Abstract
Bone morphogenetic proteins (BMPs) are members of the TGF-β superfamily and play a critical role in skeletal development, bone formation and stem cell differentiation. Disruptions in BMP signaling result in a variety of skeletal and extraskeletal anomalies. BMP9 is a poorly characterized member of the BMP family and is among the most osteogenic BMPs, promoting osteoblastic differentiation of mesenchymal stem cells (MSCs) both in vitro and in vivo. Recent findings from various in vivo and molecular studies strongly suggest that the mechanisms governing BMP9-mediated osteoinduction differ from other osteogenic BMPs. Many signaling pathways with diverse functions have been found to play a role in BMP9-mediated osteogenesis. Several of these pathways are also critical in the differentiation of other cell lineages, including adipocytes and chondrocytes. While BMP9 is known to be a potent osteogenic factor, it also influences several other pathways including cancer development, angiogenesis and myogenesis. Although BMP9 has been demonstrated as one of the most osteogenic BMPs, relatively little is known about the specific mechanisms responsible for these effects. BMP9 has demonstrated efficacy in promoting spinal fusion and bony non-union repair in animal models, demonstrating great translational promise. This review aims to summarize our current knowledge of BMP9-mediated osteogenesis by presenting recently completed work which may help us to further elucidate these pathways.
Keywords: BMP, BMP9, bone regeneration, IGF, osteogenesis, TGF-β, Wnt, signal transduction, mesenchymal stem cells
Introduction
Bone morphogenetic proteins (BMPs) are members of the TGF-β superfamily and play a critical role in skeletal development, bone formation and stem cell differentiation [1,2]. These factors were discovered when it was found that demineralized bone could induce de novo bone formation [3,4]. At least 15 different BMPs have been identified in humans, and disruptions in BMP signaling result in a variety of skeletal and extraskeletal anomalies [5,6]. BMP9, also known as growth differentiation factor 2 or GDF-2, is a relatively poorly characterized member of the BMP family that was first isolated from fetal mouse liver cDNA libraries. BMP9 is highly expressed in the developing mouse liver and stimulates hepatocyte proliferation [7]. It also induces and maintains the cholinergic phenotype within basal forebrain neurons, inhibits hepatic glucose production, inhibits critical enzymes of lipid metabolism, and helps maintain the homeostasis of iron metabolism [8-10]. BMP9 is also a synergistic factor for hematopoietic progenitor cell generation [11].
BMP9 is among the most osteogenic BMPs, promoting osteoblastic differentiation of mesenchymal stem cells (MSCs) both in vitro and in vivo [1,12-16]. We have demonstrated that BMP9 regulates a distinct set of downstream targets that likely play a role in osteoinduction and will be discussed later in this review [12-16]. Unique among members of the TGF-β superfamily, the mature BMP9 protein retains the N-terminal pro-region that is generally cleaved in other BMPs prior to secretion. Retainment of the pro-region does not result in functional inhibition of BMP9 and may in fact stabilize the mature protein after secretion [5,6,17-23]. Also unlike other BMPs, BMP9 has poor affinity for BMPR-IA, a receptor that generally transduces BMP signaling [17]. Taken together, these findings strongly suggest that the mechanisms governing BMP9-mediated osteoinduction of MSCs may differ from other osteogenic BMPs. While BMP9 has been demonstrated as one of the most osteogenic BMPs, little is known about the detailed mechanisms responsible for its functions. This review aims to summarize our current knowledge of BMP9-mediated osteogenesis, which may help us to further elucidate these pathways.
Bone morphogenetic proteins (BMPs) and osteogenesis
While the specific molecular mechanisms underlying BMP-mediated osteogenesis are somewhat poorly characterized, various studies have nonetheless demonstrated that BMPs play a critical role in osteogenic differentiation; adenoviral, retroviral and recombinant BMP overexpression have been shown to induce bone formation in animal models [24-44]. Because of the osteoinductive effects first demonstrated in these studies, recombinant BMPs are now increasingly being utilized clinically in bone regeneration applications. Recombinant BMP2 (rhBMP2) and BMP7 (rhBMP7) have been extensively studied and are widely used to augment bone healing, with better fusion rates than autografts and few associated complications [32,40,42,43,45-52]. While many studies have successfully demonstrated the osteo-regenerative effects of BMP2 and BMP7 andpaved the way for successful use of these BMPs in the clinical arena, it is unknown if these two members are actually the most osteogenic BMPs.
Osteogenic BMPs include 2, 4, 6, 7 and 9, with BMP9 demonstrating the most potent osteogenic activity both in vitro and in vivo (Figure 1) [14,53-61]. Exposure of MSCs to these osteogenic BMPs results in increased expression of osteoblast-specific markers, including connective tissue growth factor (CTGF), inhibitor of DNA binding (Id), the early osteogenic marker alkaline phosphatase (ALP), the late osteogenic markers osteocalcin and osteopontin and Cbfa1/Runx2 [13-16,56,62-64]. Previously, the osteogenic activity of all BMPs could not be analyzed since the recombinant form of each BMP was not available. However, we conducted a comprehensive analysis of both the in vitro and in vivo osteogenic activity of 14 BMPs using adenoviral-mediated gene delivery into mesenchymal stem cells (MSCs), bone marrow stromal cells with the ability to differentiate along osteogenic, chondrogenic, adipogenic and myogenic lineages [1,14,54,56,65,66]. Our results show BMP2, BMP6 and BMP9 as the most osteogenic BMPs (Figure 1A and 1B) [1,14,56]. While BMP9 demonstrated the most potent osteoinduction in our analysis [1,56], it remains one of the least studied and most poorly characterized BMPs and thus merits further investigation. The findings of our comprehensive analysis of BMPs in bone formation suggest that BMP9 may represent a more effective strategy for the augmentation of bone regeneration than the BMPs currently used in the clinical setting.
Figure 1.
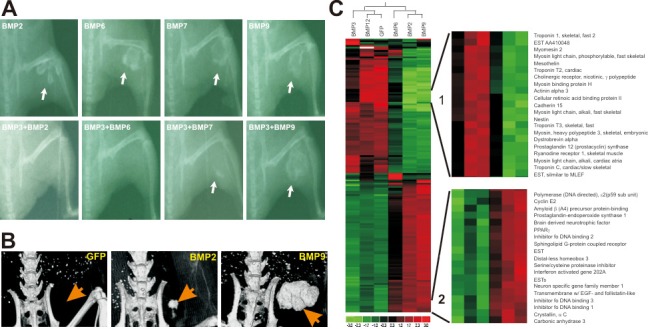
BMP9 induces osteogenesis of MSCs. A. Osteogenic AdBMPs (BMP2, -6, -7 or 9)-transduced C2C12 MSCs were injected intramuscularly alone (top row) or with AdBMP3 (bottom row), and representative radiographs are seen at three weeks. Reproduced from Gene Therapy 11: 1312-1320 (2004) [1]. B. MicroCT analysis of tissue masses at five weeks following subcutaneous injection of GFP, BMP2 or BMP9-transduced MSCs. Reproduced from Stem Cells and Development 18: 545-558 (2009) [56]. C. Microarray and clustering analysis of BMP-transduced C2C12 MSCs. Representative sub-hierarchical clusters of down-regulated genes (1) and down-regulated genes (2) are shown. Reproduced from Journal of Cellular Biochemistry 90:1149-1165 (2003) [15].
BMP9 induces osteogenic differentiation and bone regeneration
Several recent investigations have reported the osteogenic nature of BMP9 and implicated its role in osteoblastic differentiation and bone regeneration. Xu et al. demonstrated that adenoviral-mediated overexpression of BMP9 in C3H10T1/2 MSCs intensively increased alkaline phosphatase (ALP) activity, an early marker of osteogenic differentiation, as well as calcium deposition as indicated by Alizarin Red S staining [67], findings consistent with several other previous studies [11,14,68]. Cheng et al. demonstrated that adenoviral-mediated overexpression of BMP9 in C3H10T1/2 MSCs resulted in a 181-fold increase in ALP activity nine days after infection, with significant increases in ALP activity observed as early as five days post-infection [12]. Increased ALP was also seen in BMP9-stimulated preosteoblastic C2C12 cells and osteoblastic TE85 cells as early as three days after infection. RT-PCR of BMP9-stimulated C3H10T1/2 and C2C12 cells demonstrated increased expression of the late osteoblastic marker osteocalcin, and Alizarin Red immunohistochemical staining of BMP9-stimulated C3H10T1/2 cells demonstrated mineralized osteoid nodules. Furthermore, Kang et al. demonstrated that induction of the aforementioned osteogenic markers was significantly higher in BMP9-treated cells than in BMP2 or BMP7 treated cells, findings consistent with the study by Cheng et al. [1,12] . Kang et al. also found that BMP3, a known inhibitor of the well-characterized BMP2- and BMP7-mediated osteogenesis, did not inhibit BMP9-mediated bone formation, with samples demonstrating multiple foci of woven trabecularbone similar to BMP9 injection alone (Figure 1A) [1]. This finding suggests that BMP9-mediated osteogenesis may occur via a distinct mechanism from other osteogenic BMPs.
Non-adenoviral delivery of BMP9 has also demonstrated potent osteoinduction of MSCs [69-71]. In the first study to successfully utilize non-viral, ultrasound-based osteogenic gene delivery to form bone tissue in vivo, Sheyn et al. demonstrated that direct sonoporation of rhBMP9 into mouse quadriceps muscles caused the formation of ectopic bone tissue as demonstrated by osteocalcin-dependent luciferase (Luc) expression, micro-CT and histology [71]. Aslan et al. demonstrated that nucleofection, a novel electropermeabilization-based technique, of human MSCs (hMSCs) with BMP9 caused bone formation at four weeks post-injection and significantly increased calcium deposition in vitro [69]. Bergeron et al. demonstrated the osteoinductive effects of a peptide derived from BMP9 (pBMP9); treatment of MC3T3-E1 preosteoblastic cells with pBMP9 induced downstream phosphorylation of Smad uninhibited by noggin, a known extracellular antagonist of BMP2 [72]. Furthermore, pBMP9 caused a dose-dependent increase in ALP activity, and quantitative real-time PCR demonstrated activation of the osteogenic genes Runx2, Osterix, type 1 collagen a1 and osteocalcin as early as six days post-treatment [72]. A subsequent study utilized two delivery systems (DS) for pBMP9, one based on collagen and the other on chitosan. Only the chitosan DS containing BMP9 induced strong bone formation in mice quadriceps within 24 days, demonstrating the importance of the carrier in optimizing pBMP9 efficiency [73].
While multiple exogenous delivery systems have demonstrated the osteogenic properties of BMP9, several in vivo studies have confirmed BMP9 as a potent inducer of bone formation. Athymic nude mice injected with BMP9-transduced C2C12 cells into the quadriceps muscles demonstrated significant orthotopic bone formation on both X-ray and histologic evaluation [1,2,57]. Several recent studies have suggested that skeletal muscle may harbor multipotent MSCs as well as osteoblastic progenitor cells [28,33,74]. When adenovirally-delivered BMP9 (AdBMP9) was directly injected into the quadriceps muscles of athymic nude mice, there was evidence of increased osteoid matrix production and formation of mature lamellar bone compared to BMP2- and 7-treated groups. Direct intramuscular injection of AdBMP9 induced more diffuse ossification less readily detectable by X-ray, demonstrating lower efficiency of bone formation than introduction of AdBMP-transduced osteoblast progenitor cells. Similar results were seen also seen in rats in a study by Li et al [1,75]; helper-dependent adenoviral vectors were used to decrease the immune response in immune competent Sprague-Dawley rats. Helper-dependent GFP and BMP9 adenoviral vector (ADGBMP9) were used to transduce human MSCs (hMSCs) before injection into the quadriceps muscles of both athymic nude and Sprague-Dawley rats [75]. By three days after injection, ADGBMP9 induced bone formation, and by day nine, ectopic ossification was visible on CT scan, ultimately forming significant amounts of bone. Furthermore, the BMP9-induced the ectopic bone was histologically determined to be the result of normal physiologic endochondral mechanisms.
BMP9 has also demonstrated efficacy in inducing spinal fusion and fracture non-union repair in animal models, and these studies hold great promise regarding translation to the clinical arena [76,77]. Dumont et al. treated 16 athymic nude rats with a percutaneous lumbar paraspinal injection of AdBMP9-transduced hMSCs. Eight weeks after injection, CT scans and histological analysis of the lumbosacral spine demonstrated large volumes of ectopic bone formation at the injection sites and successful spinal fusion without evidence of nerve root compression or local toxicity; control groups demonstrated no evidence of osteogenesis [76]. In a 2011 study by Kimelman-Bleich et al., fracture non-union was created in the radii of C3H/HeN mice and filled with a collagen sponge which ten days later was electroporated with BMP9 plasmid [77]. Micro-CT and histologic analysis of these BMP9 electroporated radii demonstrated bone formation bridging the defect and healing of the non-union, whereas the control groups’ radii remained gapped. Li Xiang et al. recently investigated the effects of adenovirally-delivered BMP9 on the osteogenic differentiation of muscle-derived stem cells (MDSC) and bone formation in a rat radius defect repair model [78]. A 12 mm bone defect in the middle segment of the radius was introduced specifically to fit the implant length. The BMP9 treatment group demonstrated more rapid callus and larger bone formation surrounding the implant with connected ends of previously broken bone; most marrow cavities recanalized compared to the BMP2 and control treatment groups, which demonstrated slower bone formation with incomplete connection of the broken bone. Finally, Leblanc et al. demonstrated that BMP9 induced heterotopic ossification only within damaged muscle but not healthy skeletal muscle, and the addition of the soluble form of the ALK1 protein (a type I BMP receptor) inhibited osteogenesis in damaged muscles [79].
BMP9-induced bone formation shows an ossification pattern distinct from other BMPs. In a comprehensive analysis of the orthotopic bone-forming activity of BMPs, Kang et al. investigated the histology of orthotopic bone formation in athymic nude mice injected with AdBMP2, 6, 7 and 9-transduced C2C12 cells at three weeks and five weeks post-injection [1]. BMP9-treated MSCs at three weeks demonstrated variable degrees of ossification with multiple foci of immature woven bone, while BMP2 treated MSCs showed significantly less ossification and poorly developed, small foci of woven bone. Five-week samples demonstrated increased maturation in the BMP9 group with less extensive ossification in the BMP2 group. Furthermore, cartilaginous differentiation, cartilaginous matrix and bone marrow elements were significantly increased in the AdBMP9 group. Varady et al. performed a morphologic analysis of BMP9-induced osteogenesis in both athymic nude and Sprague-Dawley rats [43]. Briefly, AdBMP9 was injected into the quadriceps muscles, and morphologic analysis using light microscopy, electron microscopy, BrdU immunohistochemistry and computed tomography (CT) analysis was performed at various time points. Primitive MSCs were seen between muscle fibers beginning three days after BMP9 injection. MSCs differentiated into primitive chondroblasts secreting a loose extracellular matrix by six days, and nearly all recruited MSCs had differentiated into chondroblasts by nine days. Some areas of hypertrophic chondrocytes appearing histologically similar to the epiphyseal end plate were seen by 12 days. Cartilaginous matrix was replaced by woven bone between days 12 and 19 with mature lamellar bone seen by three months. There was no evidence of MSC proliferation or bone formation in the control groups of either athymic or Sprague-Dawley rats. Investigating the differential expression of BMPs in fresh human intramembraneous and endochondral bones as well as cell lines derived from these two types of bone, Suttapreyarsi et al. recently identified BMP9 expression in human bone for the first time [80]. BMP mRNA expression from samples of normal human intramembranous and endochondral bone was assessed using primers encoding for conserved regions of various BMPs and RT-PCR. There was no significant difference in BMP9 mRNA expression in fresh human intramembranous and endochondral bone with a trend toward increased expression in endochondral bone samples. In primary culture of human intramembraneous and endochondral-derived osteoblastic cell lines, there was again no significant difference in BMP9 expression with less of a trend toward increased expression in endochondral-derived cells. Nonetheless, further studies are necessary to determine the specific function of BMP9 in normal bone homeostasis. The aforementioned studies and associated histologic analyses illustrate that the process of BMP9-induced osteogenesis resembles the sequential physiologic phases of endochondral ossification occurring during the repair of bony fractures. These results suggest that BMP9 may very well be a more effective therapy for the induction of bone regeneration in the clinical setting than the currently utilized BMP2 and BMP7. While the specific mechanisms of BMP9-mediated osteogenesis remain to be defined, it appears that the BMP9-mediated osteogenic pathway is unique from that of other members of the BMP family.
BMP9 signaling pathway
While the specific mechanisms responsible for BMP9-mediated osteogenesis are still being determined, a considerable amount of work has been performed to elucidate the signaling pathways of the BMPs. BMP signaling transduction begins with the binding of a heterodimeric complex of two transmembrane serine/threonine kinase receptors, BMPR type 1 and BMPR type 2 [81,82]; these activated receptor kinases in turn transduce signals by phosphorylating the transcription factors Smad1, 5 and/or 8 [4]. Phosphorylated Smads then form a heterodimeric complex with Smad4 within the nucleus, activating transcription of target genes [81,83,84]. BMP9-mediated osteogenesis likely occurs by overlapping yet unique signaling pathways from other osteogenic members of the BMP family. While BMP9 induces phosphorylation of the Smad pathway like other osteogenic members of the BMP family, the extracellular antagonist noggin does not inhibit BMP9 signal transduction as it does with other BMPs [72]. Similarly, we demonstrated that BMP3, an inhibitor of BMP2 and BMP7-mediated osteogenesis, has no inhibitory effect on BMP9-mediated bone formation (Figure 1A) [1]. Subsequent studies investigating BMP9-mediated osteogenesis have identified unique signaling pathways that are essential for BMP-mediated osteoinduction.
TGF-β/BMP type I and type II receptors required for BMP9 signaling
BMP binding to the heterodimeric complex of BMPR type 1 and type 2 facilitates cross-phosphorylation of the type I receptor by the constitutively active type II receptor, leading to downstream signaling [85]. While ALK1, ALK5 and endoglin have been described as potential BMP9 type I receptors, recent studies have further investigated the receptors necessary for BMP9-mediated osteogenesis [17,86-88]. Luo et al. performed a comprehensive analysis of seven functional type I receptors in BMP9-mediated osteogenic differentiation of MSCs [11]. Although most of the seven type I receptors are expressed in MSCs, dominant negative mutations of these seven type I receptors demonstrated that only ALK1 and ALK2 mutants effectively inhibit BMP9-mediated osteogenic differentiation in vitro and ectopic bone formation in vivo. ALK1 and 2 were found to directly interact with BMP9 as assessed by protein fragment complementation, and RNA-inferrence (RNAi) silencing of ALK1 and 2 inhibited BMP9-induced BMPR-Smad activity and osteogenic differentiation of MSCs in vitro and in vivo. These results strongly suggest that ALK1 and ALK2 are the type I TGF-β receptors responsible for BMP9 osteogenic signaling.
Four different types of type II TGF-β receptors have been identified: TGFβ-RII, ActRII, ActRIIB and BMPRII. Multiple studies have shown that type II TGF-β receptors are largely responsible for the osteogenic activity of BMPs, and Wu et al. investigated the specific type II receptors necessary for BMP9-mediated osteogenesis [68]. Dominant negative (DN) type II TGF-β receptors were constructed and introduced into C3H10T1/2 MSCs. DN-BMPRII and DN-ActRII decreased BMP9-induced ALP activity, Smad binding element (SBE)-controlled reporter activity, expression of downstream Smad6 and Smad7, BMP9-induced bone mineralization in vitro and ectopic bone formation in vivo with less mature osteogenesis and smaller bony masses. Additionally, RNAi demonstrated that BMPRII and ActRII inhibited BMP9-induced ALP activity. These results strongly suggest that BMPRII and ActRII are the functional type II TGF-β receptors facilitating BMP9 osteogenic signaling.
Townson et al. recently performed a thermodynamic analysis of BMP9 and BMP10 interactions with ALK1 and type II receptors, finding that BMP9, but not BMP10, had a significant preference in type II receptor binding to ActRIIB [89]. BMP9 bound to ActRIIB with a 30-fold greater affinity than binding to BMPRII, and a 300-fold greater affinity than binding to ActRIIA. The crystal structure of a ternary complex of BMP9 with the extracellular domains of ALK1 and ActRIIB revealed that the high specificity of ALK1 for BMP9 was determined by the specific orientation of this type I receptor to BMP9, leading to novel ligand-receptor interactions and explaining how BMP9 may be able to discriminate between low and high affinity type II receptors.
Smad pathway
Upon binding specific cell-surface receptor kinases, BMP-mediated signal transduction begins with phosphorylation of Smads and subsequent heterodimer formation. Xu et al. demonstrated that, like other osteogenic BMPs, BMP9 promotes activation of Smad1/5/8 [67]. Furthermore, activation of Smads was found to be necessary for BMP9-mediated osteogenic differentiation of C3H10T1/2 cells. Levels of phosphorylated Smad1/5/8 were simultaneously enhanced in BMP9-treated C3H10T1/2 cells, indicating that BMP9 does indeed activate the Smad pathway; levels of phosphorylated Smads, p38 and ERK1/2 were detected by Western blot. Conversely, RNAi was used to knockdown Smad4, resulting in reduced formation of Smad heterodimers and a subsequently reduced nuclear translocation of Smad1/5/8. This disrupted BMP9-induced osteogenic differentiation and prevented commitment of C3H10T1/2 cells to the osteoblastic lineage. Furthermore, RNAi knockdown of Smad4 inhibited BMP9-induced ALP activity and calcium deposition, further suggesting that Smad signaling is required for BMP9-induced osteogenic differentiation of MSCs. Moreover, inhibition of p38 decreased BMP9-activated Smad signaling in C3H10T1/2 cells, while ERK1/2 inhibition stimulated Smad signaling. The findings of this study indicate that activation of the Smads pathway is critical in BMP9-induced osteogenic differentiation of MSCs.
Mediators of BMP9-induced osteogenic signaling
Inhibitors of differentiation (Ids) HLH factors
Id genes were first identified in developing myoblasts as inhibitors of the binding of basic helixloop-helix (bHLH) transcription factors to muscle-specific genes [90-92]. bHLH proteins function as critical regulators of tissue-specific gene expression by forming obligate dimers, binding to basic DNA domains and activating transcription of target genes containing the CANNTG region within the promoter. Id proteins dimerize with bHLH proteins, and resultant heterodimers are unable to bind DNA and modulate transcription. Four Id genes have been identified in mammals, and only Id-1 and Id-3 demonstrate ubiquitous expression [90-92]. Peng et al. used expression profiling analysis of MSCs to demonstrate that Id-1, -2 and -3 genes were among the most significantly upregulated upon BMP9 stimulation (Figure 1C) [16]. Expression of Id genes was induced during the early stages of BMP9 stimulation and returned to basal levels three days after stimulation. Surprisingly, both RNAi-mediated knockdown and constitutive overexpression of these three Id genes significantly diminished BMP9-induced osteogenic differentiation. Overexpression was also associated with increased cell proliferation and decreased osteoblastic differentiation. Furthermore, BMP9-induced Id expression was shown to be dependent on Smad4 signaling. The results of this study suggest that Id proteins likely play an important role in BMP9-induced osteogenic differentiation, and a balanced regulation of Id expression with downregulation during terminal differentiation of committed osteoblasts critical in osteoblast lineage-specific MSC differentiation.
Connective tissue growth factor (CTGF)
Connective tissue growth factor (CTGF) is a member of the CCN (Cyr61, CTGF and Nov) family of secreted cysteine-rich multimodular proteins [93-98] and plays a critical role in bone formation, chondrocyte maturation and embryogenesis [99]. Knockout of CTGF in embryo is lethal secondary to significantly decreased extracellular matrix and chondrocyte production with resultant skeletal dysmorphism [100-102]. Luo et al. demonstrated that CTGF plays a functional role in BMP9-induced osteoblastic differentiation [13]. Expression profiling analysis of BMP9-stimulated C3H10T1/2 MSCs demonstrated CTGF as among the most upregulated genes. Expression of CTGF was induced during the early stages of BMP9 stimulation, returning to basal levels five days after stimulation. Similar to Id genes, both siRNA knockdown and constitutive overexpression of CTGF diminished BMP9-mediated osteogenic differentiation. Exogenous expression of CTGF promoted cell migration and recruitment of MSCs, and since expression was upregulated during the early stages of BMP9-mediated osteogenic stimulation, it is likely that CTGF plays a critical role in the regulation of proliferation and recruitment of osteoprogenitor cells. Conversely, CTGF expression was downregulated as pre-osteoblasts become committed to the osteogenic lineage. The results of these studies indicate that, similar to Id proteins, balanced regulation of CTGF expression is likely critical in BMP9-induced osteogenic differentiation.
Hairy/enhancer of split-related repressor protein 1 (Hey1) bHLH factor
Hey1 (also known as Hesr1, HRT1, CHF2 and HERP2) is a nuclear protein of the Hairy/Enhancer of split-Related (HERP) family of basic helix-loop-helix transcriptional repressors. The HERP family of transcription factors is a direct target of Notch signaling, a pathway which is implicated in cell fate decision [2]. Hey1 has also been implicated in embryonic heart development, neurogenesis and somitogenesis. Sharff et al. used gene expression analysis to demonstrate that Hey1 was among the most significantly upregulated genes in BMP9-stimulated MSCs, particularly during the earliest stages of osteogenic differentiation [103]. Chromatin immunoprecipitation (ChIP) analysis identified Hey1 as a direct target of the BMP9-induced Smad signaling pathway. Constitutive Hey1 expression augmented BMP9-induced osteogenic differentiation in vitro and matrix mineralization in vivo, while siRNA-mediated Hey1 silencing diminished osteogenic differentiation both in vitro and in vivo. Hey1 and the essential osteogenic transcription factor Runx2 acted synergistically in BMP9-induced osteogenic differentiation. Furthermore, silencing of Hey1 was also correlated with decreased expression of Runx2; in MSCs with knockdown of Hey1, defective osteogenic signaling was rescued by exogenous expression of Runx2, strongly suggesting that Runx2 is a downstream mediator of Hey1 signaling. Kang et al. also demonstrated that Runx2 overexpression enhanced BMP9-mediated osteogenic differentiation [56].
Crosstalk between BMP9 and other pathways during osteogenesis
Many signaling pathways with diverse functions have been found to play a role in BMP9-mediated osteogenesis. Several of these pathways are also critical in the differentiation of other cell lineages. The following summarizes recent findings describing the crosstalk between BMP9 signaling and various other pathways (Table 1). These studies will help us to better elucidate the specific mechanisms responsible for BMP9-mediated osteogenesis.
Table 1.
Factors that Affact BMP9 Osteogenic Activity
| Treatment | Experiment Setup | Experiment Metric | Experimental Results | Reference | |
|---|---|---|---|---|---|
| BMP9 + TGF-β1 | In vitro | ALP Activity | Low concentrations of rhTGF-β1 synergistically increase ALP activity, matrix mineralization, gene expression and protein expression of osteopontin, osteocalcin and COL1a2 in C3H10T1/2 cells. TGF-β1 demonstrated biphasic effect on BMP9-meidated osteogenic differentiation. | Li et al. 2012 | |
| Alizarin Red S Staining | |||||
| RT-PCR and Western Blot | |||||
| Smad Pathway Activation | TGF-β1 combined with BMP9 exhibits lower BMPR-Smad receptor activity than BMP9 alone | ||||
| BMP9 + GH | In vitro | ALP Activity | GH potentiates BMP9-induced ALP activity, osteopontin/osteocalcin, expression and calcium deposition in MMCs | Huang et al. 2012 | |
| Osteocalcin/Osteopontin Expression | JAK/STAT inhibitors blunt BMP9-GH synergy | ||||
| Alizarin Red S Staining | GH enhances BMP9-induced endochondral ossification in cultured limb explants | ||||
| Endochondral Ossification | |||||
| In vivo | Mouse | Ectopic Bone Formation | GH augments BMP9-induced ectopic bone formation with more mature bone | ||
| BMP9 + p38 inhibitor (SB203580) | In vitro | ALP Activity | PD98059 inhibits BMP9-induced ALP activity, osteocalcin expression and calcium deposition in C3H10T1/2, MEFs, C2C12 | Xu et al. 2012, Zhao et al. 2012 | |
| Osteocalcin Expression | |||||
| Alizarin Red S Staining | |||||
| Smad Pathway Activation | PD98059 suppresses BMP9-induced Smad 1/5/8 phosphorylation/nuclear translocation and Runx2 activation | ||||
| Runx2 Activity | |||||
| In Vivo | Mouse | Bone Formation | siRNA-mediated inhibition of p38 decreased osteogenic differentiation and bone formation with thinner trabeculae | ||
| BMP9 + ERK1/2 inhibitor (PD98059) | In vitro | ALP Activity | PD98059 enhances BMP9-induced ALP activity, osteocalcin expression and calcium deposition in C3H10T1/2, MEFs, C2C12 | ||
| Osteocalcin Expression | |||||
| Alizarin Red S Staining | |||||
| Smad Pathway Activation | PD98059 stimulates BMP9-induced Smad 1/5/8 phosphorylation/nuclear translocation and Runx2 activation | ||||
| Runx2 Activity | |||||
| In vivo | Mouse | Bone Formation | siRNA-mediated inhibition of ERK1/2 increased osteogenic differentiation and bone formation with thicker trabeculae | ||
| BMP9 + Wnt3a | In vitro | ALP Activity | Wnt3a enhances BMP9-induced ALP activity. Wnt antagonist FrzB inhibits BMP9-induced ALP activity. | Tang et al. 2009 | |
| Osteocalcin Expression | Knockdown of β-catenin decreases osteocalcin expression | ||||
| In vivo | Mouse | Ectopic Bone Formation | BMP9-induced ectopic bone formation and matrix mineralization are inhibited by FrzB overexpression or β-catenin knockdown | ||
| BMP9 + PPAR-γ2 | In vitro | ALP Activity | Overexpression of PPAR-γ2 enhances BMP9-induced ALP activity | Kang et al.. 2009 | |
| In vivo | Mouse | Ectopic Bone Formation | Bone formation increased with PPAR-γ2 overexpression and decreased with PPAR-γ2 knockdown | ||
| BMP9 + Runx2 | In vitro | ALP Activity | Overexpression of Runx2 enhances BMP9-induced ALP activity | ||
| In vivo | Mouse | Ectopic Bone Formation | Bone formation increased with PPAR-γ2 overexpression and decreased with PPAR-γ2 knockdown | ||
| BMP9 + IGF2 | In vitro | ALP Activity | IGF-2 potentiates BMP9-induced ALP activity, osteocalcin/osteopontin expression and calcium deposition | Chen et al. 2010 | |
| Osteocalcin/Osteo-pontin Expression | |||||
| Alizarin Red Staining | |||||
| Smad Pathway Activation | IGF-2 enhances BMP9-mediated BMPRSmad reporter activity and nuclear translocation of Smad 1/5/8 | ||||
| In vivo | Mouse | Ectopic Bone Formation | IGF-2 augments BMP9-induced ectopic bone formation and endochondral ossification in cultured limb explants | ||
| Endochondral Ossification | |||||
TGF-β1 pathway
As one of the most abundant members of the TGF-β family, TGF-β1 regulates an array of biological processes including cell proliferation, survival, differentiation and migration [104-108]. TGF-β1 also plays a critical role in the regulation of bone growth. However, unlike BMPs, TGF-β1 is unable to induce osteogenesis in MSCs [109,110] but can direct committed osteoprogenitors toward osteogenic differentiation and bone remodeling [110-112]. TGF-β1 plays a role in bone formation, osteoblast proliferation and mineralization, increasing the strength and flexibility of bone [110,113]. Because both TGF-β1 and BMPs both regulate the late phases of differentiation and mineralization of bone [18,110,114,115], it is possible that TGF-β1 may crosstalk with BMPs during the process of osteogenic differentiation. Similar to BMPs, TGF-β1 initiates signaling by binding type I and type II transmembrane receptor serine-threonine kinases and forming a complex which, upon ligand binding, cross-phosphorylates and activates, subsequently phosphorylating its effectors, Smad2/Smad3 (Figure 2). Phosphorylated Smad2/Smad 3 then complex with Smad4 before translocating into the nucleus, interacting at the promoter with various transcription factors [81,116]. TGF-β1 also induces non-Smad signaling pathways via activation of the MAPK pathway [117,118].
Figure 2.
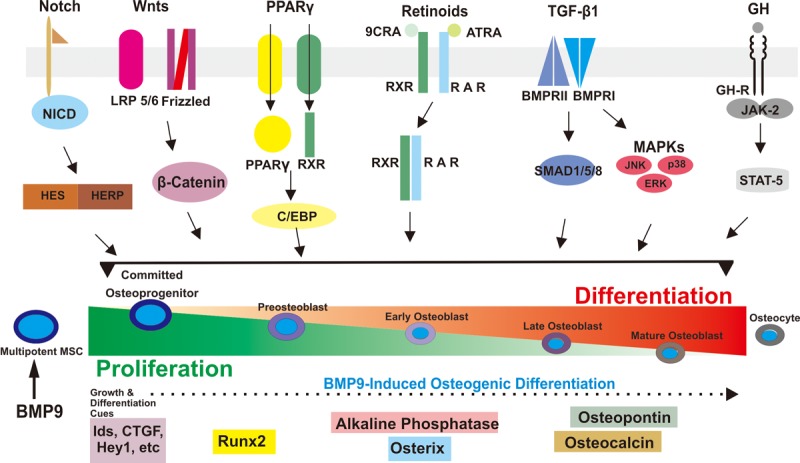
Schematic depiction of the major signaling events involved in BMP9-induced osteogenic differentiation in mesenchymal stem cells. BMP9 effectively initiates a well-coordinated cascade of signaling events, which requires the participation of other major pathways, including Wnt/β-catenin and Notch, to name a few.
Li et al. demonstrated that low concentrations of recombinant TGF-β1 (rhTGF- β1) synergistically induced expression of ALP and matrix mineralization in BMP9-transduced C3H10T1/2 cells [110]. Conversely, high concentrations of TGF-β1 inhibited BMP9-induced osteogenic activity. Real-time PCR and Western blot demonstrated that BMP9 and low concentrations of TGF-β1 potentiated expression of the late osteogenic markers osteopontin, osteocalcin and type I collagen (COL1a2), whereas high concentrations of TGF-β1 decreased expression of osteocalcin and osteopontin but not COL1a2. Cell cycle analysis demonstrated that TGF-β1 inhibited BMP9-mediated osteogenesis by restricting cells in the G0/G1 phase. Altogether, this study demonstrates that TGF-β1 likely has a biphasic effect on BMP9-induced osteogenic differentiation of MSCs.
Wnt/β-catenin signaling pathway
Wnts are a family of secreted proteins critical in skeletal development and osteoblastic differentiation [54,60,119-125]. Binding of Wnts to the Frizzled (Frz) and LRP-5/6 co-receptors results in activation of distinct signaling pathways including the canonical Wnt pathway (Figure 2) [126]. Mutations in LRP-5 adversely affect skeletal development and bone mass deposition [127], and β-catenin signaling may have roles in fracture repair [126]. Tang et al. demonstrated that the canonical Wnt/β-catenin signaling pathway plays a critical role in BMP9-induced osteogenic differentiation of MSCs [126]. Wnt3a and BMP9 enhanced one another’s ability to induce ALP activity in MSCs. Conversely, the Wnt antagonist FrzB inhibited BMP9-induced ALP activity. ChIP analysis demonstrated that BMP9 stimulation of MSCs recruited β-catenin and Runx2 to the osteocalcin promoter, whereas knockdown of β-catenin decreased expression of both early and late osteogenic markers, including ALP and osteocalcin [126]. In vivo studies demonstrated that BMP9-induced ectopic bone formation and matrix mineralization were significantly inhibited by both FrzB overexpression or β-catenin knockdown, instead forming chondrogenic matrix and immature bone [126]. The results of these studies suggest that the canonical Wnt/β-catenin pathway, likely through interactions with Runx2, is a critical mediator of BMP9-mediated osteogenic signaling.
Growth hormone (GH) pathway
Growth hormone (GH) plays a critical role in postnatal growth [128], and the regulation of its secretion within the endocrine system has been extensively described [129-135]. Briefly, GH is released by somatotrophs within the anterior pituitary when stimulated by GH releasing hormone (GHRH). Most tissues throughout the body, including non-endocrine tissues and cells, express GH receptor (GHR) [129-132,134,135]. The GH signaling pathway begins when GH binds GHR, triggering the receptor’s tyrosine kinase activity and activating JAK/STAT and other pathways, leading to multiple functions controlling growth and metabolism (Figure 2) [129,130,135]. Defects in the GH signaling pathway have been associated with postnatal growth failure [129,130,134]. Huang et al. investigated the role of BMP9-regulated GH expression in the osteogenic differentiation of murine MSCs [54,136-138]. Following BMP9 overexpression, gene expression analysis demonstrated GH as one of the most upregulated transcripts. ChIP analysis demonstrated GH as a direct target of the BMP9/Smad pathway. Exogenous GH synergized with BMP9 in the induction of early and late osteogenic markers. Moreover, BMP9 and GH co-stimulation caused a significant expansion of the growth plate of long-bone explants. While GH alone was not able to induce de novo bone formation in MSCs, co-stimulation with BMP9 and GH formed mature ectopic bone masses. The synergistic osteogenic effects of BMP9 and GH were significantly inhibited by JAK/STAT inhibitors, and GH-regulated IGF1 expression was also inhibited by JAK/STAT inhibitors. These results indicate that, as a direct target of BMP9 signaling, GH synergizes with BMP9 by activating the JAK/STAT/IGF1 pathway, causing efficient osteogenesis.
Mitogen activated protein kinases (MAPKs)
A growing body of evidence implicates mitogen activated protein kinases (MAPKs) as mediators of the intracellular signals of BMPs [5,67,139-142]. MAPKs are well-characterized protein kinases critical in regulation of gene expression, mitosis, metabolism, motility, survival, apoptosis and differentiation [143]. More than four subfamilies of MAPKs have been identified in mammals including the extracellular signal-related kinases ERK1/2, ERK5, Jun amino-terminal kinases (JNKs) and p38 MAPKs [143]. Members of the MAPKs family become activated by BMPs in response to a variety of extracellular stimuli and have different downstream targets, resulting in diverse effects in cellular responses [141,142,144,145].
Xu et al. demonstrated that BMP9 simultaneously promotes phosphorylation and thus activation of Smads, p38 and ERK1/2 [67]. p38 and ERK1/2 were found to act in opposition in regulating BMP9-mediated osteogenic differentiation via influence on the Smad signaling cascade. BMP9 was overexpressed in C3H10T1/2 cells which were then treated with selective inhibitors of p38 and ERK1/2. p38 inhibition caused inhibition of BMP9-induced ALP activity completely in a dose-dependent manner and decreased BMP9-induced calcium deposition. ERK1/2 inhibition enhanced BMP9-induced ALP activity mostly in a dose-dependent manner and increased calcium deposition. Furthermore, p38 inhibition suppressed BMP9-induced phosphorylation of Smad1/5/8 and nuclear translocation with a resultant decrease in transcriptional activity. Conversely, ERK1/2 inhibition stimulated BMP9-induced phosphorylation of Smad1/5/8 and nuclear translocation, thus enhancing transcriptional activity. The results of this study strongly suggest that p38 and ERK1/2 have opposing regulatory effects in BMP9-induced osteogenic differentiation of MSCs via the Smad signaling axis, a notion that has also been reported in other studies [146-148].
In a subsequent study, Zhao et al. examined the molecular mechanisms of p38 and ERK1/2 MAPKs on BMP9-induced osteogenic differentiation of mesenchymal progenitor cells (MPCs, including C3H10T1/2 MSCs, mouse embryonic fibroblasts and C2C12 cells), finding that BMP9 simultaneously activates p38 and ERK1/2 [89]. Similar to Xu et al., BMP9-induced ALP, osteocalcin and matrix mineralization production were inhibited with p38 inhibition and enhanced with ERK1/2 inhibition. BMP9-induced Runx2 activation and Smad signaling were also reduced by p38 inhibition but increased by ERK1/2 inhibition. Together, these in vitro studies confirm that both early and late BMP9-induced osteogenic differentiation processes are affected in opposing manners by p38 and ERK1/2 signaling pathways. In vivo studies using mouse calvarial tissue culture and subcutaneous implantation of C3H10T1/2 cells demonstrated that inhibition of p38 decreased BMP9-induced osteogenic differentiation, with thinner trabeculae, more chondrocytes and a significant number of undifferentiated MPCs. Meanwhile, siRNA-mediated inhibition of ERK1/2 significantly increased BMP9-induced osteogenic differentiation of MPCs, with increased quantity of bone formation and thicker trabeculae. Altogether, these results confirm and substantiate the findings by Su et al. that not only are p38 and ERK1/2 MAPKs activated during BMP9-induced osteogenic differentiation, but p38 and ERK1/2 also oppose one another in the regulation of BMP9-induced osteogenic differentiation [89].
Hypoxia inducible factor 1 alpha (HIF1α)
Hypoxia inducible factor 1 Alpha (HIF1α) is a regulator of angiogenesis during many developmental processes, including skeletal development [149,150]. Hu et al. recently investigated the effects of HIF1α-mediated angiogenic signaling on BMP9-regulated osteogenic differentiation of MSCs [151]. BMP9 was found to directly induce HIF1α expression in MSCs through Smad1/5/8 signaling. Exogenous over-expression of HIF1α synergized with BMP9-induced osteogenic differentiation of MSCs both in vitro as assessed by ALP activity, osteopontin and osteocalcin expression, in vivo matrix mineralization and ectopic bone formation. Conversely, siRNA-mediated silencing of HIF1α or administration of an HIF1α inhibitor significantly inhibited BMP9-induced osteogenic signaling in MSCs. HIF1α activated both angiogenic signaling (via VEGF) and osteogenic signaling pathways in MSCs during BMP9-mediated osteogenic differentiation. While angiogenesis and osteogenesis are well-coordinated processes occurring during bone development [150,152], it is unclear how they are related in during the differentiation of MSCs. This study demonstrates that osteogenic factors, such as BMP9, may induce the convergence of osteogenic and angiogenic signaling in MSC differentiation, enhancing the efficiency of bone formation and development.
Notch pathway
Notch is a transmembrane protein playing critical roles in the determination of cellular differentiation pathways and cell fate (Figure 2) [153]. Activation of notch signaling has been found to enhance BMP-induced ALP activity and calcified nodule formation in vitro [153]. Conversely, Notch inhibition causes decreased ALP activity and promoter activity of BMP target genes [154]. Notch signal transduction pathway genes are regulated by BMP2, possibly mediating the interaction of Smad and Notch signaling during osteoblastic differentiation [155]. While little is known regarding the interaction of Notch and BMP9 signaling, our preliminary findings demonstrate that Notch signaling inhibits BMP9-mediated tumorigenesis in osteosarcoma (OS) cells.
Peroxisome proliferator-activated receptor gamma (PPAR-γ)
Peroxisome proliferator-activated receptor gamma (PPAR-γ) is a crucial regulator of adipogenesis and osteogenesis [156,157]. PPAR-γ binds fatty acid derivatives and induces differentiation of preadipocytes into terminal adipocytes, and PPAR-γ2 is the predominant isoform expressed in adipose tissue (Figure 2) [30,90,92]. Overexpression of PPAR-γ in fibroblasts activates the adipogenic cascade, whereas PPAR-γ knockout mice cannot form adipose tissue [158-160]. Activating mutations of PPAR-γ in humans causes increased adipogenesis and resultant weight gain, whereas mutations inactivating PPAR-γ activity result in low body mass indices (BMI) [26,27].
Kang et al. demonstrated that overexpression of PPAR-γ2 in BMP9-stimulated MSCs promoted both osteogenic and adipogenic differentiation with mutually exclusive commitment to either lineage [56]. Conversely, both BMP9-stimulated MSCs with PPAR-γ2 knockdown and BMP-9 embryonic fibroblasts derived from PPAR-γ2-/- mice demonstrated significant decreases in osteogenic differentiation and matrix mineralization. These findings support PPAR-γ as an important regulator of BMP9-mediated osteogenesis.
IGF (insulin-like growth factor) signaling pathways
As extensively discussed earlier, BMP9 stimulation alone potently induces osteogenic differentiation in MSCs. However, several studies have shown that certain factors, most notably insulin-like growth factor 2 (IGF-2) and retinoic acids, synergistically enhance BMP9-mediated osteogenic differentiation [161,162]. IGF-2 is a member of the IGF signaling system, playing a critical role in prenatal growth and development [163]. IGF-2 signaling activates the phosphatidylinositol-3-kinase (PI3K)/AKT pathway or the MAPK pathway [164]. Chen et al. demonstrated that endogenous IGF-2 levels are relatively low in MSCs, but exogenous expression of IGF-2 potentiated BMP9-induced expression of ALP and the late osteogenic markers osteocalcin and osteopontin [161]. Furthermore, over-expression of IGF-2 enhanced BMP9-mediated induction of BMPR-Smad reporter activity and subsequent nuclear translocation of Smad1/5/8, while the PI3K inhibitor LY294002 inhibited the potentiation of IGF-2 on BMP9-mediated osteogenesis and directly inhibited BMP9 activity. In vivo, IGF-2 augmented BMP9-induced ectopic bone formation and enhanced BMP9-mediated endochondral ossification in perinatal limb explants. Exogenous expression of IGFBP3 or IGFBP4, inhibitors of IGF-2, caused the inhibition of IGF-2-mediated osteogenic effects. The results of this study demonstrate that the BMP9 signaling pathway crosstalks with IGF-2 via PI3K/AKT signaling during the osteogenic differentiation of MSCs; the therapeutic use of BMP9 in combination with IGF2 should be explored in bone regeneration applications.
Retinoid signaling pathways
Retinoic acids (RAs) play a crucial role in embryological development as well as maintenance of vital organs in adults [58,165]. RAs are ligands for two families of receptors, the RA receptors (RAR) that bind all-trans-RA (ATRA) and the Retinoid X Receptors (RXR) that bind 9-cis-RA (9CRA) (Figure 2) [166,167]. RA binding to RAR/RXR causes heterodimerization and a cascade of events resulting in recruitment of transcriptional activators and initiation of transcription [167]. Zhang et al. investigated the effects of RA signaling on BMP9-induced osteogenic differentiation [162]. In mesenchymal progenitor cells (MPCs), both ATRA and 9CRA significantly induced ALP, osteopontin and osteocalcin activity and matrix mineralization; these effects were synergistic when combined with adenoviral-mediated overexpression of BMP9. Quantitative real-time PCR (qRT-PCR) demonstrated that 9CRA and ATRA induced BMP9 expression and activates BMPR Smad-mediated transcription activity. In neonatal mouse limb explants, RAs acted with BMP9 to promote expansion of the hypertrophic chondrocyte zone. In vivo stem cell implantation studies demonstrated that RARs act synergistically with BMP9 to induce trabecular bone formation and osteoid matrix production. The results of these studies suggest that crosstalk exists between the signaling pathways facilitating BMP9-mediated osteogenesis and the retinoic acid and/or IGF signaling pathways. Furthermore, the interactions between these pathways seem to synergistically enhance osteoinduction of MSCs.
Other functions of BMP9 signaling
While BMP-9 is known to be a potent osteogenic factor, it also influences several other pathways including cancer development and angiogenesis. Studies have shown BMP9 to restrict tumor growth including prostate cancer, osteosarcoma and colon adenocarcinoma through diverse mechanisms [168-170]. However, other studies have shown BMP9 to promote cancer progression [171]. For instance, BMP9 was demonstrated to induce apoptosis of prostate cancer cells by upregulation of the pro-apoptotic protein prostate apoptosis response-4 via a Smad-dependent pathway [170]. In osteosarcoma, BMP9 acted through the PI3K/ALT pathway to inhibit cell migration and induce apoptosis of osteosarcoma cells [169]. In colon adenocarcinoma, BMP9 slowed tumor growth of via inhibitory effects on angiogenesis via the ALK1 receptor and endoglin coreceptor [168]. Meanwhile, other studies have demonstrated that BMP9 induced in vivo angiogenesis within pancreatic tumors [171]. Indeed, the effects of BMP9 on angiogenesis remain controversial, as some studies have demonstrated a proangiogenic effect [172,173] and others an antiangiogenic effect [86,174-176]. To explain this discrepancy, it has been suggested that BMP9 is pro-angiogenic at low concentrations and inhibitory at high concentrations, possibly through activation of other pathways at high concentrations with varying effects on angiogenesis [171]. Moreover, BMP9 has been demonstrated as an anti-angiogenic factor alone but pro-angiogenic when coupled with TGF-β [177,178]. From these studies, it is evident that the effects of BMP9 on angiogenesis and cancer progression remain to be fully elucidated.
BMP9 affects the processes of neurogenesis, hepatocellular regeneration, adipogenesis, chondrogenesis and myogenesis. Several studies have demonstrated BMP9 to promote the cholinergic phenotype neurologically, specifically basal forebrain cholinergic neurons through its interactions with a variety of growth factors, receptors and signaling pathways [179-182]. A prominent mediator of the pro-cholinergic effects of BMP9 is nerve growth factor, which acts in both an autocrine and paracrine fashion via the p75 receptor [181,182]. BMP9 also acts as a hepatic insulin-sensitizing substance and may play a role in hepatocellular regeneration [183,184]. BMP9 promotes adipogenesis [56] and also upregulates Sox9 expression to induce chondrogenic differentiation [185,186]. While BMP9 promotes MSC differentiation along osteogenic, adipogenic and chondrogenic lines to varying degrees, it inhibits the myogenic phenotype [15]. Overall, BMP9 has far-reaching effects beyond osteogenesis.
Concluding remarks and future directions
The investigations discussed clearly demonstrate the critical role of BMP9 in the osteogenic differentiation of MSCs. With the advent of therapeutic recombinant proteins, including other members of the BMP family, it is imperative that the mechanisms underlying BMP9-mediated osteogenesis continue to be elucidated to allow for translation to the clinical setting. The findings of many of the studies discussed here support the notion that BMP9 may provide a more effective clinical strategy for the augmentation of bone regeneration and healing than other BMPs. Studies demonstrating that BMP9-mediated osteogenesis resembles the physiologic phases of bone healing occurring during fracture repair make its translation to the clinical setting even more promising. Furthermore, several diverse signaling pathways seem to enhance BMP9-mediated osteogenesis, and further elucidation of these pathways will allow for the development of better therapies that combine BMP9 and the mediators of the other pathways.
Acknowledgements
The reported work was supported in part by research grants from the OREF and the National Institutes of Health (RCH, TCH and HHL).
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, Vanichakarn P, Park JY, Li Y, Haydon RC, He TC. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11:1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 2.Luther G, Wagner ER, Zhu G, Kang Q, Luo Q, Lamplot J, Bi Y, Luo X, Luo J, Teven C, Shi Q, Kim SH, Gao JL, Huang E, Yang K, Rames R, Liu X, Li M, Hu N, Liu H, Su Y, Chen L, He BC, Zuo GW, Deng ZL, Reid RR, Luu HH, Haydon RC, He TC. BMP-9 induced osteogenic differentiation of mesenchymal stem cells: molecular mechanism and therapeutic potential. Curr Gene Ther. 2011;11:229–240. doi: 10.2174/156652311795684777. [DOI] [PubMed] [Google Scholar]
- 3.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 4.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 5.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 6.Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
- 7.Song JJ, Celeste AJ, Kong FM, Jirtle RL, Rosen V, Thies RS. Bone morphogenetic protein-9 binds to liver cells and stimulates proliferation. Endocrinology. 1995;136:4293–4297. doi: 10.1210/endo.136.10.7664647. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Grzegorzewski KJ, Barash S, Zhao Q, Schneider H, Wang Q, Singh M, Pukac L, Bell AC, Duan R, Coleman T, Duttaroy A, Cheng S, Hirsch J, Zhang L, Lazard Y, Fischer C, Barber MC, Ma ZD, Zhang YQ, Reavey P, Zhong L, Teng B, Sanyal I, Ruben SM, Blondel O, Birse CE. An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nat Biotechnol. 2003;21:294–301. doi: 10.1038/nbt795. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Coviella I, Berse B, Krauss R, Thies RS, Blusztajn JK. Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science. 2000;289:313–316. doi: 10.1126/science.289.5477.313. [DOI] [PubMed] [Google Scholar]
- 10.Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci U S A. 2006;103:10289–10293. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo J, Tang M, Huang J, He BC, Gao JL, Chen L, Zuo GW, Zhang W, Luo Q, Shi Q, Zhang BQ, Bi Y, Luo X, Jiang W, Su Y, Shen J, Kim SH, Huang E, Gao Y, Zhou JZ, Yang K, Luu HH, Pan X, Haydon RC, Deng ZL, He TC. TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J Biol Chem. 2010;285:29588–29598. doi: 10.1074/jbc.M110.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A:1544–1552. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, Li X, Luu HH, Luo J, Montag AG, Haydon RC, He TC. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 14.Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, Sharff KA, Montag AG, Haydon RC, He TC. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25:665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 15.Peng Y, Kang Q, Cheng H, Li X, Sun MH, Jiang W, Luu HH, Park JY, Haydon RC, He TC. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. J Cell Biochem. 2003;90:1149–1165. doi: 10.1002/jcb.10744. [DOI] [PubMed] [Google Scholar]
- 16.Peng Y, Kang Q, Luo Q, Jiang W, Si W, Liu BA, Luu HH, Park JK, Li X, Luo J, Montag AG, Haydon RC, He TC. Inhibitor of DNA binding/differentiation helix-loop-helix proteins mediate bone morphogenetic protein-induced osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:32941–32949. doi: 10.1074/jbc.M403344200. [DOI] [PubMed] [Google Scholar]
- 17.Brown MA, Zhao Q, Baker KA, Naik C, Chen C, Pukac L, Singh M, Tsareva T, Parice Y, Mahoney A, Roschke V, Sanyal I, Choe S. Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J Biol Chem. 2005;280:25111–25118. doi: 10.1074/jbc.M503328200. [DOI] [PubMed] [Google Scholar]
- 18.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 19.Hill JJ, Qiu Y, Hewick RM, Wolfman NM. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: a novel protein with protease inhibitor and follistatin domains. Mol Endocrinol. 2003;17:1144–1154. doi: 10.1210/me.2002-0366. [DOI] [PubMed] [Google Scholar]
- 20.Li JZ, Li H, Sasaki T, Holman D, Beres B, Dumont RJ, Pittman DD, Hankins GR, Helm GA. Osteogenic potential of five different recombinant human bone morphogenetic protein adenoviral vectors in the rat. Gene Ther. 2003;10:1735–1743. doi: 10.1038/sj.gt.3302075. [DOI] [PubMed] [Google Scholar]
- 21.Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20:343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Li L. BMP signaling and stem cell regulation. Dev Biol. 2005;284:1–11. doi: 10.1016/j.ydbio.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Zhou L, An N, Jiang W, Haydon R, Cheng H, Zhou Q, Breyer B, Feng T, He TC. Fluorescence-based functional assay for Wnt/beta-catenin signaling activity. Biotechniques. 2002;33:1126–1128. 1130, 1132 passim. doi: 10.2144/02335dd07. [DOI] [PubMed] [Google Scholar]
- 24.Alden TD, Pittman DD, Beres EJ, Hankins GR, Kallmes DF, Wisotsky BM, Kerns KM, Helm GA. Percutaneous spinal fusion using bone morphogenetic protein-2 gene therapy. J Neurosurg. 1999;90:109–114. doi: 10.3171/spi.1999.90.1.0109. [DOI] [PubMed] [Google Scholar]
- 25.Alden TD, Pittman DD, Hankins GR, Beres EJ, Engh JA, Das S, Hudson SB, Kerns KM, Kallmes DF, Helm GA. In vivo endochondral bone formation using a bone morphogenetic protein 2 adenoviral vector. Hum Gene Ther. 1999;10:2245–2253. doi: 10.1089/10430349950017220. [DOI] [PubMed] [Google Scholar]
- 26.Baltzer AW, Lattermann C, Whalen JD, Ghivizzani S, Wooley P, Krauspe R, Robbins PD, Evans CH. Potential role of direct adenoviral gene transfer in enhancing fracture repair. Clin Orthop Relat Res. 2000:S120–125. doi: 10.1097/00003086-200010001-00016. [DOI] [PubMed] [Google Scholar]
- 27.Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, Ghivizzani SC, Robbins PD, Evans CH. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7:734–739. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- 28.Bosch P, Musgrave D, Ghivizzani S, Latterman C, Day CS, Huard J. The efficiency of muscle-derived cell-mediated bone formation. Cell Transplant. 2000;9:463–470. doi: 10.1177/096368970000900403. [DOI] [PubMed] [Google Scholar]
- 29.Breitbart AS, Grande DA, Mason JM, Barcia M, James T, Grant RT. Gene-enhanced tissue engineering: applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg. 1999;42:488–495. [PubMed] [Google Scholar]
- 30.Franceschi RT, Wang D, Krebsbach PH, Rutherford RB. Gene therapy for bone formation: in vitro and in vivo osteogenic activity of an adenovirus expressing BMP7. J Cell Biochem. 2000;78:476–486. doi: 10.1002/1097-4644(20000901)78:3<476::aid-jcb12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Gazit D, Turgeman G, Kelley P, Wang E, Jalenak M, Zilberman Y, Moutsatsos I. Engineered pluripotent mesenchymal cells integrate and differentiate in regenerating bone: a novel cell-mediated gene therapy. J Gene Med. 1999;1:121–133. doi: 10.1002/(SICI)1521-2254(199903/04)1:2<121::AID-JGM26>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 32.Krebsbach PH, Gu K, Franceschi RT, Rutherford RB. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 2000;11:1201–1210. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- 33.Lee JY, Musgrave D, Pelinkovic D, Fukushima K, Cummins J, Usas A, Robbins P, Fu FH, Huard J. Effect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. J Bone Joint Surg Am. 2001;83-A:1032–1039. doi: 10.2106/00004623-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman JR, Le LQ, Wu L, Finerman GA, Berk A, Witte ON, Stevenson S. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res. 1998;16:330–339. doi: 10.1002/jor.1100160309. [DOI] [PubMed] [Google Scholar]
- 35.Lou J, Xu F, Merkel K, Manske P. Gene therapy: adenovirus-mediated human bone morphogenetic protein-2 gene transfer induces mesenchymal progenitor cell proliferation and differentiation in vitro and bone formation in vivo. J Orthop Res. 1999;17:43–50. doi: 10.1002/jor.1100170108. [DOI] [PubMed] [Google Scholar]
- 36.Mason JM, Grande DA, Barcia M, Grant R, Pergolizzi RG, Breitbart AS. Expression of human bone morphogenic protein 7 in primary rabbit periosteal cells: potential utility in gene therapy for osteochondral repair. Gene Ther. 1998;5:1098–1104. doi: 10.1038/sj.gt.3300703. [DOI] [PubMed] [Google Scholar]
- 37.Musgrave DS, Bosch P, Ghivizzani S, Robbins PD, Evans CH, Huard J. Adenovirus-mediated direct gene therapy with bone morphogenetic protein-2 produces bone. Bone. 1999;24:541–547. doi: 10.1016/s8756-3282(99)00086-1. [DOI] [PubMed] [Google Scholar]
- 38.Okubo Y, Bessho K, Fujimura K, Iizuka T, Miyatake SI. Osteoinduction by bone morphogenetic protein-2 via adenoviral vector under transient immunosuppression. Biochem Biophys Res Commun. 2000;267:382–387. doi: 10.1006/bbrc.1999.1975. [DOI] [PubMed] [Google Scholar]
- 39.Oyama M, Tatlock A, Fukuta S, Kavalkovich K, Nishimura K, Johnstone B, Robbins PD, Evans CH, Niyibizi C. Retrovirally transduced bone marrow stromal cells isolated from a mouse model of human osteogenesis imperfecta (oim) persist in bone and retain the ability to form cartilage and bone after extended passaging. Gene Ther. 1999;6:321–329. doi: 10.1038/sj.gt.3300839. [DOI] [PubMed] [Google Scholar]
- 40.Riew KD, Wright NM, Cheng S, Avioli LV, Lou J. Induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene in a rabbit spinal fusion model. Calcif Tissue Int. 1998;63:357–360. doi: 10.1007/s002239900540. [DOI] [PubMed] [Google Scholar]
- 41.Ripamonti U, Ramoshebi LN, Matsaba T, Tasker J, Crooks J, Teare J. Bone induction by BMPs/OPs and related family members in primates. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S116–127. [PubMed] [Google Scholar]
- 42.Sandhu HS, Khan SN, Suh DY, Boden SD. Demineralized bone matrix, bone morphogenetic proteins, and animal models of spine fusion: an overview. Eur Spine J. 2001;10(Suppl 2):S122–131. doi: 10.1007/s005860100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varady P, Li JZ, Cunningham M, Beres EJ, Das S, Engh J, Alden TD, Pittman DD, Kerns KM, Kallmes DF, Helm GA. Morphologic analysis of BMP-9 gene therapy-induced osteogenesis. Hum Gene Ther. 2001;12:697–710. doi: 10.1089/104303401300057423. [DOI] [PubMed] [Google Scholar]
- 44.Whang K, Tsai DC, Nam EK, Aitken M, Sprague SM, Patel PK, Healy KE. Ectopic bone formation via rhBMP-2 delivery from porous bioabsorbable polymer scaffolds. J Biomed Mater Res. 1998;42:491–499. doi: 10.1002/(sici)1097-4636(19981215)42:4<491::aid-jbm3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 45.Bostrom MP, Camacho NP. Potential role of bone morphogenetic proteins in fracture healing. Clin Orthop Relat Res. 1998:S274–282. doi: 10.1097/00003086-199810001-00028. [DOI] [PubMed] [Google Scholar]
- 46.Cheng SL, Lou J, Wright NM, Lai CF, Avioli LV, Riew KD. In vitro and in vivo induction of bone formation using a recombinant adenoviral vector carrying the human BMP-2 gene. Calcif Tissue Int. 2001;68:87–94. [PubMed] [Google Scholar]
- 47.Gerhart TN, Kirker-Head CA, Kriz MJ, Holtrop ME, Hennig GE, Hipp J, Schelling SH, Wang E. Healing segmental femoral defects in sheep using recombinant human bone morphogenetic protein. Clin Orthop Relat Res. 1993:317–326. [PubMed] [Google Scholar]
- 48.Heckman JD, Boyan BD, Aufdemorte TB, Abbott JT. The use of bone morphogenetic protein in the treatment of non-union in a canine model. J Bone Joint Surg Am. 1991;73:750–764. [PubMed] [Google Scholar]
- 49.Helm GA, Alden TD, Beres EJ, Hudson SB, Das S, Engh JA, Pittman DD, Kerns KM, Kallmes DF. Use of bone morphogenetic protein-9 gene therapy to induce spinal arthrodesis in the rodent. J Neurosurg. 2000;92:191–196. doi: 10.3171/spi.2000.92.2.0191. [DOI] [PubMed] [Google Scholar]
- 50.Lee AR, Wilkins AC, Leather C, Brenton AG. Translational energy spectra for single-electron capture by O2+ in He, Ne, and Ar. Phys Rev A. 1994;50:1149–1154. doi: 10.1103/physreva.50.1149. [DOI] [PubMed] [Google Scholar]
- 51.Partridge K, Yang X, Clarke NM, Okubo Y, Bessho K, Sebald W, Howdle SM, Shakesheff KM, Oreffo RO. Adenoviral BMP-2 gene transfer in mesenchymal stem cells: in vitro and in vivo bone formation on biodegradable polymer scaffolds. Biochem Biophys Res Commun. 2002;292:144–152. doi: 10.1006/bbrc.2002.6623. [DOI] [PubMed] [Google Scholar]
- 52.Varady P, Li JZ, Alden TD, Kallmes DF, Williams MB, Helm GA. CT and radionuclide study of BMP-2 gene therapy-induced bone formation. Acad Radiol. 2002;9:632–637. doi: 10.1016/s1076-6332(03)80307-0. [DOI] [PubMed] [Google Scholar]
- 53.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 54.Deng ZL, Sharff KA, Tang N, Song WX, Luo J, Luo X, Chen J, Bennett E, Reid R, Manning D, Xue A, Montag AG, Luu HH, Haydon RC, He TC. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–2021. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 55.Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney Int. 2000;57:2207–2214. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 56.Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, Chen J, Bi Y, He BC, Park JK, Jiang W, Tang Y, Huang J, Su Y, Zhu GH, He Y, Yin H, Hu Z, Wang Y, Chen L, Zuo GW, Pan X, Shen J, Vokes T, Reid RR, Haydon RC, Luu HH, He TC. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18:545–559. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo J, Sun MH, Kang Q, Peng Y, Jiang W, Luu HH, Luo Q, Park JY, Li Y, Haydon RC, He TC. Gene therapy for bone regeneration. Curr Gene Ther. 2005;5:167–179. doi: 10.2174/1566523053544218. [DOI] [PubMed] [Google Scholar]
- 58.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 59.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 60.Tang N, Song WX, Luo J, Haydon RC, He TC. Osteosarcoma development and stem cell differentiation. Clin Orthop Relat Res. 2008;466:2114–2130. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagner ER, He BC, Chen L, Zuo GW, Zhang W, Shi Q, Luo Q, Luo X, Liu B, Luo J, Rastegar F, He CJ, Hu Y, Boody B, Luu HH, He TC, Deng ZL, Haydon RC. Therapeutic Implications of PPAR-gamma in Human Osteosarcoma. PPAR Res. 2010;2010:956427. doi: 10.1155/2010/956427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He TC. Distinct osteogenic activity of BMPs and their orthopaedic applications. J Musculoskelet Neuronal Interact. 2005;5:363–366. [PubMed] [Google Scholar]
- 63.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 64.Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000;21:393–411. doi: 10.1210/edrv.21.4.0403. [DOI] [PubMed] [Google Scholar]
- 65.Aubin JE. Regulation of osteoblast formation and function. Rev Endocr Metab Disord. 2001;2:81–94. doi: 10.1023/a:1010011209064. [DOI] [PubMed] [Google Scholar]
- 66.He BC, Chen L, Zuo GW, Zhang W, Bi Y, Huang J, Wang Y, Jiang W, Luo Q, Shi Q, Zhang BQ, Liu B, Lei X, Luo J, Luo X, Wagner ER, Kim SH, He CJ, Hu Y, Shen J, Zhou Q, Rastegar F, Deng ZL, Luu HH, He TC, Haydon RC. Synergistic antitumor effect of the activated PPARgamma and retinoid receptors on human osteosarcoma. Clin Cancer Res. 2010;16:2235–2245. doi: 10.1158/1078-0432.CCR-09-2499. [DOI] [PubMed] [Google Scholar]
- 67.Xu DJ, Zhao YZ, Wang J, He JW, Weng YG, Luo JY. Smads, p38 and ERK1/2 are involved in BMP9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. BMB Rep. 2012;45:247–52. doi: 10.5483/bmbrep.2012.45.4.247. [DOI] [PubMed] [Google Scholar]
- 68.Wu N, Zhao Y, Yin Y, Zhang Y, Luo J. Identification and analysis of type II TGF-{beta} receptors in BMP-9-induced osteogenic differentiation of C3H10T1/2 mesenchymal stem cells. Acta Biochim Biophys Sin (Shanghai) 2010;42:699–708. doi: 10.1093/abbs/gmq075. [DOI] [PubMed] [Google Scholar]
- 69.Aslan H, Zilberman Y, Arbeli V, Sheyn D, Matan Y, Liebergall M, Li JZ, Helm GA, Gazit D, Gazit Z. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006;12:877–889. doi: 10.1089/ten.2006.12.877. [DOI] [PubMed] [Google Scholar]
- 70.Santos JL, Pandita D, Rodrigues J, Pego AP, Granja PL, Tomas H. Non-viral gene delivery to mesenchymal stem cells: methods, strategies and application in bone tissue engineering and regeneration. Curr Gene Ther. 2011;11:46–57. doi: 10.2174/156652311794520102. [DOI] [PubMed] [Google Scholar]
- 71.Sheyn D, Kimelman-Bleich N, Pelled G, Zilberman Y, Gazit D, Gazit Z. Ultrasound-based nonviral gene delivery induces bone formation in vivo. Gene Ther. 2008;15:257–266. doi: 10.1038/sj.gt.3303070. [DOI] [PubMed] [Google Scholar]
- 72.Bergeron E, Senta H, Mailloux A, Park H, Lord E, Faucheux N. Murine preosteoblast differentiation induced by a peptide derived from bone morphogenetic proteins-9. Tissue Eng Part A. 2009;15:3341–3349. doi: 10.1089/ten.TEA.2009.0189. [DOI] [PubMed] [Google Scholar]
- 73.Bergeron E, Leblanc E, Drevelle O, Giguere R, Beauvais S, Grenier G, Faucheux N. The evaluation of ectopic bone formation induced by delivery systems for bone morphogenetic protein-9 or its derived peptide. Tissue Eng Part A. 2012;18:342–352. doi: 10.1089/ten.TEA.2011.0008. [DOI] [PubMed] [Google Scholar]
- 74.Lee JY, Peng H, Usas A, Musgrave D, Cummins J, Pelinkovic D, Jankowski R, Ziran B, Robbins P, Huard J. Enhancement of bone healing based on ex vivo gene therapy using human muscle-derived cells expressing bone morphogenetic protein 2. Hum Gene Ther. 2002;13:1201–1211. doi: 10.1089/104303402320138989. [DOI] [PubMed] [Google Scholar]
- 75.Li JZ, Hankins GR, Kao C, Li H, Kammauff J, Helm GA. Osteogenesis in rats induced by a novel recombinant helper-dependent bone morphogenetic protein-9 (BMP-9) adenovirus. J Gene Med. 2003;5:748–756. doi: 10.1002/jgm.412. [DOI] [PubMed] [Google Scholar]
- 76.Dumont RJ, Dayoub H, Li JZ, Dumont AS, Kallmes DF, Hankins GR, Helm GA. Ex vivo bone morphogenetic protein-9 gene therapy using human mesenchymal stem cells induces spinal fusion in rodents. Neurosurgery. 2002;51:1239–1244. doi: 10.1097/00006123-200211000-00020. discussion 1244-1235. [DOI] [PubMed] [Google Scholar]
- 77.Kimelman-Bleich N, Pelled G, Zilberman Y, Kallai I, Mizrahi O, Tawackoli W, Gazit Z, Gazit D. Targeted gene-and-host progenitor cell therapy for nonunion bone fracture repair. Mol Ther. 2011;19:53–59. doi: 10.1038/mt.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X, Chen L, Ke ZY, Yin LJ, Deng ZL. BMP9-Induced Osteogenic Differentiation and Bone Formation of Muscle-Derived Stem Cells. Journal of Biomedicine and Biotechnology. 2012;2012:7. doi: 10.1155/2012/610952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leblanc E, Trensz F, Haroun S, Drouin G, Bergeron E, Penton CM, Montanaro F, Roux S, Faucheux N, Grenier G. BMP9-Induced muscle heterotopic ossification requires changes to the skeletal muscle microenvironment. J Bone Miner Res. 2010 doi: 10.1002/jbmr.311. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 80.Suttapreyasri S, Koontongkaew S, Phongdara A, Leggat U. Expression of bone morphogenetic proteins in normal human intramembranous and endochondral bones. Int J Oral Maxillofac Surg. 2006;35:444–452. doi: 10.1016/j.ijom.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 81.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 82.Yamashita H, Ten Dijke P, Heldin CH, Miyazono K. Bone morphogenetic protein receptors. Bone. 1996;19:569–574. doi: 10.1016/s8756-3282(96)00259-1. [DOI] [PubMed] [Google Scholar]
- 83.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 84.Wrana JL. Regulation of Smad activity. Cell. 2000;100:189–192. doi: 10.1016/s0092-8674(00)81556-1. [DOI] [PubMed] [Google Scholar]
- 85.Yamashita H, Miyazono K. [Bone morphogenetic protein (BMP) receptors and signal transduction] . Nippon Rinsho. 1999;57:220–226. [PubMed] [Google Scholar]
- 86.Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, Lowik CW, ten Dijke P. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120:964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]
- 87.Shao ES, Lin L, Yao Y, Bostrom KI. Expression of vascular endothelial growth factor is coordinately regulated by the activin-like kinase receptors 1 and 5 in endothelial cells. Blood. 2009;114:2197–2206. doi: 10.1182/blood-2009-01-199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Upton PD, Davies RJ, Trembath RC, Morrell NW. Bone morphogenetic protein (BMP) and activin type II receptors balance BMP9 signals mediated by activin receptor-like kinase-1 in human pulmonary artery endothelial cells. J Biol Chem. 2009;284:15794–15804. doi: 10.1074/jbc.M109.002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao Y, Song T, Wang W, Wang J, He J, Wu N, Tang M, He B, Luo J. P38 and ERK1/2 MAPKs Act in Opposition to Regulate BMP9-Induced Osteogenic Differentiation of Mesenchymal Progenitor Cells. PLoS One. 2012;7:e43383. doi: 10.1371/journal.pone.0043383. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90.Kreider BL, Benezra R, Rovera G, Kadesch T. Inhibition of myeloid differentiation by the helix-loop-helix protein Id. Science. 1992;255:1700–1702. doi: 10.1126/science.1372755. [DOI] [PubMed] [Google Scholar]
- 91.Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113:3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- 92.Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003;13:410–418. doi: 10.1016/s0962-8924(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 93.Blom IE, Goldschmeding R, Leask A. Gene regulation of connective tissue growth factor: new targets for antifibrotic therapy? Matrix Biol. 2002;21:473–482. doi: 10.1016/s0945-053x(02)00055-0. [DOI] [PubMed] [Google Scholar]
- 94.Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- 95.Brigstock DR, Goldschmeding R, Katsube KI, Lam SC, Lau LF, Lyons K, Naus C, Perbal B, Riser B, Takigawa M, Yeger H. Proposal for a unified CCN nomenclature. Mol Pathol. 2003;56:127–128. doi: 10.1136/mp.56.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 97.Moussad EE, Brigstock DR. Connective tissue growth factor: what’s in a name? Mol Genet Metab. 2000;71:276–292. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- 98.Planque N, Perbal B. A structural approach to the role of CCN (CYR61/CTGF/NOV) proteins in tumourigenesis. Cancer Cell Int. 2003;3:15. doi: 10.1186/1475-2867-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ihn H. Pathogenesis of fibrosis: role of TGF-beta and CTGF. Curr Opin Rheumatol. 2002;14:681–685. doi: 10.1097/00002281-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 101.Kennedy L, Liu S, Shi-Wen X, Chen Y, Eastwood M, Sabetkar M, Carter DE, Lyons KM, Black CM, Abraham DJ, Leask A. CCN2 is necessary for the function of mouse embryonic fibroblasts. Exp Cell Res. 2007;313:952–964. doi: 10.1016/j.yexcr.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 102.Zuo GW, Kohls CD, He BC, Chen L, Zhang W, Shi Q, Zhang BQ, Kang Q, Luo J, Luo X, Wagner ER, Kim SH, Restegar F, Haydon RC, Deng ZL, Luu HH, He TC, Luo Q. The CCN proteins: important signaling mediators in stem cell differentiation and tumorigenesis. Histol Histopathol. 2010;25:795–806. doi: 10.14670/hh-25.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharff KA, Song WX, Luo X, Tang N, Luo J, Chen J, Bi Y, He BC, Huang J, Li X, Jiang W, Zhu GH, Su Y, He Y, Shen J, Wang Y, Chen L, Zuo GW, Liu B, Pan X, Reid RR, Luu HH, Haydon RC, He TC. Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. J Biol Chem. 2009;284:649–659. doi: 10.1074/jbc.M806389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- 105.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. Embo J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 107.Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci U S A. 2003;100:8430–8435. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen YJ, Wurtz T, Wang CJ, Kuo YR, Yang KD, Huang HC, Wang FS. Recruitment of mesenchymal stem cells and expression of TGF-beta 1 and VEGF in the early stage of shock wave-promoted bone regeneration of segmental defect in rats. J Orthop Res. 2004;22:526–534. doi: 10.1016/j.orthres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 109.Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-beta1 to the bone. Endocr Rev. 2005;26:743–774. doi: 10.1210/er.2004-0001. [DOI] [PubMed] [Google Scholar]
- 110.Li RD, Deng ZL, Hu N, Liang X, Liu B, Luo J, Chen L, Yin L, Luo X, Shui W, He TC, Huang W. Biphasic effects of TGFbeta1 on BMP9-induced osteogenic differentiation of mesenchymal stem cells. BMB Rep. 45:509–514. doi: 10.5483/bmbrep.2012.45.9.053. [DOI] [PubMed] [Google Scholar]
- 111.Kassem M, Kveiborg M, Eriksen EF. Production and action of transforming growth factor-beta in human osteoblast cultures: dependence on cell differentiation and modulation by calcitriol . Eur J Clin Invest. 2000;30:429–437. doi: 10.1046/j.1365-2362.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 112.Yamada T, Kamiya N, Harada D, Takagi M. Effects of transforming growth factor-beta1 on the gene expression of decorin, biglycan, and alkaline phosphatase in osteoblast precursor cells and more differentiated osteoblast cells. Histochem J. 1999;31:687–694. doi: 10.1023/a:1003855922395. [DOI] [PubMed] [Google Scholar]
- 113.Knippenberg M, Helder MN, Doulabi BZ, Bank RA, Wuisman PI, Klein-Nulend J. Differential effects of bone morphogenetic protein-2 and transforming growth factor-beta1 on gene expression of collagen-modifying enzymes in human adipose tissue-derived mesenchymal stem cells. Tissue Eng Part A. 2009;15:2213–2225. doi: 10.1089/ten.tea.2007.0184. [DOI] [PubMed] [Google Scholar]
- 114.Alliston T, Choy L, Ducy P, Karsenty G, Derynck R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. Embo J. 2001;20:2254–2272. doi: 10.1093/emboj/20.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maeda S, Hayashi M, Komiya S, Imamura T, Miyazono K. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. Embo J. 2004;23:552–563. doi: 10.1038/sj.emboj.7600067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 117.Hocevar BA, Brown TL, Howe PH. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. Embo J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, Brown M, Lazar MA. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995;270:23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 119.Bergwitz C, Wendlandt T, Kispert A, Brabant G. Wnts differentially regulate colony growth and differentiation of chondrogenic rat calvaria cells. Biochim Biophys Acta. 2001;1538:129–140. doi: 10.1016/s0167-4889(00)00123-3. [DOI] [PubMed] [Google Scholar]
- 120.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 121.Fischer L, Boland G, Tuan RS. Wnt signaling during BMP-2 stimulation of mesenchymal chondrogenesis. J Cell Biochem. 2002;84:816–831. doi: 10.1002/jcb.10091. [DOI] [PubMed] [Google Scholar]
- 122.Glass DA 2nd, Karsenty G. Molecular bases of the regulation of bone remodeling by the canonical Wnt signaling pathway. Curr Top Dev Biol. 2006;73:43–84. doi: 10.1016/S0070-2153(05)73002-7. [DOI] [PubMed] [Google Scholar]
- 123.Glass DA 2nd, Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology. 2007;148:2630–2634. doi: 10.1210/en.2006-1372. [DOI] [PubMed] [Google Scholar]
- 124.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 125.Wang J, Wynshaw-Boris A. The canonical Wnt pathway in early mammalian embryogenesis and stem cell maintenance/differentiation. Curr Opin Genet Dev. 2004;14:533–539. doi: 10.1016/j.gde.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 126.Tang N, Song WX, Luo J, Luo X, Chen J, Sharff KA, Bi Y, He BC, Huang JY, Zhu GH, Su YX, Jiang W, Tang M, He Y, Wang Y, Chen L, Zuo GW, Shen J, Pan X, Reid RR, Luu HH, Haydon RC, He TC. BMP-9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J Cell Mol Med. 2009;13:2448–2464. doi: 10.1111/j.1582-4934.2008.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 128.Huang E, Zhu G, Jiang W, Yang K, Gao Y, Luo Q, Gao JL, Kim SH, Liu X, Li M, Shi Q, Hu N, Wang L, Liu H, Cui J, Zhang W, Li R, Chen X, Kong YH, Zhang J, Wang J, Shen J, Bi Y, Statz J, He BC, Luo J, Wang H, Xiong F, Luu HH, Haydon RC, Yang L, He TC. Growth hormone synergizes with BMP9 in osteogenic differentiation by activating the JAK/STAT/IGF1 pathway in murine multilineage cells. J Bone Miner Res. 2012;27:1566–1575. doi: 10.1002/jbmr.1622. [DOI] [PubMed] [Google Scholar]
- 129.Blair JC, Savage MO. The GH-IGF-I axis in children with idiopathic short stature. Trends Endocrinol Metab. 2002;13:325–330. doi: 10.1016/s1043-2760(02)00631-8. [DOI] [PubMed] [Google Scholar]
- 130.Brooks AJ, Waters MJ. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol. 2010;6:515–525. doi: 10.1038/nrendo.2010.123. [DOI] [PubMed] [Google Scholar]
- 131.Hull KL, Harvey S. Growth hormone: roles in male reproduction. Endocrine. 2000;13:243–250. doi: 10.1385/ENDO:13:3:243. [DOI] [PubMed] [Google Scholar]
- 132.Hull KL, Harvey S. Growth hormone: a reproductive endocrine-paracrine regulator? Rev Reprod. 2000;5:175–182. doi: 10.1530/ror.0.0050175. [DOI] [PubMed] [Google Scholar]
- 133.Park P, Cohen P. Insulin-like growth factor I (IGF-I) measurements in growth hormone (GH) therapy of idiopathic short stature (ISS) Growth Horm IGF Res. 2005;15(Suppl A):S13–20. doi: 10.1016/j.ghir.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 134.Tanaka H. Growth hormone and bone diseases. Endocr J. 1998;45(Suppl):S47–52. doi: 10.1507/endocrj.45.suppl_s47. [DOI] [PubMed] [Google Scholar]
- 135.Thomas MJ. The molecular basis of growth hormone action. Growth Horm IGF Res. 1998;8:3–11. doi: 10.1016/s1096-6374(98)80316-x. [DOI] [PubMed] [Google Scholar]
- 136.Aubin JE. Advances in the osteoblast lineage. Biochem Cell Biol. 1998;76:899–910. [PubMed] [Google Scholar]
- 137.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 138.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 139.Celil AB, Campbell PG. BMP-2 and insulin-like growth factor-I mediate Osterix (Osx) expression in human mesenchymal stem cells via the MAPK and protein kinase D signaling pathways. J Biol Chem. 2005;280:31353–31359. doi: 10.1074/jbc.M503845200. [DOI] [PubMed] [Google Scholar]
- 140.Chang SF, Chang TK, Peng HH, Yeh YT, Lee DY, Yeh CR, Zhou J, Cheng CK, Chang CA, Chiu JJ. BMP-4 induction of arrest and differentiation of osteoblast-like cells via p21 CIP1 and p27 KIP1 regulation. Mol Endocrinol. 2009;23:1827–1838. doi: 10.1210/me.2009-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, Roman-Roman S. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. 2001;28:491–498. doi: 10.1016/s8756-3282(01)00415-x. [DOI] [PubMed] [Google Scholar]
- 142.Noth U, Tuli R, Seghatoleslami R, Howard M, Shah A, Hall DJ, Hickok NJ, Tuan RS. Activation of p38 and Smads mediates BMP-2 effects on human trabecular bone-derived osteoblasts. Exp Cell Res. 2003;291:201–211. doi: 10.1016/s0014-4827(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 143.Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 145.Verheyen EM. Opposing effects of Wnt and MAPK on BMP/Smad signal duration. Dev Cell. 2007;13:755–756. doi: 10.1016/j.devcel.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 146.Heffron DS, Mandell JW. Opposing roles of ERK and p38 MAP kinases in FGF2-induced astroglial process extension. Mol Cell Neurosci. 2005;28:779–790. doi: 10.1016/j.mcn.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 147.Payne KA, Meszaros LB, Phillippi JA, Huard J. Effect of phosphatidyl inositol 3-kinase, extracellular signal-regulated kinases 1/2, and p38 mitogen-activated protein kinase inhibition on osteogenic differentiation of muscle-derived stem cells. Tissue Eng Part A. 2010;16:3647–3655. doi: 10.1089/ten.tea.2009.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shields JM, Mehta H, Pruitt K, Der CJ. Opposing roles of the extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades in Ras-mediated downregulation of tropomyosin. Mol Cell Biol. 2002;22:2304–2317. doi: 10.1128/MCB.22.7.2304-2317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wan C, Shao J, Gilbert SR, Riddle RC, Long F, Johnson RS, Schipani E, Clemens TL. Role of HIF-1alpha in skeletal development. Ann N Y Acad Sci. 2010;1192:322–326. doi: 10.1111/j.1749-6632.2009.05238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hu N, Jiang D, Huang E, Liu X, Li R, Liang X, Kim SH, Chen X, Gao JL, Zhang H, Zhang W, Kong YH, Zhang J, Wang J, Shui W, Luo X, Liu B, Cui J, Rogers MR, Shen J, Zhao C, Wang N, Wu N, Luu HH, Haydon RC, He TC, Huang W. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. J Cell Sci. 2012 doi: 10.1242/jcs.114231. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tezuka K, Yasuda M, Watanabe N, Morimura N, Kuroda K, Miyatani S, Hozumi N. Stimulation of osteoblastic cell differentiation by Notch. J Bone Miner Res. 2002;17:231–239. doi: 10.1359/jbmr.2002.17.2.231. [DOI] [PubMed] [Google Scholar]
- 154.Nobta M, Tsukazaki T, Shibata Y, Xin C, Moriishi T, Sakano S, Shindo H, Yamaguchi A. Critical regulation of bone morphogenetic protein-induced osteoblastic differentiation by Delta1/Jagged1-activated Notch1 signaling. J Biol Chem. 2005;280:15842–15848. doi: 10.1074/jbc.M412891200. [DOI] [PubMed] [Google Scholar]
- 155.de Jong DS, Steegenga WT, Hendriks JM, van Zoelen EJ, Olijve W, Dechering KJ. Regulation of Notch signaling genes during BMP2-induced differentiation of osteoblast precursor cells. Biochem Biophys Res Commun. 2004;320:100–107. doi: 10.1016/j.bbrc.2004.05.150. [DOI] [PubMed] [Google Scholar]
- 156.Giaginis C, Tsantili-Kakoulidou A, Theocharis S. Peroxisome proliferator-activated receptors (PPARs) in the control of bone metabolism. Fundam Clin Pharmacol. 2007;21:231–244. doi: 10.1111/j.1472-8206.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 157.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66:236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 159.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 160.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 161.Chen L, Jiang W, Huang J, He BC, Zuo GW, Zhang W, Luo Q, Shi Q, Zhang BQ, Wagner ER, Luo J, Tang M, Wietholt C, Luo X, Bi Y, Su Y, Liu B, Kim SH, He CJ, Hu Y, Shen J, Rastegar F, Huang E, Gao Y, Gao JL, Zhou JZ, Reid RR, Luu HH, Haydon RC, He TC, Deng ZL. Insulin-like growth factor 2 (IGF-2) potentiates BMP9-induced osteogenic differentiation and bone formation. J Bone Miner Res. 2010;25:2447–2459. doi: 10.1002/jbmr.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang W, Deng ZL, Chen L, Zuo GW, Luo Q, Shi Q, Zhang BQ, Wagner ER, Rastegar F, Kim SH, Jiang W, Shen J, Huang E, Gao Y, Gao JL, Zhou JZ, Luo J, Huang J, Luo X, Bi Y, Su Y, Yang K, Liu H, Luu HH, Haydon RC, He TC, He BC. Retinoic acids potentiate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PLoS One. 2010;5:e11917. doi: 10.1371/journal.pone.0011917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Randhawa R, Cohen P. The role of the insulin-like growth factor system in prenatal growth. Mol Genet Metab. 2005;86:84–90. doi: 10.1016/j.ymgme.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 164.O’Dell SD, Day IN. Insulin-like growth factor II (IGF-II) Int J Biochem Cell Biol. 1998;30:767–771. doi: 10.1016/s1357-2725(98)00048-x. [DOI] [PubMed] [Google Scholar]
- 165.Ducy P, Starbuck M, Priemel M, Shen J, Pinero G, Geoffroy V, Amling M, Karsenty G. A Cbfa1-dependen t genetic pathway controls bone formation beyond embryonic development. Genes Dev. 1999;13:1025–1036. doi: 10.1101/gad.13.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 167.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 168.Castonguay R, Werner ED, Matthews RG, Presman E, Mulivor AW, Solban N, Sako D, Pearsall RS, Underwood KW, Seehra J, Kumar R, Grinberg AV. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J Biol Chem. 2011;286:30034–30046. doi: 10.1074/jbc.M111.260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li B, Yang Y, Jiang S, Ni B, Chen K, Jiang L. Adenovirus-mediated overexpression of BMP-9 inhibits human osteosarcoma cell growth and migration through downregulation of the PI3K/AKT pathway. Int J Oncol. 2012;41:1809–1819. doi: 10.3892/ijo.2012.1617. [DOI] [PubMed] [Google Scholar]
- 170.Ye L, Kynaston H, Jiang WG. Bone morphogenetic protein-9 induces apoptosis in prostate cancer cells, the role of prostate apoptosis response-4. Mol Cancer Res. 2008;6:1594–1606. doi: 10.1158/1541-7786.MCR-08-0171. [DOI] [PubMed] [Google Scholar]
- 171.van Meeteren LA, Thorikay M, Bergqvist S, Pardali E, Stampino CG, Hu-Lowe D, Goumans MJ, ten Dijke P. Anti-human activin receptor-like kinase 1 (ALK1) antibody attenuates bone morphogenetic protein 9 (BMP9)-induced ALK1 signaling and interferes with endothelial cell sprouting. J Biol Chem. 2012;287:18551–18561. doi: 10.1074/jbc.M111.338103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, Lee SJ, Bidart M, Feige JJ, Bailly S. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119:6162–6171. doi: 10.1182/blood-2012-01-407593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Suzuki Y, Ohga N, Morishita Y, Hida K, Miyazono K, Watabe T. BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J Cell Sci. 2010;123:1684–1692. doi: 10.1242/jcs.061556. [DOI] [PubMed] [Google Scholar]
- 174.David L, Mallet C, Keramidas M, Lamande N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor. Circ Res. 2008;102:914–922. doi: 10.1161/CIRCRESAHA.107.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Park JE, Shao D, Upton PD, Desouza P, Adcock IM, Davies RJ, Morrell NW, Griffiths MJ, Wort SJ. BMP-9 induced endothelial cell tubule formation and inhibition of migration involves Smad1 driven endothelin-1 production. PLoS One. 2012;7:e30075. doi: 10.1371/journal.pone.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yao Y, Jumabay M, Ly A, Radparvar M, Wang AH, Abdmaulen R, Bostrom KI. Crossveinless 2 regulates bone morphogenetic protein 9 in human and mouse vascular endothelium. Blood. 2012;119:5037–5047. doi: 10.1182/blood-2011-10-385906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cunha SI, Pardali E, Thorikay M, Anderberg C, Hawinkels L, Goumans MJ, Seehra J, Heldin CH, ten Dijke P, Pietras K. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. J Exp Med. 2010;207:85–100. doi: 10.1084/jem.20091309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cunha SI, Pietras K. ALK1 as an emerging target for antiangiogenic therapy of cancer. Blood. 2011;117:6999–7006. doi: 10.1182/blood-2011-01-330142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lopez-Coviella I, Follettie MT, Mellott TJ, Kovacheva VP, Slack BE, Diesl V, Berse B, Thies RS, Blusztajn JK. Bone morphogenetic protein 9 induces the transcriptome of basal forebrain cholinergic neurons. Proc Natl Acad Sci U S A. 2005;102:6984–6989. doi: 10.1073/pnas.0502097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lopez-Coviella I, Mellott TJ, Schnitzler AC, Blusztajn JK. BMP9 protects septal neurons from axotomy-evoked loss of cholinergic phenotype. PLoS One. 2011;6:e21166. doi: 10.1371/journal.pone.0021166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schnitzler AC, Lopez-Coviella I, Blusztajn JK. Differential modulation of nerve growth factor receptor (p75) and cholinergic gene expression in purified p75-expressing and non-expressing basal forebrain neurons by BMP9. Brain Res. 2008;1246:19–28. doi: 10.1016/j.brainres.2008.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schnitzler AC, Mellott TJ, Lopez-Coviella I, Tallini YN, Kotlikoff MI, Follettie MT, Blusztajn JK. BMP9 (bone morphogenetic protein 9) induces NGF as an autocrine/paracrine cholinergic trophic factor in developing basal forebrain neurons. J Neurosci. 2010;30:8221–8228. doi: 10.1523/JNEUROSCI.5611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Caperuto LC, Anhe GF, Cambiaghi TD, Akamine EH, do Carmo Buonfiglio D, Cipolla-Neto J, Curi R, Bordin S. Modulation of bone morphogenetic protein-9 expression and processing by insulin, glucose, and glucocorticoids: possible candidate for hepatic insulin-sensitizing substance. Endocrinology. 2008;149:6326–6335. doi: 10.1210/en.2008-0655. [DOI] [PubMed] [Google Scholar]
- 184.Sosa I, Cvijanovic O, Celic T, Cuculic D, Crncevic-Orlic Z, Vukelic L, Zoricic Cvek S, Dudaric L, Bosnar A, Bobinac D. Hepatoregenerative role of bone morphogenetic protein-9. Med Sci Monit. 2011;17:HY33–35. doi: 10.12659/MSM.882108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Blunk T, Sieminski AL, Appel B, Croft C, Courter DL, Chieh JJ, Goepferich A, Khurana JS, Gooch KJ. Bone morphogenetic protein 9: a potent modulator of cartilage development in vitro. Growth Factors. 2003;21:71–77. doi: 10.1080/0897719031000148822. [DOI] [PubMed] [Google Scholar]
- 186.Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189:275–284. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]


