Abstract
Neuromuscular diseases affect skeletal muscle and/or nervous control resulting in direct disruption of skeletal muscle and muscle pathology, or nervous system disruption which indirectly disrupts muscle function. Stem cell-based therapy is well-recognized as a promising approach for several types of diseases including those affecting the neuromuscular system. To design a successful therapeutic strategy, it is important to choose the most appropriate stem cell type. Skeletal muscle progenitor cells (SMPCs), also called myogenic progenitors, can contribute to muscle regeneration, differentiate into skeletal muscles, and are valuable cells for therapeutic application. Different types of stem/progenitor cells, including satellite cells, side population cells, muscle derived stem cells, mesenchymal stem cells, myogenic pericytes, and mesoangioblasts, have been identified as possible cell resources of SMPCs. Furthermore, recent advances in stem cell biology allow us to use embryonic stem cells and induced pluripotent stem cells for SMPC derivation. When skeletal muscle is chosen as a target of cell transplantation, the possible criteria for choosing the “best” progenitor/stem cell include preparation strategies, efficiency of intramuscular integration, method of cellular delivery, and functional improvement of the muscle after cell transplantation. Here, we discuss recent findings on various types of SMPCs and their promise for future clinical translation in neuromuscular diseases.
Keywords: Neuromuscular diseases, cell-based therapy, skeletal muscle progenitor cells (SMPCs), pluripotent stem cells (PSCs), transplantation
Introduction
Stem cells have received much attention in recent years because of their potential use in cell-based therapies designed to treat human diseases with no cure. In mammals, stem cells are broadly categorized into two types: pluripotent stem cells (PSCs), which include embryonic stem cells (ESCs) as well as induced pluripotent stem cells (iPSCs), and tissue-specific stem/progenitor cells. PSCs possess the potential to differentiate into virtually any specialized cell. Tissue-specific stem/progenitor cells, also called adult stem cells, intrinsically reside in various tissues of the body and can maintain, generate, and replace terminally differentiated cells within their specific tissue. While tissue-specific stem/progenitor cells normally contribute to growth and repair of resident tissue following differentiation, it has become evident that these cells can also differentiate into other cell types [1,2]. Numerous studies have demonstrated effective protocols to differentiate stem cells into various cell lineages, and have functionally examined the differentiated cells in animal models of human diseases or injury [3-6].
For neuromuscular diseases such as muscular dystrophy, stem cell-based therapy targeting degenerating muscles is a promising approach to recover skeletal muscle function either directly, by relieving intrinsic pathology, or indirectly, by relieving nerve pathology. Skeletal muscle stem/progenitor cells (SMPCs) are one of the most valuable cell types for this approach. As myogenic progenitors, SMPCs can contribute to muscle regeneration and differentiate into skeletal muscle. Various SMPCs have been isolated from pre- or post-natal muscles as well as non-muscle somatic tissues, and, recently, PSCs have been used to propagate SMPCs using distinct protocols for muscle differentiation. Among these cells, satellite cells, which are present in postnatal muscles and contribute to muscle growth and regeneration, can be defined as resident SMPCs. In contrast, tissue-specific stem/progenitor cells or pluripotent stem cells-derived myogenic progenitors can be described as de novo SMPCs.
In this review, we compare different types of stem/progenitor cells which show potential benefits for neuromuscular diseases. We also discuss the potential use of these cells as resources for SMPCs and their therapeutic applications in neuromuscular diseases.
Variations of cellular resources for SMPC preparation
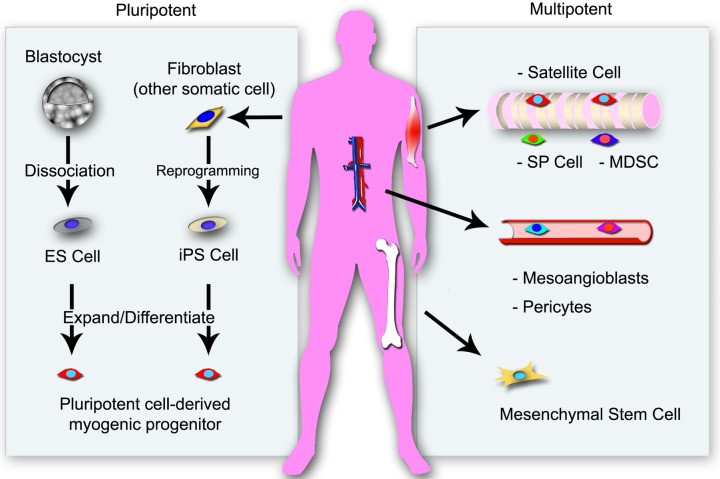
To date, different types of SMPCs have been isolated from various sources including adult tissues and pluripotent stem cells (Figure 1). When designing a cell-based therapy, the choice of cell type depends on the pathological condition to be treated and the environment within the target tissue. The survival of the transplanted cells depends on the tissue-environment into which they are transplanted. For example, fully-differentiated myoblasts from adult skeletal muscle have a low survival rate in dystrophic muscle when compared to undifferentiated stem/progenitor cells [7,8]. This suggests that exogenous fully-differentiated cells like myoblasts cannot adjust well to the dystrophic muscle environment. Therefore, undifferentiated SMPCs might be a better tool for intramuscular delivery than other types of stem/progenitor cells in the case of dystrophic skeletal muscle.
Figure 1.
Various resources for skeletal muscle stem/progenitor cells. Satellite cells are located beneath the basement membrane of skeletal muscle fibers and are naturally committed to differentiation into cells of the muscle lineage. Mesenchymal stem cells (MSCs) and pluripotent stem cells (PSCs) can differentiate into muscle progenitor cells and can be programmed ex vivo to activate their muscle differentiation capacity. Muscle side population (SP) cells and muscle-derived stem cells (MDSCs) localize to an interstitial site between muscle fibers. Mesoangioblasts and pericytes, are associated with blood vessel wall. These non-muscle cells can contribute to muscle regeneration without genetic modifications or chemicals treatment for muscle cell lineage differentiation.
Here we propose four requirements defining the appropriateness of a stem cell pool for the preparation of SMPCs for therapeutic application: (a) easy isolation from accessible tissue sources, (b) differentiation capacity for muscle cell lineages including SMPCs with or without genetic modification, (c) ability to be transplanted into muscle, and (d) possibility for systemic delivery including the ability to reach and integrate into the target site in pathological muscles. Each of the following stem cell pools have been shown to fulfill some or all of these requirements and are therefore potentially useful for therapeutic application against neuromuscular degenerative diseases.
Satellite cells
In postnatal and adult skeletal muscles, regenerative capacity is primarily based on the presence of satellite cells. These cells are localized under the basement membrane of muscle fibers [9]. In adult muscles, satellite cells are mitotically quiescent; however, they are activated in response to stress induced by weight bearing or by trauma such as muscle injury [10,11]. The descendants of activated satellite cells, called myogenic precursor cells or myoblasts, undergo multiple rounds of division, known to be controlled by hepatocyte growth factor (HGF), before fusion and terminal differentiation [10,12]. Satellite cells are biologically, biochemically, and genetically distinct from their daughter myoblasts [13]. Activated satellite cells also generate progeny to restore a pool of quiescent satellite cells [10,14]. These cells symmetrically or asymmetrically divide into myoblasts and daughter satellite cells. Symmetric satellite cell/satellite cell and asymmetric satellite cell/myoblast divisions are regulated by cell polarity and contact with the muscle fiber membrane. Apical-basal and planar cell divisions result in asymmetric and symmetric self-renewal, respectively [14,15]. Both quiescent and activated satellite cells express Pax7, which can serve as a priming factor for satellite cell myogenesis [16]. Myf5 is only expressed in activated satellite cells [17]. The cell origin of mammalian satellite cells is the Pax3/Pax7-expressing cell population that arises when the myotome (the first skeletal muscle compartment in the somite) is formed from the central dermomyotome (the epithelium of somite) [18,19]. Satellite cells can be isolated in culture from postnatal and adult muscles while maintaining their myogenic potential. Isolated satellite cells are naturally committed to become myoblasts with the expression of a muscle determinant factor MyoD [20] and then terminally differentiate into multinucleated myotubes in vitro.
Applications of satellite cells and myoblasts for cell-based therapy in neuromuscular diseases have been investigated. When isolated satellite cells are transplanted into degenerating or pathological muscle, the grafted cells can contribute to muscle fiber reconstruction. Several methods have been developed to prepare satellite cells for muscle transplantation in rodent studies [7,21,22]. These methods are mainly based on the fluorescence activated cell sorting (FACS) isolation using satellite cell-specific surface proteins allowing isolation of satellite cells from adult muscle biopsies. Satellite cells are known to express cell surface proteins such as M-cadherin, Syndecan-4 and C-X-C chemokine receptor type 4 (CXCR4) [21-24]. These protein markers have been used for FACS [25,26]. In another study, satellite cells could be isolated from the diaphragm of transgenic mice over-expressing green fluorescence protein (GFP)-tagged Pax3 (Pax3GFP/+) by FACS [19]. The integration of purified satellite cells into the dystrophic muscles in a mouse model of muscular dystrophy (mdx mice) and their subsequent differentiation resulted in significant increase in dystrophin-expressing muscle fibers and contractile function [21,27]. However, only a small percentage of transplanted highly-purified satellite cells (3-5%) have been observed to re-locate into their comfortable cellular place between basement and plasma membrane to prepare for future myotraumas such as muscle injury [7,21,27].
While promising results have been demonstrated using satellite cells in animal studies, therapeutic applications of satellite cells are still challenging in human patients. According to a previous observation in aged mice [28], the number of satellite cells may be low in the skeletal muscles of aged humans. As muscle biopsy is the first choice to isolate human satellite cells, satellite cells isolated from biopsied tissues would be few and not enough for cell sorting. To resolve this disadvantage, a new culture technique would be necessary to expand a small number of human satellite cells to a large scale and maintain them in an immature state. For example, a hydrogel microenvironment may be useful for clonal cell expansion and maintaining the undifferentiated status of isolated satellite cells [27,29]. Hydrogel substrate covered in a microwell mimics the elasticity of skeletal muscle and induces cell division without differentiation of mouse satellite cells [29]. Alternatively, a free-floating spherical culture, also known as myosphere culture, might also prove to be a powerful method to maintain and expand satellite cells from biopsied human skeletal muscle [30]. In myosphere culture, cell/cell contact, which is important for satellite cells, is continuously maintained, allowing them to comfortably expand in the spheres.
Satellite cell or myoblast transplantation is a possible treatment for neuromuscular diseases such as muscular dystrophy. In most cases of neuromuscular disease, whole body muscle atrophy and muscle degeneration is observed. Therefore, a systemic injection of stem/progenitor cells into the blood stream would be the most attractive method for cell delivery. However, it has been reported that satellite cells delivered systemically are trapped along the walls of blood vessels [31] and cannot cross the vessel wall and migrate into the skeletal muscle [32]. Despite this complication, satellite cells and myoblasts are still one of the most promising cell types for neuromuscular diseases.
Side Population (SP) cells
Side population (SP) cells are another type of myogenic cells in postnatal and adult skeletal muscles. Muscle SP cells have been isolated from mouse skeletal muscle using a similar method to that used for the purification of bone marrow SP cells [33,34]. Muscle SP cells are different from bone marrow SP cells with respect to expression of surface protein markers. When isolated using high Hoechst dye concentration, the majority of mouse muscle SP cells are positive for stem cell antigen-1 (Sca-1) and negative for the hematopoietic SP markers CD45, CD43, and c-kit [35,36].
Mouse muscle SP cells are able to survive and integrate into the skeletal muscle of mice with muscular dystrophy following transplantation. Interestingly, these SP cells could be integrated into the satellite cell position following transplantation [1,35]. However, intramuscular integration of muscle SP cells occurs with very low frequency [37]. To accelerate therapeutic applications of muscle SP cells, more studies are required to merge knowledge on how to better propagate and manipulate these cells.
Muscle Derived Stem Cells (MDSCs)
A population of early myogenic progenitors, also called muscle derived stem cells (MDSCs), has been isolated from mouse skeletal muscle based on their adhesion characteristics to collagen-coated flasks [38,39]. These cells can be purified using a modified pre-plating technique from postnatal mouse skeletal muscles. Muscle biopsy samples are mechanically disrupted and then digested by a series of enzymes. The cells are plated and separated into slowly adhering (presumably enriched MDSCs) or rapidly adhering cell (fibroblasts and myoblasts) fractions. MDSCs are a unique cell population whose characteristics are distinct from satellite cells and myoblasts [39-41]. Satellite cells typically express Pax7, whereas MDSCs are more heterogeneous but express Sca-1 consistently and often express CD34. Both in vitro and in vivo studies demonstrated that MDSCs can self-renew and differentiate into multiple lineages, and have the potential to regenerate various adult tissues [41-43]. Strikingly, MDSCs display a superior regenerative capacity relative to satellite cells following transplantation into dystrophic muscle in mdx mice [39,40]. MDSCs are at least partially immuno-privileged, as the transplantation of MDSCs results in robust dystrophin expression in mdx mice over 3 months after injection [39]. Compared to satellite cells, MDSCs may be able to overcome some of the challenges associated with transplantation such as immunorejection, poor cell survival, and the limited distribution of the transplanted cells.
Human MDSCs can be isolated from muscle biopsies of human adults by a modified preplating technique and unfractionated enzymatic digestion [44] which can accelerate their possible application for cell-based therapy in patients. However, further studies are still required to determine whether these cells are applicable for clinical translation.
Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells (MSCs) are also considered as an alternative candidate for cell-based therapy targeting degenerating or dystrophic muscles. MSCs are found in bone marrow and other mesenchymal tissues. Interestingly, similar cells were found in the perivasucular region of blood vessels [45]. These cells are easy to harvest and can be expanded in vitro to clinically relevant numbers while retaining normal karyotype and differentiation capacity [46]. Additionally, MSCs are distinct from hematopoietic stem cells and able to differentiate into various tissues such as bone, cartilage, and fat.
Several studies have demonstrated that MSCs can also differentiate into SMPCs in vitro and that these differentiated cells can integrate into the muscle [46-49]. Naïve MSCs are not able to differentiate into muscle cell lineages under normal culture conditions; however, Wakitani et al., using rat MSCs, showed that these cells can differentiate into muscle cell lineages when treated with 5’-Azacytidine, a chemical analogue of the cytosine nucleoside used in DNA and RNA [49]. Another study successfully demonstrated that activation of Notch signaling pathway using overexpression of Notch-intracellular domain (NICD) can promote skeletal muscle differentiation in both human and rat MSCs [47]. Human MSCs-derived SMPCs prepared by overexpression of Pax3 and then implanted did not attenuate dystrophic symptoms in mdx mice although the cells did integrate into muscles [48]. Failure of functional recovery might have been caused by lower muscle integration efficiency of Pax3- dependent SMPCs than Notch-dependent SMPCs (about 10% vs. about 15%~50%). Thus, detailed evaluation including functional analysis is needed for clinical application of MSCsderived SMPCs. On the other hand, murine MSCs-derived SMPCs, generated by overexpression of Pax3, failed to induce functional recovery of dystrophic muscle following intramuscular injection due to unknown mechanisms despite their local muscle integration [48].
Recent pre-clinical and clinical studies demonstrated a number of potential therapeutic applications for human MSCs targeting various diseases such as myocardial infarction, stroke, and graft-versus-host disease [50]. These cells are an attractive resource for SMPC preparation because many clinical-grade MSC lines are already currently available; however, it is still necessary to evaluate whether MSCs and MSC-derived SMPCs can promote functional benefits in animal models of neuromuscular diseases.
Mesoangioblasts
Mesoangioblasts are a type of mesodermal stem cell first identified in the wall of the mouse embryonic aorta [51]. These cells were able to proliferate on a feeder layer as a single clone and differentiate into various types of solid mesoderm [52]. Mesoangioblasts are known to have an unlimited life span and express angiogenic cell markers such as CD34, Sca-1, and Fetal Liver Kinase 1 (Flk-1) [51]. Cells resembling mesoangioblasts were also isolated from vessels of postnatal tissues in mouse, dog, and human [53]. These postnatal mesoangioblasts are similar to their embryonic counterparts in terms of proliferation and differentiation potency; however, some lines of mesoangioblasts, in canine and human for example, proliferate to a limited extent and undergo senescence.
Therapeutic benefits of wild-type or genetically-modified mesoangioblasts have been tested in animal models with muscular dystrophy. Intraarterial delivery of wild-type or genetically corrected mesoangioblasts was tested targeting dystrophic muscle of α-sarcoglycan-null mice and showed a significant functional amelioration of the dystrophic phenotype [54]. In a canine model of Duchenne muscular dystrophy, intra-arterial delivery of wild-type canine mesoangioblasts resulted in an extensive recovery of dystrophin expression, normal muscle morphology and a remarkable clinical amelioration of active motility [54]. When mesoangioblasts are autologously delivered into the blood circulation, the injected cells can migrate outside the vessel and integrate into dystrophic muscles [31]. In addition, dramatic improvement of dystrophic symptoms in mdx mice by transplantation of mesoangioblasts expressing full-length of human dystrophin supports the validity of these cells for clinical applications. Taken together, mesoangioblasts possess a strong ability to differentiate into muscle and are useful for systemic delivery. Both of these traits strongly support the possible application of mesangioblasts for treating patients with neuromuscular diseases.
Pericytes
While mesoangioblasts are isolated from fetal aorta, similar cells are present in the postnatal micro-vasculature. These cells were originally identified as periendothelial cells in small blood vessels and called pericytes, which are defined by their location, morphology and molecular markers [55]. Pericytes expressing alkaline phosphatase can differentiate into muscle cell lineages when cells are transplanted into skeletal muscle. The grafted cells are recognized as mural cells embedded within the vascular basement membrane [56]. Also, recently, endogenous pericytes were observed to integrate into skeletal muscle in mice [57]. Human pericytes can clonally be isolated from micro-vasculatures in adult skeletal muscles and used for intravenous injection. Interestingly, human muscle-derived pericytes can locate into the satellite cell position with Pax7 expression after intra-arterial injection into immuno-deficient mdx (scid-mdx) mice [56]. This suggests that human muscle-derived pericytes can convert to SMPCs in skeletal muscles without ex vivo trans-differentiation induction. Indeed, pericytes express Myf5, which is a priming factor of muscle differentiation [56]. However, pericytes are not available for systemic delivery, resulting in limitation of this cell type on therapeutic applications [56].
Embryonic Stem Cells (ESCs)
Embryonic stem cells (ESCs) are a powerful cellular resource for SMPC preparation [58]. ESCs are derived from the inner cell mass of the blastocyst, an early-stage embryo and are able to differentiate into all derivatives of the three primary germ layers: ectoderm, endoderm, and mesoderm [59-62]. Mouse ESCs can spontaneously differentiate into myoblasts through embryoid body (EB) formation [58]. Currently, three major protocols are used to prepare and concentrate myogenic precursors from mouse or human ESCs: 1) direct isolation of myogenic cells from EBs using FACS and specific cell surface markers [63,64], 2) ectopic expression of myogenic genes and isolation of muscle cell lineages using FACS [65], and 3) specific induction of muscle differentiation with defined chemicals such as retinoic acid [66]. Human ESC-derived myoblasts can be transplanted and successfully integrated in the hind limb muscle of NOD-scid mice which show immunedeficiency due to a lack of both T and B cells [63]. Differentiated SMPCs from mouse EB culture were concentrated using FACS with an antibody against a specific antigen of quiescent mouse satellite cells (SM/C-2.6) [67,68]. SM/C-2.6-positive cells resemble satellite cells and maintain self-renewability after intramuscular transplantation in mdx mice [64]. Another group used a doxycycline-inducible system in which they overexpressed Pax3 in mouse ESCs. The Pax3 gene was integrated into an inducible locus on the X chromosome of ESCs and its expression could be induced by doxycycline (iPax3). After expanding the cells in culture, Pax3 expression was induced in the EBs and the SMPC population was sorted by presence of platelet derived growth factor receptor alpha (PDGFR-α) and absence of Flk-1 [65]. Mdx mice that received the iPax3 -derived SMPCs either systemically or locally regained dystrophin expression in himdlimb muscles (tibialis anterior muscles, TA) and exhibited functional recovery of muscle force [65]. Together, these findings support the idea that ESCs are a powerful resource for SMPCs generation and for subsequent use for treatment of neuromuscular diseases.
On the other hand, several concerns still remain surround human ES cells. The use of ESCs should be considered during development of stem cell-based therapies. Firstly, the use of human embryos for research using ESCs is a hot topic currently and is high on the ethical and political agenda in many countries. Despite the potentially strong benefit of using human ESCs for the treatment of neuromuscular diseases, their use remains controversial because of their derivation from early embryos. Secondly, when transplantating ESCs into patients as part of a therapy, it should be noted that ESCs possess an ability to form tumors including teratomas [69]. To enhance the safety of ESCs for potential clinical use, development of effective procedures would be necessary to reduce or eliminate ability to cause tumors when transplanted the differentiated cells from ESCs.
Induced Pluripotent Stem Cells (iPSCs)
In 2006, it was first reported that over-expression of four different genes, Oct4, Sox2, c-Myc and Klf4, can induce dedifferentiation of mouse skin-derived fibroblasts to ESC-like reprogrammed cells, now referred to as induced-pluripotent stem cells (iPSCs) [70]. One year later, human iPSCs were generated from human primary fibroblasts using similar protocols [71,72]. Like ESCs, iPSCs, have the potential to differentiate into any cell lineage. Evidence also exists that iPSCs favor differentiation into the lineage from which they were derived due to residual epigenetic modifications [73,74].
SMPCs can be generated from both mouse and human iPSCs, and these iPSCs-derived SMPCs represent a promising therapeutic tool for neuromuscular diseases. Mouse iPSCs can spontaneously differentiate into SMPCs through EB formation. This progenitor population can be enriched using the FACS targeting the SM/C-2.6 antigen [67]. When mouse iPSC-derived SMPCs were transplanted into the TA muscle of mdx mice, they integrated and increased dystrophin expression in the grafted muscle for over 24 weeks. These data suggest that injected SMPCs continuously contribute to muscle regeneration once localized to the satellite cell position [67]. Since SM/C-2.6 antigen is a specific marker for mouse satellite cells, humanspecific antigens will be needed in order to identify and enrich human SMPCs [68]. Recently, it was demonstrated that the conditional expression of PAX7 using doxycycline derives greater numbers of SMPCs from human iPSCs [75]. Human iPSC-derived SMPCs have been observed to integrate well into the TA muscle of mdx mice and localized to the satellite cell compartment [75]. A future challenge using human iPSCs will be to derive SMPCs without genetic modification.
As iPSCs can be prepared using the somatic cells of a patient, the SMPCs differentiated from patient-specific iPSCs are able to survive in the skeletal muscles of the same patient without immunosuppressive treatments. This huge benefit will accelerate further developments of effective protocols for SMPC preparation from iPSCs and their clinical applications. Additionally, the use of patient-specific iPSCs and SMPCs overcomes both the ethical and immunological concerns intrinsic with use of ESCs. However, like ESCs, possible risks of tumor formation still remain following transplantation.
Summary and conclusion
Stem cell-based therapy targeting skeletal muscle is promising as a therapeutic approach for several types of neuromuscular disease. Much valuable knowledge has been accumulated in preclinical applications of myogenic progenitors for neuromuscular diseases [76,77].
As discussed above, various types of myogenic progenitors have been identified with the potential to contribute to muscle regeneration. Although satellite cells are considered one of the most promising cells for therapeutic applications, insufficient isolation from human biopsied muscle tissues has limited their further application for cell-based therapies. SMPCs can be converted from non-muscle cells such as MSCs using genetic modification. However, human MSC-derived SMPCs failed to promote functional recovery in dystrophic muscles when these cells were transplanted into TA muscle. This suggests that genetic modification approaches are yet immature to convert fatedecided cells to useful SMPCs. On the other hand, recent advances in stem cell biology allow us to apply PSCs, namely ESCs and iPSCs, for SMPC preparation. PSCs appear to be the most powerful resource to prepare SMPCs, because PSC-derived SMPCs can improve dystrophic symptoms and survive for more than 20 weeks in mdx mice [67]. A recent study demonstrated the presence of tumor-forming cells originating from PSCs, which express stagespecific embryonic antigen-5 (SSEA-5) [78]. These tumor forming cells can be removed with monoclonal antibody against to SSEA-5 [78]. Removal of SSEA-5-positive cells would decrease a risk of tumor formation after transplantation of PSCs-derived SMPCs. An efficient protocol, particularly without genetic modification, will need to be established for SMPC derivation from human PSCs.
Another concern around SMPC application is how to deliver the progenitor cells to the damaged muscle in patients. To evaluate the ability and efficiency of myogenic progenitors to integrate into muscle, isolated cells are systemically or intramuscularly transplanted in animal models of neuromuscular diseases. Systemic delivery would be ideal for clinical applications, because it is a non-invasive procedure which would cause less stress to patients. Highly purified satellite cells can integrate into almost all muscle fibers (over 90% of muscle fibers can be integrated) and re-locate to the satellite cell position of muscle fibers when cells are transplanted into injured muscle [7,21,27]. However, satellite cells cannot pass through blood vessel wall following intravascular injection, indicating that these cells are not suitable for systemic delivery [31]. By contrast, some non-muscle progenitors, such as mesangioblasts and MSCs, can be used for systemic delivery [37,56]. Preliminary clinical trials using systemic delivery of mesoangioblasts to treat muscular dystrophy have been ongoing in Italy since 2009. Most recently phase II-III clinical trials were engaged in 2011 [79]. Interestingly, one report exists showing that mouse ESC-derived SMPCs can be systemically delivered into injured mouse skeletal muscle through intravenous and intra-arterial injection, suggesting the PSC-derived SMPCs may be applicable for systemic delivery in future clinical trials [65]. It should carefully be determined whether non-muscle cells can result in better intramuscular integration and functional improvements in pathological conditions than SMPCs.
In conclusion, stem cell-based therapies, particularly those targeting skeletal muscle, represent a challenging but promising attack to defeat many neuromuscular diseases. Selection of stem/progenitor cells is very important for this therapeutic approach. Currently, SMPCs are a better cell type for muscle transplantation than non-muscle cells because of their efficiency of muscle integration. For generating SMPCs, PSC-derived SMPCs appear to be a more effective cell type than SMPCs derived from adult stem cells isolated from somatic tissue such as satellite cells because of their availability for systemic delivery.
Acknowledgements
This work was supported by grants from the ALS Association and the University of Wisconsin Foundation. This was also supported in part by grant 9U54TR000021 to the UW ICTR from NCATS/NIH, and by funds from the UW Stem Cell and Regenerative Medicine Center Pilot Grant Program.
References
- 1.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. discussion 702-693. [DOI] [PubMed] [Google Scholar]
- 3.Dani C, Smith AG, Dessolin S, Leroy P, Staccini L, Villageois P, Darimont C, Ailhaud G. Differentiation of embryonic stem cells into adipocytes in vitro. J Cell Sci. 1997;110:1279–1285. doi: 10.1242/jcs.110.11.1279. [DOI] [PubMed] [Google Scholar]
- 4.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 5.Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001;12:559–563. doi: 10.1097/00001756-200103050-00025. [DOI] [PubMed] [Google Scholar]
- 6.Peljto M, Wichterle H. Programming embryonic stem cells to neuronal subtypes. Curr Opin Neurobiol. 2011;21:43–51. doi: 10.1016/j.conb.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 8.Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- 9.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bischoff R, Heintz C. Enhancement of skeletal muscle regeneration. Dev Dyn. 1994;201:41–54. doi: 10.1002/aja.1002010105. [DOI] [PubMed] [Google Scholar]
- 11.Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003;35:1151–1156. doi: 10.1016/s1357-2725(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 12.Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- 13.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 14.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Grand F, Jones AE, Seale V, Scime A, Rudnicki MA. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 17.Crist CG, Montarras D, Buckingham M. Muscle Satellite Cells Are Primed for Myogenesis but Maintain Quiescence with Sequestration of Myf5 mRNA Targeted by microRNA-31 in mRNP Granules. Cell Stem Cell. 2012;11:118–126. doi: 10.1016/j.stem.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Gros J, Manceau M, Thome V, Marcelle C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–958. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- 19.Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tapscott SJ, Davis RL, Thayer MJ, Cheng PF, Weintraub H, Lassar AB. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- 21.Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukada S, Uezumi A, Ikemoto M, Masuda S, Segawa M, Tanimura N, Yamamoto H, Miyagoe-Suzuki Y, Takeda S. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells. 2007;25:2448–2459. doi: 10.1634/stemcells.2007-0019. [DOI] [PubMed] [Google Scholar]
- 23.Bornemann A, Schmalbruch H. Immunocytochemistry of M-cadherin in mature and regenerating rat muscle. Anat Rec. 1994;239:119–125. doi: 10.1002/ar.1092390202. [DOI] [PubMed] [Google Scholar]
- 24.Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan- 4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001;239:79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4:217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conboy MJ, Cerletti M, Wagers AJ, Conboy IM. Immuno-analysis and FACS sorting of adult muscle fiber-associated stem/precursor cells. Methods Mol Biol. 2010;621:165–173. doi: 10.1007/978-1-60761-063-2_11. [DOI] [PubMed] [Google Scholar]
- 27.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day K, Shefer G, Shearer A, Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol. 2010;340:330–343. doi: 10.1016/j.ydbio.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell selfrenewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Y, Li Y, Chen C, Stoelzel K, Kaufmann AM, Albers AE. Human skeletal muscle-derived stem cells retain stem cell properties after expansion in myosphere culture. Exp Cell Res. 2011;317:1016–1027. doi: 10.1016/j.yexcr.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, Bottinelli R, Cossu G. Cell therapy of alpha-sarcoglycan null dystrophic mice through intraarterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 32.Cossu G. Towards a cell therapy for muscular dystrophy. In: Hug K, Hermerén G, editors. Translational Stem Cell Research. New York: Humana Press; 2011. pp. 55–63. [Google Scholar]
- 33.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggestthat hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3:1337–1345. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 35.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 36.Motohashi N, Uezumi A, Yada E, Fukada S, Fukushima K, Imaizumi K, Miyagoe-Suzuki Y, Takeda S. Muscle CD31(-) CD45(-) side population cells promote muscle regeneration by stimulating proliferation and migration of myoblasts. Am J Pathol. 2008;173:781–791. doi: 10.2353/ajpath.2008.070902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachrach E, Li S, Perez AL, Schienda J, Liadaki K, Volinski J, Flint A, Chamberlain J, Kunkel LM. Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc Natl Acad Sci U S A. 2004;101:3581–3586. doi: 10.1073/pnas.0400373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, Peault B, Cummins J, Huard J. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501–1509. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 39.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deasy BM, Gharaibeh BM, Pollett JB, Jones MM, Lucas MA, Kanda Y, Huard J. Long-term self-renewal of postnatal muscle-derived stem cells. Mol Biol Cell. 2005;16:3323–3333. doi: 10.1091/mbc.E05-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Usas A, Maciulaitis J, Maciulaitis R, Jakuboniene N, Milasius A, Huard J. Skeletal muscle-derived stem cells: implications for cellmediated therapies. Medicina (Kaunas) 2011;47:469–479. [PubMed] [Google Scholar]
- 42.Cao B, Zheng B, Jankowski RJ, Kimura S, Ikezawa M, Deasy B, Cummins J, Epperly M, Qu-Petersen Z, Huard J. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5:640–646. doi: 10.1038/ncb1008. [DOI] [PubMed] [Google Scholar]
- 43.Deasy BM, Jankowski RJ, Huard J. Muscle-derived stem cells: characterization and potential for cell-mediated therapy. Blood Cells Mol Dis. 2001;27:924–933. doi: 10.1006/bcmd.2001.0463. [DOI] [PubMed] [Google Scholar]
- 44.Vella JB, Thompson SD, Bucsek MJ, Song M, Huard J. Murine and human myogenic cells identified by elevated aldehyde dehydrogenase activity: implications for muscle regeneration and repair. PLoS One. 2011;6:e29226. doi: 10.1371/journal.pone.0029226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 46.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 47.Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 48.Gang EJ, Darabi R, Bosnakovski D, Xu Z, Kamm KE, Kyba M, Perlingeiro RC. Engraftment of mesenchymal stem cells into dystrophin-deficient mice is not accompanied by functional recovery. Exp Cell Res. 2009;315:2624–2636. doi: 10.1016/j.yexcr.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 50.Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, Ponzetto C, Cossu G. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147:869–878. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 53.Tonlorenzi R, Dellavalle A, Schnapp E, Cossu G, Sampaolesi M. Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr Protoc Stem Cell Biol. 2007;Chapter 2:Unit 2B.1. doi: 10.1002/9780470151808.sc02b01s3. [DOI] [PubMed] [Google Scholar]
- 54.Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 55.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 57.Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 58.Rohwedel J, Maltsev V, Bober E, Arnold HH, Hescheler J, Wobus AM. Muscle cell differentiation of embryonic stem cells reflects myogenesis in vivo: developmentally regulated expression of myogenic determination genes and functional expression of ionic currents. Dev Biol. 1994;164:87–101. doi: 10.1006/dbio.1994.1182. [DOI] [PubMed] [Google Scholar]
- 59.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 60.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 62.Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22:1987–1997. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 64.Chang H, Yoshimoto M, Umeda K, Iwasa T, Mizuno Y, Fukada S, Yamamoto H, Motohashi N, Miyagoe-Suzuki Y, Takeda S, Heike T, Nakahata T. Generation of transplantable, functional satellite-like cells from mouse embryonic stem cells. FASEB J. 2009;23:1907–1919. doi: 10.1096/fj.08-123661. [DOI] [PubMed] [Google Scholar]
- 65.Darabi R, Gehlbach K, Bachoo RM, Kamath S, Osawa M, Kamm KE, Kyba M, Perlingeiro RC. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med. 2008;14:134–143. doi: 10.1038/nm1705. [DOI] [PubMed] [Google Scholar]
- 66.Ryan T, Liu J, Chu A, Wang L, Blais A, Skerjanc IS. Retinoic Acid enhances skeletal myogenesis in human embryonic stem cells by expanding the premyogenic progenitor population. Stem Cell Rev. 2012;8:482–493. doi: 10.1007/s12015-011-9284-0. [DOI] [PubMed] [Google Scholar]
- 67.Mizuno Y, Chang H, Umeda K, Niwa A, Iwasa T, Awaya T, Fukada S, Yamamoto H, Yamanaka S, Nakahata T, Heike T. Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells. FASEB J. 2010;24:2245–2253. doi: 10.1096/fj.09-137174. [DOI] [PubMed] [Google Scholar]
- 68.Fukada S, Higuchi S, Segawa M, Koda K, Yamamoto Y, Tsujikawa K, Kohama Y, Uezumi A, Imamura M, Miyagoe-Suzuki Y, Takeda S, Yamamoto H. Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp Cell Res. 2004;296:245–255. doi: 10.1016/j.yexcr.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 69.Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 71.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 72.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 73.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, Natesan S, Wagers AJ, Melnick A, Evans T, Hochedlinger K. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M, Perlingeiro RC. Human ES- and iPS-derived myogenic progenitors restore dystrophin and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10:610–619. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blau HM. Cell therapies for muscular dystrophy. N Engl J Med. 2008;359:1403–1405. doi: 10.1056/NEJMcibr0805708. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki M, McHugh J, Tork C, Shelley B, Hayes A, Bellantuono I, Aebischer P, Svendsen CN. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol Ther. 2008;16:2002–2010. doi: 10.1038/mt.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, Mosley AR, Inlay MA, Ardehali R, Chavez SL, Pera RR, Behr B, Wu JC, Weissman IL, Drukker M. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011;29:829–834. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hug K, Hermerén Gr. Translational stem cell research: issues beyond the debate on moral status of the human embryo. New York: Humana Press; 2011. [Google Scholar]