Abstract
Exogenous insulin administration and oral anti-diabetic drugs are the primary means of treating diabetes. However, tight glycaemic control, with its inherent risk of hypoglycaemia, is required to prevent the microvascular and macrovascular complications of the disease. While islet or pancreas transplantations offer a longer-term cure, their widespread application is not possible, primarily because of a lack of donor tissue, the burden of life-long immunosuppression, and eventual graft rejection. The rapid increase in the incidence of diabetes has promoted the search for alternative cell-based therapies. Here we review recent advances in the directed differentiation of both endocrine and non-endocrine progenitors towards an islet-like phenotype.
Keywords: Endocrine, progenitor cells, islet cells, insulin production, diabetes mellitus
The need for cell-based therapies for diabetes
The pancreatic islet is a complex microorganism, secreting insulin under tight physiological control. Insulin deficiency due to disturbances of insulin production/action lies at the heart of diabetes. This disease is a growing global healthcare problem, with incidence currently estimated at 346M. Whilst exogenous insulin administration is the primary mechanism of controlling blood glucose levels in type 1 diabetes, it is increasingly being introduced for those with type 2 diabetes where it has been shown to aid beta-cell survival and function [1]. However, the administration of exogenous insulin does not restore the physiological regulation of hyperglycaemia. Furthermore, strict adherence to the treatment regime is required to achieve a state close to glucose homeostasis and prevent many of the long-term complications of diabetes including cardiovascular disorders, nephropathies, and diabetic retinopathy. In addition, pharmacological induction of glycaemic control brings an inherent risk of hypoglycaemia. Thus, recent work in the field of diabetes research has focused on establishing cellular-based therapies that avoid the need for exogenous insulin delivery. Perhaps the most obvious approach is the replacement of pancreatic tissue or isolated islets by transplantation [2,3]. However, a lack of donor material and the burden of immunosuppression, coupled with high levels of graft rejection, have meant that four decades after Ballinger and Lacy successfully transplanted isolated islets in rodents [4], islet transplantations are not routine [2,3,5].
This review discusses recent developments in the production of insulin-secreting beta-cells from progenitor cells and outlines the varied approaches that have been used to differentiate both endocrine and non-endocrine progenitors towards insulin-producing cells.
Islet-cell development during embryogenesis
The pancreas arises from embryonic fusion of the dorsal and ventral primordial [6]. The endocrine portion of the adult pancreas is comprised of approximately 1 million islets of Langerhans that account for around 2-3% of pancreatic mass, but which receive approximately 30% of pancreatic blood supply [7]. Islets are anatomically complex microorgans comprised of heterogeneous cell types made up of insulin secreting beta-cells, glucagon-secreting alpha-cells, somatostatin-secreting delta-cells and polypeptide-secreting PP cells [7]. The 3D architecture of islets is important to normal function and is a crucial part of embryonic development. In the mature pancreas, islet architecture is maintained by cell adhesion molecules including E-cadherin, but in the developing pancreas, the emergence of endocrine cells from the epithelial trunk of the pancreas is dependent on a reduction in E-cadherin expression [8].
Endocrine progenitor cells co-express all endocrine hormones prior to final maturation into cells expressing a single hormone [6]. In rodent models, glucagon-secreting cells are initially detected, followed by insulin-producing cells which co-express glucagon. Mature insulin-secreting beta-cells and glucagon-releasing alpha-cells are evident from embryonic day 14 in mice [9,10] and from the end of the first trimester in humans [11]. Somatostatin secreting delta-cells develop soon afterwards, while PP-releasing cells are observed shortly before the end of gestation when clustering of cells to form 3D islets is also observed [9,10].
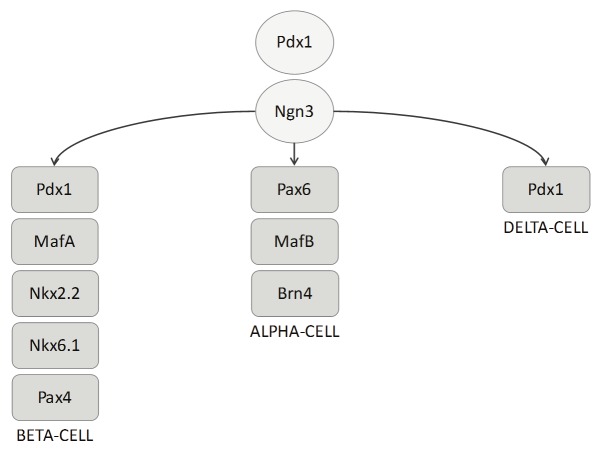
Endocrine cells are derived from Pdx-1 (pancreatic and duodenal homeobox 1) positive progenitor cells (Figure 1). Disruption of Pdx-1 results in regression of the pancreas immediately after bud formation [12]. Similarly, loss-offunction mutations causes maturity onset diabetes of the young (MODY) and pancreatic agenesis. During embryogenesis, both endocrine and exocrine progenitors are positive for Pdx-1, but on maturation, Pdx-1 is generally found in the beta-cell and delta-cells only [13]. The development of endocrine cells is regulated by the bHLH (basic helix loop helix) transcription factor Ngn-3 (Neurogenin 3). Inhibition of Ngn-3 during embryonic development hampers endocrine differentiation [14]. The further development of specific islet hormone expressing cells is regulated by an additional range of transcription factors [15]. In particular, beta-cell differentiation is largely regulated by NKX2.2 (NK2 homeobox 2) [16]. However, very recent evidence suggests a role for Grg3 (groucho-related gene 3, also known as Tle3), which encodes a member of the Groucho/TLE family of co-repressors and regulates differentiation of progenitors into mature cell types [8]. Metzger et al. [8] show that Grg3 is expressed in beta-cells and that Grg3-null mice display limited differentiation of endocrine cell types. This was attributed to delamination defects of early endocrine progenitors from the trunk epithelium. Interestingly, Grg3 normally suppresses E-cadherin gene expression, thereby promoting emergence of endocrine cells from the trunk epithelium [8]. These findings suggest an important role for Grg3 in beta-cell development.
Figure 1.
Transcriptional regulation of endocrine development. All pancreatic cells originate from pdx-1 positive cells. The transcription factor Ngn-3 is required for differentiation into an endocrine phenotype. Further development into alpha, beta, or delta-cells cells is closely controlled by a range of transcription factors as indicated in the Figure.
Production of beta-cells from endocrine progenitor cells
The expansion of all endocrine cells from Pdx-1 positive progenitors is Ngn-3 dependent [15]. Recent observations in mouse embryos suggest that Ngn-3 expression occurs in two distinct phases at approximately E8.5-E11.0 and later, at E12.0. Ngn-3 expression correlates with the first and second transitions of endocrine development, which appear to be governed by post-transcriptional regulation [17]. Indeed, mice deficient in Ngn-3 die of hyperglycaemia and related complications shortly after birth because of a total absence of endocrine islet cells [15]. Adult islet cells also express low levels of Ngn-3, which is thought to contribute to the much debated phenomenon of beta-cell replication [18].
Beta-cells respond to a plethora of hormones, neurotransmitters, nutrients, drugs and second messengers [19]. Beta-cell activity responds to physiological requirements and conditions such as pregnancy and obesity, which are associated with altered beta-cell mass and insulin secretion, can have a profound effect on normal beta-cell function [7,20]. Evidence from animal studies suggests that beta-cells can self-duplicate to redress physiological imbalances (see review by Gonez and Knight [21]). However, the mechanisms behind this process are poorly understood and widely debated.
The promotion of beta-cell regeneration by existing beta-cells is supported by pancreatic ductal cells [22]. The junction between the ductal epithelium and adjacent acinar cells houses centroacinar/terminal ductal cells (CA/TD). CA/TD are negative for endocrine cell markers, but positive for progenitor cell transcripts including nestin and Sox9 and can spontaneously develop into endocrine or exocrine phenotypes whilst retaining glucose-induced insulin secretion [23]. Moreover, it was suggested that these cells contribute to the preservation of tissue homeostasis in the murine pancreas [23].
The direct development of labeled pancreatic ductal cells into beta-cells over a 4 week period was confirmed after duct ligation in mice [24,25]. Contrastingly, other work suggests that ductal cells contribute to beta-cell neogenesis before, but not after, birth [26,27] and debate continues as whether ductal cells are true progenitors of pancreatic beta cells. The presence of true endocrine progenitors in the adult pancreas is therefore highly debatable. Limited expression of constitutive Ngn-3 in the adult pancreas implies that beta-cell neogenesis is reliant on existing beta-cells and that the endocrine pancreas has a limited ability to self-renew. In a pioneering experiment, Dor et al. [28] used an innovative linage-tracing technique to label pre-existing beta-cells in mice with a tamoxifen-inducible transgene driving the expression of Cre recombinase under the control of the rat insulin promoter (RIP-Cre-ER mice). After breeding with a reporter mouse, the offspring were injected with tamoxifen. The labelling index remained constant with age suggesting that de novo beta-cells originated from pre-existing labelled beta-cells. This study, along with a series of confirmatory reports that followed, suggests that beta-cell beget beta-cells and that the adult endocrine pancreas has limited self-renewal capacities.
However, in the same year, Seaberg et al. [29] reported the exciting discovery of rare single clonal-cells in adult mouse pancreatic tissue. The culture of endocrine islet cell clusters gave rise to a range of endocrine, exocrine and neural cells. In other words, these cells carried an intriguing capacity to differentiate into two different linages: pancreatic (insulin-, glucagon-, amylase-, and somatostatin-positive cells) and neural (neuron-like, astrocyte-like, and ligodendrocyte-like cells). These newly identified cells were called pancreas-derived multipotent precursor (PMP) cells. Importantly, PMP-derived insulin-positive cells exhibited glucose-dependent calcium-responsiveness; a unique attribute of pancreatic beta-cells. Insulin-positive PMP cells also expressed beta-cell markers including GLUT2 and C-peptide [29]. This work provided the first evidence for the existence of an intrinsic progenitor population in the adult pancreas.
Smukler and colleagues [30] extended these findings to human tissue to successfully isolate PMPs. Using state-of-the-art genetic lineage-tracing techniques, it was shown that PMP cells are of pancreatic and not neural origin. Transplantation of both mouse and [29] human PMPs into diabetic mice alleviated hyperglycaemia [30]. Immunofluorescent staining showed that PMP cells produced insulin in vivo. This observation may explain the discrepancies between the findings of Dor et al. and Seaberg et al. Since PMPs produce insulin, it is possible that the PMP cells were stained in the Dor et al. experiment upon injection of tamoxifen, remained in the pancreas and eventually differentiated into beta-cells. Their differentiation from labeled PMP to labeled beta-cell would therefore, not alter the labeling index.
Other studies have also verified that beta-cells are not the only source of beta-cell neogenesis in the adult islet. After injection with streptozotocin and 90% pancreatectomy, the rat pancreas was shown to regenerate [31]. Furthermore, Glucagon-like-peptide 1 (GLP-1) treatment of pancreatic ductal cells enhances beta-cell proliferation and reduces apoptosis, in vivo and in vitro [32]. Activation of the GLP-1 receptor is thought to improve islet neogenesis and promote beta-cell proliferation through enhances Pdx-1 activity in ductal progenitor cells and may therefore be of interest in future regenerative therapies [32].
Directed differentiation on non-endocrine progenitor cells towards a bet-cell phenotype
The ability of the liver to regenerate and proliferate makes it an ideal source of material for cell-based therapies and is a particularly attractive source of cells for autologous transplantation. Furthermore, the liver and pancreas share a common embryonic origin (See Figure 2) in the endoderm. After hepatectomy or loss of liver mass, the hepatocyte population rapidly expands to regenerate the liver. However, inhibition of the normal proliferative processes in the liver results in the production of a well-documented hepatic progenitor cell population called oval cells which can be found in the portal triads next to the canals of Hering [33-35]. While oval cells traditionally differentiate towards hepatocytes and cholangiocytes [35], they may be directed towards a pancreatic lineage if cultured under specific conditions via a process known as transdetermination (Figure 3) [36]. Culture of oval cells in high glucose medium [37] or in extracellular matrix proteins such as laminin or fibronectin [38] has been reported to produce islet cell phenotypes. Furthermore, chemical activation of oval cells by 3,5-diethoxycarbonyl-1,4-dihydrocollidine protects against streptozotocin-induced diabetes by increasing endocrine islet cell proliferation and promoting the differentiation of oval cells to insulin-positive cells [39]. Additionally, islet-like cells have been reported in the biliary tree. The intrahepatic biliary epithelial cell populations express insulin when transduced with Pdx1 or NeuroD1 [40], while downregulation of Ngn-3 leads to the appearance of islet-like cells all along the biliary tree [41,42].
Figure 2.
The process of transdifferentiation. Transdifferentiation, also referred to as lineage reprogramming, describes the conformation of one cell to an entirely different phenotype. It differs from dedifferentiation, whereby a differentiated cell reverts to its predecessor.
Figure 3.
Common embryonic origin of liver and pancreatic cells. Hepatic and pancreatic cells share a common embryonic origin in the endoderm making hepatic progenitor cells an ideal source of material for directed differentiation towards a beta-cell phenotype.
Hepatic expression of the Pdx-1 gene in the liver of streptozotocin-induced diabetic mice produces insulin-positive cells in the liver [43,44]. Pdx-1 is auto-inducing and promotes its own expression, which might account for the prolonged lifespan of liver-to-pancreas transdifferentiated cells [45]. However, this approach limited by the toxicity associated with adenoviral delivery of the Pdx-1 gene [43] and, secondly, by the high level of mortality associated with Pdx-1 expression in the liver which lead to hepatic dysmorphogenesis [34] and autodigestion of hepatic cells which coexpressed exocrine enzymes and insulin [44]. In an attempt to overcome this complication, Kojima and colleagues used a transcription factor located downstream of Pdx-1 called B2/NeuroD to induce the neogenesis of islet cells expressing all four major islet hormones in the liver [44]. In a similar vein, the adenoviral delivery of Ngn3 in combination with a beta-cell growth factor called betacellulin to the liver of streptozotocin-induced diabetic mice resulted in the production of islet-like cells releasing insulin, glucagon, somatostatin, and pancreatic polypeptide [46]. In both studies, the resulting islet-like cells were reported to display glucose-stimulated insulin secretion and, following in vivo transplantation, reversed streptozotocin-induced diabetes for extended periods of time. Importantly, the beta-like cells derived following viral transfection of Ngn3 and betacellulin were found to originate from liver oval cells by lineage tracing [44,46]. Very recently, it was found that transcription factors found in adult pancreatic cells, most notably NKX6.1, which has been shown to be essential in alpha- and beta-cell development in a variety of organisms [47-49], promotes Pdx-1-induced liver to beta-cell reprogramming, and such approaches may provide an alternative means of directing hepatic cells to a beta-cell phenotype [50].
Alternative strategies and future perspectives
The conversion of non-islet cells to islet hormone-secreting cells is a difficult and daunting prospect. Significant advances have been made in recent years, but the move towards a cellular based therapy that would replace the need for exogenous insulin administration is still some way off.
The potential sources of new cell-derived transplantable material for the treatment of diabetes are numerous. Although this review focuses on the use of progenitor cells, the therapeutic potential of Embryonic Stem Cells (ESC) has also received significant attention. From the landmark work of Lumelsky and colleagues [51] at the beginning of the last decade, the amount and intensity of research in this field has increased significantly. However, at present, the differentiation of an ESC towards a true beta-cell phenotype, capable of de novo insulin secretion and glucose responsiveness, has not been achieved [24,25]. Furthermore, the unacceptable risk of teratoma formation [52] and widespread concerns about the use of embryonic material means that the therapeutic application of ESC will remain elusive for some time.
Induced pluripotent stem cells (iPSC) mimic ESC in terms of telomerase activity and methylation of gene promoters [53,54]. However, iPSC have the theoretical advantages of posing a lesser risk of rejection since they can be isolated directly from the patient themselves. Recent studies report the conversion of iPSC derived from fibroblasts to a beta-cell phenotype positive for insulin, C-peptide, GLUT2 and MafA [55]. Nonetheless, research into iPSC derived beta-cells is still in its infancy. Most of the resultant ‘beta-cells’ have been found to co-express glucagon simultaneously; a result reminiscent of immature beta-cells in the early stages of development. Furthermore, a uniform protocol has not been established, which is a common problem with all methods attempting to generate beta-cells from alternative sources. However, the differentiation of iPSC using cytokines and small molecules has yielded different percentages of insulin positive cells, ranging from 0.4% to 16.9%.
Despite the shortcomings associated with stem cell-based approaches for islet-cell generation, the need for a genuine cell replacement therapy for diabetes persists. The vast majority of research has focused on the production of insulin secreting cells. Since homotypic cell interactions appear ample in the maintenance of normal patterns of insulin secretion [56-58], the production of pure beta-cell populations may well prove sufficient to restore glucose homeostasis. Islet and whole pancreas transplantations are not a realistic large scale solution. Therefore, the directed differentiation of non-islet cell types may offer the only large scale alternative to produce a viable cure. Recently however, the generation of pseudoislets from an electrofusion derived human beta-cell line has been reported [59], which may offer yet another potential approach to dealing with the lack of donor material.
While current progenitor-based protocols require development, this research field is still in its infancy and is rapidly expanding with new developments appearing on almost a monthly basis. Moreover, rapid advances in molecular and cellular biology provide the continual promise of exciting new mechanistic insights into cell development, differentiation, and survival. Accordingly, stem cell-based approaches offer great potential for the future and, with perseverance, may ultimately provide a cure for diabetes [60].
Acknowledgements
Arif Abed was supported by a studentship from the EPSRC funded Doctoral Training Centre in Regenerative Medicine at Keele, Loughborough and Nottingham.
References
- 1.Ryan EA, Imes S, Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028–1032. doi: 10.2337/diacare.27.5.1028. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman DB, Baker MS, Chen X, Leventhal JR, Stuart FP. Sequential kidney/islet transplantation using prednisone-free immunosuppression. Am J Transplant. 2002;2:674–677. doi: 10.1034/j.1600-6143.2002.20715.x. [DOI] [PubMed] [Google Scholar]
- 3.Ricordi C, Fraker C, Szust J, Al-Abdullah I, Poggioli R, Kirlew T, Khan A, Alejandro R. Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation. 2003;75:1524–1527. doi: 10.1097/01.TP.0000058813.95063.7A. [DOI] [PubMed] [Google Scholar]
- 4.Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery. 1972;72:175–186. [PubMed] [Google Scholar]
- 5.Shapiro AM, Nanji SA, Lakey JR. Clinical islet transplant: current and future directions towards tolerance. Immunol Rev. 2003;196:219–236. doi: 10.1046/j.1600-065x.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 6.Peck AB, Cornelius JG, Schatz D, Ramiya VK. Generation of islets of Langerhans from adult pancreatic stem cells. J Hepatobiliary Pancreat Surg. 2002;9:704–709. doi: 10.1007/s005340200097. [DOI] [PubMed] [Google Scholar]
- 7.McClenaghan NH. Physiological regulation of the pancreatic {beta}-cell: functional insights for understanding and therapy of diabetes. Exp Physiol. 2007;92:481–496. doi: 10.1113/expphysiol.2006.034835. [DOI] [PubMed] [Google Scholar]
- 8.Metzger DE, Gasperowicz M, Otto F, Cross JC, Gradwohl G, Zaret KS. The transcriptional corepressor Grg3/Tle3 promotes pancreatic endocrine progenitor delamination and beta-cell differentiation. Development. 2012;139:1447–1456. doi: 10.1242/dev.072892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development. 1993;118:1031–1039. doi: 10.1242/dev.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 10.Herrera PL, Huarte J, Sanvito F, Meda P, Orci L, Vassalli JD. Embryogenesis of the murine endocrine pancreas; early expression of pancreatic polypeptide gene. Development. 1991;113:1257–1265. doi: 10.1242/dev.113.4.1257. [DOI] [PubMed] [Google Scholar]
- 11.Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI, Hanley NA. Beta cell differentiation during early human pancreas development. J Endocrinol. 2004;181:11–23. doi: 10.1677/joe.0.1810011. [DOI] [PubMed] [Google Scholar]
- 12.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 13.Shao S, Fang Z, Yu X, Zhang M. Transcription factors involved in glucose-stimulated insulin secretion of pancreatic beta cells. Biochem Biophys Res Comm. 2009;384:401–404. doi: 10.1016/j.bbrc.2009.04.135. [DOI] [PubMed] [Google Scholar]
- 14.Prasadan K, Tulachan S, Guo P, Shiota C, Shah S, Gittes G. Endocrine-committed progenitor cells retain their differentiation potential in the absence of neurogenin-3 expression. Biochem Biophys Res Comm. 2010;396:1036–1041. doi: 10.1016/j.bbrc.2010.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gradwohl G, Dierich A, LeMeur M, Guillemot F. Neurogenin3 is Required for the Development of the Four Endocrine Cell Lineages of the Pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guney MA, Gannon M. Pancreas cell fate. Birth Defects Res C Embryo Today. 2009;87:232–248. doi: 10.1002/bdrc.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villasenor A, Chong DC, Cleaver O. Biphasic Ngn3 expression in the developing pancreas. Dev Dyn. 2008;237:3270–3279. doi: 10.1002/dvdy.21740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Jensen JN, Seymour PA, Hsu W, Dor Y, Sander M, Magnuson MA, Serup P, Gu G. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc Natl Acad Sci U S A. 2009;106:9715–9720. doi: 10.1073/pnas.0904247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly C, McClenaghan NH, Flatt PR. Role of islet structure and cellular interactions in the control of insulin secretion. Islets. 2011;3:41–47. doi: 10.4161/isl.3.2.14805. [DOI] [PubMed] [Google Scholar]
- 20.Xue Y, Liu C, Xu Y, Yuan Q, Xu K, Mao X, Chen G, Wu X, Brendel MD, Liu C. Study on pancreatic islet adaptation and gene expression during pregnancy in rats. Endocrine. 2010;37:83–97. doi: 10.1007/s12020-009-9273-0. [DOI] [PubMed] [Google Scholar]
- 21.Gonez LJ, Knight KR. Cell therapy for diabetes: stem cells, progenitors or beta-cell replication? Mol Cell Endocrinol. 2010;323:55–61. doi: 10.1016/j.mce.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Bonner-Weir S, Toschi E, Inada A, Reitz P, Fonseca SY, Aye T, Sharma A. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Pediatr Diabetes. 2004;5:16–22. doi: 10.1111/j.1399-543X.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 23.Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci U S A. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonner-Weir S, Inada A, Yatoh S, Li WC, Aye T, Toschi E, Sharma A. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans. 2008;36:353–356. doi: 10.1042/BST0360353. [DOI] [PubMed] [Google Scholar]
- 25.Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solar M, Cardalda C, Houbracken I, Martín M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, Ferrer J. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Kopinke D, Murtaugh LC. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol. 2010;10:38. doi: 10.1186/1471-213X-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 29.Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 30.Smukler SR, Arntfield ME, Razavi R, Bikopoulos G, Karpowicz P, Seaberg R, Dai F, Lee S, Ahrens R, Fraser PE, Wheeler MB, van der Kooy D. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell. 2011;8:281–293. doi: 10.1016/j.stem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Finegood DT, Weir GC, Bonner-Weir S. Prior streptozotocin treatment does not inhibit pancreas regeneration after 90% pancreatectomy in rats. Am J Physiol. 1999;276:E822–827. doi: 10.1152/ajpendo.1999.276.5.E822. [DOI] [PubMed] [Google Scholar]
- 32.Xia B, Zhan XR, Yi R, Yang B. Can pancreatic duct-derived progenitors be a source of islet regeneration? Biochem Biophys Res Comm. 2009;383:383–385. doi: 10.1016/j.bbrc.2009.03.114. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Shin JS, Kim HJ, Fisher RC, Lee MJ, Kim CW. Streptozotocin-induced diabetes can be reversed by hepatic oval cell activation through hepatic transdifferentiation and pancreatic islet regeneration. Lab Invest. 2007;87:702–712. doi: 10.1038/labinvest.3700561. [DOI] [PubMed] [Google Scholar]
- 34.Yechoor V, Liu V, Paul A, Lee J, Buras E, Ozer K, Samson S, Chan L. Gene therapy with neurogenin 3 and betacellulin reverses major metabolic problems in insulin-deficient diabetic mice. Endocrinology. 2009;150:4863–4873. doi: 10.1210/en.2009-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yechoor V, Chan L. Minireview: beta-cell replacement therapy for diabetes in the 21st century:manipulation of cell fate by directed differentiation. Mol Endocrinol. 2010;24:1501–1511. doi: 10.1210/me.2009-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, Petersen BE, Peck AB. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc Natl Acad Sci U S A. 2002;99:8078–8083. doi: 10.1073/pnas.122210699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 38.Leite AR, Correa-Giannella ML, Dagli ML, Fortes MA, Vegas VM, Giannella-Neto D. Fibronectin and laminin induce expression of islet cell markers in hepatic oval cells in culture. Cell Tissue Res. 2007;327:529–537. doi: 10.1007/s00441-006-0340-z. [DOI] [PubMed] [Google Scholar]
- 39.Kim S, Shin JS, Kim HJ, Fisher RC, Lee MJ, Kim CW. Streptozotocin-induced diabetes can be reversed by hepatic oval cell activation through hepatic transdifferentiation and pancreatic islet regeneration. Lab Invest. 2007;87:702–712. doi: 10.1038/labinvest.3700561. [DOI] [PubMed] [Google Scholar]
- 40.Nagaya M, Katsuta H, Kaneto H, Bonner-Weir S, Weir GC. Adult mouse intrahepatic biliary epithelial cells induced in vitro to become insulin-producing cells. J Endocrinol. 2009;201:37–47. doi: 10.1677/JOE-08-0482. [DOI] [PubMed] [Google Scholar]
- 41.Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Kuhara T, Horiguchi M, Koizumi M, Fujimoto K, Doi R, Wright CV, Chiba T. Loss of the major duodenal papilla results in brown pigment biliary stone formation in pdx1 null mice. Gastroenterology. 2006;130:855–867. doi: 10.1053/j.gastro.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 42.Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- 43.Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, Chan L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9:596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 44.Miyatsuka T, Kaneto H, Kajimoto Y, Hirota S, Arakawa Y, Fujitani Y, Umayahara Y, Watada H, Yamasaki Y, Magnuson MA, Miyazaki J, Hori M. Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem Biophys Res Commun. 2003;310:1017–1025. doi: 10.1016/j.bbrc.2003.09.108. [DOI] [PubMed] [Google Scholar]
- 45.Godfrey KJ, Mathew B, Bulman JC, Shah O, Clement S, Gallicano GI. Stem cell-based treatments for Type 1 diabetes mellitus: bone marrow, embryonic, hepatic, pancreatic and induced pluripotent stem cells. Diabet Med. 2012;29:14–23. doi: 10.1111/j.1464-5491.2011.03433.x. [DOI] [PubMed] [Google Scholar]
- 46.Henseleit KD, Nelson SB, Kuhlbrodt K, Hennings JC, Ericson J, Sander M. NKX6 transcription factor activity is required for alpha- and beta-cell development in the pancreas. Development. 2005;132:3139–3149. doi: 10.1242/dev.01875. [DOI] [PubMed] [Google Scholar]
- 47.Nelson SB, Schaffer AE, Sander M. The transcription factors Nkx6.1 and Nkx6.2 possess equivalent activities in promoting beta-cell fate specification in Pdx1+ pancreatic progenitor cells. Development. 2007;134:2491–2500. doi: 10.1242/dev.002691. [DOI] [PubMed] [Google Scholar]
- 48.Binot AC, Manfroid I, Flasse L, Winandy M, Motte P, Martial JA, Peers B, Voz ML. Nkx6.1 and nkx6.2 regulate alpha- and beta-cell formation in zebrafish by acting on pancreatic endocrine progenitor cells. Dev Biol. 2010;340:397–407. doi: 10.1016/j.ydbio.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 49.Gefen-Halevi S, Rachmut IH, Molakandov K, Berneman D, Mor E, Meivar-Levy I, Ferber S. NKX6.1 promotes PDX-1-induced liver to pancreatic beta-cells reprogramming. Cell Reprogram. 2010;12:655–664. doi: 10.1089/cell.2010.0030. [DOI] [PubMed] [Google Scholar]
- 50.Soria B, Roche E, Berna G, Leon-Quinto T, Reig JA, Martin F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157–162. doi: 10.2337/diabetes.49.2.157. [DOI] [PubMed] [Google Scholar]
- 51.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 52.Gerecht-Nir S, Osenberg S, Nevo O, Ziskind A, Coleman R, Itskovitz-Eldor J. Vascular development in early human embryos and in teratomas derived from human embryonic stem cells. Biol Reprod. 2004;71:2029–2036. doi: 10.1095/biolreprod.104.031930. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 54.Maherali N, Hochedlinger K. Induced pluripotency of mouse and human somatic cells. Cold Spring Harb Symp Quant Biol. 2008;73:157–162. doi: 10.1101/sqb.2008.73.017. [DOI] [PubMed] [Google Scholar]
- 55.Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 56.Kelly C, Guo H, McCluskey JT, Flatt PR, McClenaghan NH. Comparison of insulin release from MIN6 pseudoislets and pancreatic islets of Langerhans reveals importance of homotypic cell interactions. Pancreas. 2010;39:1016–1023. doi: 10.1097/MPA.0b013e3181dafaa2. [DOI] [PubMed] [Google Scholar]
- 57.Brereton H, Carvell MJ, Persaud SJ, Jones PM. Islet alpha-cells do not influence insulin secretion from beta-cells through cell-cell contact. Endocrine. 2007;31:61–65. doi: 10.1007/s12020-007-0004-0. [DOI] [PubMed] [Google Scholar]
- 58.Hauge-Evans AC, Squires PE, Persaud SJ, Jones PM. Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli: enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes. 1999;48:1402–1408. doi: 10.2337/diabetes.48.7.1402. [DOI] [PubMed] [Google Scholar]
- 59.Guo-Parke H, McCluskey JT, Kelly C, Hamid MH, McClenaghan NH, Flatt PR. Configuration of electrofusion derived human insulin-secreting cell line as pseudoislets enhances functionality and therapeutic utility. J Endocrinol. 2012;214:257–265. doi: 10.1530/JOE-12-0188. [DOI] [PubMed] [Google Scholar]
- 60.Kelly C, Flatt CC, McClenaghan NH. Stem cell-based approaches for the treatment of diabetes. Stem Cells Int. 2011;2011:424986. doi: 10.4061/2011/424986. [DOI] [PMC free article] [PubMed] [Google Scholar]