Abstract
Response to Gram-negative bacteria (GNB) is partially mediated by the recognition of GNB-derived endotoxin (E) by host cells. Potent host response to E depends on the sequential interaction of E with lipopolysaccharide binding protein (LBP), CD14, MD-2 and Toll-Like Receptor 4 (TLR4). While CD14 facilitates the efficient transfer of E monomers to MD-2 and MD-2·TLR4, activation of MD-2·TLR4 can occur in the absence of CD14, through an unknown mechanism. Here we show that incubation of purified E aggregates (Eagg, Mr ≥ 20 million) in phosphate buffered saline (PBS) with ≥ 0.1% albumin in the absence of divalent cations Ca2+ and Mg2+, yields E·albumin complexes (Mr ~70,000). E·albumin transfers E monomers to sMD-2 or sMD-2·TLR4 ectodomain (TLR4ecd) with a “Kd” of ~4 nM and induces MD-2·TLR4-dependent, CD14-independent cell activation with a potency only 10-fold less than that of monomeric E·CD14 complexes. Our findings demonstrate for the first time a mechanistic basis for delivery of endotoxin monomers to MD-2 and for activation of TLR4 that is independent of CD14.
Keywords: Albumin, Endotoxin, MD-2, Toll-Like Receptor 4, CD14
INTRODUCTION
Infection by even small numbers of Gram-negative bacteria (GNB) typically elicits a rapid and robust innate immune response, characterized by the production of pro-inflammatory cytokines (e.g., TNFα, IL-6) and the recruitment and activation of phagocytes at the site of infection.1 Endotoxin [E, Lipopolysaccharide (LPS), Lipooligosaccharide (LOS)] is a unique, abundant glycolipid located in the outer leaflet of the outer membrane of GNB (GNBom).2 Potent host inflammatory responses to endotoxin are mediated by Toll-like receptor 4 (TLR4) and its obligatory co-receptor MD-2, which are expressed on the surface of multiple host cell types.3, 4 While exposure to endotoxin facilitates the mobilization of the immune system against invading GNB, continued exposure to high concentrations of endotoxin or the inability to resolve endotoxin-induced inflammation, can result in severe immunopathologies such as sepsis.5, 6
Potent host response to endotoxin involves the ordered interactions of endotoxin with Lipopolysaccharide Binding Protein (LBP), membrane-bound CD14 (mCD14) or soluble CD14 (sCD14) and extracellular soluble MD-2 (sMD-2) or MD-2 bound to TLR4 (MD-2·TLR4).3, 7–14 LBP binds with high affinity to endotoxin-rich surfaces [e.g., GNBom, aggregates of purified endotoxin (Eagg)], and likely alters the arrangement of endotoxin within this interface.10, 15 LBP increases the exposure of the normally concealed hydrophobic lipid A moiety of endotoxin, catalyzing extraction of individual endotoxin molecules by soluble or membrane associated CD14.16–19 This results in the formation of monomeric E·CD14.7, 10 E·CD14 can then rapidly deliver the endotoxin monomer to sMD-2 or to MD-2·TLR4, which results in cellular activation.8, 11, 12, 14, 20
While CD14 is required for maximal potency of response to endotoxin, activation of TLR4 by endotoxin can occur in the absence of CD14 through an unknown mechanism.21–24 Given what is known about the requirements for activation of MD-2·TLR4 by endotoxin, it is likely that a CD14-indepenent mechanism also requires the extraction and transfer of an endotoxin monomer from endotoxin-rich interfaces (e.g., GNBom, Eagg) to MD-2·TLR4.8, 20, 25 This could involve an LBP-independent mechanism to destabilize packing of endotoxin monomers in endotoxin-rich surfaces and a host protein that, like CD14, acts as an endotoxin monomer acceptor/donor (i.e., “CD14-surrogate”). This CD14-surrogate would be capable of shuttling endotoxin monomers from disrupted interfaces to MD-2(·TLR4), thereby activating TLR4.
We now show that albumin can serve as a CD14 surrogate for delivery of endotoxin monomers to MD-2(·TLR4) and induces CD14-independent TLR4 activation. The rate-limiting step is extraction and transfer of endotoxin monomers from endotoxin-rich interfaces, as in the CD14-dependent pathway. Unlike CD14, transfer of endotoxin monomers to albumin is not promoted by LBP but rather by depleting the divalent cations (Ca2+ and Mg2+) necessary to stabilize the dense packing of endotoxin monomers within endotoxin-enriched supra-molecular assemblies.26–28 These results provide new insights concerning the mechanism and molecular requirements of endotoxin recognition by MD-2·TLR4.
EXPERIMENTAL PROCEDURES
Materials
LBP and sCD14 were gifts from Xoma (Berkeley, CA) and Amgen Corp. (Thousand Oaks, CA), respectively. Acyloxyacyl hydrolase (AOAH) was a gift from Dr. R. Munford (NIAID, Bethesda, MD). Human serum albumin (HSA) was obtained as an endotoxin-free 25% stock solution from Baxter Healthcare (Glendale, CA). HEK293 cells stably expressing TLR4 (HEK TLR4) and their parental cell line (HEK293) were a gift from Dr. J. Chow (Eisai Research Institute, Andover, MA). L-929 cell conditioned medium was generated as previously described.29 [3H]LOS (25,000 cpm/pmol) was isolated from an aceE mutant of Neisseria meningitidis serogroup B after metabolic labeling as described previously.30 [14C]LPS, Rc and S chemotypes, were isolated in the same way after metabolic labeling of aceE Escherichia coli CL99 (a galE mutant of E. coli O111:B4) during growth in medium supplemented with 1,2-[14C]acetate ± 2 mM galactose (manuscript in preparation).31 Chromatography matrices (Sephacryl HR S200 and Ni2+ FF-Sepharose) were purchased from GE Healthcare (Piscataway, NJ). Anti-FLAG M2-agarose was purchased from Sigma. Monoclonal antibody (mAb) specific for human serum albumin (anti-HSA) [15C7] (ab10241) and control mouse IgG2b [MPC-11] (ab18469) were purchased from Abcam.
Production of Recombinant Proteins
Recombinant human FLAG-TLR4 ectodomain was generated by transient transfection of HEK293T cells as previously described.14 In brief, HEK293T cells were grown in Dulbecco’s modified eagle’s medium (DMEM) supplemented with 10% fetal calf serum. Cells in T75 flasks (~80% confluent) were transfected with 12 μg of an expression vector containing cDNA encoding amino acids 24-631 of TLR4, corresponding to the predicted ectodomain of TLR4 (TLR4ecd) linked to a FLAG tag (pFLAG-CMV-TLR4ecd), using PolyFect reagent (Qiagen). After 12 h, flasks were rinsed in PBS and 8 ml of serum-free medium (293 SFM, Invitrogen) was added. Medium containing expressed protein was collected 24–48 h later. Medium was concentrated 10–20 fold using Millipore Centricon Plus-70 prior to use.
Recombinant human His6-MD-2 was generated as previously described.15 Briefly, cDNA encoding MD-2 was inserted into pBAC3 (Novagen) to promote the secretion of MD-2 linked to a His6 tag. The generated baculovirus was then amplified in Sf9 cells and used to infect High Five™ (Invitrogen) insect cells in serum-free medium for protein production. Conditioned medium containing secreted His6-MD-2 was used to generate LOS·MD-2 in co-capture experiments and bioassays.
Preparation of [3H]LOSagg and [3H]LOS·protein complexes
[3H]LOS (or LPS)agg, [3H]LOS·sCD14 and [3H]LOS·MD-2 were generated as previously described.8–10, 30 Briefly, [3H]LOSagg (Mr ≥ 20 million) was obtained after hot phenol extraction of [3H]LOS followed by ethanol precipitation of [3H]LOSagg and ultracentrifugation. Monomeric [3H]LOS·sCD14 (Mr ~60,000) was prepared by treatment of [3H]LOSagg for 30 min at 37°C with substoichiometric amounts of LBP (molar ratio 200:1 of LOS:LBP) and equimolar (to LOS) amounts of sCD14 followed by size-exclusion chromatography (Sephacryl S200, 1.6 × 70 cm column) in PBS, pH 7.4, 0.03% HSA to isolate monomeric [3H]LOS·sCD14 complexes. [3H]LOS·MD-2 (Mr ~25,000) was generated by treatment of [3H]LOS·sCD14 with ESF921 (Expression Systems) insect cell conditioned media containing His6-MD-2 for 30 min at 37°C followed by isolation of [3H]LOS·MD-2 by S200 chromatography. [3H]LOS·albumin was generated from sonicated (15 min) [3H]LOSagg that were then incubated overnight at 37°C in PBS (no Ca2+/Mg2+) supplemented with 1.0% HSA. Alternatively, sonicated [3H]LOSagg were incubated overnight at 37°C in 100 mM Tris-HCl/5 mM EDTA (pH 8.0) supplemented with 1.0% HSA. [3H]LOS·albumin was isolated by size-exclusion chromatography (Sephacryl S200).
Co-capture of [14C]LOS·albumin with anti-HSA IgG
Mouse monoclonal anti-HSA (non-reactive with bovine serum albumin) or isotype-matched control mouse IgG2b was coated on 96-well plates at a concentration of 30 μg/ml in 100 μl of 0.1 M sodium carbonate buffer (pH 9.5) by incubation overnight at 4°C with shaking. The buffered solution was then removed and the coated wells washed × 3 with PBS/0.05% Tween-20 (200 μl) followed by incubation for 2 h at 25 °C with 100 μl of 3 nM [14C]LOS·albumin or 3 nM [14C]LOS·MD-2 in PBS/1.0% BSA. After this incubation, the supernatant containing [3H]LOS·protein complexes that did not bind to the antibody-coated well was removed from the well and transferred to additional antibody-coated wells to increase the anti-HSA mAb-dependent absorption of [3H]LOS·albumin. This was repeated for a total of 5 incubations. After the final incubation, the supernatant was removed and 100 μl of aqueous 5% SDS solution was added to each of the washed wells that had been exposed to [3H]LOS·protein complex. The plates were then warmed on a heating block for 10 min to elute the bound [3H]LOS·protein. The radioactivity in the recovered eluate was measured via liquid scintillation spectroscopy.
AOAH treatment of [3H]LOSagg and [3H]LO-S·protein complexes
[3H]LOSagg or the indicated [3H]LOS·protein (0.5 ng LOS) complexes were incubated in Hank’s buffered salt solution with Ca2+ or Mg2+ (pH 7.4), 0.1% HSA with or without AOAH (7.5 nU/sample, 5.0 ng/μl) for 2 h at 37°C. The extent of deacylation of [3H]LOS by AOAH was monitored by separation of released [3H]-free fatty acids from partially deacylated and remaining intact [3H]LOS by ethanol precipitation of the latter. Ethanol-soluble radioactivity representing released [3H]fatty acids was measured by liquid scintillation spectroscopy. AOAH can release only two of the six fatty acids in LOS.15 Hence, the extent of partial deacylation of [3H]LOS by AOAH was calculated as % of total cpm recovered in the ethanol-soluble fraction × 3.
HEK293 Activation Assay
HEK293 TLR4 cells or their parental cell line were seeded in a 96-well plate (1×105 cells/well) in DMEM/10%FBS/Ciprofloxacin (10 μg/ml) and incubated overnight at 37°C in 5% CO2 and 95% humidity. The following day, cells were washed twice with warm PBS (pH 7.4) and the indicated treatments were added in 200μl of DMEM/0.1% HSA. The cells were incubated with the indicated treatments for 18 h at 37°C. Activation of cells was assessed by measuring accumulation of extracellular IL-8 by ELISA (BD Biosciences).
Alternatively, HEK293 TLR4 cells were seeded in a 96-well plate (5×104 cells/well) in DM-EM/10% FBS/Ciprofloxacin (10 μg/ml) and incubated overnight at 37°C in 5% CO2 and 95% humidity. The following day, the incubation medium was aspirated and cells were transfected with 0.5 μg/well of either empty vector (pCis-CK) or an MD-2 expression vector (MD-2-FLAG-His6; pEF-BOS) with PolyFect reagent (Qiagen) per manufacturer’s protocol.32 The following day, the transfection medium was aspirated, 200 μl/well of fresh DMEM/10% FBS/Ciprofoxacin was added and the cells were incubated for an additional 24 h to allow for maximal expression of plasmid products. The cells were then washed and treated as described above.
Preparation and activation of CD14KO Bone Marrow Derived Macrophages (BMDM)s by LOSagg + LBP and LOS·protein complexes
Preparation of BMDM was carried out as previously described.29, 33 Briefly, tibias and femurs from C57BL/6 CD14KO mice were removed following isoflurane asphyxiation and cervical dislocation and the bones were flushed with DMEM using a 23-gauge needle. The flushed cells were grown on 100 × 15 mm dishes in DMEM/10% Heat-inactivated FBS/20% L-929 cell condition medium/Ciprofloxacin (10 μg/ml) for 7 d at 37°C in 5% CO2. On day 7, growth medium was aspirated, cells were dislodged by incubation with versene, seeded in a 48-well plate (2.5×105 cells/well) in DMEM/10% Heat-inactivated FBS/Ciprofloxacin (10 μg/ml), and incubated overnight at 37°C. The following day, cells were washed twice with warm PBS (pH 7.4) and incubated with the indicated treatments for 18 h at 37°C in DMEM/0.1% HSA. Activation of cells was assessed by measuring accumulation of extracellular TNF by ELISA (BD Biosciences).
Reaction of His6-sMD-2 or His6-sMD-2/FLAG-TLR4ecd with either [3H]LOS·albumin or [3H]LOS·sCD14
[3H]LOS·albumin or [3H]LOS·sCD14 were incubated for 3 h at 37 °C at the indicated concentrations with insect cell conditioned medium containing or lacking His6-sMD-2 (4 μl, 0.2 nM active protein), or a combination of 8 μl of insect cell medium containing MD-2 with 25 μl of HEK293 cell medium containing FLAG-TLR4ecd (His6-sMD-2/FLAG-TLR4ecd, 0.15 nM active complex) brought to 0.2 ml (His6-sMD-2 samples) or 0.4 ml (His6-sMD-2/FLAG-TLR4ecd samples) with PBS (no Ca2+/Mg2+)/1.0% HSA, pH 7.4. Following this incubation Ni2+ FF-Sepharose beads (30 μl) to capture [3H]LOS·His6-sMD-2 or anti-FLAG M2-agarose beads (30 μl) to capture [3H]LOS-·His6-sMD-2/FLAG-TLR4ecd were added and incubated at 25°C with rotation for 45 min. Following this incubation, beads were spun-down via centrifugation at 1000×g for 2 min. The supernatant was collected and beads were washed 2x in PBS (no Ca2+/Mg2+)/1.0% HSA, pH 7.4. [3H]LOS was quantified in the recovered supernatant, wash and beads via liquid scintillation spectroscopy. Radioactivity recovered in the beads was converted to molar amounts of product (i.e., [3H]LOS·MD-2 or ([3H]LOS·MD-2·TLR4ecd)2 formed based on the known specific radioactivity of the [3H]LOS.14 There was no specific capture of [3H]LOS from [3H]LOS·albumin or [3H]LOS·CD14 by anti-FLAG M2 agarose when incubations were carried out with media containing FLAG-TLR4ecd alone. Binding and Scatchard analyses were performed using Prism 5 (Graphpad) Software as described previously.14
RESULTS
LBP/CD14-independent disaggregation of LOSagg and formation of LOS·sMD-2 are promoted by limiting divalent cations (Ca2+/Mg2+)
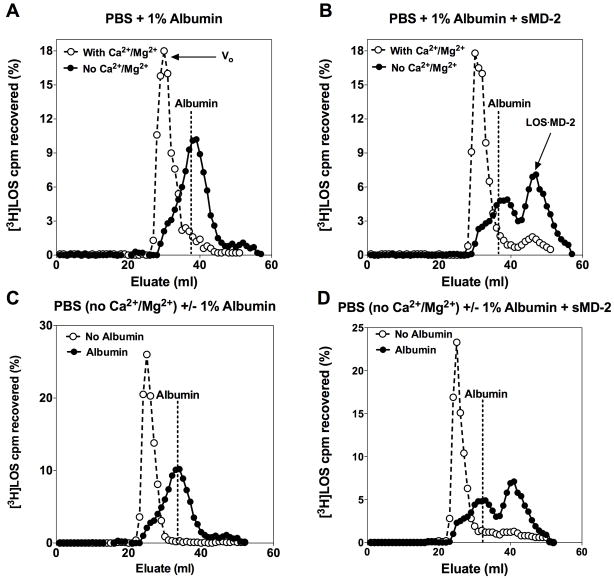
The dense packing of endotoxin monomers within the GNBom or Eagg depends on the presence of Ca2+ and/or Mg2+ to reduce electrostatic repulsion between neighboring polyanionic endotoxin molecules.26–28, 34 Binding of these divalent cations to endotoxin can be reduced by either treatment of endotoxin-rich surfaces with Tris/EDTA (pH 8.0) or by prolonged exposure to media depleted of these divalent cations.35–37 We initially chose the latter conditions to determine whether limiting divalent cations could lead to LBP/CD14-independent disaggregation of purified high molecular weight LOSagg and transfer of an endotoxin monomer to sMD-2. To facilitate quantitative analysis, we utilized uniformly radiolabeled [3H]LOS (Mr ≥ 20 million) sonicated for 15 min and then further incubated at 37°C in PBS ± Ca2+/Mg2+ (see Experimental Procedures) and supplemented with 1.0% HSA. Size-exclusion (Sephacryl S200) chromatography was utilized to determine the aggregation state of the [3H]LOS (Fig. 1A). In the presence of divalent cations, nearly all of the [3H]LOS eluted in the void volume (V0), reflecting [3H]LOSagg of Mr > 250,000. However, in conditions of limiting Ca2+ and Mg2+, more than half of the [3H]LOS eluted later, at approximately the same elution volume as albumin alone (Fig. 1A). Thus, conversion of the large [3H]LOSagg (Mr ≥ 20 million) to much smaller (Mr ~70,000) complexes was greatly enhanced by incubation in medium lacking Ca2+ and Mg2+. Maximum conversion of the large LOS aggregates to Mr ~70,000 required incubations for up to 18 h (Fig. S1). All subsequent incubations intended to generate the Mr ~70,000 complex were therefore carried out for 18 h.
Fig. 1.
LBP/CD14-independent disaggregation of [3H]LOSagg and formation of [3H]LOS·MD-2 requires limiting divalent cations and the presence of albumin. Purified [3H]LOSagg (2 nM) were incubated in PBS with or without Ca2+/Mg2+ for 18 h at 37°C in the presence of 1.0% HSA (A,B) followed by 3 h incubation at 37°C with added sMD-2-containing insect cell conditioned medium (100 ng active protein/ml incubation mixture) (B). Purified [3H]LOSagg (2 nM) were incubated in PBS without Ca2+/Mg2+ for 18 h at 37°C in the presence or absence of 1.0% albumin (C,D) followed by 3 h incubation at 37°C with added sMD-2-containing insect-cell conditioned medium (see above) (D). Products were resolved via size-exclusion (Sephacryl S200) chromatography utilizing a 1.6/30 cm column. Radioactivity ([3H]LOS) in the recovered fractions was analyzed via liquid scintillation counting. Data shown are representative of > 3 independent experiments with overall [3H]LOS recovery ≥ 70%. Dotted lines indicate peak of elution volume of albumin. Void-volume (V0, ~30 mL), representing [3H]LOSagg is indicated in (A). Arrow indicates elution of the [3H]LOS·MD-2 complex peak at 47 mL in (B) and (D).
To test whether limiting divalent cations also promoted LBP/CD14-independent transfer of endotoxin monomers to sMD-2, [3H]LOS was incubated with insect cell conditioned medium containing sMD-2 after pre-incubation of [3H]LOS in PBS/1.0% HSA/± Ca2+ and Mg2+. A new, later eluting (i.e. lower Mr) complex containing [3H]LOS was formed to a much greater extent after the [3H]LOSagg were pre-incubated in PBS without divalent cations (Fig. 1B). This later eluting complex corresponded to monomeric [3H]LOS·MD-2 (Mr ~25,000) as determined by: 1) co-elution with purified LOS·MD-2; 2) absence of this complex when [3H]LOSagg pre-incubated with PBS/1.0% HSA/± Ca2+ and Mg2+ were incubated in control insect cell conditioned medium lacking sMD-2 (Fig. S2A); and 3) dose-dependent activation of HEK TLR4 cells with potency equal to that of purified LOS·MD-2 (Fig. S2B). These data show that under conditions LOSagg are destabilized when divalent cations are limiting, and can form smaller complexes similar in size to albumin. These smaller endotoxin-containing complexes are capable of delivering an E monomer to sMD-2, thus forming LOS·MD-2.
LBP/CD14-independent disaggregation of LOSagg and formation of LOS·MD-2 requires presence of albumin
Albumin plays an essential role in LBP/CD14-dependent activation of MD-2·TLR4 by facilitating LBP-catalyzed extraction and transfer of endotoxin monomers to CD14 from endotoxin-rich surfaces and subsequent transfer of the endotoxin monomer from CD14 to sMD-2 or MD-2·TLR4.10 To test whether albumin was necessary for the observed LBP/CD14-independent disaggregation of LOSagg and formation of LOS·MD-2, these experiments were repeated in the presence or absence of albumin (Fig 1C, D). As shown, albumin is required for both conversion of [3H]LOSagg to smaller (Mr ~70,000) complexes (Fig. 1C) and formation of monomeric [3H]LOS·MD-2 (Fig. 1D), from [3H]LOSagg incubated in PBS without Ca2+ and Mg2+ in the presence of sMD-2. This demonstrates that generation of the Mr ~70,000, endotoxin-containing complex depends on both the destabilization of LOSagg by limiting divalent cations and the presence of albumin (Fig. 1). A similar conversion of the large LOSagg complexes to the smaller Mr ~70,000 complex was observed following incubation of the [3H]LOSagg in a divalent cation-chelating buffer, 100 mM Tris-HCl/5mM EDTA (pH 8.0), supplemented with albumin (Fig. S1). Near maximal generation of the Mr 70,000 complex was achieved with molar ratios of albumin to LOS as low as ~1:1 after overnight incubation at 37°C (data not shown). Aggregates of rough (Rc chemotype) and smooth (S chemotype) LPS similarly generated a Mr ~70,000 complex under the above conditions (Fig. S3A,B), which could react with sMD-2, independent of LBP/CD14, to form LPS·MD-2 complexes (Fig. S3C). Whereas LBP catalytically promotes extraction and transfer of endotoxin monomers from endotoxin aggregates to CD14, extraction and transfer of endotoxin monomers to albumin was not promoted by the presence of LBP (Fig. S4).10, 15, 17
Mr ~70,000 complex is the donor of [3H]LOS monomers to sMD-2
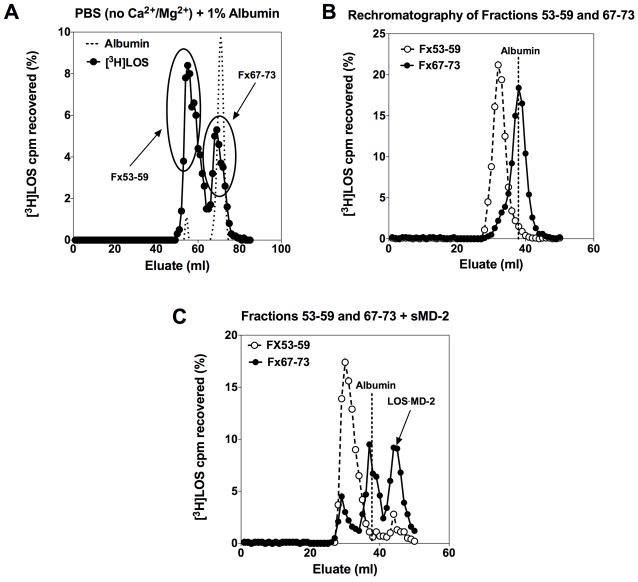
To determine if the Mr ~70,000 complex could directly transfer endotoxin monomers to sMD-2, this complex was isolated by Sephacryl S200 chromatography after pre-incubation of [3H]LOSagg in PBS/1.0% HSA (no Ca2+/Mg2+). A longer (1.6 × 7.0 cm) S200 column was used to improve resolution of the Mr ~70,000 complex (second [3H]LOS-containing peak) from the larger [3H]LOSagg eluting in the V0 (Fig. 2A). Pooled fractions from the up- and down-slopes of the first (Fx 53-59, corresponding to the V0) and second (Fx 67-73) [3H]LOS-containing peaks were re-analyzed by Sephacryl S200 chromatography to confirm the stability and enrichment of the larger [3H]LOSagg in the V0 and the Mr ~70,000 complex in the second peak (Fig. 2B). These pooled fractions were then incubated with sMD-2 at 37°C and the formation of monomeric [3H]LOS·MD-2 was evaluated by Sephacryl S200 chromatography (Fig. 2C). The pooled [3H]LOS-containing fractions (Fx 67-73) highly enriched in the Mr ~70,000 complex reacted with sMD-2 to form monomeric [3H]LOS·MD-2 (Mr ~25,000). In contrast, very little [3H]LOS·MD-2 was formed under the same incubation conditions when the pooled fractions (Fx 53-59) highly enriched in larger [3H]LOSagg were used (Fig. 2C). Under these conditions, the Mr ~70,000 complex was the preferred donor of monomeric [3H]LOS to sMD-2. The small amount of [3H]LOS·MD-2 formed when incubations were carried out with pooled fractions 53–59 may reflect contaminating, incompletely resolved Mr ~70,000 complex (Fig. 2C).
Fig. 2.
Mr ~70,000 complex is a CD14-independent donor of [3H]LOS monomer(s) to sMD-2, yielding [3H]LOS·MD-2. Purified [3H]LOSagg (2 nM) were incubated in PBS (no Ca2+/Mg2+)/1.0% HSA for 18 h at 37°C (A) and reaction products were resolved via size-exclusion (Sephacryl S200) chromatography utilizing a 1.6/70 cm column. The indicated pooled fractions from peaks corresponding to LOSagg or Mr ~70,000 were collected. To test for the purity and stability of these two different [3H]LOS-containing species, an aliquot of each pool was analyzed by S200 size-exclusion chromatography utilizing a 1.6/30 cm column (B). An aliquot of the pooled fractions were then incubated with (see Fig. 1 legend) sMD-2-containing insect-cell conditioned medium for 3 h at 37°C. The reaction products were resolved utilizing Sephacryl S200 (1.6/30 cm column) (C). Data shown are representative of > 3 independent experiments with [3H]LOS recovery at ≥ 75%. Dotted lines indicate peak of albumin elution (A,B,C). Arrow indicates elution of the [3H]LOS·MD-2 complex in (C).
The Mr ~70,000 complex contains E (LOS) monomer(s) complexed to albumin (LOS·albumin)
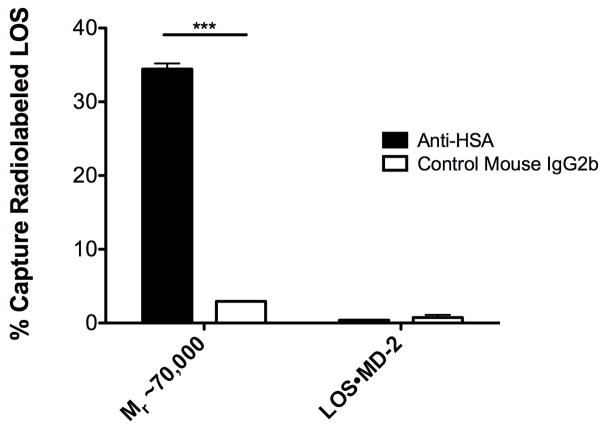
The dependence for generation of the Mr ~70,000 complex on the presence of albumin and the similar elution profile under size exclusion chromatography to that of albumin (Fig. 2A, B) strongly suggested that the Mr ~70,000 peak represents a complex of [3H]LOS monomer(s) (Mr ~5,000) and albumin (Mr ~65,000). To test this possibility more directly, the Mr ~70,000 complex was purified followed by assay of the ability of radiolabeled LOS in this complex to be captured by an immobilized anti-HSA mAb compared to an unrelated, isotype-matched mouse IgG2b. As shown in Figure 3, the anti-HSA mAb captured nearly 40% of the radiolabeled LOS contained in the added Mr ~70,000 complex (i.e., pooled fractions of 67–73; Fig. 2A), while virtually no capture was seen in wells coated with control mouse IgG2b (Fig. 3). There was no capture of [3H]LOS·MD-2 by immobilized anti-HSA (or by the control mouse IgG2b; Fig. 3), further demonstrating the specificity of the co-capture by anti-HSA mAb of the radiolabeled LOS in the Mr ~70,000 complex.
Fig. 3.
Specific co-capture of radiolabeled LOS in the Mr ~70,000 complex with anti-HSA antibody. Purified fractions of radiolabeled Mr ~70,000 or LOS·MD-2 (3 nM) were incubated in 96-well plates pre-coated with either anti-HSA Ab or a control mouse IgG2b as described in Experimental Proceedures. Absorbed radiolabeled LOS was eluted with 2% SDS and measured via liquid scintillation spectroscopy. Data is expressed as % capture of total radiolabeled LOS added. *** = p < 0.0001, as calculated by student’s t-test. Data shown are representative of > 3 independent experiments.
LOS in the Mr ~70,000 complex (LOS·albumin) is susceptible to deacylation by acyloxyacyl hydrolase (AOAH)
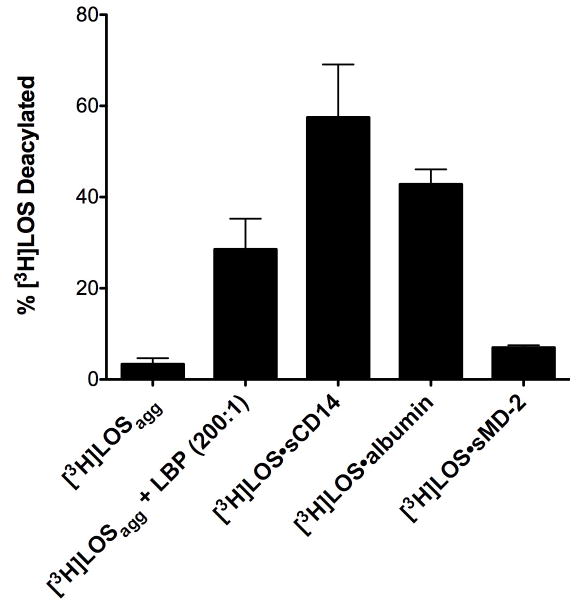
In the LBP/CD14-dependent pathway of TLR4 activation by endotoxin, endotoxin monomer transfer occurs from Eagg or GNBom treated with very low amounts of LBP (e.g., E:LBP, 200:1 mol/mol) to CD14 and from monomeric E·CD14 to sMD-2 (or to MD-2·TLR4). At each of these steps, endotoxin in the endotoxin donor (i.e., E·LBP (200:1), E·CD14) shows markedly increased susceptibility to the deacylase AOAH.15 This may reflect increased accessibility of the lipid A fatty acyl chains to AOAH, a property which may favor endotoxin monomer transfer from one endotoxin binding protein to another. The ability of albumin to deliver an endotoxin monomer to MD-2 shown above (Fig. 2C) indicates that endotoxin (LOS) is bound to albumin in a way that makes endotoxin monomer transfer possible and might therefore manifest increased susceptibility to AOAH. As previously shown, LOS within unmodified LOS aggregates are relatively refractory to AOAH (Fig. 4) under physiological buffer conditions (see Experimental Procedures).15 In contrast, LOS in the Mr ~70,000 complex (LOS·albumin), as in other complexes (e.g., LOSagg·LBP (200:1) and LOS·CD14) that favor LOS monomer transfer, is much more susceptible to AOAH (Fig. 4). Note that the [3H]LOS within the monomeric LOS·MD-2 complex is also relatively insensitive to AOAH, paralleling the much less facile transfer of LOS from MD-2 and consistent with the sequestration of the acyloxyacyl-linked fatty acyl chains of endotoxin within the hydrophobic cavity of MD-2.20, 25
Fig. 4.
LOS in the Mr ~70,000 complex is susceptible to deacylation by AOAH. [3H]LOS or the indicated [3H]LOS·protein (0.5 ng LOS) complexes were incubated in HBSS/0.1% HSA with AOAH for 2 h at 37°C. The extent of LOS deacylation was determined as described in Experimental Procedures. Radioactivity recovered in the supernatant after ethanol precipitation from [3H]LOS containing samples incubated without AOAH was < 5% of the total added radioactivity and was subtracted to calculate the % [3H]LOS deacylation by AOAH. The results shown represent the mean ± SEM of three or more determinations.
MD-2/TLR4 dependent, CD14-independent cell activation by LOS·albumin
The ability of LOS·albumin to deliver endotoxin (LOS) monomers to sMD-2 (Fig. 1, 2), generating bioactive LOS·MD-2 (Fig. S2) strongly suggested that LOS·albumin complexes could provide a CD14-independent mechanism for cell activation. To test this hypothesis, we made use of HEK293 cells (which lack MD-2 & CD14) and tested the ability of LOS·albumin to activate HEK cells that stably express TLR4 (HEK TLR4) in the presence or absence of MD-2, monitoring secretion of IL-8 as an indicator of cell activation. Figure 5 shows that LOS·albumin induced robust activation of HEK TLR4 cells provided that MD-2 was also present, either as added sMD-2 in insect cell conditioned medium (Fig. 5A) or by transient transfection of HEK TLR4 cells with an expression vector encoding MD-2 (Fig. 5B). Little or no cell activation by LOS·albumin was observed toward either parental HEK293 cells lacking TLR4 regardless of whether sMD-2 was provided (Fig. 5A) or HEK TLR4 cells without sMD-2 (“empty vector”) or co-expressed MD-2 (Fig. 5B). These findings demonstrate that LOS·albumin can activate cells that lack CD14 in an MD-2·TLR4-dependent manner.
Fig. 5.
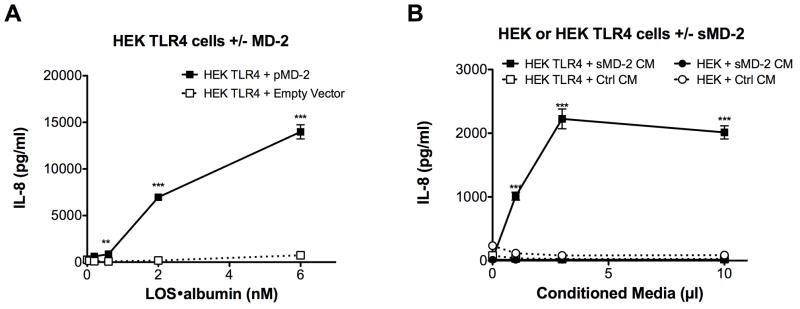
CD14-independent, MD-2 dependent activation of HEK/TLR4 by LOS·albumin. HEK293 cells stably expressing TLR4 (HEK TLR4) or the parental HEK293 cell line (HEK) were incubated with LOS·albumin (2 nM) and increasing amounts of either sMD-2-containing insect cell conditioned medium (sMD-2 CM) or insect cell conditioned medium lacking sMD-2 (Ctrl CM) (A) for 18 h at 37°C. Alternatively, HEK TLR4 cells were transiently transfected with either an expression vector encoding human MD-2 (HEK TLR4 + pMD-2) or an empty control vector (HEK TLR4 + Empty Vector). Following transfection, cells were incubated with increasing amounts of LOS·albumin for 18 h at 37°C (B). Accumulation of IL-8 in culture supernatants was quantified by ELISA as a marker of activation. *** = p < 0.0001, ** = p < 0.005, as calculated by t-test. Data shown are representative of > 3 independent experiments.
Comparison of LOS·albumin vs. LOS·sCD14 potency in MD-2·TLR4-dependent activation of HEK293 cells
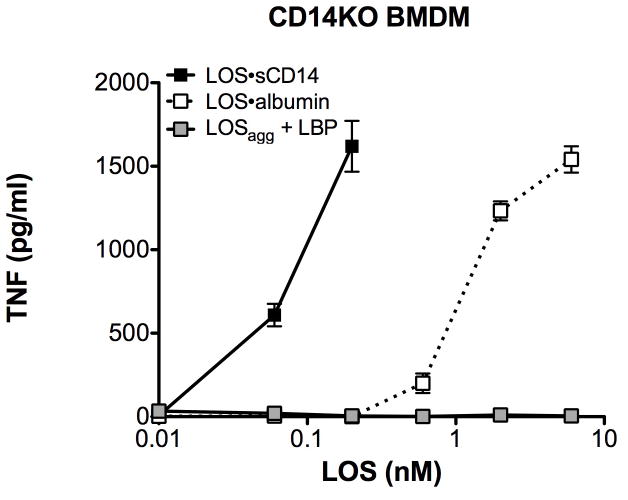
The above findings indicate that LOS·albumin, much like monomeric LOS·CD14 complexes, can induce MD-2·TLR4-dependent cell activation.7, 8 To better gauge the relative potency of the LOS·albumin complexes, we directly compared effects of increasing amounts of LOS·albumin vs. LOS·sCD14 on activation of HEK/TLR4 cells co-incubated with sMD-2 (Fig. 6A) or co-expressing MD-2 (Fig. 6B). Under these conditions, LOS·albumin was ~10-fold less potent than LOS·sCD14 in inducing MD-2·TLR4-dependent cell activation (e.g., levels of IL-8 produced, respectively, by cells stimulated with 2 and 6 nM LOS·albumin were roughly equal to that induced by 200 and 600 pM LOS·sCD14). In addition, BMDMs from CD14KO mice, which naturally express MD-2/TLR4, were activated by LOS·sCD14 and to a similar extent by a 10-fold higher concentration of LOS·albumin (Fig. 7). In contrast to LOS·sCD14 or LOS·albumin, LOSagg + LBP induced little or no activation of either HEK 293 cells expressing MD-2 and TLR4 or CD14KO BMDMs (Fig. 7), paralleling the inability of LOSgg + LBP to efficiently transfer LOS monomers to MD-2 or MD-2·TLR4 in the absence of CD14.8, 14
Fig. 6.
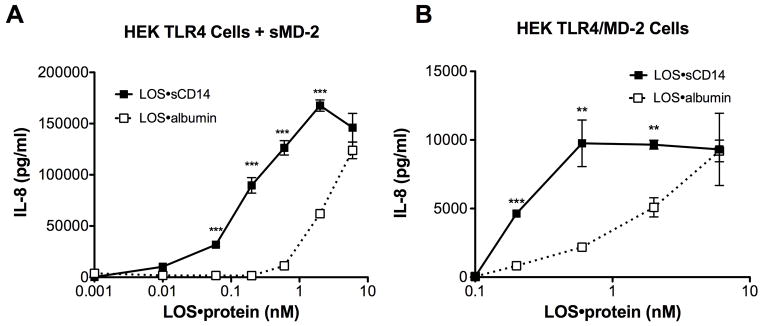
Comparison of LOS·albumin vs. LOS·sCD14 potency in MD-2·TLR4-dependent activation of HEK cells. HEK TLR4 cells were incubated with increasing amounts of LOS·sCD14 or LOS·albumin in the presence of sMD-2-containing insect cell conditioned medium (A). Alternatively, HEK TLR4 cells were transfected with an expression vector encoding human MD-2 (HEK TLR4/MD-2) and then incubated with increasing amounts of either LOS·sCD14 or LOS·albumin (B). Accumulation of IL-8 in culture supernatants was quantified by ELISA. *** = p < 0.0001, ** = p < 0.005, as calculated by t-test. Data shown are representative of > 3 independent experiments.
Fig. 7. CD14-independent activation of CD14KO BMDMs by LOS·albumin.
CD14KO BMDMs were incubated with increasing amounts of LOS·albumin, LOS·sCD14 or LOSagg + LBP (10 ng/ml of LBP) at 37°C for 18 h. Accumulation of TNF in the culture supernatants was quantified by ELISA. Results shown respresent mean ± SEM of triplicate samples.
Comparison of [3H]LOS monomer transfer from [3H]LOS·albumin or [3H]LOS·sCD14 to sMD-2(·TLR4ecd)
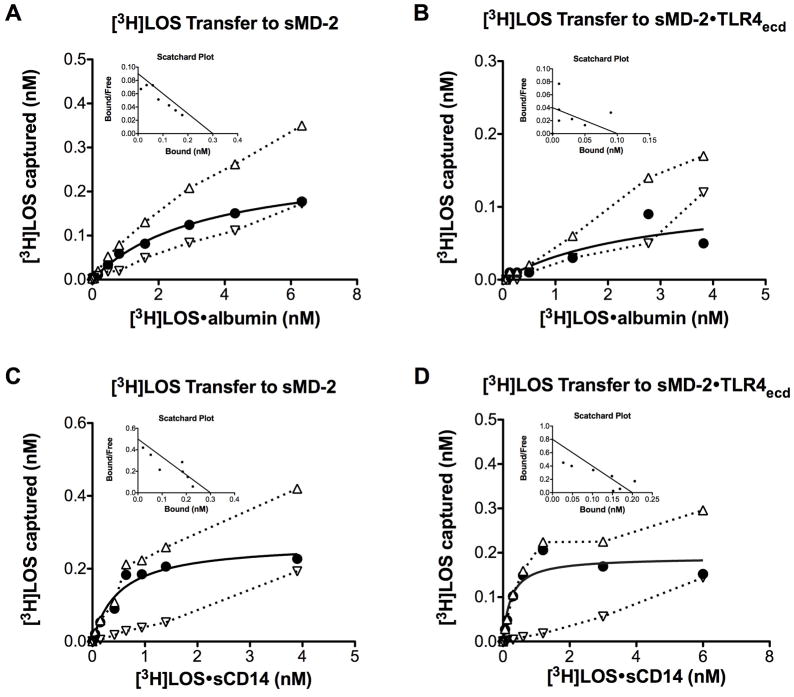
The lower potency of LOS·albumin vs. LOS·sCD14 in inducing MD-2·TLR4-dependent cell activation (Fig. 6) could reflect lower efficiency (e.g., higher Kd) of transfer of LOS monomer from LOS·albumin vs. LOS·sCD14 to sMD-2 and/or MD-2·TLR4. To test this hypothesis, we took advantage of our ability to sensitively, specifically, and quantitatively measure LOS transfer from [3H]LOS·albumin and from [3H]LOS·sCD14 to His6-sMD-2 and to His6-MD-2·FLAG-TLR4ecd by co-capture assays utilizing Ni2+ Sepharose beads to capture complexes containing His6-MD-2 or anti-FLAG Ab-coated agarose beads to capture complexes containing FLAG-TLR4ecd (Fig. 8).14, 32 Preliminary experiments demonstrated that the rate of [3H]LOS transfer was slower from [3H]LOS·albumin than from [3H]LOS·sCD14 (Fig. S5). Therefore, the apparent “Kd” of transfer was measured after 3 h incubation, a time sufficient for maximum transfer of LOS from both LOS·albumin and LOS·sCD14 to sMD-2 and sMD-2·TLR4ecd. Scatchard analysis indicated an apparent Kd of 4.0 ± 0.3 nM (n=4) for [3H]LOS transfer from [3H]LOS·albumin to His6-MD-2 (Fig. 8A) vs. an apparent Kd of 0.9 ± 0.2 nM (n=4) for [3H]LOS transfer from [3H]LOS·sCD14 to His6-MD-2. Similarly, the apparent Kd of [3H]LOS transfer to His6-MD-2·FLAG-TLR4ecd was higher (nearly 9-fold) from [3H]LOS·albumin (2.7 ± 0.1 nM; Fig. 8B) than from [3H]LOS·sCD14 (0.3 ± 0.1 nM) (Fig. 8D). The lower potency of LOS·albumin vs. LOS·sCD14 in stimulating MD-2·TLR4-dependent cell activation thus roughly parallels the lower efficiency of LOS transfer from LOS·albumin vs. LOS·sCD14 to sMD-2 and to sMD-2·TLR4ecd.
Fig. 8.
Analysis of [3H]LOS transfer from [3H]LOS·albumin or [3H]LOS·sCD14 to His6-sMD-2 or His6-sMD-2·FLAG-TLR4ecd. [3H]LOS capture to Ni2+ FF-sepharose was measured after incubation of increasing concentrations of [3H]LOS·albumin (A) or [3H]LOS·sCD14 (C) with either His6-sMD-2 (0.2 nM) containing insect cell conditioned medium (total capture, --△--) or control insect cell conditioned medium lacking sMD-2 (non-specific capture, --▽--) in PBS (no Ca2+/Mg2+)/1% HSA for 3 h at 37°C. [3H]LOS co-capture to anti-FLAG agarose was measured after incubation of increasing concentration of [3H]LOS·albumin (B) or [3H]LOS·sCD14 (D) with medium containing His-6-sMD-2·FLAG-TLR4ecd (0.15 nM, total capture, --△--) or medium containing FLAG-TLR4ecd (0.15 nM, non-specific capture, --▽--) alone. Specific capture (solid line) was calculated as the difference between total and non-specific capture and used for Scatchard analysis (figure inserts) as described in Experimental Procedures. Data shown are representative of > 3 independent experiments.
DISCUSSION
Previous work by our lab and others has shown that mammalian cell activation by endotoxin depends on the sequential interaction of endotoxin with specific host proteins and the delivery of endotoxin monomers to MD-2·TLR4.3, 9, 14, 19, 38, 39 The best studied and most efficient of these mechanisms involves formation of monomeric E·CD14 complexes in a process dependent on the presence of LBP and albumin.8, 10, 17, 19 However, activation of TLR4 by endotoxin can occur in the absence of CD14.21–24 Since activation of MD-2·TLR4 by endotoxin depends on the binding of a monomer of endotoxin to MD-2, this CD14-independent mechanism would still likely involve the alteration of endotoxin-rich surfaces to favor the extraction and binding of an endotoxin monomer by a CD14-surrogate host protein, which would then transfer the monomeric endotoxin to MD-2(·TLR4).3, 8, 11, 40, 41 Here we show for the first time a mechanistic basis for activation of MD-2·TLR4 by endotoxin in the absence of CD14 and LBP utilizing apparently monomeric E·albumin complexes.
It has been known for some time that albumin interacts with endotoxin, although the nature and physiological consequences of these interactions have remained relatively uncharacterized.42–45 Studies investigating the physical and chemical determinants of endotoxin association with albumin have suggested that binding of endotoxin to albumin is primarily mediated by hydrophobic interactions of the fatty acyl chains of lipid A with one or more hydrophobic fatty acid binding sites within albumin.42, 46 As such, the uptake of endotoxin by albumin may reflect albumin’s role as a relatively non-specific transporter of various hydrophobic molecules rather than a specific and dedicated endotoxin carrier function. Fluorescent displacement assays utilizing dansylsarcosine and warfarin have suggested that binding of lipid A by albumin occurs in domain III of the protein.42 Domain III contains two high-affinity fatty acyl chain-binding pockets, capable of accommodating long-chain (>12 C) fatty acids.47–50 However, the mechanism by which albumin gained access to the fatty acids of lipid A, which are normally sequestered within the GNBom or within aggregates/micelles of purified lipid A (endotoxin), and the biological properties of the resulting endotoxin·albumin complexes could not be elucidated from these earlier studies.15, 17, 19, 28
We have now shown that depletion or sequestration of the divalent cations necessary for the dense packing of the highly negatively charged endotoxin monomers within endotoxin-rich interfaces such as Eagg (Mr ≥ 20 million) favors the extraction and delivery of endotoxin to albumin and formation of stable E·albumin complexes (Mr ~70,000) (Figs. 1–3). Near maximal generation of the Mr ~70,000 E·albumin complex can occur with molar ratios of endotoxin to albumin approaching 1:1, suggesting that the Mr ~70,000 complex contains 1 mol of endotoxin (LOS, Mr ~5,000) with 1 mol of albumin (Mr ~65,000). Importantly, E·albumin is capable of donating endotoxin to sMD-2 or MD-2 bound to TLR4, forming bioactive E·MD-2(·TLR4) complexes (Fig 2, S2). Endotoxin bound to albumin is susceptible to partial deacylation by AOAH, as is endotoxin bound to CD14, suggesting the acyloxyacyl linkage of the branched fatty acids of the lipid A moiety of endotoxin is at least partially exposed to the aqueous environment when endotoxin is bound to either protein (Fig. 4).15 Partial exposure of lipid A acyl chains may be a necessary characteristic of endotoxin bound to proteins that promote transfer of endotoxin monomers to downstream endotoxin binding proteins which interact substantially with the acyl chains of lipid A, such as MD-2.15, 51 Indeed, like monomeric E·sCD14, apparently monomeric E·albumin complexes are capable of activating cells that express TLR4 in the presence of sMD-2 or cells that express MD-2·TLR4, in an LBP/mCD14-independent manner (Fig. 5).9 Taken together, these data show that albumin functions as a CD14-surrogate, binding endotoxin monomers released from endotoxin-rich interfaces and delivering those endotoxin monomers to MD-2(·TLR4), resulting in TLR4 activation.
Our HEK293 cell activation data show that E·albumin is ~10 fold less potent in its ability to activate MD-2·TLR4 than E·sCD14 (Fig. 6). This correlates with a ~4–10 fold higher apparent Kd of endotoxin transfer from E·albumin vs. E·sCD14 to MD-2(·TLR4) and slower transfer kinetics of endotoxin monomers to MD-2(·TLR4) (Fig. 8, S5). These differences may reflect more favorable endotoxin presentation by CD14 and/or a role for specific protein-protein (e.g., CD14-MD-2) contacts needed for initial docking of the endotoxin monomer donor to MD-2 and efficient endotoxin transfer.8, 52–54
The differences (~10-fold) in the potency of MD-2·TLR4 activation by E·albumin vs. E·sCD14 are not nearly as pronounced as differences (~1000-fold) previously observed in vitro and in vivo when comparing endotoxin potency in CD14-containing vs. CD14-free settings.22, 23, 55 One possible explanation is that, in those earlier studies, the rate-limiting step in CD14-independent pro-inflammatory action of endotoxin was the extraction and delivery of endotoxin monomers to albumin, rather than the activity per se of E·albumin. Our findings clearly show that neither albumin alone nor albumin in the presence of LBP promotes appreciable formation of bioactive monomeric E·albumin complexes (Fig. S4). Therefore, different biological conditions may be required for extraction and delivery of endotoxin monomers to albumin and subsequent E monomer delivery to MD-2 (Fig. 9). In our study, transfer of endotoxin monomers to albumin was accomplished by manipulating incubation media to deplete endotoxin-rich interfaces of stabilizing divalent cations. We speculate that similar disruption of endotoxin packing within the GNBom might be effected at extravascular neutrophil-rich infection sites where cationic antimicrobial proteins, such as BPI, cathelicidins and defensins, accumulate and target anionic groups in endotoxin that normally bind Ca2+ and Mg2+.56–61 The interactions of these proteins with endotoxin might also reduce LBP-endotoxin interactions, inhibiting LBP-dependent delivery of endotoxin to CD14 and thereby favoring transfer of endotoxin to albumin (which would be present in abundance).62 The lower levels of plasma lipoproteins at extravascular (vs. intravascular) sites could favor delivery of endotoxin monomers to MD-2(·TLR4) by reducing competing endotoxin binding reactions with lipoproteins.63 This may be particularly important for E·albumin complexes, given the slower rate of transfer of E monomers from albumin (vs. CD14) to MD-2 (Fig. S5).
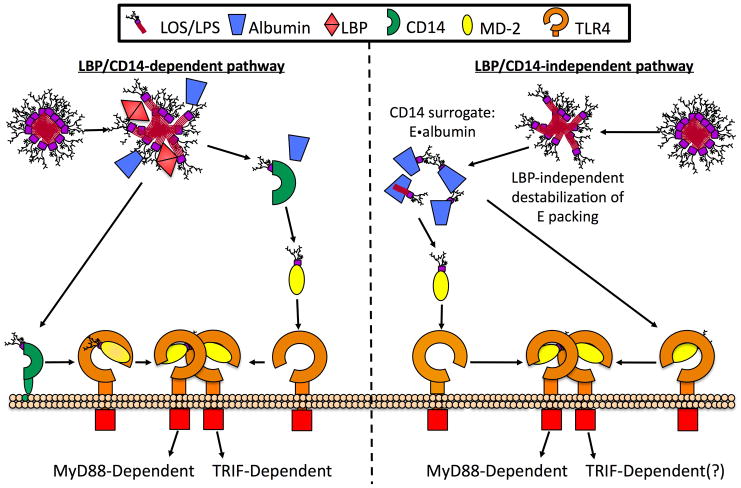
Fig. 9.
LBP/CD14-independent activation of host cells by E·albumin. Maximum potency of TLR4 activation occurs through the LBP/CD14-dependent pathway (on left) when LBP alters the dense packing of endotoxin within Eagg and catalyzes the extraction of an endotoxin monomer by mCD14 or sCD14, which then transfers those monomers to MD-2(·TLR4). Alternatively, LBP/CD14-independent activation of host cells by endotoxin (on right) can occur following destabilization of endotoxin aggregates by limiting or chelating divalent cations or perhaps by the action of host defense proteins such as the complement membrane attack complex or host cationic antimicrobial peptides. This leads to the extraction and binding of endotoxin by albumin. Albumin can serve as a CD14-surrogate by transferring endotoxin monomers to MD·2(·TLR4), resulting in activation of TLR4. Since activation of TLR4 by E·albumin occurs in the absence of CD14, it is possible that the resulting intracellular signaling would proceed predominantly through the MyD88-dependent pathway.
TLR4 is the only TLR capable of triggering two distinct intracellular signaling pathways: the MyD88-dependent pathway, which utilizes the adaptor molecules MyD88 and Mal and results in production of pro-inflammatory cytokines such as TNF; and the TRIF-dependent pathway, which signals through the adaptor molecules TRIF and TRAM and results in the production of type 1 interferons.64–67 The concerted action of these pathways helps produce an effective and appropriate immune response during most GNB infections. The mechanisms that govern the initiation and magnitude of activation of each TLR4 signaling pathway remain unclear. A role for CD14 in facilitating TRIF-dependent TLR4 signaling in response to endotoxin has been shown, but these observations have thus far been limited to macrophages from wt and CD14-null mice activated with a narrow range of endotoxin species (i.e., lipid A and Re LPS).68 The ability of E·albumin to activate MD-2·TLR4 independently of CD14, combined with the capacity of E·albumin to bind both R and S species of endotoxin (Fig. S3) should provide a means for a more comprehensive analysis of mammalian host cell TLR4 activation by endotoxin in the absence of CD14 and the effects of this stimulation on subsequent activation of TRIF and/or MyD88-dependent signaling.
Supplementary Material
Footnotes
This work was supported by U.S. Public Health Service Grant A105732 (to J.P.W.), a Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development grant (to T.L.G.), and the University of Iowa Dean’s Graduate Fellowship and a Training in Mechanisms of Parasitism grant T32 A10075 (to G.E.).
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3:169–176. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 3.Shimazu R, Akashi S, Ogata H, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarember KA, Godowski PJ. Tissue expression of human Toll-Like receptors and differential regulation of Toll-Like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 6.Doi K, Leelahavanichkul A, Yuen PST, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119:2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gioannini TL, Teghanemt A, Zarember KA, Weiss JP. Regulation of interactions of endotoxin with host cells. J Endotoxin Res. 2003;9:401–408. doi: 10.1179/096805103225002773. [DOI] [PubMed] [Google Scholar]
- 8.Gioannini TL, Teghanemt A, Zhang D, et al. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci USA. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gioannini TL, Teghanemt A, Zhang D, Levis EN, Weiss JP. Monomeric endotoxin:protein complexes are essential for TLR4-dependent cell activation. J Endotoxin Res. 2005;11:117–123. doi: 10.1179/096805105X35198. [DOI] [PubMed] [Google Scholar]
- 10.Gioannini TL, Zhang D, Teghanemt A, Weiss JP. An essential role for albumin in the interaction of endotoxin with lipopolysaccharide-binding protein and sCD14 and resultant cell activation. J Biol Chem. 2002;277:47818–47825. doi: 10.1074/jbc.M206404200. [DOI] [PubMed] [Google Scholar]
- 11.Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 12.Re F, Strominger JL. Monomeric recombinant MD-2 binds Toll-like receptor 4 tightly and confers lipopolysaccharide responsiveness. J Biol Chem. 2002;277:23427–23432. doi: 10.1074/jbc.M202554200. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler-Heitbrock HWL, Ulevitch RJ. CD14: Cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]
- 14.Prohinar P, Re F, Widstrom R, et al. Specific high affinity interactions of monomeric endotoxin. protein complexes with Toll-like receptor 4 ectodomain. J Biol Chem. 2007;282:1010–1017. doi: 10.1074/jbc.M609400200. [DOI] [PubMed] [Google Scholar]
- 15.Gioannini TL, Teghanemt A, Zhang D, et al. Endotoxin-binding proteins modulate the susceptibility of bacterial endotoxin to deacylation by acyloxyacyl hydrolase. J Biol Chem. 2007;282:7877–7884. doi: 10.1074/jbc.M605031200. [DOI] [PubMed] [Google Scholar]
- 16.Lee JD, Kravchenko V, Kirkland TN, et al. Glycosyl-phosphatidylinositol-anchored or integral membrane forms of CD14 mediate identical cellular responses to endotoxin. Proc Natl Acad Sci USA. 1993;90:9930–9934. doi: 10.1073/pnas.90.21.9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hailman E, Lichenstein HS, Wurfel MM, et al. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301–304. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- 19.Gioannini T, Weiss J. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol Res. 2007;39:249–260. doi: 10.1007/s12026-007-0069-0. [DOI] [PubMed] [Google Scholar]
- 20.Park BS, Song DH, Kim HM, Choi B-S, Lee H, Lee J-O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 21.Golenbock DT, Liu Y, Millham FH, Freeman MW, Zoeller RA. Surface expression of human CD14 in Chinese hamster ovary fibroblasts imparts macrophage-like responsiveness to bacterial endotoxin. J Biol Chem. 1993;268:22055–22059. [PubMed] [Google Scholar]
- 22.Haziot A, Ferrero E, Köntgen F, et al. Resistance to endotoxin shock and reduced dissemination of Gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 23.Lee JD, Kato K, Tobias PS, Kirkland TN, Ulevitch RJ. Transfection of CD14 into 70Z/3 cells dramatically enhances the sensitivity to complexes of lipopolysaccharide (LPS) and LPS binding protein. J Exp Med. 1992;175:1697–1705. doi: 10.1084/jem.175.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber M, Kalis C, Keck S, et al. R-form LPS, the master key to the activation ofTLR4/MD-2-positive cells. Eur J Immunol. 2006;36 (3):701–711. doi: 10.1002/eji.200535593. [DOI] [PubMed] [Google Scholar]
- 25.Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 26.Bayer ME, Leive L. Effect of ethylenediaminetetraacetate upon the surface of Escherichia coli. J Bacteriol. 1977;130:1364–1381. doi: 10.1128/jb.130.3.1364-1381.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shands JW, Chun PW. The dispersion of Gram-negative lipopolysaccharide by deoxycholate. Subunit molecular weight. J Biol Chem. 1980;255:1221–1226. [PubMed] [Google Scholar]
- 28.Snyder S, Kim D, McIntosh TJ. Lipopolysaccharide bilayer structure: effect of chemotype, core mutations, divalent cations, and temperature. Biochemistry. 1999;38:10758–10767. doi: 10.1021/bi990867d. [DOI] [PubMed] [Google Scholar]
- 29.Johnson CR, Kitz D, Little JR. A method for the derivation and continuous propagation of cloned murine bone marrow macrophages. J Immunol Methods. 1983;65:319–332. doi: 10.1016/0022-1759(83)90127-8. [DOI] [PubMed] [Google Scholar]
- 30.Giardina PC, Gioannini T, Buscher BA, et al. Construction of acetate auxotrophs of Neisseria meningitidis to study host-meningococcal endotoxin interactions. J Biol Chem. 2001;276:5883–5891. doi: 10.1074/jbc.M009273200. [DOI] [PubMed] [Google Scholar]
- 31.Goldman RC, White D, Orskov F, et al. A surface polysaccharide of Escherichia coli O111 contains O-antigen and inhibits agglutination of cells by O-antiserum. J Bacteriol. 1982;151:1210–1221. doi: 10.1128/jb.151.3.1210-1221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prohinar P, Rallabhandi P, Weiss JP, Gioannini TL. Expression of functional D299G. T399I polymorphic variant of TLR4 depends more on coexpression of MD-2 than does wild-type TLR4. J Immunol. 2010;184:4362–4367. doi: 10.4049/jimmunol.0903142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutterwala FS, Noel GJ, Clynes R, Mosser DM. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 1997;185:1977–1985. doi: 10.1084/jem.185.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schindler M, Osborn MJ. Interaction of divalent cations and polymyxin B with lipopolysaccharide. Biochemistry. 1979;18 (20):4425–4430. doi: 10.1021/bi00587a024. [DOI] [PubMed] [Google Scholar]
- 35.Elsbach P, Weiss J, Kao L. The role of intramembrane Ca2+ in the hydrolysis of the phospholipids of Escherichia coli by Ca2+-dependent phospholipases. J Biol Chem. 1985;260:1618–1622. [PubMed] [Google Scholar]
- 36.Leive L. A nonspecific increase in permeability in Escherichia coli produced by EDTA. Proc Natl Acad Sci USA. 1965;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akashi S, Shimazu R, Ogata H, et al. Cutting Edge: Cell surface expression and lipopolysaccharide signaling via the Toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 39.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 40.Resman N, Vasl J, Oblak A, et al. Essential roles of hydrophobic residues in both MD-2 and toll-like receptor 4 in activation by endotoxin. J Biol Chem. 2009;284:15052–15060. doi: 10.1074/jbc.M901429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teghanemt A, Zhang D, Levis EN, Weiss JP, Gioannini TL. Molecular basis of reduced potency of underacylated endotoxins. J Immunol. 2005;175:4669–4676. doi: 10.4049/jimmunol.175.7.4669. [DOI] [PubMed] [Google Scholar]
- 42.David SA, Balaram P, Mathan VI. Characterization of the interaction of lipid A and lipopolysaccharide with human serum albumin: implications for an endotoxin carrier function for albumin. J Endotoxin Res. 1995;2:99–106. [Google Scholar]
- 43.de Haas CJC, van Leeuwen HJ, Verhoef J, van Kessel KPM, van Strijp JAG. Analysis of lipopolysaccharide (LPS)-binding characteristics of serum components using gel filtration of FITC-labeled LPS. J Immunol Methods. 2000;242:79–89. doi: 10.1016/s0022-1759(00)00207-6. [DOI] [PubMed] [Google Scholar]
- 44.Galanos C, Rietschel ET, Lüderitz O, Westphal O, Kim YB, Watson DW. Biological activities of lipid A complexed with bovine-serum albumin. Eur J Biochem. 1972;31:230–233. doi: 10.1111/j.1432-1033.1972.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 45.Rietschel ET, Kim YB, Watson DW, Galanos C, Luderitz O, Westphal O. Pyrogenicity and immunogenicity of lipid A complexed with bovine serum albumin or human serum albumin. Infect Immun. 1973;8:173–177. doi: 10.1128/iai.8.2.173-177.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jürgens G, Müller M, Garidel P, et al. Investigation into the interaction of recombinant human serum albumin with Re-lipopolysaccharide and lipid A. J Endotoxin Res. 2002;8:115–126. doi: 10.1179/096805102125000263. [DOI] [PubMed] [Google Scholar]
- 47.Curry S, Mandelkow H, Brick P, Franks N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat Struct Biol. 1998;5:827–835. doi: 10.1038/1869. [DOI] [PubMed] [Google Scholar]
- 48.Petitpas I, Grüne T, Bhattacharya AA, Curry S. Crystal structures of human serum albumin complexed with monounsaturated and polyunsaturated fatty acids. J Mol Biol. 2001;314:955–960. doi: 10.1006/jmbi.2000.5208. [DOI] [PubMed] [Google Scholar]
- 49.Simard JR, Zunszain PA, Hamilton JA, Curry S. Location of high and low affinity fatty acid binding sites on human serum albumin revealed by NMR drug-competition analysis. J Mol Biol. 2006;361:336–351. doi: 10.1016/j.jmb.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 50.Sugio S, Kashima A, Mochizuki S, Noda M, Kobayashi K. Crystal structure of human serum albumin at 2. 5 Å resolution. Protein Eng Des Sal. 1999;12:439–446. doi: 10.1093/protein/12.6.439. [DOI] [PubMed] [Google Scholar]
- 51.Koraha J, Tsuneyoshi N, Kimoto M, Gauchat J-F, Nakatake H, Fukudome K. Comparison of Lipopolysaccharide-Binding Functions of CD14 and MD-2. Clin Diagn Lab Immunol. 2005;12:1292–1297. doi: 10.1128/CDLI.12.11.1292-1297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juan TS, Hailman E, Kelley MJ, Wright SD, Lichenstein HS. Identification of a domain in soluble CD14 essential for lipopolysaccharide (LPS) signaling but not LPS binding. J Biol Chem. 1995;270:17237–17242. doi: 10.1074/jbc.270.29.17237. [DOI] [PubMed] [Google Scholar]
- 53.Juan TS, Kelley MJ, Johnson DA, et al. Soluble CD14 truncated at amino acid 152 binds lipopolysaccharide (LPS) and enables cellular response to LPS. J Biol Chem. 1995;270:1382–1387. doi: 10.1074/jbc.270.3.1382. [DOI] [PubMed] [Google Scholar]
- 54.Shapiro RA, Cunningham MD, Ratcliffe K, et al. Identification of CD14 residues involved in specific lipopolysaccharide recognition. Infect Immun. 1997;65:293–297. doi: 10.1128/iai.65.1.293-297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perera PY, Vogel SN, Detore GR, Haziot A, Goyert SM. CD14-dependent and CD14-independent signaling pathways in murine macrophages from normal and CD14 knockout mice stimulated with lipopolysaccharide or taxol. J Immunol. 1997;158:4422–4429. [PubMed] [Google Scholar]
- 56.Elass-Rochard E, Legrand D, Salmon V, et al. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the Lipopolysaccharide-Binding Protein. Infect Immun. 1998;66:486–491. doi: 10.1128/iai.66.2.486-491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levy O. A Neutrophil-derived anti-infective molecule: Bactericidal/Permeability-Increasing Protein. Antimicrob Agents Chemother. 2000;44:2925–2931. doi: 10.1128/aac.44.11.2925-2931.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagaoka I, Hirota S, Niyonsaba Fo, et al. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-α by blocking the binding of LPS to CD14+ cells. J Immunol. 2001;167:3329–3338. doi: 10.4049/jimmunol.167.6.3329. [DOI] [PubMed] [Google Scholar]
- 59.Risso A. Leukocyte antimicrobial peptides: multifunctional effector molecules of innate immunity. J Leukocyte Biol. 2000;68:785–792. [PubMed] [Google Scholar]
- 60.Rosenfeld Y, Papo N, Shai Y. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. J Biol Chem. 2006;281:1636–1643. doi: 10.1074/jbc.M504327200. [DOI] [PubMed] [Google Scholar]
- 61.Weiss J. Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): structure, function and regulation in host defence against Gram-negative bacteria. Biochem Soc Trans. 2003;31:785–790. doi: 10.1042/bst0310785. [DOI] [PubMed] [Google Scholar]
- 62.Tobias PS, Soldau K, Iovine NM, Elsbach P, Weiss J. Lipopolysaccharide (LPS)-binding proteins BPI and LBP form different types of complexes with LPS. J Biol Chem. 1997;272:18682–18685. doi: 10.1074/jbc.272.30.18682. [DOI] [PubMed] [Google Scholar]
- 63.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11:225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 64.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 65.Fitzgerald KA, Rowe DC, Barnes BJ, et al. LPS-TLR4 Signaling to IRF-3/7 and NF-κB involves the Toll adapters TRAM and TRIF. J Exp Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 67.Ostuni R, Zanoni I, Granucci F. Deciphering the complexity of Toll-like receptor signaling. Cell Mol Life Sci. 2010;67:4109–4134. doi: 10.1007/s00018-010-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang Z, Georgel P, Du X, et al. CD14 is required for MyD88-independent LPS signaling. Nature. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.