Abstract
The vertebrate intestinal epithelium is renewed continuously from stem cells at the base of the crypt in mammals or base of the fold in fish over the life of the organism. As stem cells divide, newly formed epithelial cells make an initial choice between a secretory or enterocyte fate. This choice has previously been demonstrated to involve Notch signaling as well as Atonal and Her transcription factors in both embryogenesis and adults. Here, we demonstrate that in contrast to the atoh1 in mammals, ascl1a is responsible for formation of secretory cells in zebrafish. ascl1a−/− embryos lack all intestinal epithelial secretory cells and instead differentiate into enterocytes. ascl1a−/− embryos also fail to induce intestinal epithelial expression of deltaD suggesting that ascl1a plays a role in initiation of Notch signaling. Inhibition of Notch signaling increases the number of ascl1a and deltaD expressing intestinal epithelial cells as well as the number of developing secretory cells during two specific time periods: between 30 and 34 hpf and again between 64 and 74 hpf. Loss of enteroendocrine products results in loss of anterograde motility in ascl1a−/− embryos. 5HT produced by enterochromaffin cells is critical in motility and secretion within the intestine. We find that addition of exogenous 5HT to ascl1a−/− embryos at near physiological levels (measured by differential pulse voltammetry) induce anterograde motility at similar levels to wild type velocity, distance, and frequency. Removal or doubling the concentration of 5HT in WT embryos does not significantly affect anterograde motility, suggesting that the loss of additional enteroendocrine products in ascl1a−/− embryos also contributes to intestinal motility. Thus, zebrafish intestinal epithelial cells appear to have a common secretory progenitor from which all subtypes form. Loss of enteroendocrine cells reveals the critical need for enteroendocrine products in maintenance of normal intestinal motility.
Keywords: Intestine, ascl1a, Enteroendocrine, Enterocyte, Enterochromaffin, Goblet cell, Serotonin, Notch, Motility, Spatotemporal mapping, Smooth muscle, Enteric neurons, Differential pulse voltammetry
Introduction
The intestinal epithelium is a unique vertebrate tissue that continually renews itself throughout the life of the organism. Epithelial cells originate from stem cells at the base of the crypt in mammals (Spence et al., 2011) or the base of the fold in zebrafish (Ng et al., 2005; Wallace et al., 2005b) and differentiate into enterocytes, goblet, enteroendocrine, or paneth cells as they move out of the stem cell compartment. As cells reach the tip, they undergo apoptosis and are shed into the lumen. Due to the constant turnover of epithelial cells, typical neural innervation is not common; instead, axons terminate near the epithelial cells (Gershon, 2003). In order for luminal conditions to be communicated to enteric neurons and surrounding tissue, a variety of specialized secretory cells differentiate within the epithelium and release their products to the basal surface (Gunawardene et al., 2011; Moran et al., 2008). These secretions alter motility and intestinal absorption.
During mammalian intestinal epithelial development, cells in the developing proliferative compartments at the base of the villi express Atoh1/Math1. Loss of atoh1/math1 prevents differentiation of secretory cells (Yang et al., 2001). In contrast, Hes1 is expressed along the villi, is excluded from secretory cells, and loss of hes1 results in increased secretory cell differentiation (Jensen et al., 2000). Notch signaling components are expressed in the embryonic intestine and loss of either atoh1/math1 or hes1 results in misregulation of Delta ligands (Jensen et al., 2000; Yang et al., 2001). This suggests a model of lateral inhibition in which epithelial cells expressing atoh1/math1 differentiate into secretory cells. Differentiating secretory cells then express Notch ligands to induce the enterocyte fate in surrounding cells by activating the Notch signaling pathway. Activation of the Notch receptor results in cleavage of the intracellular domain (ICD), which enters the nucleus to interact with RBP-Jk resulting in the activation of downstream genes such as hes1, which in turn represses genes specifying the enteroendocrine fate (Fre et al., 2011; Hartenstein et al., 2010; Schonhoff et al., 2004).
This mechanism of choice between secretory cells and enterocytes has been conserved over a wide range of species. Loss of Notch signaling in zebrafish fails to specify enterocytes and many more secretory cells develop (Crosnier et al., 2005). In the adult Drosophila intestine, Notch signaling is required to produce enterocytes, while low Notch levels produce secretory cells (Bardin et al., 2010; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006, 2007).
Notch continues to play an important role in the adult intestine where signaling is required for both maintenance of the stem cell compartment and making the choice between the secretory or enterocyte fate. Recent work demonstrates that stem cells expressing a Leucine-rich repeat containing G protein–coupled receptor 5 (Lgr5) cluster around Paneth cells (Sato et al., 2011). Paneth cells are required for proliferation and maintenance of the stem cell compartment and are a source of Wnt3, Notch ligand Dll4, EGF, and TGF-A (Sato et al., 2011). Loss of Notch signaling results in absence of crypt proliferation and differentiation of stem cells into goblet cells (Van Es et al., 2005). In addition, as during embryogenesis, atoh1/math1 is required for epithelial cells to enter the secretory fate (Van Es et al., 2010). Notch signaling is induced in cells entering the enterocyte fate with up regulation of hes1 and repression of atoh1/math1.
After initial differentiation into enterocyte and secretory lineages, there is further restriction into specific subsets of each cell type. Neurogenin3 specifies the enteroendocrine lineage (Jenny et al., 2002), while other secretory cells differentiate into goblet or Paneth cells. Goblet cells produce mucin to lubricate the lumen and provide a barrier to microbial invasion. Enteroendocrine stem cells go on to differentiate into approximately 10 different lineages (Schonhoff et al., 2004). Subtypes of enteroendocrine cells are dispersed throughout the epithelium (Sjolund et al., 1983) and use G protein coupled receptors, similar to oral taste receptors, to sense luminal contents, resulting in the stimulation of mediator release (Iwatsuki and Uneyama, 2012; Raybould, 2010). Mediators from enteroendocrine cells act on mucosal enteric neurons and surrounding tissue to alter secretory and motor activity within the intestine (Hansen and Witte, 2008; Mawe et al., 2006; Spiller, 2011). While enterocytes develop specific regionalization along the proximal to distal axis, specific classes of these cells are not as well defined.
One well-studied enteroendocrine lineage is enterochromaffin (EC) cells, which synthesize and secrete serotonin (5HT). EC cells act as transducers, releasing 5HT in response to stretch and pressure (Heredia et al., 2009) as well as luminal contents (Kidd et al., 2008), resulting in altered motility and stimulation of mucosal secretions. In mammals, fourteen 5HT receptors have been identified: 5HT1A, 5HT1B, 5HT1P, 5HT2A, 5HT2B, 5HT3, and 5HT4 are expressed within the digestive system (Hansen and Witte, 2008). 5HT receptors are located on both intrinsic and extrinsic neurons as well as surrounding tissue including smooth muscle (Hansen, 2003; Hansen and Witte, 2008; Mawe et al., 2006). Binding to 5HT1P, 5HT3, and 5HT4 is excitatory while binding to 5HT1A is inhibitory (Gershon and Tack, 2007; Hansen and Witte, 2008).
Here we investigate the role of one of the zebrafish acheatescute like family members, ascl1a, in specification of the intestinal epithelial secretory lineage. ascl1a expression suggests a role in specification of secretory cells. We find that ascl1a null mutants do not develop secretory cells and the entire epithelium differentiates into enterocytes. ascl1 has been shown to play a role in avian, mammalian, and zebrafish delta expression (Hans and Campos-Ortega, 2002; Mizuguchi et al., 2006; Nelson et al., 2009). We find that ascl1a mutants fail to initiate deltaD expression within the intestinal epithelium, suggesting that ascl1a expressing cells activate Notch signaling in neighboring cells. Here we investigate whether Notch signaling is active throughout the entire period of intestinal ascl1a expression. In addition, ascl1a mutants have a loss of anterograde motility. Replacement of 5HT initiates motility with the same velocity, distance, and frequency found in wild type embryos. Pharmacological removal of 5HT in wild type embryos does not, however, result in loss of anterograde motility.
Materials and methods
Fish Stocks
Fish maintenance and matings were performed as previously described (Westerfield, 1993). AB wild type fish were used for most procedures (Westerfield, 1993). ascl1a null mutants were obtained from Matthias Hammerschmidt and described in (Pogoda et al., 2006). Embryos kept for motility experiments were treated with pronase (Roche) at the end of the first day of embryogenesis and washed out at the beginning of the second day of embyrogenesis in order to remove the chorion. Embryos were allowed to grow with pigment and E3 (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4) was exchanged each day for optimal growth.
Immunohistochemistry
Embryos were fixed in 4% formaldehyde for a period of either 2 hours or overnight. Fixed embryos were then permeabilized with Proteinase K (Sigma) or Collagenase (Sigma) in PBS (0.(186.5 mM NaCl; 2.68 × 10−2 mM KCl; 1 mM Na2HPO4 (dibasic); 6.95 mM NaH2PO4-H2O (monobasic)) for 20 min at room temperature. Primary antibody was added and incubated at 4 °C overnight. Embryos were then washed and incubated with secondary antibody (1:500, Molecular Probes-Invitrogen) for two hours. Embryos for desmin and acetylated tubulin antibody incubation were permeabilized as previously described with phospholipase A (Sigma) (Akhtar et al., 2009). Primary antibodies are rabbit serotonin (5HT) (1:500, Sigma), mouse 2F11 (1:1000, AbCam), rabbit anti-type IIb sodium-phosphate co-transporter (1:100 dilution, a gift of A. Werner), rabbit anti-desmin (1:100 dilution, Sigma), mouse anti-acetylated tubulin (1:100 dilution, Sigma), mouse anti-HuC/D (1:50 dilution, Molecular Probes), mouse anti-ZO1 (1:100 dilution, gift of Dr. S. Tsukita and T. Obara), rabbit anti-sodium/potassium ATPase (1:100 dilution, Developmental Studies Hybridoma Bank).
For Wheat Germ Agglutinin experiments (1:100, Vector Laboratories) embryos were permeabilized with Collagenase and incubated overnight in PBST (186.5 mM NaCl; 2.68 × 10−2 mM KCl; 1 mM Na2HPO4 (dibasic); 6.95 mM NaH2PO4-H2O (monobasic); 0.1% Tween-20). Embryos were visualized on a Nikon TE200 inverted microscope using a Hamamatsu Orca camera with IP lab software.
At least three independent experiments were performed for all immunohistochemical detections.
Embryo visualization
The digestive system was visualized as described previously (Olden et al., 2008). For immunohistochemistry, intestines were dissected and mounted separately in Vectashield (Vector Laboratories). For RNA in situ hybridization, skin and yolk were removed and visualized ventrally in glycerol. Both intestine dissection and yolk removal were used in order to determine the total number of ascl1a, deltaD, and 2F11 expressing cells throughout the entire intestine for embryos exposed to DAPT and DMSO.
RNA in situ hybridization
Whole mount RNA in situ hybridization was done as previously described (Wallace and Pack, 2003). Antisense probes were transcribed from ascl1a (Allende and Weinberg, 1994), deltaD, phox2b (Elworthy et al., 2005). At least three independent experiments were performed for all RNA in situ hybridizations.
Protein detection
ascl1a−/− embryos and WT siblings were grown to 5 dpf. Brains and intestines from mutants and WT were dissected into TPER Tissue Protein Extraction Reagent (Pierce) with Halt Protease Inhibitor Cocktail and EDTA (Pierce) with double the recommended concentration in order to prevent protein degradation. Also, in order to prevent sample degradation, dissected samples were manually disrupted in the extraction buffer every two to three dissections. Protein gel electrophoresis and western blots were performed and blots washed as previously described (Hongay and Orr-Weaver, 2011) with the following changes. Protein samples were run on Bio-Rad TGX Stain-Free precast gels and visualized on the UV setting in the BioRad Chemidoc MP Imaging System to estimate total protein per lane. β-mercaptoethanol was not added for protein loading as it has been previously reported to interfere with the anti-DeltaD antibody (Wright et al., 2011). β-actin antibody does not detect the epitope in non-reducing conditions and equivalent quantities of extract from the same sample were run on a gel with β-mecaptoethanol as an additional loading control to the Bio-Rad TGX Stain-Free precast gels. Blocking and antibody incubations were done in NAP blocker™ (G-Biosciences). The mouse anti-Delta D (Abcam, zdd2) and mouse anti-β-Actin antibodies (Sigma) were used 1:10,000 and incubations were done overnight at 4 °C. For detection, the blots were incubated overnight at 4 °C with anti-mouse biotin (Jackson Laboratories) followed by anti-mouse streptavidin-HRP (Vectastain Elite ABC system, Vector Laboratories). Molecular weight markers are Precision Plus Protein Kaleidoscope Standards (BioRad). Protein detection was achieved with femtoCHROMO™-–HRP (G-Biosciences) and visualized using the colorimetric setting in the BioRad Chemidoc MP Imaging System.
Spatiotemporal mapping
Motility of 5 dpf embryos was recorded at one-second intervals for 10 min using a Flex camera (Research Diagnostics) on a Lecia MZ12.5 stereomicroscope with Spot software. Recordings were then analyzed using Volumetry software written by Grant Hennig at the University of Nevada to create Spatiotemporal maps (STMaps) (Hennig et al., 1999). STMaps and analysis in zebrafish have been previously described (Holmberg et al., 2007). Briefly, intestinal contractions result in a change in intestine diameter appearing as a distortion on STMaps. A number of values such as frequency of contraction (cycles/min), velocity (mm/s), and distance (mm) of contractions can then be determined. Velocity and distance are determined with a best fit line drawn over the contraction. At least 10 embryos are recorded for a mean value with standard error. One-way analysis of variance was used to reveal significant differences between the groups. Post-hoc tests were performed to determine either equal or unequal variances using the Fisher test and the appropriate T test was utilized (for equal or unequal variances). The Bonferroni correction was then applied to determine the appropriate level of significance for multiple T tests.
DAPT treatment
Notch signaling was inhibited in vivo using N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT) (Geling et al., 2002). DAPT (Sigma) was diluted to 50 μM in embryo media. Embryos were incubated for a particular time period and then washed out into embryo media. For DAPT exposures beginning at a specific time point until 5 dpf, the DAPT was replaced each day. Control embryos were exposed to equivalent concentrations of dimethyl sulfoxide (DMSO) (Sigma).
Differential pulse voltammetry
Instrumentation
Differential pulse voltammetry experiments were performed using a CH Instrument electrochemical analyzer potentiostat (CH Instruments Inc.) and carried out using a conventional electrochemical setup with the chitosan modified CFME as the working electrode, a Ag/AgCl/3 M NaCl (BAS MF-2052, RE-5B) as the reference electrode, and a platinum wire as the counter electrode.
Preparation of the carbon fiber microelectrodes
Single carbon fiber microelectrodes were produced as described previously (Ozel et al., 2011). Modification with 1% chitosan improves selectivity by restricting access to high concentration substances such as ascorbic acid.
In vivo electrochemical measurements in live embryos
Five dpf embryos were placed in a pocket in agarose. Chitosan modified microelectrodes were implanted in the distal portion of the intestine utilizing a Narishige (MO-155) micromanipulator under a Nikon SMZ 1000 stereomicroscope. Electrochemical measurements were carried out in E3 medium. Differential pulse voltammetry (DPV) was used for in vivo detection of serotonin in the potential range: 0–0.6 V at a scan increment of 4.0 mV. The pulse period, width and amplitude were 200 ms, 50 ms and 50 mV, respectively. Serotonin is characterized by a well-defined peak with a maximum at ~0.37 V. All differential pulse voltammograms were analyzed after background subtraction. Background current values were recorded in E3. 5HT detection with the implanted microelectrode is performed on live embryos.
Results
ascl1a is expressed in intestinal epithelial cells in a pattern suggesting a role in secretory cell specification
While ascl1a expression has been extensively characterized during zebrafish brain development, expression in the developing intestine has not been well characterized. To determine whether ascl1a is expressed when it might play a role in specification of epithelial secretory cells, we characterized expression in the developing intestine. As with detection of other genes in the digestive system, levels of tissue permeabilization need to be adjusted in order to detect both central nervous system and digestive expression. To confirm that these permeabilization conditions produce specific staining, we compared anti-sense and sense probes for RNA in situ hybridization. While these conditions generate robust staining in both the intestine and central nervous system with anti-sense probes, sense probes incubated for the same time have no staining in the embryo demonstrating that detection of ascl1a expression is specific.
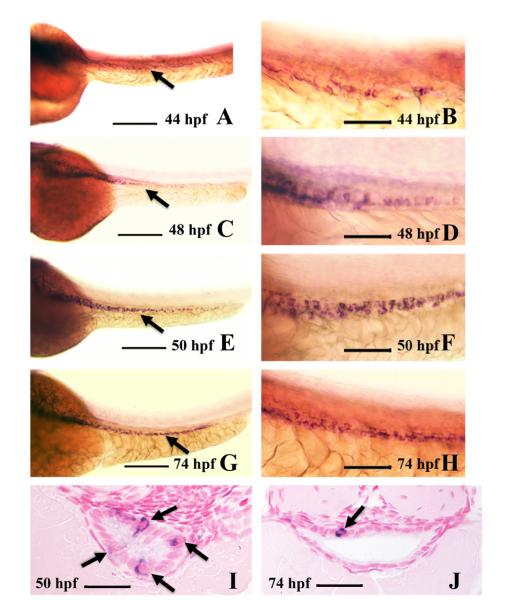
We find that ascl1a is expressed in a few individual intestinal epithelial cells at 44 hpf primarily in the proximal half of the intestine (Fig. 1A and B). The number of epithelial cells expressing ascl1a at 48 hpf increases and expands throughout the entire intestine (Fig. 1C). ascl1a expression is present in intestinal epithelial cells from proximal to distal, but the cells do not appear to be clustered (Fig. 1D). The number of ascl1a expressing cells increases and becomes more intense by 50 hpf (Fig. 1E and F). Multiple ascl1a expressing cells can often be observed in single sections by 50 hpf (Fig. 1I). At 74 hpf, ascl1a is expressed in fewer epithelial cells (Fig. 1G and H), with single cells more often found in cross sections (Fig. 1J). By 82 hpf, ascl1a is no longer expressed within the intestinal epithelium.
Fig. 1.
Intestinal expression of ascl1a. ascl1a is expressed in a few intestinal epithelial cells at 44 hpf throughout the length of the intestine ((A), arrow points to intestine). Higher magnification (B) shows individual epithelial cells expressing ascl1a, which is sparse and light at this stage. The number of cells expressing ascl1a increases by 48 hpf ((C), arrow points to intestine) and higher magnification (D). This changes at 50 hpf (E), when ascl1a is expressed more evenly throughout the intestinal epithelium, often in clusters of cells (higher magnification in (F)). In cross section at 50 hpf a single section will often display multiple epithelial cells expressing ascl1a (arrows in (I)). Expression of ascl1a is still strong in the intestinal epithelium at 74 hpf ((G), arrow points to intestine) but cells are often less clustered. Cross sections more frequently display single epithelial cells expressing ascl1a (arrow in (J)). All whole mount images ((A)–(H)) are oriented anterior to left and posterior to right. Scale bars: (A), (C), (E), and (G) 200 μm; (B), (D), (F), and (H) 50 μm; (I) and (J) 20 μm.
ascl1a expression might also be expected in enteric neural precursors as this gene is expressed in a portion of mammalian enteric neural precursors (Blaugrund et al., 1996; Lo et al., 1991). Zebrafish enteric precursors migrate along the lateral sides of the intestine during the second and third day of embryogenesis creating a chain-like migration pattern (Olden et al., 2008;Shepherd et al., 2004). Enteric neural precursor migration creates a distinct and reproducible pattern of cells on both sides of the intestine. Both in whole mount and in cross section, we do not observe expression of ascl1a at any stage in the enteric precursors that migrate along the lateral sides of the intestine. ascl1a expression instead appears to be contained within the intestinal epithelium. In ascl1a null mutants there is, however, a loss of ninety percent of the enteric neurons as observed at the end of embryogenesis (see below). ascl1a could act earlier before enteric precursors reach the intestine. Alternatively, loss of epithelial ascl1a expression may result in a lack of signals required for stimulation of enteric neural development.
As demonstrated previously, intestinal epithelial cells have a high proliferation rate which drops dramatically by the beginning of the third day. Epithelial differentiation markers appear towards the later half of the third day (Ng et al., 2005; Wallace et al., 2005a). As ascl1a expression in individual epithelial cells during the second and third day of embryogenesis encompasses periods before and during secretory cell specification, this gene may play a role in specification of secretory cells.
acsl1a null mutants lack all secretory cells within the intestinal epithelium
To further define a role for ascl1a in specification of intestinal epithelial secretory cells, we analyzed ascl1a−/− embryos. Previously, a null ascl1a mutant was identified which generates a premature stop codon, truncating the protein at amino acid 69 before any of the basic helix-loop-helix region is translated (Pogoda et al., 2006). ascl1a null mutants result in loss of all endocrine cells within the adenohypophysis (Pogoda et al., 2006). In mammals, the major secretory cells within the intestinal epithelium consist of goblet, enteroendocrine, and paneth cells. In the zebrafish intestine, there is presently no evidence for the presence of Paneth cells but there are goblet and a variety of enteroendocrine cells (Ng et al., 2005; Njagi et al., 2010; Wallace et al., 2005a).
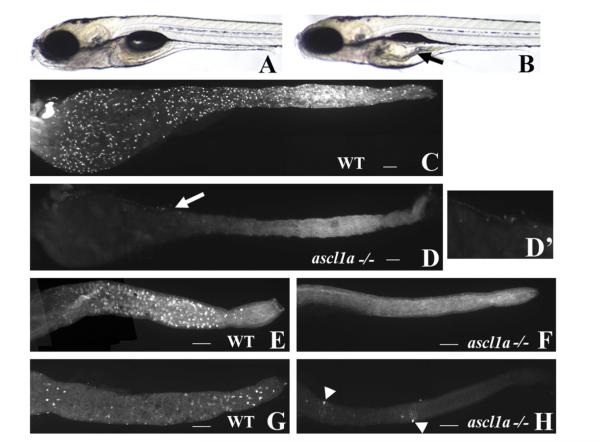
ascl1a−/− embryos have a distinct phenotype at 5 dpf that includes an enlarged proximal intestine and lack of an inflated swim bladder (Fig. 2B compare to WT in Fig. 2A). Embryos displaying this phenotype were confirmed to be ascl1a−/− using previously described PCR primers (Pogoda et al., 2006). We analyzed 5 dpf ascl1a−/− embryos for the presence of the general secretory cell marker 2F11 as well as goblet and enterochromaffin cells. The 2F11 monoclonal antibody was previously identified as a pan-secretory marker, which labels secretory cells throughout the entire intestinal epithelium (Crosnier et al., 2005). ascl1a−/− embryos show no 2F11 labeled secretory cells throughout the entire intestine (Fig. 2D compare to WT in Fig. 2C). Goblet cells were identified with fluorescently labeled Wheat Germ Agglutinin (WGA), which labels the mucin in the apical portion of the cells. In wild type embryos, goblet cells are present only in the distal intestine (Fig. 2E), but in ascl1a−/− embryos there is a complete absence of goblet cells (Fig. 2F). Immunohistochemistry using anti-serotonin (5HT) antibody demonstrates that enterochromaffin cells are present only in the distal intestine in wild type embryos (Fig. 2G). In ascl1a−/− embryos, there is a complete lack of enterochromaffin cells (Fig. 2H) but a few 5HT enteric neurons differentiate along the length of the intestine (arrowheads in Fig. 2H).
Fig. 2.
ascl1a−/− 5 dpf embryos lack intestinal epithelial secretory cells. At 5 dpf WT embryos have a narrow diameter throughout the intestinal lumen (A). ascl1a−/− embryos (B) develop an increased luminal diameter in the proximal intestine (arrow), fail to completely utilize the yolk, and fail to inflate the swim bladder while the distal intestine appears normal. The 2F11 intestinal epithelial pan-secretory cell marker reveals numerous secretory cells distributed throughout the length of the 5 dpf intestine (C). Within ascl1a−/− 5 dpf embryos, secretory cells are absent throughout the entire intestine (D). In this preparation residual pancreatic cells are observed on the lateral side of the proximal intestine (arrow in (D) and region enlarged in (D′)). Markers for individual subtypes of secretory cells are also absent in the intestinal epithelium (only the distal intestine is shown in panels (E)–(H)). Goblet cells are distributed only throughout the distal WT intestine (E) as demonstrated by the fluorescently labeled lectin, wheat germ agglutinin but are absent from ascl1a−/− embryos (F). Serotonin (5HT) containing enterochromaffin cells are distributed only throughout the distal intestinal epithelium along with 5HT enteric neurons as demonstrated by anti-5HT immunohistochemistry (G). In ascl1a−/− embryos, enterochromaffin cells are absent; however, there are a few remaining 5HT enteric neurons (arrowheads (H)). Scale bar represents 100 μm.
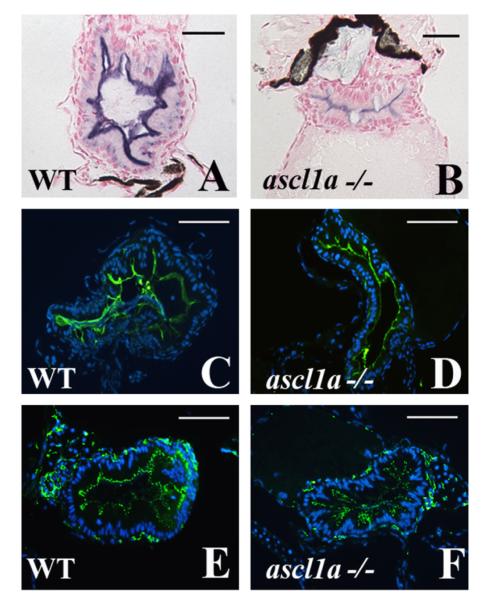
As there are no secretory cells within the 5 dpf ascl1a−/− intestinal epithelium, we verified that the cells are instead differentiating into enterocytes. We analyzed three markers of enterocyte maturation and differentiation; alkaline phosphatase, sodium phosphate transporter, and ZO1. Alkaline phosphatase is a traditional marker for enterocyte maturation and has recently been shown to detoxify LPS, a major component of gram negative bacterial membranes (Bates et al., 2007). ZO1 is a component of tight junctions, which form at the apical/lateral surfaces of enterocyte sheets. The sodium phosphate transporter moves inorganic phosphate from the lumen to maintain homeostasis. Each of these markers is present in intestinal epithelial cells of ascl1a−/− embryos in normal distribution from proximal to distal (Fig. 3B, D and F compare to WT in Fig. 3A, C, and E). These results suggest that ascl1a is required for secretory cell formation but enterocytes still differentiate when expression is lost.
Fig. 3.
Intestinal enterocytes differentiate with characteristic markers in ascl1a−/− 5 dpf embryos. Enterocytes differentiate within the ascl1a−/− intestinal epithelium as demonstrated by apical alkaline phosphatase (blue in (B) compared to WT in (A)), apical sodium phosphate transporter (NaPi) (green in (D) compared to WT in (C)), and the tight junction marker ZO-1 (green in (F) compared to WT in (E)). Apical alkaline phosphatase in ascl1a−/− is typically less than WT but NaPi and ZO-1 are present at the same intensity. Scale bar represents 50 μm except in (A) and (B) where the bar is 20 μm.
ascl1a null mutants fail to initiate intestinal epithelial expression of deltaD
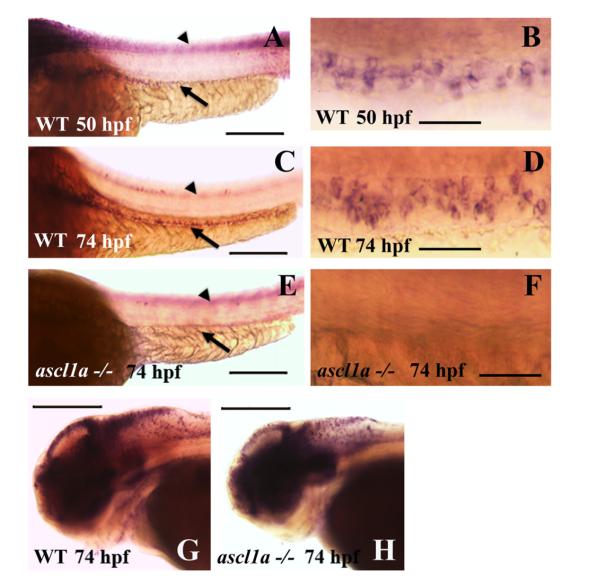
Regulation of deltaD expression by ascl1a appears to be dependent on a specific group of E boxes within the HII control region (Hans and Campos-Ortega, 2002). We therefore determined whether loss of ascl1a also results in loss of deltaD expression within the intestinal epithelium. At 74 hpf, DeltaD protein has been found to be present in a punctate distribution within developing secretory cells (Crosnier et al., 2005). While we observe a similar distribution of DeltaD within the intestine, this pattern is not useful for determining number and distribution of Delta D cells in whole mount embryos as the protein is not present around the periphery of the entire cell. deltaD RNA expression is an effective means to determine the numbers of cells expressing the gene. deltaD is lightly expressed in isolated cells throughout the proximal to distal extent of the intestinal epithelium on the second (50 hpf; Fig. 4A and B) and more intensely during the third day of embryogenesis (74 hpf;Fig. 4C and D). The number and distribution of cells has similarities to intestinal ascl1a expression but is delayed. Groups of clustered ascl1a cells form at 50 hpf in the intestinal epithelium but are more isolated by 74 hpf. In contrast, deltaD intestinal epithelial cells begin as more small clusters and individual cells at 50 hpf (Fig. 4B), but increase in number and form larger clusters at 74 hpf. This time delay would be expected if ascl1a plays a role in initiation of deltaD expression.
Fig. 4.
Intestinal expression of deltaD in WT and ascl1a−/− embryos. deltaD expression in the intestinal epithelium begins on the second day of embryogenesis by 50 hpf ((A), arrow: intestinal expression; arrowhead: neural tube expression out of focal plane) with stronger expression in the proximal intestine. Magnified view of 50 hpf deltaD expression (B) demonstrates small clusters and some isolated intestinal epithelial cells. deltaD expression becomes stronger and more even throughout the intestinal epithelium at 74 hpf ((C): arrow; arrowhead: neural tube expression out of focal plane). Magnified view of 74 hpf (D) demonstrates that deltaD is now expressed in larger clusters and higher numbers of intestinal epithelial cells. ascl1a−/− embryos lack expression of deltaD within the intestinal epithelium at 74 hpf (arrow in (E)) but retain neural tube expression (arrowhead in (E) out of focal plane). Magnified view of ascl1a−/− (F) shows absence of deltaD expression in intestinal epithelium. Within the same ascl1a−/− embryos, deltaD expression in the brain is altered but remains strong (compare WT in (G) to ascl1a−/− in (H)). Anterior is to the left and posterior to the right in all panels. Scale bars: (A), (C), (E), (G), and (H) 200 μm; (B), (D), and (F) 50 μm.
With loss of ascl1a expression in null mutants, we expect a concomitant loss of epithelial deltaD expression. We performed this assay at 74 hpf when intestinal epithelial expression of deltaD expression is stronger. ascl1a−/− embryos do not display an observable phenotype before 5 dpf so we analyzed deltaD expression in embryos of mixed genotype at 74 hpf from a heterozygote cross of two ascl1a carriers (+/−). All of the embryos retain central nervous expression in both the brain and neural tube (Fig. 4C, E, G, and H) but a quarter of the embryos have no intestinal expression of deltaD (Fig. 4 E and F). We confirmed that embryos lacking intestinal deltaD expression are ascl1a null by genotyping the embryos with previously described PCR primers (Pogoda et al., 2006).
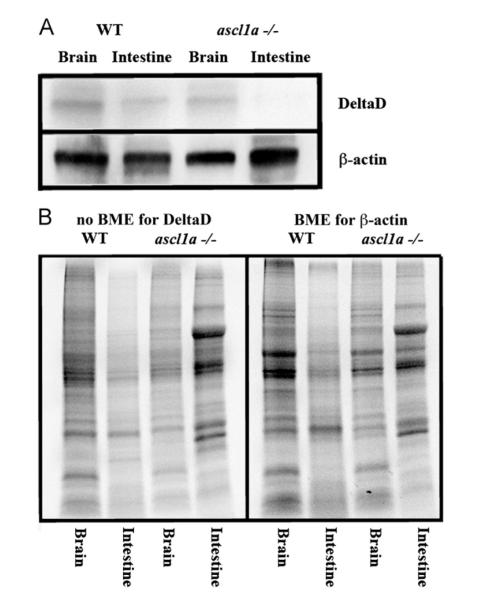
Loss of deltaD mRNA expression in ascl1a −/− intestines should also result in loss of protein expression. To determine if ascl1a loss of function embryos lack intestinal DeltaD, we dissected equivalent numbers of intestines and brains from both mutant and wild type siblings at 5 dpf (when the phenotype becomes visible) for western analysis. Similar to loss of deltaD mRNA expression in ascl1a −/− embryos, we find a loss of DeltaD in the mutant intestine but not the brain (Fig. 5). In contrast, wild type siblings have DeltaD in both intestine and brain (Fig. 5). Both the lack of DeltaD mRNA and protein expression suggests that ascl1a plays a role in initiating and/or maintaining intestinal deltaD expression. ascl1a is required for secretory cell differentiation. This suggests that high levels of deltaD are expressed in cells differentiating as secretory cells which would then activate Notch in surrounding cells to limit the number of secretory cells by lateral inhibition.
Fig. 5.
Intestinal expression of DeltaD in WT and ascl1a−/− embryos. (A) Both mutant and WT 5 dpf intestines and brains were dissected for western blot. Brains for both WT and ascl1a−/− demonstrate similar levels of DeltaD and the loading control β-actin. ascl1a−/− intestines lack DeltaD while there are similar levels of β-actin compared to WT. DeltaD is approximately 100 kD while β-actin is 55 kD. (B) Total protein as visualized by induced fluorescence on stain-free gel. Proteins are visualized after electrophoresis but before transfer to membrane as an alternative loading control to β-actin. The protein gel for DeltaD was run without β-mecaptoethanol (BME) and shown to the left while protein gel for β-actin was run with β-mecaptoethanol (BME) and is shown to the right. The same volume of protein from the same sample was loaded on both the BME and no BME gels.
Notch gamma-secretase inhibition changes the number of ascl1a expressing cells
Previously, experiments have identified increases in secretory cell formation where either Notch signaling is absent throughout all of embryogenesis or between the first and third dpf (Crosnier et al., 2005; Van Es et al., 2005; Zecchin et al., 2007). While these results indicate that Notch signaling is required for limiting the number of intestinal epithelial secretory cells, it is unknown whether Notch signaling is required continuously or only during specific periods in order to accomplish this reduction. Expression of ascl1a suggests that Notch signaling may be active during the during the entire period of 44–82 hpf.
To determine when signaling is required, we took the approach of inhibiting Notch signaling for specific periods during embryogenesis. Inhibition of Notch signaling should result in an increase the number of intestinal epithelial cells specified as secretory cells only when signaling is active. The increased number of cells specified as secretory cells should also express ascl1a. If Notch signaling is active throughout the entire period of ascl1a expression, then there should be increases in the number of ascl1a expressing cells any time Notch is inhibited between 44 and 82 hpf. Alternatively, if Notch signaling is only required for distinct periods, then increases in ascl1a expressing cells will only occur when signaling is inhibited for specific periods.
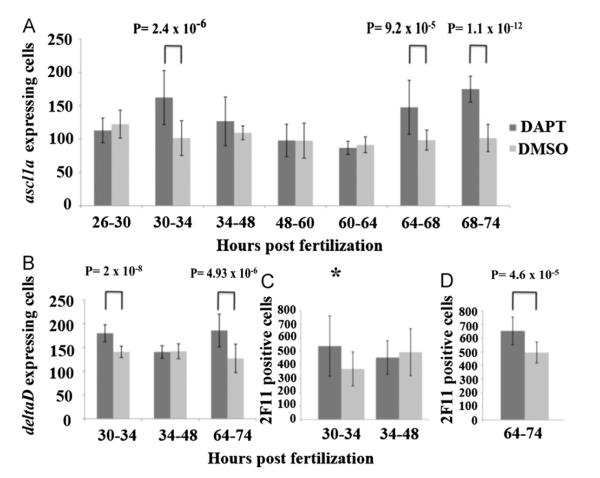
Notch signaling was inhibited for specific periods with the addition of DAPT followed by washout into E3. DAPT inhibits gamma secretase and prevents cleavage of the Notch intracellular domain (NICD), preventing activation of Notch target genes (Geling et al., 2002). We first surveyed the time span between the first to third days of embryogenesis with DAPT inhibition for 12–14 h periods. Embryos exposed to DAPT were analyzed for ascl1a expression at 74 hpf towards the end of acsl1a expression in the intestinal epithelium. In this initial survey, we found that Notch signaling only increases ascl1a expressing epithelial cells during specific 12–18 h periods between 30–48 hpf and 60–74 hpf. These periods with increased numbers of ascl1a expressing epithelial cells were divided into four hour blocks to identify more specific times when Notch signaling is required. Inhibition of Notch signaling resulted in significant increases in the number of ascl1a only during the periods of 30–34 hpf and again during 64–74 hpf (Fig. 6A). There were no significant increases in ascl1a expressing cells during the other time periods (Fig. 6A), suggesting that Notch signaling is only active in secretory cell specification from 30 to 34 hpf and again during 64–74 hpf.
Fig. 6.
Effects of inhibition of Notch signaling within the intestinal epithelium. Embryos from three independent experiments were exposed to the gamma-secretase inhibitor DAPT, or DMSO as a control, for 4–14 h periods followed by washout and return to embryo media. Embryos were then assayed for increases in the number of ascl1a (A), deltaD (B) (by RNA in situ hybridization), or 2F11 (using immunohistochemistry). (C) expressing cells throughout the entire intestine at 74 hpf. For ascl1a and deltaD RNA in situ hybridization, the yolk was removed and intestinal cells were counted. Embryos were assayed (D) for increases in secretory cells (2F11) after Notch inhibition between 64 and 74 hpf and at 96 hpf instead of at 74 hpf. In panels A through (C), a two-way analysis of variance showed a significant difference between time and DAPT inhibition; (A) F(6,238)=16.162, p=0.00, (B) F(2,96)=15.504, p=0.00, (C) F(1,56)=5.086, p=0.028. In post-hoc tests, either equal or unequal variances were identified using the Fisher test and the appropriate T test was utilized (equal or unequal variances). Two-tailed student T tests with unequal variances (Welch’s T test) are used for 30–34 hpf, 34–48 hpf, and 64–68 hpf in panel (A) while the rest have equal variances. p values from the appropriate T test are indicated above the time periods with a significant difference when compared to DMSO control group after Bonferroni correction ((A) p<0.0071; (B) p<0.0167; (C) p<0.025). While the p value for 30–34 hpf in panel (C) (p=0.029) is not significant using the Bonferroni correction, the value is just above the level of significance p<0.025) and labeled with an asterisk to indicate a trend of increased numbers of secretory cells. Each of the other time periods do not result in significantly different values when compared to the DMSO controls. Black lines on bars represent standard deviation. Total number of individuals tested from each of the three independent experiments are as follows; Panel (A) 26–30 DAPT: n=22, DMSO: n=21 30–34 DAPT: n=20, DMSO: n=23 34–48 DAPT: n=20, DMSO: n=14 48–60 DAPT: n=14, DMSO: n=17 60–64 DAPT: n=14, DMSO: n=18 64–68 DAPT: n=18, DMSO: n=15 68–74 DAPT: n=20, DMSO: n=16 Panel (B) 30–34 DAPT: n=15, DMSO: n=18 34–48 DAPT: n=16, DMSO: n=17 64–74 DAPT: n=19, DMSO: n=17 Panel (C) 30–34 DAPT: n=20, DMSO: n=11 34–48 DAPT: n=15, DMSO: n=14 Panel (D) 64–74 DAPT: n=15, DMSO: n=15.
Notch gamma-secretase inhibition increases the number of deltaD expressing cells
If ascl1a expression plays a role in initiation of deltaD expression, then periods of Notch inhibition with DAPT with increases in ascl1a intestinal epithelial cells should also increase numbers of deltaD expressing cells. In the previous section, we find increases in ascl1a intestinal epithelial cells only when Notch is inhibited between 30–34 hpf and 64–74 hpf. Therefore, the number of intestinal epithelial cells expressing deltaD should also only increase during these two periods when Notch is active.
We inhibited Notch signaling using DAPT for three periods during 30–34 hpf, 34–48 hpf, and 64–74 hpf and assayed numbers of intestinal epithelial cells expressing deltaD at 74 hpf. Similar to observed increases in ascl1a expressing cells, we find increases in deltaD expressing cells only when Notch is inhibited between 30–34 and 64–74 but not during 34–48 hpf (Fig. 6B). This suggests that additional intestinal epithelial cells become specified as secretory cells and initiate deltaD expression. This lends further support to the idea that Notch signaling is only active during discrete periods rather than continuously during secretory cell specification.
Notch gamma-secretase inhibition increases the number of epithelial secretory cells by the midpoint of embryogenesis
If Notch signaling is only active during the periods of 30–34 and again between 64 and 74 hpf as suggested by increases in ascl1a and deltaD during these periods, then Notch inhibition should only increase the number of secretory cells during the same periods. We used DAPT to inhibit Notch signaling for three time periods at 30–34 hpf, 34–48 hpf, and 64–74 hpf. Inhibition for the period of 34–48 hpf was used to demonstrate a lack of increased numbers of secretory cells.
To identify differentiating secretory cells early in embryogenesis, we used the pan-secretory monoclonal antibody 2F11 at 74 hpf, which is the first time this marker is present in the intestinal epithelium. During this period other markers of secretory cell differentiation are not yet expressed. We find increases in secretory cells when Notch signaling is inhibited between 30 and 34 hpf but are not statistically significant. The p value generated in the T test is just above the level of significance with application of the Bonferroni correction (p=0.029 with a level of significance p<0.025). This suggests a trend of increased secretory cell numbers that might become significant with an increased sample size. As expected, we find no significant increase in the numbers of secretory cells upon Notch inhibition from 34 to 48 hpf (Fig. 6C). Inhibition of Notch signaling between 64 and 74 hpf increases secretory cell numbers however, at 74 hpf most of the epithelium appears to express the 2F11 epitope. Since embryos were fixed just after DAPT inhibition, we thought that this might be affecting 2F11 expression. To allow additional time between DAPT inhibition and fixation, we again inhibited Notch signaling with DAPT but allowed the embryos to develop until 96 hpf. At 96 hpf, we find that 2F11 is expressed in cells with characteristic secretory cell shape and no longer throughout the entire epithelium. Comparison of Notch inhibition between 64 and 74 hpf and DMSO control embryos demonstrates a significant increase in secretory cells at 96 hpf (Fig. 6D). Therefore, upon Notch inhibition with DAPT between 30–34 hpf and 64–74 hpf, we find increases in cells expressing ascl1a and deltaD as well as increases in the overall number of intestinal epithelial secretory cells.
Notch gamma-secretase inhibition changes the final subtype composition of secretory cells in the 5 dpf intestinal epithelium
Embryos which underwent Notch inhibition during 30–34 and again during 64–74 hpf were grown to 5 dpf and analyzed for changes in secretory cell numbers. In addition to the 2F11 antibody, we utilized markers of differentiated secretory cells for enterochromaffin and goblet cells. 5HT was used to identify enterochromaffin cells while wheat germ agglutinin (WGA) was used to identify mucin in goblet cells. Embryos exposed to DAPT only during the 30–34 hpf or 64–74 hpf period did not have significant changes in the number or type of secretory cells at 5 dpf. We hypothesized that there may be a compensatory affect within the epithelium after Notch inhibition by DAPT is relieved, resulting in subsequent restoration of the wild type secretory to enterocyte ratio.
To determine whether increased numbers of secretory cells could be retained until the end of embryogenesis, Notch inhibition using DAPT was continued until 5 dpf. We began DAPT exposure beginning around the times when we observe increases in ascl1a, deltaD, or secretory cell numbers at 74 hpf (30–60 hpf) or when Notch signaling appears to not be active (34 hpf). We again used 2F11 antibody and markers of differentiated secretory cells for enterochromaffin (5HT) and goblet cells (WGA).
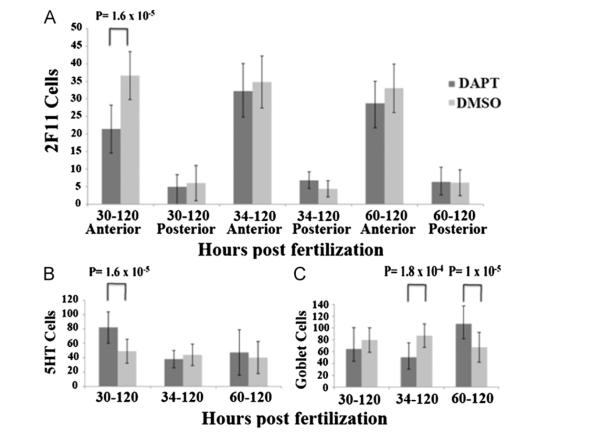
With continuous Notch inhibition until 5 dpf, we do not find much change in overall numbers of secretory cells beginning at any of the three time points as demonstrated by similar numbers of 2F11 cell counts (Fig. 7A). Due to the large number of secretory cells in the 5 dpf intestinal epithelium, we sampled the number of secretory cells in both the proximal and distal intestine. Using 2F11, we recorded a representative sampling of secretory cells, measuring 100 μm from each end of the intestine and then counted cells for the next 200 μm. 2F11 demonstrates slight but significant decreases in the proximal epithelium when exposure begins at 30–60 hpf. The distal epithelium has no significant increase in 2F11 cells (Fig. 7A). Individual secretory subtypes however, do change more significantly.
Fig. 7.
Effects of Notch inhibition on secretory cells at the end of embryogenesis. Embryos from three independent experiments were exposed to the gamma-secretase inhibitor DAPT, or DMSO as a control, beginning at three time points then continually until 5 dpf. Embryos were assayed for the pan-secretory cell marker 2F11 (A), enterochromaffin cells (B), goblet cells (C). In panels (A) through (C), a two-way analysis of variance showed a significant difference between time and DAPT inhibition; (A) Proximal F(2,140)=11.09, p=0.00 (B) F(2,131)=7.148, p=0.00 (C) F(2,118)=20.95, p=0.00. There was no significant interaction between time and DAPT inhibition in distal measurements in panel (A) F(2,150)=1647, p=0.96. In post-hoc tests, either equal or unequal variances were identified using the Fisher test and the appropriate T test was utilized (equal or unequal variances). Two-tailed student T test with unequal variances (Welch’s T test) are used for 30–120 hpf in panel (B) while the rest have equal variances. P values from the appropriate T test are indicated above the time periods with a significant difference when compared to DMSO control group. Each of the other time periods do not result in significantly different values when compared to the DMSO controls. Black lines on bars represent standard deviation. Total number of individuals tested from each of the three independent experiments are as follows; Panel (A) 30–120 hpf DAPT n=12, DMSO n=24 34–120 hpf DAPT n=15, DMSO n=26 60–120 hpf DAPT n=31, DMSO n=29 Panel (B) 30–120 hpf DAPT n=22, DMSO n=25 34–120 hpf DAPT n=12, DMSO n=16 6–120 hpf DAPT n=26, DMSO n=23 Panel (C) proximal 30–120 hpf DAPT n=17, DMSO n=25 34–120 hpf DAPT n=11, DMSO n=16 60–120 hpf DAPT n=46, DMSO n=43 distal 30–120 hpf DAPT n=17, DMSO n=25 34–120 hpf DAPT n=11, DMSO n=16 60–120 hpf DAPT n=46, DMSO n=43.
Enterochromaffin (EC) cells demonstrate a significant increase when exposure begins at 30 hpf. There is no significant change when exposure begins at 34 or 60 hpf (Fig. 7B). With specific staining for goblet cells using wheat germ agglutinin, goblet cells decrease by 42% when exposure begins at 34 hpf, but then increases by 37.2% when exposure begins at 60 hpf (Fig. 7C).
The increase in goblet cells with DAPT exposure beginning at 60 hpf is similar to inhibition of Notch receptor cleavage with another gamma secretase inhibitor, DBZ. In the adult mammalian intestine, conditional inhibition of Notch receptor cleavage transforms the transit amplifying compartment of the crypt into postmitotic goblet cells accompanied with a complete loss of mitotic proliferation (Van Es et al., 2005). Notch signaling during the later half of embryogenesis may be initiating formation of the future zebrafish adult stem cell compartment in a manner similar to the mammalian adult crypt.
Absence of intestinal secretory cells results in loss of distal intestinal motility
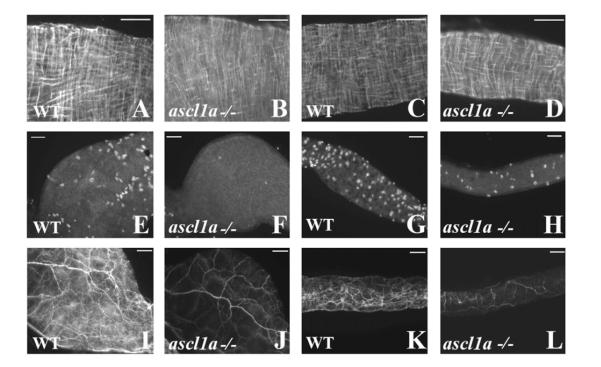
In the adult intestine, motility requires the presence of functional smooth muscle and an enteric neural network coupled with interstitial cells of cajal (ICC) (Hennig et al., 2010; Huizinga et al., 2011, 2009). As a result, we began our investigation of intestinal motility with analysis of smooth muscle and enteric neurons in 5 dpf ascl1a −/− embryos. In acsl1a −/− embryos, using desmin immunohistochemistry, we observe differentiated circular and longitudinal smooth muscle with a wild type appearance in both the proximal (Fig. 8B compared to Fig. 8A) and distal intestine (Fig. 8D compared to Fig. 8C). However, recording the number of enteric neurons throughout the entire intestine, we find that ascl1a −/− embryos have only 9.6% of the neurons found in WT 5 dpf embryos (WT 407.77±56.4; n=10 and ascl1a −/− 39.4±12.9; n=9, p=2×10−9 from 2 tailed Welch’s student T test for unequal variances). Even though the enteric neurons are severely reduced, they appear to be evenly spaced throughout the length of the entire ascl1a −/− intestine (proximal Fig. 8F compared to Fig. 8E; distal Fig. 8H compared to Fig. 8G). Lower numbers of enteric neurons in ascl1a −/− intestines correspond to a significantly reduced neuronal fiber density (proximal Fig. 8J compared to Fig. 8I; distal Fig. 8L compared to Fig. 8K). Many of the proximal neurites appear to be extrinsic as they arise from large neurons that travel into the intestine through the esophagus. The paucity of enteric neurons is not likely to form normal reflex circuits. However, even without normal enteric neural circuits, smooth muscle could retain the ability to generate intestinal motility.
Fig. 8.
Smooth muscle and enteric neurons in ascl1a−/− at 4–5 dpf. Smooth muscle within the digestive system appears normal from proximal ((B) compared to (A)) to distal ((D) compared to (C)) in ascl1a−/− 5 dpf embryos as determined by desmin immunohistochemistry. Neuronal cell bodies highlighted by Elav 1 are numerous in both the proximal (E) and distal (G) WT intestine at 5 dpf but have been severely reduced in the proximal (F) and distal (H) ascl1a−/− intestine. Neurite density is also severely reduced in both the proximal (J) and distal (L) ascl1a−/− intestine when compared to WT proximal (I) and distal (K) as shown with acetylated tubulin. Scale bars represent 50 μm.
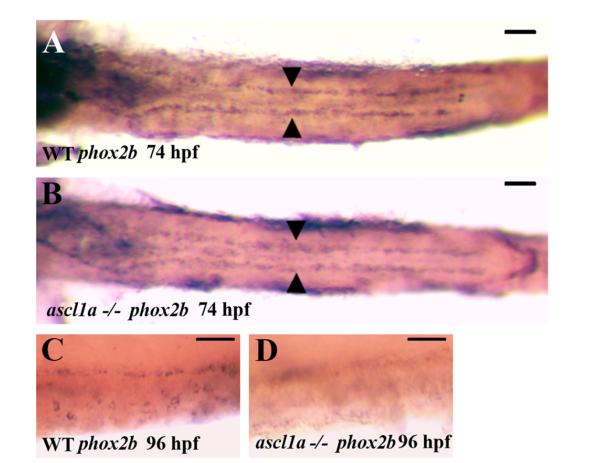
Loss of the majority of enteric neurons may result from a failure of enteric neural precursors to proliferate and migrate through the intestine. Alternatively, enteric neural precursors may proliferate and migrate to the correct position but fail to differentiate. To begin to distinguish between these alternatives, we used expression of phox2B, which is expressed in enteric neural precursors as they migrate through the intestine. We are unable to distinguish ascl1a −/− embryos phenotypically at 74 hpf so we observed phox2B expression in the entire progeny from crosses between heterozygotes. At 74 hpf, enteric neural precursors have migrated along the length of the intestine and have begun circumferential migration in the proximal portion (Olden et al., 2008). We removed the yolk to visualize phox2b and find no apparent differences between any of the embryos. To confirm that ascl1a −/− embryos are present in this group, each of the embryos were imaged and then genotyped with previously characterized primers (Pogoda et al., 2006). ascl1a −/− and WT have the same migration pattern at 74 hpf and appear to have similar numbers of enteric precursors (compare Fig. 9A and B).
Fig. 9.
Migration of enteric neural precursors within ascl1a−/− embryos. At 74 hpf enteric neural precursors, as demonstrated by phox2b RNA in situ hybridization, have migrated in two lateral lines to the distal end of the digestive system (A). In genotyped ascl1a−/− embryos, enteric neural precursors also migrate in similar duplicate lateral lines to reach the distal intestine at 74 hpf (B) similar to WT (A). At 96 hpf, phox2b is distributed around the circumference of the WT intestine (C) while they are no longer present in the ascl1a−/− intestine at the same time (D). Scale bars represent 50 μm.
ascl1a −/− embryos can be distinguished phenotypically at 4 dpf with a smaller but similar enlargement of the proximal intestine than observed at 5 dpf (Fig. 2B). We find that phox2B expression is lost within ascl1a −/− embryos (Fig. 9C compared to Fig. 9D), suggesting that enteric neural precursors have been lost by this stage. Staining with acridine orange at 96 hpf demonstrates no observable cell death within either wild type or ascl1a −/− intestines, suggesting that enteric precursors have already under-gone apoptosis at this point. This would indicate that enteric neural precursors enter the intestine and migrate to the correct position by 74 hpf, but the majority fail to undergo differentiation in ascl1a −/− embryos. Enteric neural precursors may require products from secretory cells for proper differentiation.
Subtypes of enteroendocrine cells secrete products that are also able to alter intestinal motility by acting on mucosal enteric neurons and surrounding tissue (Gunawardene et al., 2011; Hansen and Witte, 2008; Mawe et al., 2006; Spiller, 2011). Due to the loss of all secretory cells in ascl1a −/− embryos we investigated whether there were alterations in intestinal motility. Motility within the intestine was analyzed using volumetry to generate spatiotemporal maps for determining frequency, velocity, distance, interval, and direction of intestinal contractions. As shown previously, spontaneous intestinal contractions originate at a point just distal to the end of the swim bladder (Holmberg et al., 2007; Kuhlman and Eisen, 2007). From this origin, one contraction proceeds retrograde and the other anterograde (Fig. 10A). The retrograde contraction travels through the intestinal bulb and appears to be involved primarily in mixing. The anterograde contraction travels to the end of the intestine and appears to be similar to the mammalian migrating motor complex (MMC), which is involved in propulsion of luminal contents to the anus. Both of these contractions are spontaneous, as the embryos have not yet been fed. As demonstrated in Table 1 (and Supplemental Fig. 1), WT 5 dpf embryos have regular retrograde and anterograde contractions that consistently travel the entire distance of these regions. Retrograde contractions occur more frequently than anterograde contractions but travel at similar velocities.
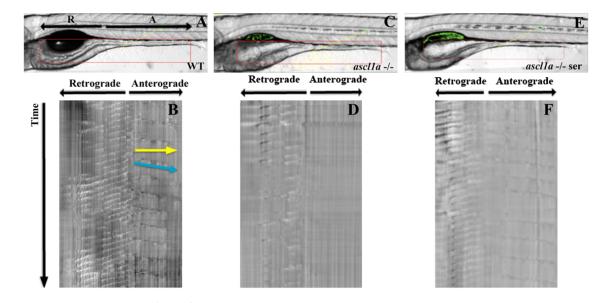
Fig. 10.
Anterograde intestinal motility in ascl1a−/− 5 dpf embryos. Spatiotemporal maps (STMaps) were generated by drawing a box around the area of interest (red box in (A), (C), and (E)). Just distal to the swim bladder there is a region that generates both retrograde and anterograde contractions. Length and start position of retrograde and anterograde contractions are indicated by arrows (R-retrograde and A-anterograde). (B) WT STMap generated from area of interest in panel (A). Individual contractions are identified by changes in opacity, which create darker bands and travel downwards over time (indicated at side of STMap). Velocity is calculated using the slope of the line representing the contraction (indicated by blue line), and distance is calculated by measuring the length of the line (indicated by yellow line). Motility is recorded for ten minutes. The origin of retrograde and anterograde contractions are indicated at the top of the panel. Retrograde contractions are more frequent than anterograde contractions. ascl1a−/− embryos (C) with a similar region of interest (red box) generate an STMap (D) with only retrograde contractions and an absence of anterograde contractions. Upon addition of serotonin to ascl1a−/− embryos (E), anterograde contractions (F) are restored at a similar frequency, distance, and velocity to WT (compare anterograde contractions in (F)–(B)).
Table 1.
Changes in 5 dpf intestinal motility analyzed with volumetry. Motility is separated into retrograde and anterograde contractions with average differences in frequency of contraction (cycles/min), distance (μm), and velocity (μm/s) (n=10 for each group). WT retrograde contractions occur at almost double the frequency of anterograde contractions, but travel about half the distance. Velocity for both retrograde and anterograde contractions are similar. While there is not much change in frequency and distance of retrograde ascl1a−/− contractions, anterograde contractions are absent. Anterograde contractions are replaced by occasional movement, which appears to involve pressure release from the enlarged proximal intestine. The entire distal intestine opening is represented by high velocity of contraction but similar distance to WT. Anterograde contractions are restored to frequency, distance, and velocity similar to WT in ascl1a−/− embryos with the addition of 5HT at near physiological concentrations. Mean value is represented followed by standard deviation. This table shares the WT data with Table 2. A one-way analysis of variance revealed significant differences between the groups; retrograde frequency F(4,1190)=3.793, p=0.00, distance F(4,1901)=87.06, p=0.00, velocity F(4,1901)=14.16, p=0.00; anterograde frequency F(4,524)=4.685, p=0.00, distance F(4,558)=12.92, p=0.00, velocity F(4,558)=78.72, p=0.00. In post-hoc tests, ascl1a−/− or ascl1a−/− with 5HT were compared to WT to determine either equal or unequal variances using the Fisher test and the appropriate T test was utilized (for equal of unequal variances). Values that differ significantly after Bonferroni correction (p<0.01). from WT are indicated in bold with p value from T test
| Retrograde | Frequency | Distance | Velocity |
|---|---|---|---|
| WT | 2.8±1.6 | 174.6±58.2 | 9.2±4 |
| ascl1a−/− | 2.8±1.3 | 170.7±60.3 | 10.4±5.3 (P=3.7×10−4) |
| ascl1a−/− 5HT | 2.9±1.7 | 172.6±61.7 | 9.2±4.7 |
|
| |||
| Anterograde | Frequency | Distance | Velocity |
|
| |||
| WT | 1.6±1.5 | 371.8±58 | 11.5±2.7 |
| ascl1a−/− | 0.05±0.16 (P=1.1×10−20) | 335.8±34.2 | 37.1±7.4 (P=6.1×10−3) |
| ascl1a−/− 5HT | 1.9±3.2 | 318.1±56.1 (P=2.5×10−10) | 12.0±4.0 |
In ascl1a −/− 5 dpf embryos, retrograde contractions are present with a similar frequency, distance, and velocity to WT embryos (Fig. 10D and Table 1). In contrast, there is a complete absence of anterograde contractions. Instead of contractions, nearly the entire distal lumen will infrequently increase in diameter (four events occur in the entire group of n=10 compared to 10–12 per WT embryo), which appears to be a way of venting what appears to be increased proximal pressure due to the remaining retrograde contractions (Table 1 and Supplemental Fig. 2). This enlargement of the intestinal diameter does not appear to involve smooth muscle contraction. Loss of anterograde motility could be due to nonfunctional smooth muscle, reduction of enteric neurons, or loss of secretory cells in the intestinal epithelium.
5HT initiates distal intestinal motility in ascl1a −/− 5 dpf embryos
In both embryonic and adult intestines enteroendocrine cells receive and interpret information from the lumen and relay the information to enteric neurons and surrounding tissue. Enterochromaffin cells (EC) produce and release serotonin (5HT) upon interaction with luminal chemicals or stretch (Spiller, 2011) and alter motility upon binding a variety of 5HT receptors (Gershon and Tack, 2007; Hansen, 2003; Hansen and Witte, 2008; Mawe et al., 2006). Within the zebrafish intestine, EC cells are present only within the distal intestine and correspond to the region that lacks motility. While ascl1a −/− 5 dpf embryos lack all secretory cells, loss of EC cells may play a critical role in distal motility. We previously demonstrated that the 5HT concentration can be quantified in live embryos using the electrochemical method of differential pulse voltammetry (DPV) with chitosan modified microelectrodes implanted in the distal portion of the intestine (Ozel et al., 2011). DPV entails insertion of the carbon fiber probe into the intestine, which may lyse some EC cells thereby releasing 5HT. However, as EC cells are not densely packed, probe insertion is not likely to consistently lyse many EC cells suggesting that part of the 5HT concentration is normally present within the extracellular matrix. This basal 5HT concentration may initiate a myogenic reflex that is absent with loss of 5HT in ascl1a −/−.
If loss of distal intestinal motility is due to low 5HT concentration, then replacement of 5HT should alter motility in ascl1a −/− 5 dpf embryos. ascl1a −/− embryos were placed in a 300 μM 5HT E3 solution for one hour and then washed out. As demonstrated previously, DPV recordings before addition of 5HT in ascl1a −/− embryos demonstrate an average 5HT concentration of 2.09 (±73.27) nM (n=9), estimated using a calibration curve. Variation in 5HT concentration is likely due to the variability of inserting the probe near one of the remaining 5HT containing enteric neurons within (Fig. 2H). Once ascl1a −/− embryos are incubated in 5HT, voltammetric responses record an average concentration of 5HT of 55.15 (±10.9) nM (n=9) (WT concentration is 30.8 (±3.4) nM (n=3).
Increased 5HT tissue concentration in ascl1a −/− embryos results in anterograde contractions in addition to the retrograde contractions (Fig. 10F) that are not significantly different than WT values for distance, velocity, and frequency (Table 1 and Supplemental Fig. 3). The standard deviation in the frequency measurement is high due to increased variability of the number of anterograde contractions per embryo. However, the distance and velocity of anterograde contractions show similar variability to WT values. Anterograde contractions are initiated at the same location as WT embryos, just distal to the end of the swim bladder, and proceed to the anus. Within the proximal intestine, there is no significant change in frequency, velocity or distance of retrograde contractions. This suggests that smooth muscle is functional and 5HT receptors are present which alter motility in the distal intestine. In contrast, no distal contractions develop when ascl1a −/− embryos are soaked in a solution without 5HT.
Pharmacological alteration of 5HT does not mimic loss of distal motility in ascl1a−/−
Increased 5HT concentration results in wild type values of anterograde motility in ascl1a −/− embryos (except for a decrease in distance traveled by the contraction) suggesting that EC cells are able to initiate and drive anterograde motility. However, all of the other enteroendocrine products are also absent. To determine whether loss of 5HT is the sole factor for initiation and maintenance of anterograde motility, we pharmacologically manipulated 5HT in WT embryos and recorded their intestinal motility to determine if there are changes in frequency, velocity, or distance. We recently demonstrated that application of reserpine, which blocks the vesicular monoamine transporter, lowers the concentration of 5HT to undetectable levels within the live zebrafish intestine using DPV (Njagi et al., 2010). In contrast, fluvoxamine, a serotonin reuptake inhibitor (SSRI), doubles the WT concentration of 5HT within the intestine (Njagi et al., 2010).
While there are some significant changes when 5HT is removed, we do not observe the loss of the anterograde motility seen in ascl1a −/−. We do observe changes in retrograde contractions, which suggests that altering 5HT concentrations can modify this motility (Table 2), but not alter it far from WT values. Therefore, the products of other secretory cells are likely to produce overlapping or compensatory effects, allowing WT motility even when one of the signals is removed. Loss of other secretory products is also required for loss of distal intestinal motility.
Table 2.
Changes in 5 dpf intestinal motility with pharmacological modulation of 5HT concentration. WT 5 dpf embryos were incubated with either reserpine, which blocks the vesicular monoamine transporter and lowers 5HT concentration to undetectable levels, or fluvoxamine, a serotonin reuptake inhibitor (SSRI) which doubles the intestinal concentration of 5HT. These embryos were then analyzed by volumetry for frequency, (cycles/min), distance (μm), and velocity (μm/s) of contractions (n=10 for each group). The distance of anterograde intestinal contractions are altered by loss of 5HT in otherwise WT embryos, but not frequency or velocity. Only the distance of retrograde contractions demonstrate significant changes. The concentration of 5HT is doubled by fluvoxamine. Average value is listed followed by standard deviation. A one-way analysis of variance revealed significant differences between the groups with results as listed in Table 1. In post-hoc tests, reserpine or fluvoxamine were compared to WT to determine either equal or unequal variances using the Fisher test and the appropriate T test was utilized (for equal of unequal variances). Values that differ significantly after Bonferroni correction (p<0.01) from WT are indicated in bold with P value from T test
| Retrograde | Frequency | Distance | Velocity |
|---|---|---|---|
| WT | 2.8±1.6 | 174.6±58.2 | 9.2±4 |
| Reserpine | 2.7±1.1 | 191.0±57 (P=2.4×10−5) | 8.5±3.4 (P=6.3×10−3) |
| Fluvoxamine | 3.2±1.9 (P=9.5×10−3) | 244.0±82.4 (P=8.2×10−38) | 10.1±3.6 (P=1.1×10−3) |
|
| |||
| Anterograde | Frequency | Distance | Velocity |
|
| |||
| WT | 1.6±1.5 | 371.8±58 | 11.5±2.7 |
| Reserpine | 1.5±1.6 | 340.0±70.2 (P=1.9×10−5) | 11.1±2.5 |
| Fluvoxamine | 2.11±1.7 (P=5.7×10−3) | 363.9±57.3 | 13.9±3.6 (P=1.8×10−10) |
Discussion
Here we demonstrate that ascl1a is a key transcription factor in differentiation of zebrafish intestinal secretory cells. Loss of ascl1a results in complete loss of all intestinal epithelial secretory cells, which instead differentiate into enterocytes (Fig. 11A). Loss of ascl1a also results in loss of deltaD expression within the intestinal epithelium suggesting ascl1a expression is necessary to initiate and possibly maintain Notch signaling (Fig. 11C). Similar to mammalian intestinal development, zebrafish also has a common progenitor for all intestinal epithelial secretory cells that is dependent on a proneural basic helix-loop-helix (bHLH) transcription factor for initial specification.
Fig. 11.
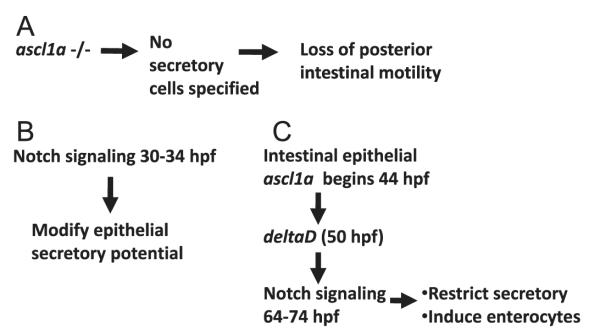
Model for formation of intestinal epithelial secretory cells and requirement for motility. (A) We find that ascl1a expression is a critical step in specification of secretory cells. Loss of secretory cells results in loss of distal but not proximal intestinal motility. We find Notch signaling is active at two points in specification of intestinal epithelial cells as either secretory or enterocytes. (B) In this model, we propose that Notch signaling between 30 and 34 hpf is involved in the potentiation of intestinal epithelial cells to become either secretory cells or enterocytes via lateral induction. (C) Later, ascl1a is expressed in cells with secretory potential, inducing deltaD to activate Notch signaling in surrounding cells. We propose that this stage involves classical lateral inhibition to restrict the number of secretory cells.
In mammals, atoh1/math1 is the atonal family member required to specify secretory cells (Yang et al., 2001). In this study, we find that ascl1a is the ascl family member that specifies intestinal epithelial secretory cells in zebrafish. While the proneural bHLH transcription factors are related genes, ascl and atonal family members have only 45% homology while ascl members have 70% homology (Bertrand et al., 2002). The ascl and atoh families of transcription factors share conserved function across invertebrates to vertebrates (Bertrand et al., 2002). ascl and atonal family members have conserved expression domains and functions between zebrafish and mammals (Cau and Wilson, 2003; Lucas et al., 2006; Madelaine and Blader, 2011; Mueller and Wullimann, 2003; Wullimann and Mueller, 2002, 2004). Between zebrafish and mammals, ascl and atoh appear to have switched roles in intestinal epithelial secretory cell specification while retaining homologous roles in other tissues.
Role of Notch signaling and ascl1a in epithelial development during mid-embryogenesis
In this study, we find that Notch signaling is active in intestinal epithelial development during 64–74 hpf. DAPT inhibition of gamma secretase during this period demonstrates statistically significant increases in ascl1a and deltaD expressing cells as well as the pan-secretory marker 2F11. We find that ascl1a intestinal epithelial expression during this period appears to be involved in initiation of expression of the Notch ligand, deltaD (Fig. 11C).
Within zebrafish, ascl1a has been shown to be capable of direct regulation of deltaD and is dependent on a specific group of E boxes within the HII control region (Hans and Campos-Ortega, 2002). Regulation of Delta ligands by ascl1 also occurs in other systems. ascl1 is required for delta expression within the mouse and chicken retinal progenitors as well as formation of mouse spinal sensory interneurons (Mizuguchi et al., 2006; Nelson et al., 2009).
ascl1a expression combined with initiation of deltaD suggests Notch signaling is involved in classic lateral inhibition during this period (Fig. 11C). During the second day of embryogenesis, all of the intestinal epithelial cells expressing ascl1a would have the potential to differentiate into secretory cells. Up-regulation of ascl1a in one or more cells would then increase deltaD expression. Increased DeltaD levels would initiate Notch signaling in surrounding cells to specify enterocytes. Initially, there would be a larger group of intestinal epithelial cells with the potential to become secretory cells. Notch signaling would, therefore, restrict the number of secretory cells to a few in each group. In this scenario, lack of Notch signaling would cause all of the ascl1a expressing epithelial cells to differentiate into secretory cells.
Role of Notch signaling during early epithelial development
We also observe Notch signaling within the intestinal epithelium between 30 and 34 hpf. DAPT inhibition of gamma secretase during this period causes statistically significant increases in ascl1a and deltaD expressing cells as well as increases in cells expressing the pan-secretory cell marker 2F11. ascl1a is required for secretory cell specification; however, this is ten hours before expression of ascl1a is first detected within the intestinal epithelium at 44 hpf. We suggest then that Notch signaling during this period is instead involved in the formation of pro-secretory domains within the intestinal epithelium that later have the potential to become secretory when ascl1a is expressed.
Previously, in the Notch signaling defective mutant mindbomb (mib), all epithelial cells appear to become secretory cells as the entire epithelium expresses 2F11 (Crosnier et al., 2005). If the secretory fate is the default state, Notch signaling between 30 and 34 hpf might then specify pro-enterocyte domains which would not express ascl1a later during the second day of embryogenesis (Fig. 11B). Inhibition of Notch signaling during this period would allow the entire epithelium to form as a pro-secretory domain. If the entire epithelium becomes a pro-secretory domain, many additional secretory cells will begin differentiating. Notch signaling through lateral inhibition would still be able to specify enterocytes between 64 and 74 hpf.
Between 30 and 34 hpf the intestinal epithelium has just begun to form a tube with six to seven cells lining the proximal lumen and only three to four in the distal lumen (Wallace and Pack, 2003). Therefore whether Notch signaling is specifying a secretory or enterocyte fate during this stage, the epithelial cells that receive Notch signaling will need to maintain their specification as they proliferate since this period of Notch signaling occurs within a short four hour window.
Alteration of secretory cells during continuous Notch inhibition
Inhibition of Notch signaling produces increased numbers of secretory cells at mid-embryogenesis. Increased secretory cell numbers should also be observed at the end of embryogenesis. However, embryos inhibited for the previous 4–8 h period and grown to 5 dpf revealed no significant change in the number of secretory cells. Normal numbers of secretory cells at 5 dpf might be due to compensatory mechanisms or incomplete Notch inhibition. We hypothesized that continuous inhibition of Notch signaling until the end of embryogenesis might maintain increases in secretory cell numbers. Embryos inhibited continuously after the initial exposure to DAPT resulted in nearly the same number of secretory cells as the WT control exposed to DMSO as observed with the pan-secretory cell marker, 2F11.
We suggest that DAPT may not provide enough continuous Notch inhibition over the course of embryogenesis to significantly alter the total number of secretory cells. A similar lack of complete Notch signaling after DAPT treatment was observed during lateral line formation (Matsuda and Chitnis, 2010). In these experiments, loss of all Notch signaling due to Delta ligand in the mindbomb (mib) mutant removes expression of the Notch target gene, her4. Exposure to DAPT or morpholino knockdown of notch3 combined with a notch1a mutant does not result in loss of her4 expression. In contrast, DAPT exposure combined with notch3 morpholino and notch1a mutant does result in loss of her4 expression similar to the mib phenotype. This suggests that even low levels of Notch signaling are able to induce target gene expression albeit at what may be a lower level. Lower levels of Notch signaling, however, may be enough to cause significant changes in the numbers of various subtypes of secretory cells. We observe a significant increase in enterochromaffin cells when Notch inhibition begins at 30 hpf. In contrast, there is a significant decrease in goblet cell numbers when Notch inhibition begins at 34 hpf with a subsequent increase beginning at 60 hpf.
Alterations in numbers of secretory cell subtypes could be due to differential effects of Notch inhibition as there are four different Notch receptors in zebrafish. Each of the receptors might also perform different time dependent tasks or only act on certain secretory cell subtypes. Within the embryonic zebrafish intestine, previously identified secretory cells, such as goblet and pancreatic polypeptide cells, develop within specific proximal or distal boundaries (Njagi et al., 2010; Wallace et al., 2005a). We find that wild type boundaries between proximal and distal secretory cell domains are maintained even with Notch signaling inhibition. Notch receptors limited to proximal or distal domains would then be able to differentiate a subset of secretory cells.
While there appears to be a common secretory precursor it is likely that there are further differentiation steps, creating lineages that would only differentiate into a subset of secretory cells. Previously, enterocytes and subtypes of secretory cells have been observed to begin differentiation at different times during embryogenesis (Ng et al., 2005; Njagi et al., 2010; Wallace et al., 2005a). The subtypes of secretory precursors may also be affected by Notch signaling and respond differently to Notch inhibition, further altering numbers of secretory cell subtypes.
Role of secretory cells in intestinal motility
In ascl1a mutants, we find that loss of all secretory cells within the intestinal epithelium results in a loss of motility in the distal intestine. This suggests that products of enteroendocrine cells play a significant role in initiating and driving motility in the embryonic intestine (Fig. 11A). ascl1a mutants also lose the majority of enteric neurons. Previously, however, it has been demonstrated that enteric neurons do not exert significant control over frequency, velocity, and distance of contractions during embryogenesis (Holmberg et al., 2007). In addition, extrinsic innervation remains intact in ascl1a −/− embryos, which may provide some neural regulation. Within mammals, serotonin (5HT) producing secretory cells (enterochromaffin cells—EC) play a critical role in intestinal motility. EC cells release 5HT upon stimulation by touch or stretch and bind receptors on neurons and surrounding tissue. As a result, EC cells play a role in the activation of enteric neural reflexes involved in both contraction and luminal secretion (Hansen and Witte, 2008; Heredia et al., 2009; Mawe et al., 2006; Spiller, 2011). Zebrafish also have a distal complement of EC cells in combination with 5HT enteric neuronal subtypes which produce a homogeneous regional concentration of 5HT within the intestine as detected with differential pulse voltammetry (Njagi et al., 2010).
We find that increasing 5HT to near physiological levels in ascl1a mutant embryos initiates the same velocity and frequency as wild type contractions, but decreases the distance traveled by anterograde contractions. While neurites from extrinsic neurons still appear to be present in ascl1a mutants, only one-tenth of the expected enteric neurons are present. So few intrinsic neurons suggest incomplete reflex circuits are present and 5HT receptors on smooth muscle instead induce motility upon introduction to the system.
The mechanism by which 5HT initiates and maintains motility in ascl1a −/− embryos is currently unknown. Within the adult intestine, as discussed above, 5HT is released at specific points from ECs in response to stretch or pressure. With exposure of the entire intestine to 5HT, there are no longer point sources of 5HT secretion and we may be exposing other intestinal cells that are not normally exposed to 5HT. Within mammals, affects of 5HT alteration are complex with low level increases in 5HT concentration increasing propulsive motility while higher concentration slow motility due to desensitization of receptors (Linden et al., 2003; Wade et al., 1996). While 5HT concentrations in this work are higher than physiological levels, we only observe increased contractility in ascl1a −/− embryos after a short exposure. Longer exposure or higher concentrations to 5HT might desensitize receptors and slow motility. Further work needs to be done to determine how 5HT is initiating motility in ascl1a −/− embryos.
While we find that 5HT is sufficient to stimulate motility within the distal intestine, it is not the only substance that drives WT motility. If 5HT were the sole enteroendocrine product with the ability to induce motility, then removing 5HT from the WT intestine should reduce or remove contractions completely. After removal of 5HT at 5 dpf with the vesicular monoamine transport blocker reserpine, we observed only a moderate decrease in distance that the anterograde contraction travels. We suggest that distal contractions can be initiated and modulated by 5HT, but as in mammalian systems other enteroendocrine cell products are also involved in motility.
ascl1a mutants lose anterograde contractions; however, retrograde contractions continue with similar frequency, velocity, and distance. In the 5 dpf intestine, there is a region just distal to the swim bladder which originates both the anterograde and retrograde contractions. In ascl1a mutants, this region continues to contract in a similar manner to WT embryos but only propagates retrograde contractions. The anterograde contraction appears to begin but does not continue from this region. The position where these contractions originate may be a segment that is able to initiate contractile activity independently of enteroendocrine products or enteric neurons. There still could be a contribution from extrinsic innervation. Combination of this segment and the resulting retrograde contractions may be part of an endogenous pacemaker activity that can be modified by enteroendocrine products and enteric and extrinsic neurons. Future work will be needed to determine the role of this region in initiating motility within the intestine.
In conclusion, ascl1a plays a role in differentiation of a common secretory cell progenitor within the zebrafish intestinal epithelium. Loss of ascl1a function results in loss of all intestinal epithelial secretory cells and loss of distal but not proximal motility. 5HT initiates motility in the distal intestine but is not the sole enteroendocrine product to induce contractility. Additionally, electrochemistry on zebrafish embryos provides a unique opportunity to rapidly determine quantitative changes in physiological concentrations of many of these products in the live embryo. The relative ease with which motility can be measured and altered with compounds added to the media provide an interesting vertebrate system to investigate the role of enteroendocrine cells in intestinal motility.
Supplementary Material
Acknowledgements
We are very grateful to Matthias Hammerschmidt for providing the ascl1a mutant line, Grant Hennig for allowing us to use the Volumetry software and Adam Rich for helping train us on use of the software. We also thank James Schulte and Robert Dowman for additional statistical analysis. This work was supported by NIDDK (1R15DK089474-01 to K.N.W.).
Footnotes
Appendix A. Supporting information Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ydbio.2013.01.013.
References
- Akhtar T, Li J, Olden T, Wallace KN. Use of phospholipase A(2) for antigen retrieval in zebrafish whole-mount immunohistochemistry. Zebrafish. 2009 doi: 10.1089/zeb.2009.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allende ML, Weinberg ES. The expression pattern of two zebrafish achaete-scute homolog (ash) genes is altered in the embryonic brain of the cyclops mutant. Dev. Biol. 1994;166:509–530. doi: 10.1006/dbio.1994.1334. [DOI] [PubMed] [Google Scholar]
- Bardin AJ, Perdigoto CN, Southall TD, Brand AH, Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development. 2010;137:705–714. doi: 10.1242/dev.039404. Cambridge, England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007;2:371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat. Rev. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Blaugrund E, Pham TD, Tennyson VM, Lo L, Sommer L, Anderson DJ, Gershon MD. Distinct subpopulations of enteric neuronal progenitors defined by time of development, sympathoadrenal lineage markers and Mash-1-dependence. Development. 1996;122:309–320. doi: 10.1242/dev.122.1.309. (Cambridge, England) [DOI] [PubMed] [Google Scholar]
- Cau E, Wilson SW. Ash1a and Neurogenin1 function downstream of floating head to regulate epiphysial neurogenesis. Development. 2003;130:2455–2466. doi: 10.1242/dev.00452. [DOI] [PubMed] [Google Scholar]
- Crosnier C, Vargesson N, Gschmeissner S, Ariza-Mc N, Aughton L, Morrison A, Lewis J. Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development. 2005;132:1093–1104. doi: 10.1242/dev.01644. (Cambridge, England) [DOI] [PubMed] [Google Scholar]
- Elworthy S, Pinto JP, Pettifer A, Cancela ML, Kelsh RN. Phox2b function in the enteric nervous system is conserved in zebrafish and is sox10-dependent. Mech. Develop. 2005;122:659–669. doi: 10.1016/j.mod.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Fre S, Bardin A, Robine S, Louvard D. Notch signaling in intestinal homeostasis across species: the cases of Drosophila, zebrafish and the mouse. Exp. Cell Res. 2011 doi: 10.1016/j.yexcr.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–694. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon MD. Plasticity in serotonin control mechanisms in the gut. Curr. Opin. Pharmacol. 2003;3:600–607. doi: 10.1016/j.coph.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int. J. Exp. Pathol. 2011;92:219–231. doi: 10.1111/j.1365-2613.2011.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans S, Campos-Ortega JA. On the organisation of the regulatory region of the zebrafish deltaD gene. Development. 2002;129:4773–4784. doi: 10.1242/dev.129.20.4773. (Cambridge, England) [DOI] [PubMed] [Google Scholar]
- Hansen MB. Neurohumoral control of gastrointestinal motility. Physiol. Res. 2003;52:1–30. [PubMed] [Google Scholar]
- Hansen MB, Witte AB. The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol. (Oxford, England) 2008;193:311–323. doi: 10.1111/j.1748-1716.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- Hartenstein V, Takashima S, Adams KL. Conserved genetic pathways controlling the development of the diffuse endocrine system in vertebrates and Drosophila. Gen. Comp. Endocrinol. 2010;166:462–469. doi: 10.1016/j.ygcen.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J. Physiol. 1999;517(2):575–590. doi: 10.1111/j.1469-7793.1999.0575t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Spencer NJ, Jokela-Willis S, Bayguinov PO, Lee HT, Ritchie LA, Ward SM, Smith TK, Sanders KM. ICC-MY coordinate smooth muscle electrical and mechanical activity in the murine small intestine. Neurogastroenterol. Motil. 2010;22:e138–151. doi: 10.1111/j.1365-2982.2009.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology. 2009;136:1328–1338. doi: 10.1053/j.gastro.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg A, Olsson C, Hennig GW. TTX-sensitive and TTX-insensitive control of spontaneous gut motility in the developing zebrafish (Danio rerio) larvae. J. Exp. Biol. 2007;210:1084–1091. doi: 10.1242/jeb.000935. [DOI] [PubMed] [Google Scholar]
- Hongay CF, Orr-Weaver TL. Drosophila inducer of MEiosis 4 (IME4) is required for Notch signaling during oogenesis. Proc. Nat. Acad. Sci. U.S.A. 2011;108:14855–14860. doi: 10.1073/pnas.1111577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Martz S, Gil V, Wang XY, Jimenez M, Parsons S. Two independent networks of interstitial cells of cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Front. Neurosci. 2011;5:93. doi: 10.3389/fnins.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Zarate N, Farrugia G. Physiology, injury, and recovery of interstitial cells of cajal: basic and clinical science. Gastroenterology. 2009;137:1548–1556. doi: 10.1053/j.gastro.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki K, Uneyama H. Sense of taste in the gastrointestinal tract. J. Pharmacol Sci. 2012;118:123–128. doi: 10.1254/jphs.11r08cp. [DOI] [PubMed] [Google Scholar]
- Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell ,fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat. Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G260–272. doi: 10.1152/ajpgi.00056.2008. [DOI] [PubMed] [Google Scholar]
- Kuhlman J, Eisen JS. Genetic screen for mutations affecting development and function of the enteric nervous system. Dev. Dyn. 2007;236:118–127. doi: 10.1002/dvdy.21033. [DOI] [PubMed] [Google Scholar]
- Linden DR, Chen JX, Gershon MD, Sharkey KA, Mawe GM. Serotonin availability is increased in mucosa of guinea pigs with TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G207–216. doi: 10.1152/ajpgi.00488.2002. [DOI] [PubMed] [Google Scholar]
- Lo LC, Johnson JE, Wuenschell CW, Saito T, Anderson DJ. Mammalian achaete-scute homolog 1 is transiently expressed by spatially restricted subsets of early neuroepithelial and neural crest cells. Genes Dev. 1991;5:1524–1537. doi: 10.1101/gad.5.9.1524. [DOI] [PubMed] [Google Scholar]
- Lucas ME, Muller F, Rudiger R, Henion PD, Rohrer H. The bHLH transcription factor hand2 is essential for noradrenergic differentiation of sympathetic neurons. Development. 2006;133:4015–4024. doi: 10.1242/dev.02574. [DOI] [PubMed] [Google Scholar]
- Madelaine R, Blader P. A cluster of non-redundant Ngn1 binding sites is required for regulation of deltaA expression in zebrafish. Dev. Biol. 2011;350:198–207. doi: 10.1016/j.ydbio.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Chitnis AB. Atoh1a expression must be restricted by Notch signaling for effective morphogenesis of the posterior lateral line primordium in zebrafish. Development. 2010;137:3477–3487. doi: 10.1242/dev.052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe GM, Coates MD, Moses PL. Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment. Pharmacol. Therap. 2006;23:1067–1076. doi: 10.1111/j.1365-2036.2006.02858.x. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Mizuguchi R, Kriks S, Cordes R, Gossler A, Ma Q, Goulding M. Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nat. Neurosci. 2006;9:770–778. doi: 10.1038/nn1706. [DOI] [PubMed] [Google Scholar]
- Moran GW, Leslie FC, Levison SE, Worthington J, McLaughlin JT. Enteroendocrine cells: neglected players in gastrointestinal disorders? Therap. Adv. Gastroenterol. 2008;1:51–60. doi: 10.1177/1756283X08093943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T, Wullimann MF. Anatomy of neurogenesis in the early zebrafish brain. Brain Res. Dev. Brain Res. 2003;140:137–155. doi: 10.1016/s0165-3806(02)00583-7. [DOI] [PubMed] [Google Scholar]
- Nelson BR, Hartman BH, Ray CA, Hayashi T, Bermingham-McDonogh O, Reh TA. Acheate-scute like 1 (Ascl1) is required for normal delta-like (Dll) gene expression and notch signaling during retinal development. Dev, Dyn. 2009;238:2163–2178. doi: 10.1002/dvdy.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AN, De Jong-Curtain TA, Mawdsley DJ, White SJ, Shin J, Appel B, Dong PD, Stainier DY, Heath JK. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev. Biol. 2005;286:114–135. doi: 10.1016/j.ydbio.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Njagi J, Ball M, Best M, Wallace KN, Andreescu S. Electrochemical quantification of serotonin in the live embryonic zebrafish intestine. Anal. Chem. 2010;82:1822–1830. doi: 10.1021/ac902465v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. (New York, N.Y. [DOI] [PubMed] [Google Scholar]
- Olden T, Akhtar T, Beckman SA, Wallace KN. Differentiation of the zebrafish enteric nervous system and intestinal smooth muscle. Genesis. 2008;46:484–498. doi: 10.1002/dvg.20429. [DOI] [PubMed] [Google Scholar]
- Ozel RE, Wallace KN, Andreescu S. Chitosan coated carbon fiber microelectrode for selective in vivo detection of neurotransmitters in live zebrafish embryos. Anal. Chim. Acta. 2011;695:89–95. doi: 10.1016/j.aca.2011.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoda HM, von der Hardt S, Herzog W, Kramer C, Schwarz H, Hammerschmidt M. The proneural gene ascl1a is required for endocrine differentiation and cell survival in the zebrafish adenohypophysis. Development. 2006;133:1079–1089. doi: 10.1242/dev.02296. (Cambridge, England) [DOI] [PubMed] [Google Scholar]
- Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton. Neurosci. 2010;153:41–46. doi: 10.1016/j.autneu.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, Van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–2644. doi: 10.1210/en.2004-0051. [DOI] [PubMed] [Google Scholar]
- Shepherd IT, Pietsch J, Elworthy S, Kelsh RN, Raible DW. Roles for GFRalpha1 receptors in zebrafish enteric nervous system development. Development. 2004;131:241–249. doi: 10.1242/dev.00912. (Cambridge, England) [DOI] [PubMed] [Google Scholar]
- Sjolund K, Sanden G, Hakanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed] [Google Scholar]
- Spence JR, Lauf R, Shroyer NF. Vertebrate intestinal endoderm development. Dev. Dyn. 2011;240:501–520. doi: 10.1002/dvdy.22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller RC. Targeting the 5-HT(3) receptor in the treatment of irritable bowel syndrome. Curr. Opin. Pharmacol. 2011;11:68–74. doi: 10.1016/j.coph.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Van Es JH, De Geest N, Van de Born M, Clevers H, Hassan BA. Intestinal stem cells lacking the Math1 tumour suppressor are refractory to Notch inhibitors. Nat. Commun. 2010;1:18. doi: 10.1038/ncomms1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J. Neurosci. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KN, Akhter S, Smith EM, Lorent K, Pack M. Intestinal growth and differentiation in zebrafish. Mech. Dev. 2005a;122:157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Wallace KN, Dolan AC, Seiler C, Smith EM, Yusuff S, Chaille-Arnold L, Judson B, Sierk R, Yengo C, Sweeney HL, Pack M. Mutation of smooth muscle myosin causes epithelial invasion and cystic expansion of the zebrafish intestine. Dev. Cell. 2005b;8:717–726. doi: 10.1016/j.devcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Wallace KN, Pack M. Unique and conserved aspects of gut development in zebrafish. Developmental biology. 2003;255:12–29. doi: 10.1016/s0012-1606(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio) M. Westerfield; Eugene, OR: 1993. [Google Scholar]
- Wright GJ, Giudicelli F, Soza-Ried C, Hanisch A, Ariza-McNaughton L, Lewis J. DeltaC and DeltaD interact as Notch ligands in the zebrafish segmentation clock. Development. 2011;138:2947–2956. doi: 10.1242/dev.066654. (Cambridge, England) [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T. Expression of Zash-1a in the postembryonic zebrafish brain allows comparison to mouse Mash1 domains. Brain Res. Gene Expr. Patterns. 2002;1:187–192. doi: 10.1016/s1567-133x(02)00016-9. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Mueller T. Identification and morphogenesis of the eminentia thalami in the zebrafish. J. Comp. Neurol. 2004;471:37–48. doi: 10.1002/cne.20011. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. (New York, N.Y. [DOI] [PubMed] [Google Scholar]
- Zecchin E, Filippi A, Biemar F, Tiso N, Pauls S, Ellertsdottir E, Gnugge L, Bortolussi M, Driever W, Argenton F. Distinct delta and jagged genes control sequential segregation of pancreatic cell types from precursor pools in zebrafish. Dev. Biol. 2007;301:192–204. doi: 10.1016/j.ydbio.2006.09.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.