Abstract
ANF-RGC is the prototype membrane guanylate cyclase, both the receptor and the signal transducer of the hormones ANF and BNP. After binding them at the extracellular domain it, at its intracellular domain, signals activation of the C-terminal catalytic module and accelerates production of the second messenger, cyclic GMP. This, in turn, controls the physiological processes of blood pressure, cardiovascular function, and fluid secretion, and others: metabolic syndrome, obesity and apoptosis. What is the biochemical mechanism by which this single molecule controls these diverse processes, explicitly of the blood pressure regulation is the subject of the present study. In line with the concept that the structural modules of ANF-RGC are designed to respond to more than one, yet distinctive signals, the study demonstrates the construction of a novel ANF-RGC-In-gene-669WTAPELL675 mouse model. Through this model, the study establishes that 669WTAPELL675 is a vital ANF signal transducer motif of the guanylate cyclase. Its striking physiological features linked with their biochemistry are that (1) it controls the hormonally-dependent cyclic GMP production in the kidney and the adrenal gland; (3) its deletion causes hypertension, and (3) cardiac hypertrophy; and (4) these mice show higher levels of the plasma aldosterone. For the first time, a mere 7-amino acid encoded motif of the mouse gene has been directly linked with the physiological control of the blood pressure regulation, a detailed biochemistry of this linkage has been established and a model for this linkage has been offered.
Keywords: ANF-RGC, membrane guanylate cyclase, cyclic GMP, blood pressure, adrenal gland, kidney, mouse model
Introduction
Characterization of the first membrane guanylate cyclase ANF-RGC (Atrial Natriuretic Factor Receptor Membrane Guanylate Cyclase) dawned a new era of the field of membrane guanylate cyclase1–3. The structural and functional uniqueness of ANF-RGC brought forth seminal principles of the cellular signal transduction (reviewed in: 4, 5). First, it terminated over two decades-long debate on its independent existence from the soluble form. Second, unlike the three-signaling-component composition of its predecessors, adenylate cyclase and G-protein-coupled-receptors, ANF-RGC was a single transduction component, being both a receptor and the signal transducer. Third, unlike the soluble form of adenylate cyclase, it was membrane bound. Fourth, in contrast to the seven-transmembrane-spanning structure of G-proteins, it was a single-transmembrane-spanning protein. Fifth, being a physiological receptor of the two most hypotensive hormones, ANF and BNP, it was a potential regulator of the cardiovascular processes, including blood pressure, natriuresis and fluid secretion. Indeed, through biochemical, physiological and gene deletion studies it is now established that ANF-RGC signal transduction system is a central component of the cardiovascular regulation. Depending upon the degree, its imbalance is a primary cause of ventricular heart failure and hypertension (reviewed in: 6–9).
With the inclusion of two other members, CNP-RGC, the receptor of C-type natriuretic peptide (CNP)10, 11, and STa-RGC, the receptor of heat stable enterotoxin, guanylin and uroguanylin12, 13, a three-member natriuretic peptide hormone surface receptor membrane guanylate cyclase family was established.
The sequential developments expanded the family into two additional branches: Ca2+-modulated, rod outer segment guanylate cyclase, ROS-GC, with two members, ROS-GC1 and ROS-GC214, and the odorant (uroguanylin) surface receptor and Ca2+-modulated subfamily with one member olfactory neuroepithelial guanylate cyclase, ONE-GC15, 16. ROS-GC subfamily primarily exists in the vision-linked neurons; it is the central component of phototransduction (reviewed in: 14, 17, 18). And is also present in the mitral cells of the olfactory bulb neurons but its physiological linkage with olfaction has not been established19. ONE-GC is expressed in the olfactory sensory neurons; it is the receptor of the odorant uroguanylin15, 20, and, indirectly, of atmospheric CO221, 22.
The common structural trait of the family is that it represents a single transmembrane-spanning protein. It is composed of modular blocks (reviewed in: 4, 5). Gathered analyses of its members indicate that it is homodimeric. The transmembrane module in each monomeric subunit, divides the protein into two roughly equal portions, extracellular and intracellular. The family contains a conserved core catalytic domain, which is a common signal translational site for the production of cyclic GMP. A striking topographical difference on the orientation of the core catalytic domain between the subfamily of ANF-RGC and those of ROS-GC and ONE-GC exists. This is caused by the presence of the C-terminal extension (CTE) segment in the latter two, which is absent in ANF-RGC and CNP-RGC. The CTE follows the core catalytic domain and contains selective Ca2+ sensor sites. In ROS-GC the diverse Ca2+ signaling pathways run both downstream and upstream yet they are translated at the common core catalytic domain23. In contrast, till recently, it was believed that the signaling pathways of the ANF-RGC receptor subfamily only flow downstream to be translated at the core catalytic domain5, 23. In a surprising change of this paradigm, two recent findings of these authors demonstrate an additional model of ANF-RGC signal transduction24, 25. In addition to being a transducer of the ANF and BNP hormonal signals, ANF-RGC is also the transducer of Ca2+ signals generated in the zona glomerulosa of the adrenal gland25. The Ca2+ signal is captured by its sensor myristoylated neurocalcin δ (NCδ), which is bound directly to the core catalytic domain. Thereby, the Ca2+ sensor element serves as a CONTROL switch of the blood pressure regulation. Deletion of this switch in the mice results in hypertension25.
This information provides a new insight into a mechanism and the function-related structure of the core catalytic module. The module, besides functioning as a central and common translational center of all the signals generated by the guanylate cyclases into the production of cyclic GMP, it also functions as a regulatory center for the Ca2+ signal. Core catalytic module, therefore, is composed of two subtle structural compartments, one for regulating and the other for translating the Ca2+ signals; importantly, the translating compartment is common for both types, hormonal and Ca2+, signals. Thus, ANF-RGC is not only the traditional transducer of the extracellularly-generated hormonal signals; it is also the transducer of the intracellular Ca2+ signal. Because the end-results of these two types of signal are the same, generation of cyclic GMP, the net physiological response to both signals is the same, i.e., both contribute to the regulation of cardiovascular function in identical fashions. In support of this concept, the latest finding of the authors demonstrates that, indeed, deletion of the Ca2+ switch in mice causes hypertension25.
The 669WTAPELL675 motif of ANF-RGC is conserved in the membrane guanylate cyclase family. In ANF-RGC, it controls transmission of the hormonal signal to the catalytic domain26. In the photoreceptor ROS-GC1 guanylate cyclase it controls the Ca2+-modulated phototransduction machinery present in the sensory neurons of rods and cones27. Thus, in this evolving concept, the same motif is vital for the operation of two very different membrane guanylate cyclase machineries, and it performs very different functions. It programs the signaling functions according to the peculiarities and cellular location of that cyclase.
This concept has been advanced in the present study. In addition to its vital role in the regulation of the processes involved in blood pressure, cardiovascular function, and fluid secretion, ANF-RGC also is linked with other biological processes: metabolic syndrome, obesity and apoptosis. How this single receptor membrane guanylate cyclase controls so many diverse processes? To begin answering this question, through gene targeting, the 7-amino acid motif, 669WTAPELL675, has been deleted from the murine ANF-RGC. The mutated protein has been analyzed for its control of the physiology of blood pressure regulation; the regulation has been explained in biochemical terms and a pictorial model for this mechanism has been offered.
EXPERIMENTAL PROCEDURES
ANF-RGC-In-gene-669WTAPELL675(−/−) mouse model
Care of the experimental animals conformed to the protocols approved by the IACUC at Salus University and was in strict compliance with the NIH guidelines.
Construction of the model
Two fragments (5'-arm, from intron 8 to 13; 3'-arm, from intron 13 to 18) of the ANF-RGC gene were amplified from the mouse genomic DNA. These were subcloned into the multiple cloning sites (separated by PGK-neo cassette flanked by two LoxP sequences) of the HSV-TK vector. The 669WTAPELL675 motif (located in exon 14 of ANF-RGC gene) was deleted from the construct using site directed mutagenesis. The 5'-arm-loxP-PGKneo-loxP-3'-arm fragment was excised from the mutated targeting vector and electroporated into mouse embryonic stem (ES) cells. G418- and ganciclovir (Ganc)-resistant ES cell clones were expanded and analyzed by long range PCR using primers with sequences homologous to regions outside the arms to identify targeted ES clones. Clones that had undergone homologous recombination were injected into C57BL/6 blastocytes. Male chimeras were bred with wild type CD1 female to determine the germline transmission and then with Hprt-Cre mice to remove the PGK neo cassette. Finally, the male mice were bread to C57BL/6 females to obtain ANF-RGC-669WTAPELL675(+/−) heterozygous mutants and finally homozygous 669WTAPELL675(−/−) mice. The targeting of the ANF-RGC 669WTAPELL675 motif is schematically shown in figure 1A. The retained Lox-P sites are used for genotyping with the following primers: Forward: 5'-CGCAGCCTTCTCAGATT and Reverse: 5'-CAGTTTTTCTGTGGATCAG. Amplification of the wild type allele yields a fragment of 371 bp and of the 669WTAPELL675 deletion allele, a fragment of 471 bp (Fig. 1B).
Figure 1. Schematic representation of construction of ANF-RGC 669WTAPELL675 motif targeted mice.
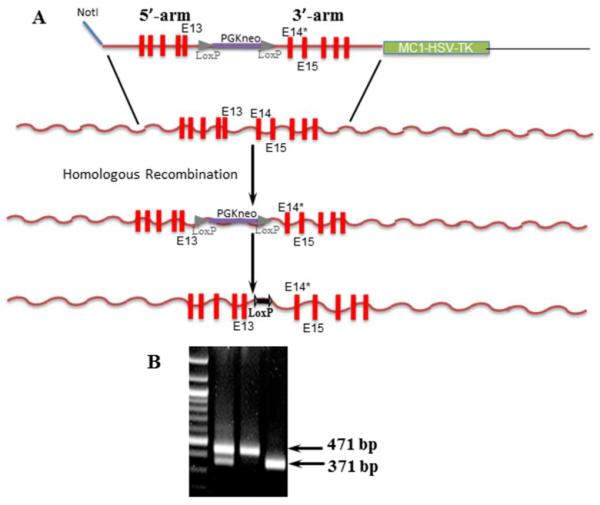
(A) Two fragments (5'-arm, from intron 8 to 13; 3'-arm, from intron 13 to 18) of the ANF-RGC were subcloned into the multiple cloning sites (separated by PGK-neo cassette flanked by two LoxP sequences) of the HSV-TK vector. The 669WTAPELL675 motif (located in exon 14 of ANF-RGC gene) was deleted from the construct using site directed mutagenesis. The 5'-arm-loxP-PGKneoloxP-3'-arm fragment was excised from the mutated targeting vector and electroporated into mouse ES cells. G418- and ganciclovir (Ganc)-resistant ES cell clones were expanded and analyzed by long range PCR using primers with sequences homologous to regions outside the arms to identify targeted ES clones. Clones that had undergone homologous recombination were processed as described in “Experimental Procedures” section. (B) Agarose gel showing genotyping for 669WTAPELL675 deletion. The retained Lox-P sites are used for genotyping. Amplification of the wild type allele yields a fragment of 371 bp and of the 669WTAPELL675 deletion allele, a fragment of 471 bp.
With this strategy, the construction of the mouse model was contracted to the Gene Targeting and Transgenic Facility of the University of Connecticut.
Membrane preparation
Tissues (heart, kidney and adrenal glands) were removed from the wild type (control), the ANF-RGC-669WTAPELL675(+/−) and 669WTAPELL675(−/−) mice. The tissues were powdered under liquid nitrogen and homogenized in a buffer consisting of 250 mM sucrose, 10 mM Tris-HCl pH 7.4, and protease inhibitor cocktail (Sigma), centrifuged at 1,000g and then at 100,000g to pellet the membrane fraction. This fraction was suspended in 50 mM Tris-HCl pH 7.4/10 mM MgCl2 buffer and used for guanylate cyclase activity assay.
ANF-RGC 699WTAPELL675 deletion mutant
This mutant was constructed as described previously26.
Expression in COS cells
COS cells maintained in DMEM medium supplemented with 10% fetal bovine serum and penicillin/streptomycin antibiotics were transfected with ANF-RGC cDNA expression construct using calcium phosphate co-precipitation technique28. 64 hr after transfection cells were washed with 50 mM Tris-HCl pH 7.4/10 mM Mg2+ buffer, homogenized and the particulate fraction pelleted by centrifugation.
Guanylate cyclase activity assay
The membrane fraction was incubated on ice-bath with ANF and ATP in the assay system containing 10 mM theophylline, 15 mM phosphocreatine, 20 μg creatine kinase and 50 mM Tris-HCl, pH 7.5. The total assay volume was 25 μl. The reaction was initiated by addition of the substrate solution (4 mM MgCl2 and 1mM GTP, final concentration) and maintained by incubation at 37 °C for 10 min. The reaction was terminated by the addition of 225 μl of 50 mM sodium acetate buffer, pH 6.2 followed by heating on a boiling water-bath for 3 min. The amount of cyclic GMP formed was determined by radioimmunoassay29.
Immunohistochemistry
Mice were sacrificed by lethal injection of ketamine/xylazine (the protocol approved by the Salus University IUCAC) and perfused through the heart, first with a standard phosphate-buffered saline (PBS) and then with freshly prepared 4% paraformaldehyde in Tris-buffered saline (TBS). The adrenal glands were removed and fixed for 1–4 hours in 4% paraformaldehyde with TBS at 4°C, cryoprotected in 30% sucrose overnight at 4°C and cut into 20 μm sections using Hacker-Bright OTF5000 microtome cryostat (HACKER Instruments and Industries Inc., Winnsboro, SC). The sections were washed with TBS, blocked in 10% normal donkey serum in TBS/0.5% Triton X-100 (TBST) for 1hr at room temperature, washed with TBST, incubated with ANF-RGC antibody (50:1) in blocking solution overnight at 4°C, washed with TBST, incubated with DyLight 488 conjugated donkey anti-rabbit antibody (100:1) for 1 hr and again washed with TTBS. Images were acquired using an inverted Olympus IX81 microscope/FV1000 Spectral laser confocal system, and analyzed using Olympus FluoView FV10-ASW software. Digital images were processed using Adobe Photoshop software.
Antibodies
Antibodies against ANF-RGC were raised in rabbits. Their specificities were described previously24, 25. The antibodies were affinity purified. Secondary antibodies conjugated to a fluorescent dye (DyLight 488) were purchased from Jackson ImmunoResearch Laboratories, Inc., West Grove, PA.
Blood pressure measurements
Systolic and diastolic blood pressure of the ANF-RGC-WTAPELL+/+ (wild type, control) or the heterozygous ANF-RGC-669WTAPELL675(+/−) and homozygous 669WTAPELL675(−/−) mice was measured every day for one week by the noninvasive computerized tail-cuff method with CODA (Kent Scientific) according to the manufacturer's protocol. An average blood pressure level of 10 sessions per day was calculated for analysis after 3 days of mice training. The mice were maintained on normal chow and drinking water available ad libitum.
Mean arterial pressure (MAP) was calculated from the values of systolic (S) and diastolic (D) pressure according to the equation: MAP ≈ (2D+S)/3.
Aldosterone level
Aldosterone levels in the plasma of the ANF-RGC-669WTAPELL675(+/+), ANF-RGC-669WTAPELL675(+/−) or ANF-RGC-669WTAPELL675(−/−) mice was determined using RIA kit and the manufacturer's protocol (Siemens Medical Solutions Diagnostics; CA, USA).
Statisticl analysis
The results are presented as mean ± SD. Differences between the three groups of mice studied, ANF-RGC-669WTAPELL675(+/+), ANF-RGC-669WTAPELL675(+/−) and 669WTAPELL675(−/−) were compared using Student's t test. A P value of <0.05 was considered significant.
RESULTS AND DISCUSSION
ANF-RGC 669WTAPELL675 motif targeted mice
All biological effects of ANF are mediated by its second messenger cyclic GMP produced by ANF-RGC. Studies in the recombinant system have shown that the 669WTAPELL675 motif of ANF-RGC is critical for the transduction of the ANF signal into increased synthesis of cyclic GMP26. To examine whether the same applies to the ANF signaling in the biological systems an in-ANF-RGC-gene 669WTAPELL675 deletion mouse model was developed. To date no ANF-RGC domain-specific genetically modified animal model is available. The only available, is the one with the entire ANF-RGC gene deleted30, 31. It was hypothesized that absence of the 669WTAPELL675 signaling motif from the ANF-RGC protein should result in a mouse phenotype where the mutant specifically lacks the ability of transmitting the ANF-dependent ATP signal from the ARM (ATP-Regulated Module) to the ANF-RGC catalytic domain. It, then, cannot accelerate production of the ANF second messenger cyclic GMP and the mouse will be inflicted with the pathologies of hypertension and cardiac and renal hypertrophies.
Viable homozygous mice, with the 669WTAPELL675 coding sequence deleted from both copies of ANF-RGC gene [669WTAPELL675(−/−)] prove that the deletion is not immediately lethal and does not interfere with the reproduction. The homo- and heterozygous mice do not differ in their appearance from their wild type littermates.
What follows is the detailed physiological analysis and biochemistry behind the 669WTAPELL675 deletion mutation phenotype in mice.
PHYSIOLOGY
669WTAPELL675 motif is critical for the cardiovascular physiology
It is well established that elimination of either ANF-RGC or ANF expression leads to increased blood pressure, cardiac and renal hypertrophy, fibrosis, to name some of the related pathological conditions in mice6, 31–35. It is also established that the 7-amino acid motif 669WTAPELL675 controls the signal transduction activity of ANF-RGC26. Does this motif control the physiological functions of ANF-RGC as well? As it will be shown, it does.
Blood pressure
After three days of mock sessions for training purposes, the systolic blood pressure of the 669WTAPELL675(+/+), 669WTAPELL675(+/−) and 669WTAPELL675(−/−) mice was measured. It was 102 ± 9 mm Hg for the wild type mice, 134 ± 17 mm Hg for the heterozygous and 159 ± 11 mm Hg for the homozygous mice (Fig. 2A). The increase was statistically highly significant (P < 0.005). Together with systolic, the diastolic pressure was recorded and from their combined values the mean arterial pressure was calculated. It was 78 ± 6 mm Hg for the wild type mice, 108 ± 11 mm Hg for the heterozygous and 127 ± 10 mm Hg for the homozygous (Fig. 2B). Similar as with the systolic pressure, the differences between the wild type and 669WTAPELL675(+/−) heterozygous mice as well as between the heterozygous and 669WTAPELL675(−/−) homozygous mice were statistically very significant (P<0.005). These results show that the progressive elevation of blood pressure directly correlates with the number of ANF-RGC gene copies with deleted the WTAPELL motif's coding sequence demonstrating, that the motif is critical for the ANF-RGC function in the blood pressure regulation.
Figure 2. Systolic (A) and mean arterial (B) blood pressure in ANF-RGC 669WTAPELL675 motif targeted mice.
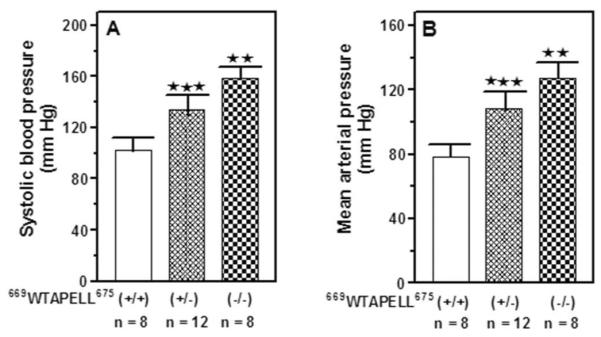
The mice used were 669WTAPELL675(+/+) wild type allele, 669WTAPELL675(+/−) heterozygous type allele and 669WTAPELL675(−/−) homozygous type allele. The mice were fed a normal-salt diet. Systolic blood pressure was measured every day for one week by the noninvasive computerized tail-cuff method. An average blood pressure level of 10 sessions a day was calculated for analysis after 3 days of training. The mean arterial blood pressure was calculated from the values of systolic and diastolic pressures as described in the “Experimental Procedures” section. Bars indicate means ± SD values for the representative genotype. n describes the number of mice analyzed for each genotype. ** indicates that the P value was <0.005 and *** indicates that the P value was <0.001.
Plasma aldosterone level
Volume homeostasis and blood pressure are influenced by the steroid hormone aldosterone secreted by the cells of zona glomerulosa of the adrenal gland. Aldosterone inhibits sodium secretion and diuresis thus increases blood pressure. Its secretion is controlled by renin angiotensin system (RAS). RAS is kept in balance through cyclic GMP generated by ANF-RGC36–38. In the adrenocortical zona glomerulosa cyclic GMP inhibits aldosterone synthesis and prevents elevation of blood pressure. The issue was:
Does the WTAPELL motif enable ANF-RGC to modulate blood pressure through this mechanism, e.g. by synthesizing cyclic GMP in quantity sufficient to inhibit aldosterone synthesis? As it will be shown, it does.
Plasma aldosterone concentrations were measured in both types of genetically modified mice (heteroand homozygous) and in their isogenic controls (wild type). The results are shown in figure 3. In the plasma of wild type mice aldosterone concentration was 147 ± 12 pg/ml; it increased to 204 ± 18 pg/ml in the heterozygous (P < 0.005) and further, up to 256 ± 22 pg/ml in the homozygous mice (P < 0.05 for the increase between hetero- and homo-zygous mice). It is therefore concluded that deletion of the 669WTAPELL675 motif from ANF-RGC correlates with the increased aldosterone concentrations in the 669WTAPELL675 targeted mice in comparison with the wild type animals.
Figure 3. Plasma aldosterone levels in ANF-RGC 669WTAPELL675 motif targeted mice.
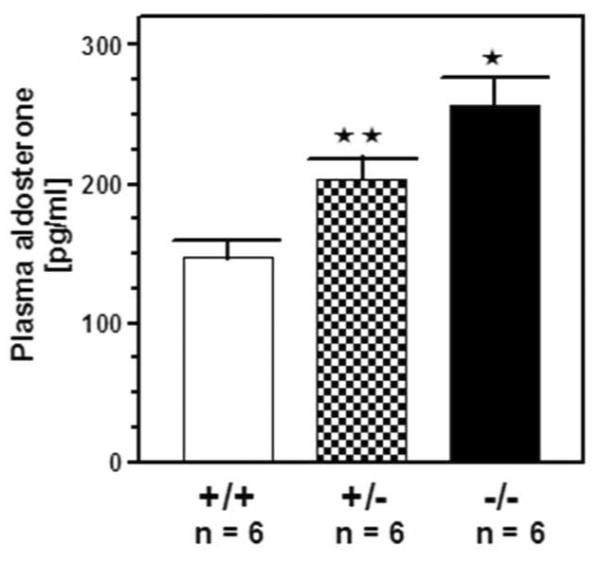
The mice used were 669WTAPELL675(+/+) wild type allele, 669WTAPELL675(+/−) heterozygous type allele and 669WTAPELL675(−/−) homozygous type allele. The mice were fed a normal-salt diet. Aldosterone concentrations were determined using Coat-A Count aldosterone RIA kit. Bars indicate means ± SD values for the representative genotype. n describes the number of mice analyzed for each genotype. ** indicates that the P value was <0.005 and * indicates P <0.05.
Cardiac hypertrophy
The final issue was: Does the chronic pressure overload lead to cardiac hypertrophy? The results show that it does.
The ratio of the heart weight (in mg) to the whole body weight (in g) of 10 weeks old 669WTAPELL675(+/+), 669WTAPELL675(+/−) and 669WTAPELL675(−/−) mice was determined and the results are shown in figure 4. The ratio was 5 ± 0.3, 5.6 ± 0.3 and 6.1 ± 0.5 for the 669WTAPELL675(+/+), 669WTAPELL675(+/−) and 669WTAPELL675(−/−) mice, respectively. Thus, progressive increase in cardiac hypertrophy correlates with the number of ANF-RGC gene copies with the deleted sequence coding for the 669WTAPELL675 motif.
Figure 4. Absence of the 669WTAPELL675 motif in ANF-RGC causes cardiac hypertrophy.
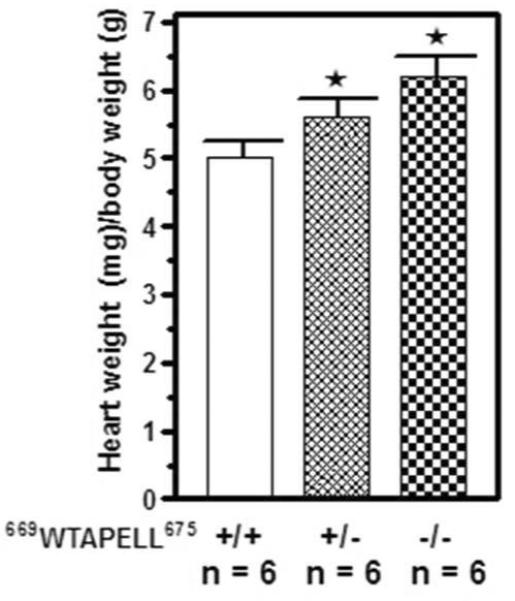
The ratio of the heart weight (in mg) to whole body weight (in g) of 10 weeks old 669WTAPELL675(+/+) wild type, 669WTAPELL675(+/−) heterozygous and 669WTAPELL675(−/−) homozygous was determined. The mice were fed a normal-salt diet. Bars indicate means ± SD values for the representative genotype. n describes the number of mice analyzed for each genotype. * indicates P <0.05.
The results presented demonstrate that deletion of the 669WTAPELL675 motif from ANF-RGC mimics the effects of the loss of ANF-RGC protein39. Thus, at the molecular level, the 669WTAPELL675 motif of ANF-RGC is the regulator of the cyclase signal transduction and its absence disables the cyclase's physiological functions and leads to cardiovascular pathologies.
BIOCHEMISTRY
What is the biochemical explanation for the inability of the mutated ANF-RGC to perform its biological functions? Three issues were considered: level of expression of the mutated cyclase vs. wild type, its basal activity, and its response to the ANF signal.
The 669WTAPELL675 encoded gene motif has no role in ANF-RGC expression in the kidney and adrenal gland
After being excreted via the atrial stretch, ANF through blood stream is transported to two key organs, adrenal gland and kidney40, where it signals the catalytic activation of ANF-RGC41–45. And these organs are critical in maintaining cardiovascular and renal homeostasis6, 46, 47.
The issue was: Does the 669WTAPELL675 encoded gene motif affect the ANF-RGC gene expression in these organs? The results show that it does not.
It was analyzed by immunocytochemistry. The kidneys and adrenal glands were removed from the wild type [669WTAPELL675(+/+)], heterozygous [669WTAPELL675(+/−)] and homozygous [669WTAPELL675(−/−)] male mice and the frozen sections of these tissues were stained with immunopurified anti ANF-RGC antibody.
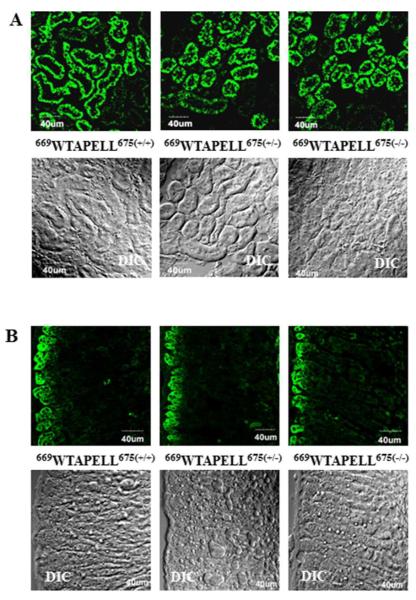
Figures 5A and 5B [panel: 669WTAPELL675(+/+)] show images generated in the kidney and the adrenal gland, respectively, of the wild type mouse. Intense immunoreactivity (green color) was observed in the renal apical and basolateral aspects of the tubular elements (Fig. 5A) and adrenocortical zona glomerulosa (Fig. 5B). These results are in agreement with previous localizations of ANF-RGC in the kidney and adrenal gland24, 25, 45. Identical pattern of immunoreactivity was observed when the kidney and adrenal gland sections from the 669WTAPELL675(+/−) and 669WTAPELL675(−/−) mice were analyzed (Fig. 5A and 5B, panels 669WTAPELL675(+/−) and 669WTAPELL675(−/−), respectively). Visual examination of the stained sections as well as of the respective differential interference contrast (DIC) images indicates that the deletion does also not affect the integrity of these tissues. These results demonstrate that deletion of the sequence coding for the 669WTAPELL675 motif from either one or two ANF-RGC gene copies does not affect the cyclase tissue-specific expression.
Figure 5. Deletion of the sequence coding for the 669WTAPELL675 motif from the ANF-RGC gene does not affect expression of the mutated cyclase in the kidney (A) and adrenal gland (B).
Cryosections of the kidney and adrenal gland from the wild type [669WTAPELL675(+/+)], heterozygous [669WTAPELL675(+/−)], and homozygous [669WTAPELL675(−/−)] mice were immuno-stained with ANF-RGC antibodies. The DIC image showing the integrity of the sections are also shown (“DIC”). Intense staining was observed in the renal apical and basolateral aspects of the tubular elements and adrenocortical zona glomerulosa.
The 669WTAPELL675 motif does not control the structure of the ANF-RGC catalytic site therefore it does not affect the basal cyclase activity in the kidney and adrenal gland
The next question was: Is the apparent unchanged expression of the ANF-RGC mutant protein accompanied by unchanged basal activity of the cyclase in the analyzed tissues? The answer is, yes.
Membrane fractions of the kidney and the adrenal gland were isolated from the 669WTAPELL675(+/+) mice, the heterozygous 669WTAPELL675(+/−) and the homozygous 669WTAPELL675(−/−) and they were assessed for the basal guanylate cyclase activity. Membranes of COS cells expressing wild type ANF-RGC and its 669WTAPELL675 deletion mutant were assayed in parallel as controls. The activities of the wild type ANF-RGC and its 669WTAPELL675 deletion mutant expressed in COS cells were virtually identical, ~23 pmol cyclic GMP min−1(mg prot)−1. The particulate guanylate cyclase activities expressed in the kidneys and the adrenal glands were also practically indistinguishable between the membranes isolated from the wild type and the genetically modified mice. They were respectively ~41 and ~78 pmol cyclic GMP min−1(mg prot)−1 for the kidney and the adrenal gland (Table 1). These results demonstrate that in both the recombinant system and in the in vivo deletion of the system 669WTAPELL675 motif does not affect the basal activity of the catalytic domain of ANF-RGC. Therefore, the motif does not control the structure of the catalytic domain.
Table 1.
Guanylate cyclase activity in the membrane fraction of the kidney and adrenal gland of the wild type and ANF-RGC 669WTAPELL675 modified mice.
| Tissue/ activity | Wild type | 669WTAPELL675(+/−) | 669WTAPELL675(−/−) |
|---|---|---|---|
| Kidney | 42 ± 4 | 40 ± 6 | 41 ± 5 |
| Adrenal gland | 78 ± 9 | 75 ± 10 | 81 ± 9 |
| COS cells | 23 ± 3 | 22 ± 3 |
The membrane fractions were prepared as described in the Experimental Procedures section. Membranes of the COS cells expressing wild type ANF-RGC and its 669WTAPELL675 deletion mutant were assayed as control. The experiment was done in triplicate and repeated three times with different membrane preparations. The results are mean ± SD from these experiments. The activity is expressed as pmol cyclic GMP min−1 (mg protein)−1.
The 669WTAPELL675 motif controls the ANF-dependent regulatory activity of ANF-RGC
The membrane fractions of the kidneys and the adrenal glands isolated from the 669WTAPELL675(+/+) mice, the heterozygous 669WTAPELL675(+/−) and the homozygous 669WTAPELL675(−/−), were evaluated for guanylate cyclase activity in the presence of increasing concentrations of ANF and 0.8 mM ATP. Recombinant ANF-RGC and its 669WTAPELL675 deletion mutant expressed individually in COS cells were treated identically as control. The results are presented in figure 6. In the wild type mice the guanylate cyclase activity was stimulated in an ANF dose-dependent fashion in excess of 9-fold in the kidney membranes and over 5-fold in the adrenal gland membranes [form 41 ± 6 to 389 ± 40 pmol cyclic GMP min−1(mg prot)−1 for the kidney (Fig. 6A: open circles) and from 78 ± 13 to 413 ± 35 pmol cyclic GMP min−1(mg prot)−1 for the adrenal (Fig. 6B: open circles)]. For both types of membranes the half maximal stimulation was at ~ 10−9 M ANF. The guanylate cyclase activity in these membranes had the ANF dose-dependent profile comparable if not identical with the activity of COS cell membranes expressing wt ANF-RGC (compare the “open circles” curves in figures 6 A and 6B with 6C). Also the calculated Hill's coefficients for the dose-response curves were very similar: 0.91 ± 0.2 for the membranes isolated from kidney and adrenal gland and 0.95 ± 0.12 for the recombinant wtANF-RGC. In the membranes isolated from the heterozygous mice (669WTAPELL675(+/−)), the cyclase remained responsive to ANF/ATP, the ANF EC50 value was again at ~10−9 M but the saturated activity was only 47% of that achieved by the wild type cyclase in kidney (Fig. 6A: closed circles) and 61% in the adrenal gland (Fig. 6B: closed circles). These values, oscillating around 50% of the wild type values, are indicative that in the heterozygous mice where the product of only one ANF-RGC gene copy is of the wild type and the other, of the deletion-mutated cyclase, only the wild type ANF-RGC is responsive to ANF/ATP and the mutant is not.
Figure 6. ANF/ATP-dependent ANF-RGC activity in the membranes of kidney (A) and adrenal gland (B) of the wild type and 669WTAPELL675 genetically modified mice and of COS cells transfected with wild type ANF-RGC or its 669WTAPELL675 deletion mutant (C).
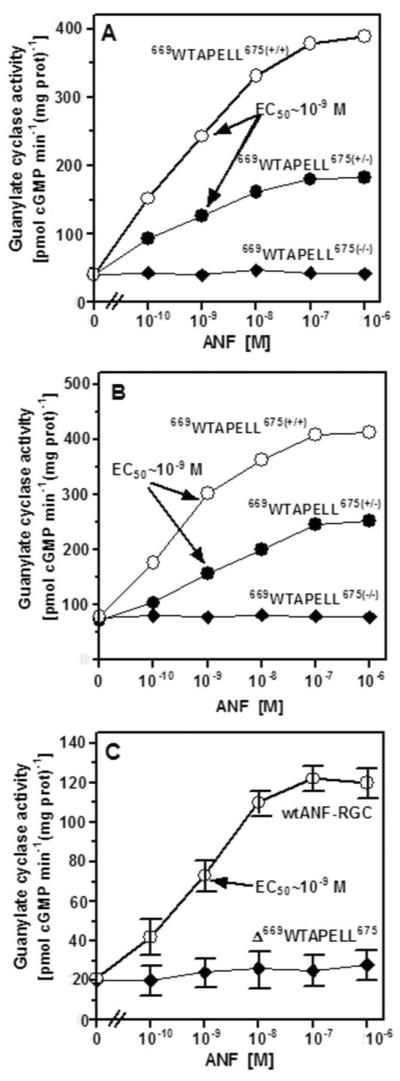
Membrane fractions of the kidneys and the adrenal glands were isolated from the wild type [669WTAPELL675(+/+)], the heterozygous [669WTAPELL675(+/−)], the homozygous [669WTAPELL675(−/−)] mice and COS cells expressing wild type ANF-RGC or its 669WTAPELL675 deletion mutant. These were evaluated for guanylate cyclase activity in the presence of indicated concentrations of ANF and 0.8 mM ATP and cyclic GMP formed was measured by radioimmunoassay. The experiment was done in triplicate and repeated three times with separate membrane preparations. The results presented (mean ± SD) are from these experiments.
The membranes of the homozygous mice (669WTAPELL675(−/−)) isolated either from the kidney (Fig. 6A: closed diamonds) or the adrenal gland (Fig. 6B: closed diamonds) were totally unresponsive to ANF/ATP; the cyclase activity at all concentrations of ANF tested was the same as in their absence. Importantly, the activity of the recombinant 669WTAPELL675 deletion mutant expressed in COS cells was also indifferent to the tested concentrations of ANF and ATP (Fig. 6C: closed diamonds).
These findings establish that the 669WTAPELL675 motif selectively controls the ANF-dependent regulatory activity of ANF-RGC; it has no control over its basal activity. These are striking properties of ANF-RGC which could not be observed in the earlier total ANF-RGC gene knock-out mouse model studies30, 31. The present model has made it possible to link the mechanism of ANF/ATP signaling of ANF-RGC activation with hypertension. It is therefore concluded that in the ANF-dependent regulatory activity of ANF-RGC the 669WTAPELL675 motif plays an essential role in the action of ANF in the regulation of blood pressure. Disruption of this motif results in hypertension.
The ANF/ATP and Ca2+-modulated ANF-RGC signaling pathways independently control blood pressure
The recent ground breaking discovery of the authors has disclosed that ANF-RGC is a bimodal signal transduction switch24, 25. In addition to being a signal transducer of the hormones, ANF and BNP, it is also a signal transducer of the [Ca2+]i. The sensor of Ca2+ is neurocalcin δ (NCδ). Importantly, the Ca2+ signal transduction pathway also controls mouse blood pressure through the control of aldosterone synthesis in the adrenal glands25. The NCδ+/− mice is hypertensive25.
The question was: Does the 669WTAPELL675 motif influence the [Ca2+]i-dependent ANF-RGC catalytic activities in the kidney and adrenal glands of the mouse? The answer is that it does not. Membranes isolated from the kidney and adrenal gland of the 669WTAPELL675 targeted wild type, heterozygous and homozygous mice and the membranes of COS cells expressing recombinant ANF-RGC and its 669WTAPELL675 deletion mutant were exposed to 4 μM NCδ and 1 μM Ca2+ (Fig. 7). For all three mouse genotypes the ANF-RGC activity was stimulated by 4.6 fold, from ~41 to 190 pmol cyclic GMP min−1(mg prot)−1 in the kidney membranes (Fig. 7A), 3.2 fold, from ~78 to 247 pmol cyclic GMP min−1(mg prot)−1, in the adrenal gland membranes (Fig. 7B), and approx. 5-fold, from 23 to 100, for both the wt and the 669WTAPELL675 motif deleted ANF-RGC expressed in COS cell membranes. These results demonstrate that the 669WTAPELL675 motif is not involved the Ca2+-modulation of ANF-RGC activity. And, importantly, the two signaling pathways, the ANF-dependent involving the 669WTAPELL675 motif and the ANF-independent Ca2+-modulated in which the 669WTAPELL675 motif is not involved, function through the generation of their second messenger cyclic GMP and through it, they both independently contribute to the regulation of blood pressure.
Figure 7. The motif 669WTAPELL675 is not involved in maintaining the integrity of the ANF-RGC catalytic domain.
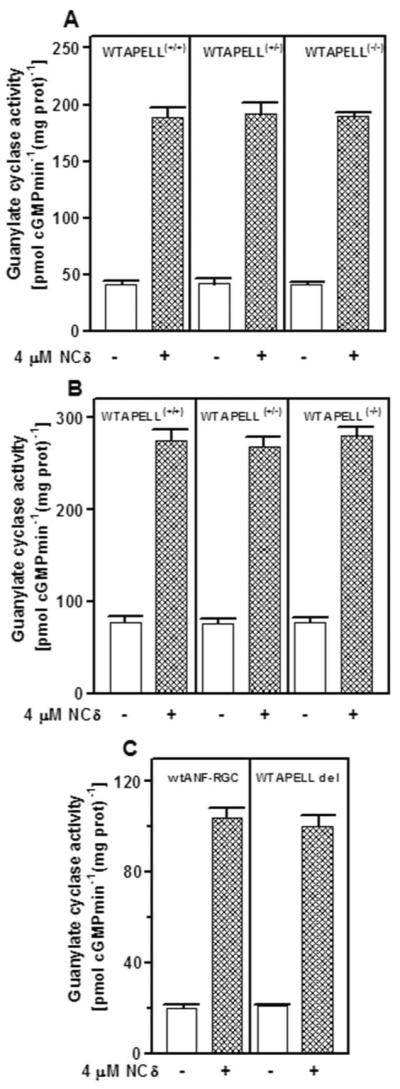
The particulate fractions of the kidney (A), adrenal gland (B) from the wild type 669WTAPELL675(+/+), heterozygous 669WTAPELL675(+/−) and homozygous 669WTAPELL675(−/−) mice and from COS cells expressing wild type ANF-RGC or its 669WTAPELL675 deletion mutant were prepared and tested for their responsiveness to 4 μM neurocalcin q in the presence of 1 μM Ca2+. The experiment was done in triplicate and repeated two times. The results shown are mean ± SD from these experiments.
CONCLUSION
Through a mouse molecular genetics approach involving a domain-specific targeting of ANF-RGC guanylate cyclase, this study directly links biochemistry of the transduction events of the 669WTAPELL675 motif with the physiological processes of blood pressure regulation. There are several striking features of this study. First and foremost, the novelty of the approach; for the first time, a domain-based ANF-RGC mouse in-gene-deletion model has been used to decode the principles of ANF-RGC signal transduction. Second, the study demonstrates that a mere 7-amino acid motif, 669WTAPELL675, of ANF-RGC controls the ANF/ATP-dependent catalytic activity of the cyclase. Third, the motif controls only the hormone-dependent regulatory activity of ANF-RGC. Fourth, absence of the motif in the ANF-RGC protein results in increased blood pressure. Fifth, the motif also is in command of the aldosterone plasma levels. Sixth, by causing chronic pressure overload, absence of the motif inflicts the mouse with cardiac hypertrophy. And, seventh, all these abnormalities are due to the dysfunctional ANF-dependent ANF-RGC signal transduction apparatuses in the adrenal and the kidney glands of the mouse.
We propose a model to explain these findings (Fig. 8).
Figure 8. ANF signaling of ANF-RGC activity linked with cardiovascular physiology: a model.
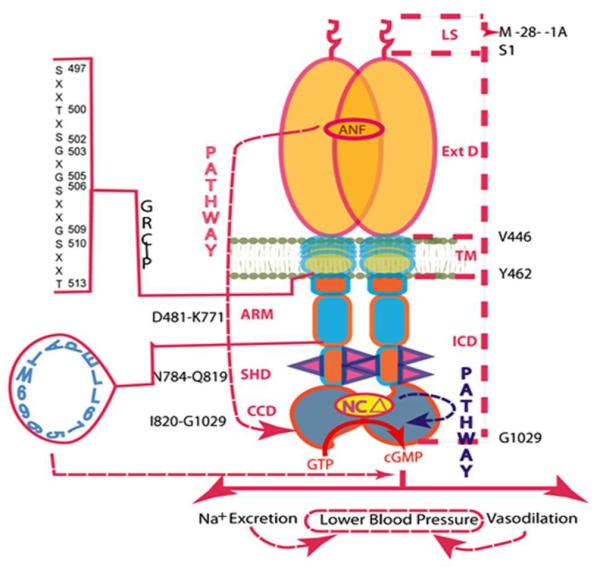
ANF-RGC is a single transmembrane spanning homodimer protein. The dashed lines on the right show the defined boundaries of its segments: LS, leader sequence; Ext D, extracellular domain; TM, transmembrane domain; ICD, intracellular domain. The functional domains housed in the intracellular domain (ICD), their designated names and the amino acid residues constituting their boundaries are indicated at the left: ARM, the ATP regulated module; SHD-signaling helix domain; CCD-core catalytic domain. The CCD exists as an antiparallel homodimer. The sequence of the ARM domain's region of the glycine rich cluster and the phosphorylation sites is shown at the left-hand side as GRC~P; the helical 669WTAPELL675 motif is shown in blue. The neurocalcin δ targeted site within the catalytic domain is shown as “NCΔ”. The red dashed arrow indicates the physiological targets affected by deletion of the 669WTAPELL675 motif of ANF-RGC.
MODEL
(i) ANF signaling of ANF-RGC, cyclic GMP production events. The signal originates by the binding of one molecule of ANF to the ECD of ANF-RGC dimer48, 49. The binding modifies the juxtamembrane region where the disulfide 423C-C432 structural motif is a key element50, 51. The signal twists the trans-membrane domain52, induces a structural change in the ARM domain and prepares it for the ATP binding53. Step1. ARM domain binds ATP what leads to a cascade of temporal and spatial changes54. They involve (1) shift in ATP binding pocket position by 3–4 Å and rotation of its floor by 15°; G505 acts as a critical PIVOT for both the shift and the rotation; (2) movement by 2–7 Å but not the rotation of its β4 and β5 strands and its loop; (3) movement of its αEF helix by 2–5 Å. This movement exposes the hydrophobic motif, 669WTAPELL675, which facilitates its direct (or indirect) interaction with the catalytic module resulting in its partial, about 50%, activation26. Step2: The six phosphorylation sites are brought from their buried to the exposed state55. ATP through a hypothetical protein kinase phosphorylates the residues and the full activation (additional 50%) of ANF-RGC is achieved. ANF-dependent cyclic GMP is generated and it functions as the second messenger of blood pressure regulation. Concomitantly, phosphorylation converts the ATP binding site from high to low affinity, ATP dissociates, and ANF-RGC returns to its ground state55. (ii) Post cyclic GMP production events. Cyclic GMP generated suppresses the RAS-dependent secretion of aldosterone through yet not fully understood mechanism. It is known, however, that it affects number of effectors of aldosterone synthesis. They include cyclic GMP-gated channels, cyclic GMP-dependent protein kinases, and cyclic GMP-regulated phosphodiesterases56–59. Lowered aldosterone level results in the increase of sodium excretion and diuresis in the kidney and maintaining physiological blood pressure.
ACKNOWLEDGMENTS
We acknowledge Guanylate Cyclase Innovative Biotechnologies (GCIB) for the support of 669WTAPELL675-deleted ANF-RGC gene mouse model.
Founding Statement The study was supported by National Heart, Blood and Lung Institute grants HL084584 and S82701
Abbreviations
- ANF
atrial natriuretic factor
- BNP
B-type natriuretic peptide
- CNP
C-type natriuretic peptide
- ANF-RGC
atrial natriuretic factor receptor guanylate cyclase
- ARM
ATP regulatory module
- GCAP
guanylate cyclase activating protein
- NCδ
neurocalcin δ
- ONE-GC
olfactory neuroepithelial guanylate cyclase
- RAS
renin-angiotensin system
- ROS-GC
rod outer segment guanylate cyclase
REFERENCES
- 1.Paul AK. Doctoral thesis. University of Tennessee; 1986. Particulate guanylate cyclase from adrenocortical carcinoma 494. Purification, biochemical and immunological characterization. [Google Scholar]
- 2.Paul AK, Marala RB, Jaiswal RK, Sharma RK. Coexistence of guanylate cyclase and atrial natriuretic factor receptor in a 180-kD protein. Science. 1987;235:1224–1226. doi: 10.1126/science.2881352. [DOI] [PubMed] [Google Scholar]
- 3.Kuno T, Andresen JW, Kamisak,i Y, Waldman SA, Chang LY, Saheki S, Leitman DC, Nakane M, Murad F. Co-purification of an atrial natriuretic factor receptor and particulate guanylate cyclase from rat lung. J Biol Chem. 1986;261:5817–5823. [PubMed] [Google Scholar]
- 4.Sharma RK. Evolution of the membrane guanylate cyclase transduction system. Mol Cell Biochem. 2002;230:3–30. [PubMed] [Google Scholar]
- 5.Sharma RK. Membrane guanylate cyclase is a beautiful signal transduction machine: overview. Mol Cell Biochem. 2010;334:3–36. doi: 10.1007/s11010-009-0336-6. [DOI] [PubMed] [Google Scholar]
- 6.Pandey KN. Guanylyl cyclase/atrial natriuretic peptide receptor-A: role in the pathophysiology of cardiovascular regulation. Can J Physiol Pharmacol. 2011;89:557–573. doi: 10.1139/y11-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martel G, Hamet P, Tremblay J. Central role of guanylyl cyclase in natriuretic peptide signaling in hypertension and metabolic syndrome. Mol Cell Biochem. 2010;334:53–65. doi: 10.1007/s11010-009-0326-8. [DOI] [PubMed] [Google Scholar]
- 8.Gardner DG, Chen S, Glenn DJ, Grigsby CL. Molecular biology of the natriuretic peptide system: implications for physiology and hypertension. Hypertension. 2007;49:419–426. doi: 10.1161/01.HYP.0000258532.07418.fa. [DOI] [PubMed] [Google Scholar]
- 9.Wedel BJ, Garbers DL. New insights on the functions of the guanylyl cyclase receptors. FEBS Lett. 1997;410:29–33. doi: 10.1016/s0014-5793(97)00358-x. [DOI] [PubMed] [Google Scholar]
- 10.Schul,z S, Singh S, Bellet RA, Singh G, Tubb DJ, Chin H, Garbers DL. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989;58:1155–1162. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- 11.Duda T, Goraczniak RM, Sitaramayya A, Sharma RK. Cloning and expression of an ATP-regulated human retina C-type natriuretic factor receptor guanylate cyclase. Biochemistry. 1993;32:1391–1395. doi: 10.1021/bi00057a001. [DOI] [PubMed] [Google Scholar]
- 12.de Sauvage FJ, Camerato TR, Goeddel DV. Primary structure and functional expression of the human receptor for Escherichia coli heat-stable enterotoxin. J Biol Chem. 1991;266:17912–17918. [PubMed] [Google Scholar]
- 13.Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, Smith CE. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci USA. 1992;89:947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch KW, Duda T, Sharma RK. Ca(2+)-modulated vision-linked ROS-GC guanylate cyclase transduction machinery. Mol Cell Biochem. 2010;334:105–115. doi: 10.1007/s11010-009-0330-z. [DOI] [PubMed] [Google Scholar]
- 15.Duda T, Sharma RK. ONE-GC membrane guanylate cyclase, a trimodal odorant signal transducer. Biochem Biophys Res Commun. 2008;36:440–445. doi: 10.1016/j.bbrc.2007.12.153. [DOI] [PubMed] [Google Scholar]
- 16.Sharma RK, Duda T. Odorant-linked ROS-GC subfamily membrane guanylate cyclase transduction system. Mol Cell Biochem. 2010;334:181–189. doi: 10.1007/s11010-009-0333-9. [DOI] [PubMed] [Google Scholar]
- 17.Koch KW, Duda T, Sharma RK. Photoreceptor specific guanylate cyclases in vertebrate phototransduction. Mol Cell Biochem. 2002;230:97–106. [PubMed] [Google Scholar]
- 18.Pugh EN, Jr., Duda T, Sitaramayya A, Sharma RK. Photoreceptor guanylate cyclases: a review. Biosci Rep. 1997;1:429–473. doi: 10.1023/a:1027365520442. [DOI] [PubMed] [Google Scholar]
- 19.Duda T, Venkataraman V, Krishnan A, Nagel,e RG, Sharma RK. Negatively calcium-modulated membrane guanylate cyclase signaling system in the rat olfactory bulb. Biochemistry. 2001;40:4654–4662. doi: 10.1021/bi0027985. [DOI] [PubMed] [Google Scholar]
- 20.Leinders-Zufall T, Cockerham E, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci USA. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci USA. 2009;10:2041–2046. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duda T, Sharma RK. Distinct ONE-GC transduction modes and motifs of the odorants: Uroguanylin and CO(2) Biochem Biophys Res Commun. 2010;391:1379–1384. doi: 10.1016/j.bbrc.2009.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma RK, Duda T. Ca(2+)-sensors and ROS-GC: interlocked sensory transduction elements: a review. Front Mol Neurosci. 2012;5:42. doi: 10.3389/fnmol.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duda T, Pertzev A, Koch KW, Sharma RK. Antithetical modes of and the Ca(2+) sensors targeting in ANF-RGC and ROS-GC1 membrane guanylate cyclases. Front Mol Neurosci. 2012;5:44. doi: 10.3389/fnmol.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duda T, Pertzev A, Sharma RK. Ca(2+) Modulation of ANF-RGC: New Signaling Paradigm Interlocked with Blood Pressure Regulation. Biochemistry. 2012;51:9394–9405. doi: 10.1021/bi301176c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duda T, Bharill S, Wojtas I, Yadav P, Gryczynski I, Gryczynski Z, Sharma RK. Atrial natriuretic factor receptor guanylate cyclase signaling: new ATP-regulated transduction motif. Mol Cell Biochem. 2009;324:39–53. doi: 10.1007/s11010-008-9983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duda T, Pertzev A, Sharma RK. 657WTAPELL663 motif of the photoreceptor ROS-GC1: a general phototransduction switch. Biochem Biophys Res Commun. 2011;408:236–241. doi: 10.1016/j.bbrc.2011.03.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook MJ, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Cold Spring Harbor Laboratory, Cold Spring Harbor; NY: 1989. [Google Scholar]
- 29.Nambi P, Aiyar NV, Sharma RK. Adrenocorticotropin-dependent particulate guanylate cyclase in rat adrenal and adrenocortical carcinoma: comparison of its properties with soluble guanylate cyclase and its relationship with ACTH-induced steroidogenesis. Arch. Biochem.Biophys. 1982;217:638–646. doi: 10.1016/0003-9861(82)90545-8. [DOI] [PubMed] [Google Scholar]
- 30.Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 32.Ellis KL, Newton-Cheh C, Wang TJ, Frampton CM, Doughty RN, Whalley GA, Ellis CJ, Skelton L, Davis N, Yandle TG, Troughton RW, Richards AM, Cameron VA. Association of genetic variation in the natriuretic peptide system with cardiovascular outcomes. J Mol Cell Cardiol. 2011;50:695–701. doi: 10.1016/j.yjmcc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Das S, Au E, Krazit ST, Pandey KN. Targeted disruption of guanylyl cyclase-A/natriuretic peptide receptor-A gene provokes renal fibrosis and remodeling in null mutant mice: role of proinflammatory cytokines. Endocrinology. 2010;151:5841–5850. doi: 10.1210/en.2010-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellmers LJ, Scott NJ, Piuhola J, Maeda N, Smithies O, Frampton CM, Richards AM, Cameron VA. Npr1-regulated gene pathways contributing to cardiac hypertrophy and fibrosis. J Mol Endocrinol. 2007;38:245–257. doi: 10.1677/jme.1.02138. [DOI] [PubMed] [Google Scholar]
- 35.Wadei HM, Textor SC. The role of the kidney in regulating arterial blood pressure. Nat Rev Nephrol. 2012;8:602–609. doi: 10.1038/nrneph.2012.191. [DOI] [PubMed] [Google Scholar]
- 36.Anand-Srivastava MB, Trachte GJ. Atrial natriuretic factor receptors and signal transduction mechanisms. Pharmacol Rev. 1993;45:455–497. [PubMed] [Google Scholar]
- 37.Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990;70:665–699. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- 38.Ganguly A. Atrial natriuretic peptide-induced inhibition of aldosterone secretion: a quest for mediator(s) Am J Physiol. 1992;263:E181–194. doi: 10.1152/ajpendo.1992.263.2.E181. [DOI] [PubMed] [Google Scholar]
- 39.Zhao D, Vellaichamy E, Somanna NK, Pandey KN. Guanylyl cyclase/natriuretic peptide receptor-A gene disruption causes increased adrenal angiotensin II and aldosterone levels. Am J Physiol Renal Physiol. 2007;293:F121–127. doi: 10.1152/ajprenal.00478.2006. [DOI] [PubMed] [Google Scholar]
- 40.de Bold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- 41.Marala RB, Sharma RK. Ubiquitous and bifunctional 180 kDa atrial natriuretic factor-dependent guanylate cyclase. Mol Cell Biochem. 1991;100:25–30. doi: 10.1007/BF00230806. [DOI] [PubMed] [Google Scholar]
- 42.Ballermann BJ, Marala RB, Sharma RK. Characterization and regulation by protein kinase C of renal glomerular atrial natriuretic peptide receptor-coupled guanylate cyclase. Biochem Biophys Res Commun. 1988;157:755–761. doi: 10.1016/s0006-291x(88)80314-0. [DOI] [PubMed] [Google Scholar]
- 43.De Léan A, Gutkowska J, McNicoll N, Schiller PW, Cantin M, Genest J. Characterization of specific receptors for atrial natriuretic factor in bovine adrenal zona glomerulosa. Life Sci. 1984;35:2311–2318. doi: 10.1016/0024-3205(84)90522-8. [DOI] [PubMed] [Google Scholar]
- 44.Lynch DR, Braas KM, Snyder SH. Atrial natriuretic factor receptors in rat kidney, adrenal gland, and brain: autoradiographic localization and fluid balance dependent changes. Proc Natl Acad Sci U S A. 1986;83:3557–3561. doi: 10.1073/pnas.83.10.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chai SY, Sexton PM, Allen AM, Figdor R, Mendelsohn FA. In vitro autoradiographic localization of ANP receptors in rat kidney and adrenal gland. Am J Physiol. 1986;250:F753–757. doi: 10.1152/ajprenal.1986.250.4.F753. [DOI] [PubMed] [Google Scholar]
- 46.de Zeeuw D, Janssen WM, de Jong PE. Atrial natriuretic factor: its (patho)physiological significance in humans. Kidney Int. 1992;41:1115–1133. doi: 10.1038/ki.1992.172. [DOI] [PubMed] [Google Scholar]
- 47.Misono KS. Atrial natriuretic factor binding to its receptor is dependent on chloride concentration: A possible feedback-control mechanism in renal salt regulation. Circ Res. 2000;6:1135–1139. doi: 10.1161/01.res.86.11.1135. [DOI] [PubMed] [Google Scholar]
- 48.Duda T, Goraczniak RM, Sharma RK. Site-directed mutational analysis of a membrane guanylate cyclase cDNA reveals the atrial natriuretic factor signaling site. Proc Natl Acad Sci U S A. 1991;88:7882–7886. doi: 10.1073/pnas.88.17.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Akker F, Zhang X, Miyagi M, Huo X, Misono KS, Yee VC. Structure of the dimerized hormone-binding domain of a guanylyl-cyclase-coupled receptor. Nature. 2000;406:101–104. doi: 10.1038/35017602. [DOI] [PubMed] [Google Scholar]
- 50.Ogawa H, Qiu Y, Ogata CM, Misono KS. Crystal structure of hormone-bound atrial natriuretic peptide receptor extracellular domain: rotation mechanism for transmembrane signal transduction. J Biol Chem. 2004;279:28625–28631. doi: 10.1074/jbc.M313222200. [DOI] [PubMed] [Google Scholar]
- 51.Duda T, Sharma RK. Two membrane juxtaposed signaling modules in ANF-RGC are interlocked. Biochem Biophys Res Commun. 2005;332:149–156. doi: 10.1016/j.bbrc.2005.04.102. [DOI] [PubMed] [Google Scholar]
- 52.Joubert S, Jossart C, McNicoll N, De Léan A. Atrial natriuretic peptide-dependent photolabeling of a regulatory ATP-binding site on the natriuretic peptide receptor-A. FEBS J. 2005;272:5572–5583. doi: 10.1111/j.1742-4658.2005.04952.x. [DOI] [PubMed] [Google Scholar]
- 53.Burczynska B, Duda T, Sharma RK. ATP signaling site in the ARM domain of atrial natriuretic factor receptor guanylate cyclase. Mol Cell Biochem. 2007;301:93–107. doi: 10.1007/s11010-006-9400-7. [DOI] [PubMed] [Google Scholar]
- 54.Duda T, Yadav P, Jankowska A, Venkataraman V, Sharma RK. Three dimensional atomic model and experimental validation for the ATP-Regulated Module (ARM) of the atrial natriuretic factor receptor guanylate cyclase. Mol Cell Biochem. 2001;217:165–172. doi: 10.1023/a:1007236917061. [DOI] [PubMed] [Google Scholar]
- 55.Duda T, Yadav P, Sharma RK. Allosteric modification, the primary ATP activation mechanism of atrial natriuretic factor receptor guanylate cyclase. Biochemistry. 2011;50:1213–1225. doi: 10.1021/bi1018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lohmann SM, Vaandrager AB, Smolenski A, Walter U, De Jonge HR. Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 57.Pfeifer A, Ruth P, Dostmann W, Sausbier M, Klatt P, Hofmann F. Structure and function of cGMP-dependent protein kinases. Rev Physiol Biochem Pharmacol. 1999;135:105–149. doi: 10.1007/BFb0033671. [DOI] [PubMed] [Google Scholar]
- 58.MacFarland RT, Zelus BD, Beavo JA. High concentrations of a cGMP-stimulated phosphodiesterase mediate ANP-induced decreases in cAMP and steroidogenesis in adrenal glomerulosa cells. J Biol Chem. 1991;266:136–142. [PubMed] [Google Scholar]
- 59.Côté M, Payet MD, Rousseau E, Guillon G, Gallo-Payet N. Comparative involvement of cyclic nucleotide phosphodiesterases and adenylyl cyclase on adrenocorticotropin-induced increase of cyclic adenosine monophosphate in rat and human glomerulosa cells. Endocrinology. 1999;140:3594–3601. doi: 10.1210/endo.140.8.6889. [DOI] [PubMed] [Google Scholar]