Abstract
Many factors are possibly involved in the inflammatory process which causes the degeneration of the arterial wall in the formation of Abdominal Aortic Aneurysms. During the last years different experimental models have been published to treat this fault of the arterial walls. Parallel the clinical treatment has evolved. With this work we have tried to develop an animal model basing on the clinical current treatment. Finally, we propose a treatment based on mesenchymal cells to disable local immune response, preventing excessive fibrosis, apoptosis, and inducing intrinsic cellular progenitors. Objective: To present a reproducible superior animal model of experimentation, intending to show that mesenchymal stem cells inserted in the sac of an artificial aneurysm are able to survive, so that they can be made accountable for a subsequent beneficial effect upon this condition. Methods: Six Landrace-White pigs, weighing around 25Kg. We generate 2 aneurysms of abdominal aorta (2x1cm) with Dacron’s patches. Later we treat the aneurysms endoscopic with a covered endograft. Finally, in one of the aneurysmal sac we introduce 1cc fibrin sealant and in another 1 cc of fibrin sealant with 10 million MSC. Animals were sacrificed at 24 hs and 1, 3, 5, 7 and 9 weeks. Samples of aneurysms were processed histologically (H&E and Masson). The injected cells were located by immunofluorescence (GFP market). Results: The surgical technique is reproducible and similar to those conducted in common clinical practice. Histological cross-section samples of cases treated with MSC and analyzed by a blind researcher present a lower inflammation reaction, or with longer evolution time than in controls. Immunofluorescence studies have detected cells marked with GFP up to three weeks after treatment. Conclusion: This reproducible animal model is similar to common clinical treatment. MSC can stand alive at least for three weeks since their implantation within an aneurysm sac. This may improve the results of conventional endovascular treatment by the stabilization of the aneurysmal sac.
Keywords: Aortic aneurysm, experimental model, adipose derived stem cells
Introduction
Arterial conditions share one common factor, which is the inflammatory response that appears in its walls as a response to different noxae. This response can translate into the accumulation of substances and degradation products in the sub-endothelial region (obstructive condition, intimal hyperplasia) or into the degradation and weakening of the dilating wall (aneurysms). The initiating or regulatory mechanisms for this process are not exactly known; however, it is known that progenitor cells play a major role in the repair and “scarring” processes of those lesions induced on the arterial wall.
The exact sequence of events which leads to aneurysm progression and rupture is not known; but it is clear that there is inflammation and proteolysis of the tunica media, which causes a critical reduction in the wall’s tensile resistance [1,2].
Many factors are possibly involved in the formation of Abdominal Aortic Aneurysms (AAA), as well as in the activation of lNF-B and AP-1 pathways, IL-6 and IL-8 hyper-expression, and the accumulation of neutrophils involved in the inflammatory process which causes the aneurysmal degeneration of the arterial wall [3]. It has also been described that intraluminal thrombus may play a direct role in aneurysm progression, through the activation of proteolysis mediated by metalloproteases and plasmin. It might also be involved in the hypoxia of tunica media, resulting in the apoptosis-necrosis of smooth muscular cells. Finally, some authors postulate the possibility of thrombus itself increasing the AAA’s wall tension [4].
The impact of hemodynamics upon aneurysm development has been proven in clinical as well as experimental trials. The infrarenal aorta has the highest proneness to degenerate into aneurysm, either because of its anatomical situation, higher peripheral resistance, higher wall stress, and a lower flow at rest than other aortic segments; or by the increase in MMP-9 expression in the aortic wall compared with proximal segments [5].
Bone marrow-derived stem cells and endothelial progenitor cells are involved in endothelial regeneration and repair processes after acute lesions. In these cases, a generalized inflammatory reaction appears, including progenitor cell mobilization, local chemotactic synthesis in the ischemic tissue, and production of humoral and neural signals which activate the migration of new progenitor cells from the bone marrow. Many progenitor cell families have been identified in peripheral blood: hematopoietic stem cells, endothelial progenitor cells, mesenchymal stromal cells, angiogenic cells and small pluripotent embryonic cells; anyway, the role of these circulating cells in endothelial repair is still uncertain. It is only known that the number of cells decreases in diabetic patients, and is increased by statin use or exercise [6-9].
When there is tissue damage, an increase in MSC (Mesenchymal Stem Cells) is observed, and these could be involved in the inflammatory process and the delivery of pro-angiogenic signals [10,11]. In recent years, the effects of MSC upon damaged regions have been proven, causing the inhibition of local immune response, preventing excessive fibrosis, apoptosis, and inducing mitosis in intrinsic cellular progenitors [12]. These immunomodulating effects are caused by reducing the functions of B and T lymphocytes and Natural Killer cells, affecting the function of dendritic cells [13]. Moreover, MSCs cause a low immunogenic effect, even upon models or patients with different HLA, due to low expression levels of HLA-I and null expression levels of HLA-II [14]. Another effect of MSCs upon affected regions is their involvement in new vessel formation [15], either by cell differentiation or by secreting pro-angiogenic stimulating factors into the bloodstream.
We present a reproducible superior animal model of experimentation, and we propose the basis for a possible way of treatment, intending to show that stem cells of the mesenchymal family inserted in the sac of an artificial aneurysm are able to survive, so that they can be made accountable for a subsequent beneficial effect upon this condition [16].
Materials and methods
Experimentation animals
Six Large-White female pigs, weighing from 22 to 28kg, underwent surgery according to the protocol approved by the CEBA (Ethical Committee for Animal Welfare) in the La Paz Universitary Hospital, and taking into account the rules in the EU Directive on Animal Experimentation (86/609/EEC), as well as Spanish regulations (RD1201/2005). Animals were housed individually, and were controlled daily by the research team and the animal house staff.
Surgical procedure
96 hours before the procedure, antibiotherapy was initiated with Ceftriaxone 40mg/Kg (i.m.) and continued during one week. 24 hours before surgery, fentanyl patches were applied to all animals, animals were fasted 12h before surgery. Before surgery, all animals received pre-medication with Ketamine: 10mg/Kg (i.m), Midazolam: 0,4mg/Kg (i.m) and Tramadol: 5mg/Kg (i.v). Once in the operating room, animals were anesthetized with Isoflurane 5%, which was also used for maintenance of anaesthesia during the operation 1,5-2%, associated with a continuous infusion of morphine, ketamine and lidocaine (12, 30 and 15mg respectively in 500cc saline solution; 10ml/Kg/h). Once the operation was completed, analgesia was managed with Tramadol 5mg/Kg (i.m.) and fentanyl patches during a week. Animals started tolerating food 12 hours after the intervention.
Surgical procedure was conducted in the supine position, i.v. infusion in the outer ear, and non-invasive monitoring of vital signs (blood pressure, temperature, heart rate and arterial oxygenation). Through median laparotomy, the following steps were taken: 1. Dissection and control of infrarenal aorta and dual iliac bifurcation; 2. Systemic heparinization with i.v. Heparin 5000 IU.; 3. Infrarenal clamping and suture of two oval-shaped Dacron patches (Vascutek, Scotland, UK) measuring 2cm length x 1cm width, separated at least by 1cm of healthy proximal aorta (up to the origin of the renal arteries), with 1cm separation between them, and 1cm to the fist aortic bifurcation. Thus we will obtain two adjoining small-sized fusiform aneurysms within 7cm of abdominal aorta; 4. Declamping and haemostasis of both patches; 5. Distal aorta control and clamping, and iliac branch clamping. A straight Dacron tube of 8mm diameter is sutured over the bifurcated distal aorta (Vascutek, Scotland, UK), which will be used to access endovascular devices; 6. Anastomotic hemostasis and preparation of area for the second endovascular period (Figure 1).
Figure 1.

Anastomotic haemostasis and preparation of area for the second endovascular period.
Following this, through fluoroscopic monitoring, endovascular treatment of aneurysms was conducted through covered Wallgraft endoprosthesis (Boston Scientific, Natich, MA,USA) of 6-8mm diameter and length adequate to cover both aneurysms without covering the renal arteries or the iliac bifurcation. We used a 9F introductor in the Dacron prosthesis previously sutured to the terminal aorta, and catheterization with hydrophillic guide of the whole aortic axis. Before completing the aneurysm exclusion, a catheter is left inside the proximal aneurysmal sac through a second 5F introductor, also placed in the Dacron prosthesis. Once the endovascular exclusion has been completed, the catheter placed in the aneurysmal sac is used as a vehicle to place the stem cells inside this sac (Figure 2).
Figure 2.

Complete aneurysm, a catheter is left inside the proximal aneurysmal sac through a second 5F introductor, also placed in the Dacron prosthesis. The catheter placed in the aneurysmal sac is used as a vehicle to place the stem cells inside this sac.
At the same time as the surgical intervention is being conducted, the cells to be injected are prepared. Aliquots of 10 million ASCs are dissolved in 1cc fibrin sealant (PF, Tissucol Duo, Baxter, Germany). We check the correct placement of the catheter through fluoroscopy, and the PF with ASCs is introduced in it. To ensure that nothing remains in the catheter lumen, 0,5cc of fresh saline solution are introduced immediately after, in order to know the exact number of cells we have implanted.
Animals were sacrificed by intravenous injection of potassium chloride at lethal doses, under inhaled anaesthesia with isoflurane at 24 hs and 1, 3, 5, 7 and 9 weeks.
Cell isolation and preparation
Adipose Tissue-Derived Stem Cells (ASC) were used, obtained from an animal of the same breed according to the protocol previously described [17]. Afterwards they were stably transfected through the GFP (Green Fluorescent Protein) gene, following the technique described for the product by the National Center for Cardiovascular Research (CNIC). Once transfected, cells were expanded in culture, and were characterized and differentiated as adipocyte, osteocyte and chondrocyte, according to the protocols described by Zuk P. el al (confirming that we are working with mesenchymal stem cells according to international criteria) [18,19]. Finally, cells were expanded in vitro and aliquots of 10 million were frozen with 10% DMSO and stored in liquid nitrogen until their use.
One week before the intervention, those aliquots required were defrosted and cultured until a sufficient number was obtained. Before being used, cells were detached from the culture through Trypsin-EDTA, and washed 3 times with buffered saline solution (PBS, Gibco).
Histological study
Samples of aneurysms (with or without cells) were collected for the histological study. Said samples were added to a buffer with 10% formalin, and subsequently soaked in paraffin. 5 micron cross-sections were prepared, which were processed through hematoxylin & eosin and trichrome staining.
Samples of the histological blocks were used for the immunofluorescence study. 5 micron cross-sections were dewaxed and rehydrated. Afterwards, anti-GFP primary antibodies (SC-8334, Santa Cruz Biotechnology, California, U.S.A.) were diluted at 1:100, pre-treated with citrate buffer (pH=6) and incubated with blocking buffer for 1 hour. This step was followed by washing and 1-hour incubation with the secondary antibody Alexa Fluor 488 (Molecular Probes, Eugene, Oregon, U.S.A.) 1:200 diluted; afterwards, they were mounted with Antifade reactive with DAPI (ProLong® Gold Molecular Probes; Eugene; Oregon, U.S.A.) and observed through a fluorescence microscope (Leica DMI6000B).
Results
No complications or adverse effects were encountered during the intervention of all animals. Once operated, animals recovered their intestinal habit and typical development rate for their age. Animals initiated their usual diet 12 hours after the intervention, and gained weight during follow-up.
Macroscopic results
When animals were sacrificed, we found the typical post-surgical fibrosis, which is a result of scarring of tissues previously operated upon, particularly, and as also commonly happens in clinical practice, in those places where foreign materials have been left, such as Dacron in this case. Initially we found no morphologic differences between aneurysms treated with ASC+PF and those non-treated, only with PF injection. However, consistency at palpation is harder in the treated aneurysmal sac compared with the untreated one in the same animal; on the contrary, the untreated one has a more elastic consistency. This major difference in consistency makes us think that in the future we must design specific studies to analyze it, as it could be a direct consequence of the higher and lower stabilization of the aneurysmal sac (Figure 3).
Figure 3.

A-C: Macroscopic view of the aneurismal sac (three weeks); B-D: Macroscopic view of the aneurysmal sac (three weeks) in 10% formalin. E: Hematoxylin & Eosin Staining Section. X4. Aneurysmal sac induced by Dacron patch.
Surgical results
The surgical technique we have described is reproducible and similar to those conducted in common clinical practice. During the intervention, we encountered no anaesthetic difficulties, bleeding was minimal, and the most important thing was the lack of complications. Therefore, we can claim that this surgical technique presented is safe and reproducible. We recommend performing a small cystostomy with a conventional Foley catheter at the beginning of the intervention, in order to facilitate surgical area exposure and being able to measure diuresis during surgery avoiding the need of catheter devices adapted to the size and anatomy of the animal. Making a Dacron access for endovascular devices does not increase surgical time to a great extent, and then allows fast access and is very safe for the animal, therefore minimizing the potential damage caused by endovascular devices designed for an adult man’s size on the arterial tree of a smaller experimentation animal, and at the same time allowing the experimentation model to be quite similar to common clinical practice in the endovascular section.
Histological results
Cross-section samples analyzed with the hematoxylin eosin technique show the lymphocytic infiltrate associated with fibrin sealant already seen in other studies. Histological cross-section samples of cases treated with MSC and analyzed by a blind researcher present a lower inflammation reaction, or with longer evolution time, than in controls (Figure 4).
Figure 4.
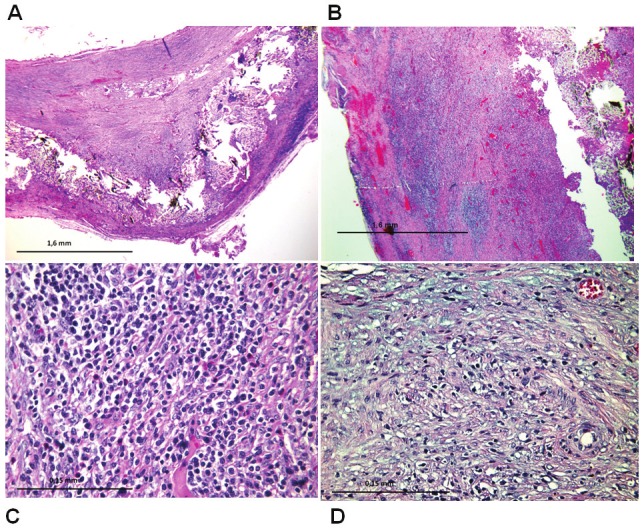
A: Treated Aneurysm: x4 magnification. Hematoxylin eosin. Aneurysm case treated. Arterial wall to be observed in which a Dacron patch has caused an aneurysm and received stem cell injection. Presents regenerative changes and mild lymphocytic inflammation. B: Figure 4A. Aneurysm control: Magnification x4. Hematoxylin eosin. Arterial wall to be observed in which aDacron patch has caused an aneurysm without injection of stem cells. Presents regenerative changes and lots of lymphocytic inflammation. C: Inflammation control: x40 magnification. Hematoxylin eosin. Lymphocytic inflammation was observed in the arterial wall. It counts 420 lymphocytes per high power field magnification x40. D: Inflammation treated: x40 magnification. Hematoxylin eosin. It is observed in the arterial wall of a case treated with stem cells, and spindle cell reparative lymphocytic inflammation lesser extent than the control case (70 linphocites by HPF x40).
Immunofluorescence studies have detected cells marked with GFP up to three weeks after the initial procedure (Figure 5). We haven’t found a positive immunofluorescence beyond this period, but in these cases we have noticed, through hematoxylin eosin staining, a fusiform disposition of aneurysmal sac cells in the circular direction of the vessel section which resembles the adjacent median layer (Figure 6).
Figure 5.
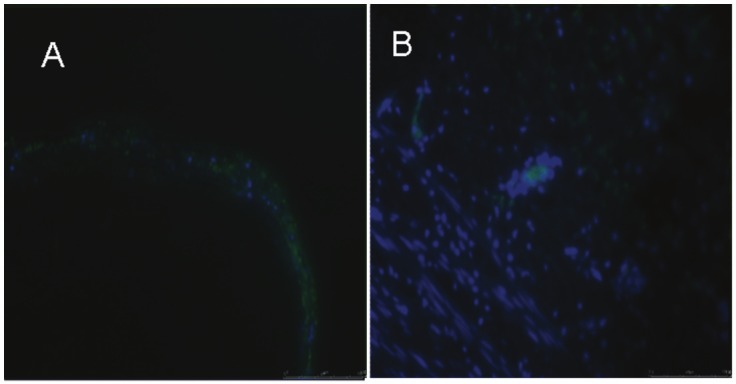
Immunofluorescence have detected cells marked with GFP (eliminate) to three weeks after the initial procedure. Section A: x 4; B: x 20.
Figure 6.

Reparative cells: x20 magnification. Hematoxylin eosin. It is observed in the arterial wall of a case treated with stem cells, spindle cell reparative cells with concentric arrangement reminiscent medial arterial wall.
Discussion
A lower perioperative morbimortality has been established for endovascular treatment not requiring such a major surgical aggression as conventional surgery, or aortic clamping; it also entails a shorter hospital stay and better tolerability by the patient. This advantage by endovascular treatment seems to be diminishing during these patients’ follow-up period, with a higher readmission rate for new complementary treatments in the endovascular group due to leaks.
There has been research in terms of the likelihood of improving the endovascular results. One of these lines of research has involved filling the treated aneurysm sac with various substances through endoprosthesis, thus avoiding those complications derived from the so-called aneurysm sac remodeling along time, which occasionally may cause changes in the position of the prosthesis, or even structural changes which cause leaks to appear, with the consequent risk of growth and rupture.
Adipose-Derived Mesenchymal Stem Cells (ASC, Adipose Stem Cells) have shown important immunological, antiinflammatory and regenerative properties. We consider that all these properties make them an ideal type of cell for AAA treatment. The idea of modulating this inflammatory response in situ, through stem cells which release immunomodulating substances, leads us to think that maybe the process might not be reversed but controlled once the aneurysm is endovascularly treated, thus improving the long-term results for this treatment.
We are not able to know whether inserted cells are able to divide and perpetuate in this niche, but we do know that their feasibility is possible, and therefore it seems sensible to think that their functionality and division ability will be possible too. We have not been able to confirm whether stem cells have migrated to tunica media with those marking techniques used, but we cannot find any other explanation for the cell fusiform disposition only found in treated cases; this could point to a possible differentiation of implanted cells.
Currently, the role of stem cells in angiogenesis and in tissue repair and inflammation seems increasingly clear. Many clinical trials intend to make use of the repairing and pro-angiogenic properties that these cells seem to have, at myocardial as well as peripheral arterial level, focusing on cardiovascular conditions, but their ways of administration are not clear. Our paper, based on an easy and reproducible animal model, shows that local administration in the aneurismal sac is possible, in some cases with the assistance of endovascular techniques.
We think that this modulating response can be equally interesting and applicable for treatment of the other patient group with severe arteriosclerosis, who develop aneurysms. In the same way as there is a new vessel formation response to ischemia, after the stimulus of a sick arterial wall there can be a process modulation, by acting as inflammatory response modulators and stabilizing the thrombus. This hypothesis, added to endovascular treatment which suppresses the hemodynamic stimulus, might optimize the final result, improving the endovascular treatment results.
As far as we know, this is the first time that intravascular as well as localized stem cell administration is used in an experimental model, through an intraluminal device, in a living being.
Acknowledgements
This study was supported by a grant from the Spanish Ministry of Health and Consumer Affairs (FIS PI11/00116) and RETIC Program (RD06/0010/0018). Special gratitude to Boston Scientific, who generously donated the stent grafts for this study.
References
- 1.Xu C, Zarins CK, Glagov S. Aneurysmal and occlusive atherosclerosis of the human abdominal aorta. J Vasc Surg. 2001;33:91. doi: 10.1067/mva.2001.109744. [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Hussien H, Hanemaaijer R, Kleemann R, Verhaaren BF, van Bockel JH, Lindeman JH. The pathophysiology of abdominal aortic aneurysm growth: corresponding and discordant inflammatory and proteolytic processes in abdominal aortic and popliteal artery aneurysms. J Vasc Surg. 2010;51:1479–87. doi: 10.1016/j.jvs.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 3.Parry DJ, Al-Barjas HS, Chappell L, Rashid ST, Ariëns RA, Scott DJ. Markers of inflammation in men with small abdominal aortic aneurysm. J Vasc Surg. 2010;52:145–51. doi: 10.1016/j.jvs.2010.02.279. [DOI] [PubMed] [Google Scholar]
- 4.Michel JB, Martin-Ventura JL, Egido J, Sakalihasan N, Treska V, Lindholt J, Allaire E, Thorsteinsdottir U, Cockerill G, Swedenborg J FAD EU consortium. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2011;90:18–27. doi: 10.1093/cvr/cvq337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ailawadi G, Knipp BS, Lu G, Roelofs KJ, Ford JW, Hannawa KK, Bishop K, Thanaporn P, Henke PK, Stanley JC, Upchurch GR Jr. A nonintrinsic regional basis for increased infrarenal aorticMMP-9 expression and activity. J Vasc Surg. 2003;37:1059. doi: 10.1067/mva.2003.163. [DOI] [PubMed] [Google Scholar]
- 6.Wojakowski W, Landmesser U, Bachowski R, Jadczyk T, Tendera M. Mobilization of stem and progenitor cells in cardiovascular diseases. Leukemia. 2011;26:23–33. doi: 10.1038/leu.2011.184. [DOI] [PubMed] [Google Scholar]
- 7.Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, Silver M, Thorne T, Losordo DW. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterialinjury. Circ Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 8.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–6. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 9.Sprengers RW, Moll FL, Teraa M, Verhaar MC JUVENTAS Study Group. Rationale and design of the JUVENTAS trial for repeated intra-arterial infusion of autologous bone marrow-derived mononuclear cells in patients with critical limb ischemia. J Vasc Surg. 2010;51:1564–8. doi: 10.1016/j.jvs.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Gyöngyösi M, Posa A, Pavo N, Hemetsberger R, Kvakan H, Steiner-Böker S, Petrási Z, Manczur F, Pavo IJ, Edes IF, Wojta J, Glogar D, Huber K. Differential effect of ischaemic preconditioning on mobilisation and recruitment of haematopoietic and mesenchymal stem cells in porcine myocardial ischaemia-reperfusion. Thromb Haemost. 2010;104:376–84. doi: 10.1160/TH09-08-0558. [DOI] [PubMed] [Google Scholar]
- 11.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010 Apr 26;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 13.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotentmesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 14.Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 15.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006 Oct;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 16.Trollope A, Moxon JV, Golledge J. Animal models of abdominal aortic aneurysm and their role in furthering management of human disease. Cardiovasc Pathol. 2011;20:114–23. doi: 10.1016/j.carpath.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 17.García-Olmo D, García-Arranz M, García LG, Cuellar ES, Blanco IF, Prianes LA, Montes JA, Pinto FL, Marcos DH, García-Sancho L. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn’s disease: a new cell-based therapy. Int J Colorectal Dis. 2003;18:451–4. doi: 10.1007/s00384-003-0490-3. [DOI] [PubMed] [Google Scholar]
- 18.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human Adipose Tissue Is a Source of Multipotent Stem Cell. Mol Biol Cell. 2002 Dec;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotentmesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–17. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]


