.3787251. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Klinkert M. Q., Theilmann L., Schaller H. Characterization of large surface proteins of hepatitis B virus by antibodies to preS-S encoded amino acids. Virology. 1986 Jan 15;148(1):15–22. doi: 10.1016/0042-6822(86)90399-5. [DOI] [PubMed] [Google Scholar]
- Rall L. B., Standring D. N., Laub O., Rutter W. J. Transcription of hepatitis B virus by RNA polymerase II. Mol Cell Biol. 1983 Oct;3(10):1766–1773. doi: 10.1128/mcb.3.10.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymondjean M., Cereghini S., Yaniv M. Several distinct "CCAAT" box binding proteins coexist in eukaryotic cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):757–761. doi: 10.1073/pnas.85.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romet-Lemonne J. L., McLane M. F., Elfassi E., Haseltine W. A., Azocar J., Essex M. Hepatitis B virus infection in cultured human lymphoblastoid cells. Science. 1983 Aug 12;221(4611):667–669. doi: 10.1126/science.6867736. [DOI] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Nucleotide sequence of an infectious molecularly cloned genome of ground squirrel hepatitis virus. J Virol. 1984 Aug;51(2):367–375. doi: 10.1128/jvi.51.2.367-375.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y., Ben-Levy R. Multiple nuclear proteins in liver cells are bound to hepatitis B virus enhancer element and its upstream sequences. EMBO J. 1987 Jul;6(7):1913–1920. doi: 10.1002/j.1460-2075.1987.tb02451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul Y., Rutter W. J., Laub O. A human hepatitis B viral enhancer element. EMBO J. 1985 Feb;4(2):427–430. doi: 10.1002/j.1460-2075.1985.tb03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A. Hepatitis B virus DNA in Kaposi sarcoma. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4861–4864. doi: 10.1073/pnas.80.15.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring D. N., Rutter W. J., Varmus H. E., Ganem D. Transcription of the hepatitis B surface antigen gene in cultured murine cells initiates within the presurface region. J Virol. 1984 May;50(2):563–571. doi: 10.1128/jvi.50.2.563-571.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibbe W., Gerlich W. H. Structural relationships between minor and major proteins of hepatitis B surface antigen. J Virol. 1983 May;46(2):626–628. doi: 10.1128/jvi.46.2.626-628.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]