Abstract
Cyclin D1 overexpression is found in more than 50% of human breast cancers and causes mammary cancer in transgenic mice. Dysregulation of cyclin D1 gene expression or function contributes to the loss of normal cell cycle control during tumorigenesis. Recent studies have demonstrated that cyclin D1 conducts additional specific functions to regulate gene expression in the context of local chromatin, promote cellular migration, and promote chromosomal instability. It is anticipated that these additional functions contribute to the pathology associated with dysregulated cyclin D1 abundance. This article discusses evidence that examines the functional roles that cyclin D1 may play in cancer with an emphasis on other cyclin family members that also may contribute to cancer and disease in a similar fashion.
Keywords: cyclins, cyclin D1, migration, CIN, stem cells
Introduction
The cyclin-dependent kinases (CDKs) are a family of serine/threonine kinases controlling progression through the cell cycle.1 The regulatory subunits of the CDKs, known as cyclins, form complexes with their catalytic partner to function as checkpoint kinases of specific proteins that regulate progression through the cell cycle. The cyclin-CDK complexes govern a linear progression of events that lead cells from a resting state (G0), growth phase (G1), through DNA replication (S), and finally to cell division (M). Abnormalities that occur in any of the phases initiate a signal that triggers a cell cycle arrest until the issue is resolved. There are some 11 cyclins found in human cells, many having subfamily members (e.g., D-type cyclin D1, D2, and D3). Cyclins partner with associated CDKs and assembly factors to affect their canonical roles in cell cycle checkpoint regulation. Several cyclins exhibit noncanonical roles that may be kinase independent. This review is focused on new and emerging roles for cyclin D1 and includes other cyclins that function in a similar manner.
Cyclin Functions in Cell Migration
Cyclin D1 plays an important role in cell cycle progression through the association with CDK4 and CDK6, which phosphorylate and inactivate the retinoblastoma protein pRb, leading to the expression of a subset of proliferation-associated E2F target genes.2,3 In addition to this canonical pRB-dependent effect in cell cycle progression, cyclin D1 functions in cellular migration, DNA damage response and repair, and chromosome stability.4-8 Metastasis is a major cause of death in cancer patients. Cellular migration is essential for tumor metastasis. Macrophages, fibroblasts, and epithelial cells have enhanced adhesion and reduced migration after depletion of cyclin D1.9-11 The cyclin D1K112E mutant that fails to activate CDK4 or CDK6 does not increase cell migration as the wild-type cyclin D1 protein. This suggests that cyclin D1 induction of cell migration is a CDK-dependent function.10,11 Some CDK4/CDK6 substrates have roles in cell adhesion, cell migration, and cytoskeletal remodeling. Phosphorylation of these substrates such as filamin A12 and Ral GEF Rgl213 by cyclin D1–CDK4 contributes to enhanced cell detachment and motility.
Cyclin D1 binding of p27Kip1 contributes to cellular migration. p27Kip1 has effects on cell migration in either a Rac- or a RhoA-dependent manner through inhibition of RhoA.14 Introduction of p27Kip1 rescued the cellular migratory defect of cyclin D1−/− cells. Cyclin D1 cannot induce migration following p27Kip1 knockdown. This suggests that cyclin D1 association with p27Kip1 may contribute to cyclin D1 functions in cell migration independent of CDK4/CDK6.11 In addition, cyclin D1 promotes cellular migration by firstly binding p27Kip1 and thereby inhibiting Rho GTPase activity and secondly by transcriptional upregulation of ROCKII and thrombospondin (TSP-1). The frequent amplification and overexpression of cyclin D1 in cancer cells15,16 and its upregulation by mitogenic growth factors, cytokines, ECM proteins, and other genes,17 which are important in malignant development, suggest that cyclin D1 may have a central role in mediating the invasion and metastasis of cancer cells by controlling Rho/ROCK signaling and expression of TSP-1.10
Alternate noncanonical roles for cyclins continue to be discovered; they have important implications for their role in cancer and metastasis. The traditional role for cyclin A2 is in the somatic cell cycle at 2 critical points, when it activates CDK2 at the onset of DNA replication and when it activates CDK1 during G2-M transition. During S phase, cyclin A2 is mostly located in the nucleus, where it regulates the initiation and progression of DNA synthesis.18 Cyclin A2 localizes to the centrosomes in the cytoplasm, where it binds to the poles of mitotic spindles in a CDK- independent manner. In recent studies, cyclin A2 regulated cytoskeletal organization and cell migration independently of its binding to CDK.19 Depletion of cyclin A2 causes a change in the distribution of actin filaments and an increase in cell migration. Cyclin A2 interacts with, and activates, RhoA, an actin regulator, which in turn negatively regulates migration. In addition, metastatic cancer cells show less cyclin A2 expression than nonspreading tumor cells.19
Cyclins in the Regulation of Transcription
As well as having defined roles in cell cycle progression, many cyclins also regulate gene transcription and mRNA processing. Over the last 20 years, a large body of work has implicated cyclin D1 in transcriptional regulation.20 Cyclin D1 physically associates with more than 30 other transcription factors20 and regulates the transcriptional activity of estrogen receptor and androgen receptor.21-23 The histone acetyltransferases P/CAF, p300, and AlB1 bind to cyclin D1.23,24 Chromatin immunoprecipitation (ChIP) demonstrated cyclin D1 association within target gene promoters, correlated with deacetylation of histone (H3), in particular at H3 lysine 9. Deacetylation of H3 Lys9 was restored upon the reintroduction of cyclin D1, with concomitant recruitment of HDAC1/HDAC3.17 Thus, cyclin D1 is recruited in the context of local chromatin to specific target genes.25-27 Cyclin D1 recruitment to genomic DNA was also associated with the shuttling of the histone acetyltransferase p300/CBP to regulate genes governing DNA damage repair signaling.26 Cyclin D1 was shown to regulate the activity of p300 in a kinase-independent manner. As p300 is regarded as a transcriptional co- integrator, cyclin D1 was proposed as a regulator of gene transcription through co-occupancy with p300 at target DNA binding sites.26
Protein-coding genes are transcribed by the transcriptional machinery, a multicomplex protein composed of transcription factors (TFIIB, -D, -E, -F, and -H), the Mediator complex, and RNA polymerase II (RNAPII).28 The Mediator complex is conserved in eukaryotes and bridges the gap between transcription factors and RNAPII.29,30 Mediator is required for the transcription of all yeast RNAPII genes.31 Several cyclins are involved in the phosphorylation of the largest subunit of RNAPII to regulate transcription: cyclin C–CDK8, cyclin H–CDK7, cyclin T–CDK9, and cyclin K–CDK12 or CDK13. Phosphorylation of RNAPII occurs in the heptapeptide (YS2PTS5PS7) repeats, referred to as the carboxy terminal domain (CTD).32 A series of phosphorylation events at S2, S5, and S7 of the CTD affects the transcriptional cycle from initiation to elongation and termination.33,34 Cyclin C–CDK8 associates with Mediator proteins Med12 and Med13 to form a subcomplex that interacts with the core Mediator complex to repress activated transcription and does so through phosphorylating the RNAPII CTD and the cyclin H subunit of TFIIH.
The cyclin H–CDK7–Mat1 (CDK-activating kinase [CAK]) complex binds TFIIH and is the principal S5 kinase that eases promoter clearance and enables the transition to transcription initiation, hence providing a direct link between the cell cycle machinery and transcription regulation.35-39 The cyclin H–CDK7–Mat1 complex also interacts directly with transcription factors to regulate their function. Using mouse embryonic fibroblasts (MEFs) and 3T3-L1 cells, phosphorylation of PPARγ by CDK7 blocks lipogenesis.40
In humans, cyclin T (T1, T2, and T2b) and CDK9 associate to form a complex termed PTEFb, which phosphorylates CTD S2 of RNAPII to regulate productive transcription elongation.41-43 Like cyclin H, the level of cyclin T does not oscillate during the cell cycle, suggesting that these cyclins perform necessary functions that are not cell cycle stage specific.44-47 Cyclin T1 expression is regulated during T-cell activation.48-51 Cyclin T–CDK9 are important regulators of several cellular processes including lymphoid development.52 Overexpression of cyclin T is sufficient to induce foci and colony formation in NIH 3T3 cells in vitro and induces tumor growth in Nu/Nu mice in vivo.52 Cyclin T likely contributes to lymphomas derived from B- and T-cell lineages possibly through the inhibition of apoptosis.
Cyclin K binds CDK12 and CDK13, likely in 2 separate complexes to regulate the phosphorylation of S2 and S5 of the RNAPII CTD.53,54 In human cells, depletion of CDK12 results in a marked reduction of CTD S2 phosphorylation. The net effect on gene expression is a downregulation in a modest number of genes; however, these genes have key roles in the DNA damage response (DDR): FANCI, FANCD2, ATR, and BRCA1.53 Consistent with the role in regulating the DDR, depletion of cyclin K–CDK12 results in sensitivity to DNA damaging agents and increased γ-H2AX foci.53 Cyclin L (L1 and L2) is closely related to cyclin K, cyclin T1, and cyclin T2. Cyclin L binds CDK11 and interacts with the family of SR splicing proteins to regulate splicing.55-57 Cyclin L is a candidate oncogene in head and neck cancer.58
Cyclins in the Regulation of Stem Cells
Considerable interest has been given to the hypothesis that stem cells are a central and critical determinant in cancer initiation, maintenance, and recurrence following treatment. The last several years have seen this hypothesis gain in popularity due to a growing body of evidence implicating breast cancer stem cells (BCSCs) as responsible for the origin and maintenance of tumors.59,60 The model proposes that due to the increased longevity of stem cells, they have the propensity to accumulate genetic lesions that could transform the stem cell from a highly controlled and regulated cell to a deregulated abnormal BCSC. The next section will highlight roles for cyclins in stem cells.
Mice lacking cyclin D1 are viable and show deficiencies that are restricted only to a limited set of tissues. Cyclin D1–null animals have hypocellular retinas due to abnormalities in retinal progenitor cell proliferation and retinal cell death.61-63 The abnormality is predicted to be caused by a protracted cell cycle in retinal progenitor cells and early cell cycle exit.64 Cyclin D1–null animals also display defects in mammary gland development; cyclin D1–null females are defective in lobuloalveolar development during pregnancy and cannot lactate.61,62 Cyclin D1–null mice are resistant to breast cancer induced by Neu and Ras oncogenes. However, animals that are lacking cyclin D1 remain fully sensitive to other oncogenic pathways of the mammary epithelium, driven, for example, by c-Myc or Wnt-1.65-67 Thus, a cyclin D1–targeted therapy could be highly effective in the treatment of human breast cancers in which the primary driver is the Ras oncogenic pathway. The requirement of cyclin D1 in normal mammary gland development appears to be kinase independent.68 Cyclin D1K112E knockin to the cyclin D1 allele rescued the female mammary gland defect; however, the knockin animals were still resistant to ErbB2-induced tumorigenesis.69 To explain this puzzling discrepancy, the Hinds laboratory conducted a systematic analysis of the progenitor cell pools in the mammary gland that are dependent on cyclin D1.68 There are 3 fundamental epithelial cell types in the mammary tissue: luminal, myoepithelial, and alveolar cell types that arise during pregnancy and undergo cell death following the cessation of lactation. All 3 cell types are thought to arise from precursor progenitor cells with self-renewal properties. One type of stem/progenitor cell can be identified with cell surface markers CD24med/CD29HI or CD24+/CD49fHI; a second type is reported to be able to establish a fully functional mammary gland upon transplantation (parity- identified mammary cells: PI-MEC).70-75 An analysis on the cyclin D1KE/KE mutant confirmed that the resistance to ErbB2-driven tumorigenesis is linked to near total absence of the PI mammary cells, making those progenitor cells the likely target for ErbB2-induced tumorigenesis. Cyclin D1 kinase activity is therefore required for mammary progenitor cells’ self-renewal and activity. Recently, acute, conditional ablation of cyclin D1 using a floxed model demonstrated the requirement for cyclin D1 in tumor maintenance.76 In an MMTV-ErbB2 mammary carcinoma model, deletion of cyclin D1 resulted in reduced tumor cell proliferation and increased cellular senescence, suggesting that the continued presence of cyclin D1 is required to maintain tumor growth in ErbB2-induced mammary carcinomas.
Cyclins appear to have specific and nonredundant roles in stem cell function. Cyclin C was originally cloned from a screen in Saccharomyces cerevisiae conducted to identify factors that could rescue G1 cyclin deficiency.77,78 Subsequently, cyclin C has been shown to promote the progression from G0 quiescence to G1 and does so in part by binding CDK3 to phosphorylate pRB.79 Recently, an interesting role for cyclin C has been uncovered in the inhibition of hematopoietic stem progenitor cell (HSPC) quiescence.80 Cyclin C expression was induced upon cytokine activation in HSPCs from human cord blood. siRNA to cyclin C increased the quiescent HSPC population, increased the long-term colony-forming ability, and increased the engraftment capacity.
Cyclin A overexpression correlates with poor prognosis in breast cancer, contributes to prostate cancer invasion and metastasis, and may contribute to colorectal carcinogenesis.81-83 Cyclin A levels increase at S-phase onset. Cyclin A binds to the partners CDK1 and CDK2 and phosphorylates targets that regulate DNA replication (e.g., MCM7).84,85 The cyclin A–CDK complex remains high through mitosis, where it functions to initiate chromosomal condensation and nuclear membrane dissolution.86-88 Cyclin A is redundant in fibroblast cell proliferation but is essential for embryonic and hematopoietic stem cells.89
Elevated cyclin H is associated with very high-risk gastrointestinal stromal tumors, and reduced or absent cyclin H expression correlates with lower proliferation in B-cell lymphoma.90,91 The transcriptional regulation elicited by the CAK complex (cyclin H–CDK7–Mat1) impacts embryonic stem (ES) cell differentiation. The complex activates CDK1, CDK2, CDK4, and CDK6 through phosphorylation of the T-loop.92-94 Loss of cyclin H function in ES cells induces the differentiation of ES cells and expansion defects of the inner cellular mass in blastocysts.95 Cyclin H represses ES differentiation possibly through phosphorylation of the negative elongation factor Spt5, an event required for the repressive effect of Spt5 on differentiation.95 CDK7 phosphorylates Spt5 in vitro, and downregulation of Spt5 leads to the same induction of the differentiation program elicited by the loss of cyclin H.95,96 This is likely transcriptionally regulated since Spt5 regulates RNA processing and transcriptional pausing at sites proximal to the promoter.
Cyclins in DNA Damage and Genomic Instability
Genomic DNA is continually subject to insults by damaging ionizing radiation, chemical carcinogens, and reactive oxygen species generated by cellular metabolism.97,98 In addition, cells are sensitized to errors from DNA replication during S phase. In order to maintain genomic integrity, the cell has several preventative mechanisms relayed through the DDR pathway. Defects in the DDR can lead to genomic instability and cancer. Several cyclin-CDK complexes are implicated in the DDR.99
Cyclin D1 abundance was shown to determine the DDR, assessed using γ-H2AX and a comet assay.6 Cyclin D1 was shown to be recruited to the sites of DNA damage, requiring the carboxy terminal exon 5, and to bind directly to RAD51 (a recombinase that drives the homologous recombination process).6 In a subsequent proteomic screening of cyclin D1, interacting proteins revealed a pool of DNA repair proteins; among them, the most notable was also RAD51. Irradiation of cells stimulated cyclin D1 binding to RAD51 and aided RAD51 recruitment to DNA damage foci in a process that was BRCA2 dependent.6 This finding was consistent with a prior finding that cyclin D1 bound BRCA1.100
Reduction of cyclin D1 levels in different types of human cancer cells (mantle cell lymphoma, breast cancer, squamous cell carcinoma, and colorectal cancer) led to the impaired recruitment of RAD51 to the damaged DNA, thus increasing the sensitivity of the cells to radiation. MEFs lacking cyclin D1 showed increased sensitivity to ionizing radiation, which is rescued upon the reintroduction of cyclin D1. This proved to be a kinase-dependent process since the expression of the cyclin D1K112E point mutant and the use of specific CDK4 and CDK6 inhibitors had no effect on the radiation sensitivity. While radiation induces comparable levels of DNA damage in both cyclin D1 control and cyclin D1–depleted cells, the amount of unrepaired DNA after radiation is higher in the cyclin D1–depleted cells.
Cyclin F is unique among the cyclins in that it contains both a cyclin and F-box domain. It does not bind or activate a known CDK and like most cyclins oscillates through the cell cycle. F-box proteins are components of SCF complexes (SKP1–Cullin–F-box); hence, cyclin F acts as a phosphorylation-dependent ubiquitin ligase.101-103 Cyclin F localizes to centrosomes and the nucleus.104 In the cytoplasmic compartment, cyclin F targets centriolar coiled-coil proteins of 110 kDa (CP110) for degradation. CP110 promotes centrosome duplication; therefore, cyclin F inhibits genomic instability by ensuring that a single centrosome duplication event occurs once per cell cycle.105,106 Additionally, cyclin F degrades nucleolar and spindle-associated protein 1 (NuSAP1) to regulate the correct mitotic spindle architecture.107 A nuclear role for cyclin F relates to its regulation of RRM2 (ribonucleotide reductase family member 2).106 RRM2 catalyzes the conversion of ribonucleotides to dNTPs necessary for replication and DNA repair. Failure of cyclin F to degrade RRM2 leads to imbalances in the dNTP pool and increased frequency of genomic mutations. Overexpression of RRM2 leads to lung cancer in mice, and elevated RRM2 in ovarian, colorectal, liver, and breast cancers is associated with poor prognosis.108-112
Downregulation of the cyclin G2 transcript has been linked to various cancers including the thyroid and oral cavity.113,114 Cyclin G1 and cyclin G2 share 53% amino acid identity; however, cyclin G1 lacks the protein-destabilizing PEST domain.115 The cyclin G1 gene has a p53 binding site and is induced in a p53-dependent manner.116 The cyclin G2 gene is a transcriptional target of the p53 homolog, p63.117 Both cyclin G1 and G2 are induced following DNA damage and maintain p53-dependent cell cycle arrest.118,119 Both cyclin G1 and cyclin G2 enhance G2-M checkpoint regulation. In the case of cyclin G1, it may promote or inhibit cell arrest or apoptosis; in the case of cyclin G2, cell cycle arrest is thought to occur through the inhibition of cyclin B1–Cdc2.120-124
Cyclins in the Regulation of Chromosomal Instability
Chromosomal instability (CIN) is a prevalent feature widely shared by cells from solid tumors and is considered a hallmark of cancer.125,126 CIN can be caused by multiple mechanisms and results in an abnormal chromosomal complement. Whether CIN is a cause or a consequence of cancer is a highly debated topic; however, CIN does occur early in cancer development and is associated with poor prognosis.127 Cyclin D1 is overexpressed in the majority of human breast tumors. Several lines of evidence suggest that, although cyclin D1 is required for tumorigenesis, cyclin D1 conveys a number of cyclin D1 kinase-independent functions. Further, several lines of evidence suggest that the oncogenic function of cyclin D1 may not correlate with its ability to phosphorylate pRB. In this regard, cyclin D1 overexpression does not correlate with pRB phosphorylation or the proliferative marker Ki67 in human breast cancer.128-132
Based on a significant number of publications from our laboratory, and others, we had proposed an alternative mechanism by which cyclin D1 may drive tumorigenesis by inducing CIN.133 Early studies showed that cyclin D1 did not induce aneuploidy in rat embryonic fibroblasts.134 A subsequent study on mouse primary hepatocytes showed that transiently expressing cyclin D1 induced abnormal mitosis, accumulation of supernumerary centrosomes, defects in the mitotic spindle, and aneuploidy.135 Copy number changes in cyclin D1 have been proposed as a biomarker for CIN in bladder cancer.135 Cyclin D1 gene amplification was only seen in CIN-positive bladder cancer samples and correlated with tumor grade. Our studies, using ChIP of cyclin D1 followed by sequencing (ChIP-Seq), demonstrated that gene regulatory elements bound by cyclin D1 are enriched for genes that govern CIN.8 Using mammary epithelial-targeted transgenics, the CIN genetic signature136 was enriched through cyclin D1 overexpression and reduced through the induction of mammary gland–targeted, inducible cyclin D1 antisense.8 FACS and spectral karyotyping demonstrated that chromosomal duplication was induced by cyclin D1 within 5 cellular divisions. Furthermore, in our studies of 2,254 patients, we showed that cyclin D1 expression correlates with the induction of CIN in luminal B breast cancer.8 These studies suggest that cyclin D1 promotes CIN as a direct consequence of inducing expression of the mitotic transcriptional program regulated by cyclin D1.
Overexpression of cyclin B1 has been reported in various human tumors, such as colorectal cancer, non–small cell lung cancer, and head and neck squamous cell carcinoma, and its upregulation is closely associated with poor prognosis in breast cancer.137-141 In addition, overexpression of cyclin B1 is related to aneuploidy and high proliferation of human mammary carcinomas.141 In yeast, overexpression of cyclin B1 and cyclin B2 leads to CIN.142 In eukaryotes, the maturation/M phase–promoting factor (MPF) regulates entry into M phase.143,144 The MPF complex is composed of cyclin B1 and CDK1 and is sufficient to induce meiotic G2-M–phase transition in immature oocytes and mitosis of somatic cells.145-148 Cyclin B1 mediates chromosome condensation and nuclear envelope dissolution; cyclin B2 mediates Golgi disassembly. The major inhibitor of MPF is protein phosphatase 2A/B55 (PP2A-B55).149,150 MPF suppresses PP2A-B55–dependent inhibition through the Greatwall kinase (Gwl).150,151 Recently, the definition of MPF has been revised to include Gwl as an essential component, at least in frog and starfish oocytes and possibly somatic cells.152 Depletion of Gwl results in G2 arrest in Drosophila and human somatic cells, and Gwl is required for MPF activity in the oocyte cytoplasm.152
The abundance of the cyclin E transcript and protein is increased in carcinomas of the lung, gastrointestinal tract, and breast in addition to lymphomas and leukemias.153-159 Increased cyclin E expression occurs in 18% to 22% of breast cancers and has been used as a prognostic marker.160 A low molecular weight hyperactive version of cyclin E exists in breast cancer and is associated with very poor prognosis.153,161,162 Cyclin E was thought to be an essential cell cycle regulatory protein involved in promoting the G1-S–phase transition.163 Cyclin E binds its catalytic subunit CDK2 and phosphorylates Rb, in a processive event following cyclin D1–CDK4/6, to release E2F transcription factors that regulate genes involved in S-phase progression. However, a cyclin E knockout model demonstrated that mitotic cell division does not require cyclin E1 and E2, challenging the key requirement for cyclin E in S-phase progression. In addition, development in CDK2-null mice was normal, and null fibroblasts demonstrated a normal cell cycle profile. However, these models do underscore several requirements for cyclin E. Endoreplication of placental trophoblast giant cells and megakaryocytes is severely impaired in cyclin E–null mice.164,165 In addition, null MEFs are resistant to oncogenic transformation, fail to progress from quiescence into S phase, and showed a defect in MCM loading onto replication forks. The absence of these key defects in the CDK2-null mouse may be due to the redundancy offered by cyclin E–CDK1 complexes. Interestingly, the G0-S phase and replication licensing functions of cyclin E were found to be kinase independent. Aberrant cyclin E expression may be caused by gene amplification, defects in the p16–Rb–cyclin D1 signaling axis, or defects in the ubiquitin-mediated degradation pathway.166-168 Increased cyclin E abundance may be a consequence of increased proliferation rates since it often correlates with proliferation indices.169 However, it may itself act as a molecular driver of transformation and do so through CIN. Induction of cyclin E in rat fibroblasts or human epithelial cells caused aneuploidy.134 In primary human cells, deregulated cyclin E expression and defective p53 led to increased ploidy and genetic instability. In a lung mouse model, high expression of a degradation-resistant cyclin E targeted to the lung frequently caused dysplasia, multiple lung adenocarcinomas, and tumors exhibiting CIN.170
Summary
The molecular interplay between the cell cycle, cyclins, and cell function is far from being fully understood. Conceptual advances in the field continue to uncover novel and interesting roles for cyclins in cellular processes that contribute to cancer and disease. In the case of cyclin D1, traditionalists place the protein solely in the RB-E2F signaling axis as a driver of proliferation. However, new functions are emerging that would directly place cyclin D1 as a contributor to cellular transformation and would better explain cyclin D1’s role in oncogenesis, particularly in the fields of stem cell regulation, DDR, and chromosomal stability (Figure 1). Uncovering a breast stem cell population that is dependent on cyclin D1 fits well with previous data that suggest that cyclin D1 is a key inhibitor of differentiation. A contribution of cyclin D1 to enhance DNA repair may protect transformed cells from excessive genomic instability and may help protect breast cancer cells when challenged with DNA-damaging therapies. Cyclin D1 promoting whole-genome chromosome instability is a new discovery. This role for cyclin D1 may be particularly important in the 15% of breast cancers with clonally selected cyclin D1 amplification in which cyclin D1 may be an early driver of oncogenesis through CIN. Future studies should focus on deciphering the key events that cyclins regulate, which instigate and perpetuate cellular transformation.
Figure 1.
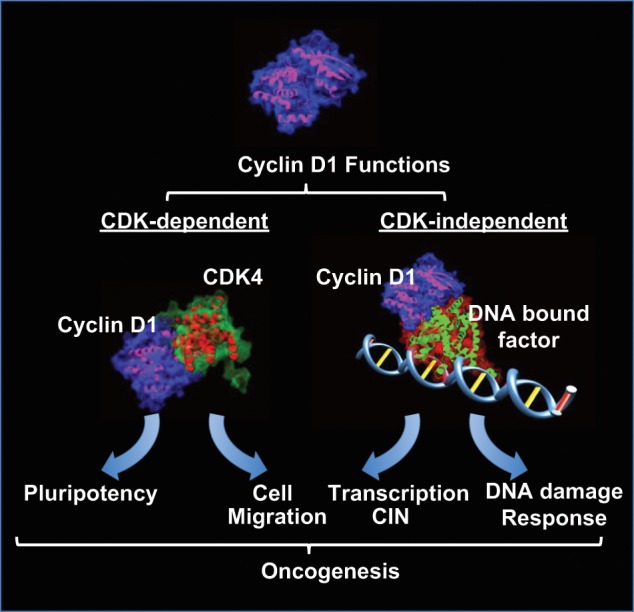
Cyclin D1 forms a holoenzyme through binding CDK4 to elicit kinase-dependent functions that regulate stem cell self-renewal and promote cellular migration. Cyclin D1 functions in a kinase-independent manner to enhance DNA repair and also binds DNA in the context of chromatin to regulate the expression of genes governing CIN. These noncanonical kinase-dependent and -independent functions may contribute to the oncogenic potential of cyclin D1.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article: This project was funded in part by a grant from the Pennsylvania Department of Health (R.G.P.) and the Breast Cancer Research Foundation (R.G.P.). This project was also funded in part from the Dr. Ralph and Marian C. Falk Medical Research Trust (R.G.P.). The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
References
- 1. Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630-41 [DOI] [PubMed] [Google Scholar]
- 2. Musgrove EA, Lee CS, Buckley MF, Sutherland RL. Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc Natl Acad Sci U S A. 1994;91:8022-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558-72 [DOI] [PubMed] [Google Scholar]
- 4. Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439-47 [DOI] [PubMed] [Google Scholar]
- 5. Li Z, Wang C, Prendergast GC, Pestell RG. Cyclin D1 functions in cell migration. Cell Cycle. 2006;5:2440-2 [DOI] [PubMed] [Google Scholar]
- 6. Li Z, Jiao X, Wang C, et al. Alternative cyclin D1 splice forms differentially regulate the DNA damage response. Cancer Res. 2010;70:8802-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jirawatnotai S, Hu Y, Michowski W, et al. A function for cyclin D1 in DNA repair uncovered by protein interactome analyses in human cancers. Nature. 2011;474:230-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casimiro MC, Crosariol M, Loro E, et al. ChIP sequencing of cyclin D1 reveals a transcriptional role in chromosomal instability in mice. J Clin Invest. 2012;122:833-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neumeister P, Pixley FJ, Xiong Y, et al. Cyclin D1 governs adhesion and motility of macrophages. Mol Biol Cell. 2003;14:2005-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Z, Wang C, Jiao X, et al. Cyclin D1 regulates cellular migration through the inhibition of thrombospondin 1 and ROCK signaling. Mol Cell Biol. 2006;26:4240-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Z, Jiao X, Wang C, et al. Cyclin D1 induction of cellular migration requires p27(KIP1). Cancer Res. 2006;66:9986-94 [DOI] [PubMed] [Google Scholar]
- 12. Zhong Z, Yeow WS, Zou C, et al. Cyclin D1/cyclin-dependent kinase 4 interacts with filamin A and affects the migration and invasion potential of breast cancer cells. Cancer Res. 2010;70:2105-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernandez RM, Ruiz-Miro M, Dolcet X, Aldea M, Gari E. Cyclin D1 interacts and collaborates with Ral GTPases enhancing cell detachment and motility. Oncogene. 2011;30:1936-46 [DOI] [PubMed] [Google Scholar]
- 14. Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beroukhim R, Mermel CH, Porter D, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899-905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Santarius T, Shipley J, Brewer D, Stratton MR, Cooper CS. A census of amplified and overexpressed human cancer genes. Nat Rev Cancer. 2010;10:59-64 [DOI] [PubMed] [Google Scholar]
- 17. Klein EA, Assoian RK. Transcriptional regulation of the cyclin D1 gene at a glance. J Cell Sci. 2008;121:3853-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 2992;11:961-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arsic N, Bendris N, Peter M, et al. A novel function for cyclin A2: control of cell invasion via RhoA signaling. J Cell Biol. 2012;196:147-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439-47 [DOI] [PubMed] [Google Scholar]
- 21. Zwijsen RM, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides RJ. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405-15 [DOI] [PubMed] [Google Scholar]
- 22. Knudsen KE, Cavenee WK, Arden KC. D-type cyclins complex with the androgen receptor and inhibit its transcriptional transactivation ability. Cancer Res. 1999;59:2297-301 [PubMed] [Google Scholar]
- 23. Reutens AT, Fu M, Wang C, et al. Cyclin D1 binds the androgen receptor and regulates hormone-dependent signaling in a p300/CBP-associated factor (P/CAF)-dependent manner. Mol Endocrinol. 2001;15:797-811 [DOI] [PubMed] [Google Scholar]
- 24. Zwijsen RM, Buckle RS, Hijmans EM, Loomans CJ, Bernards R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 1998;12:3488-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hulit J, Wang C, Li Z, et al. Cyclin D1 genetic heterozygosity regulates colonic epithelial cell differentiation and tumor number in ApcMin mice. Mol Cell Biol. 2004;24:7598-611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fu M, Wang C, Rao M, et al. Cyclin D1 represses p300 transactivation through a cyclin-dependent kinase-independent mechanism. J Biol Chem. 2005;280:29728-42 [DOI] [PubMed] [Google Scholar]
- 27. Bienvenu F, Barre B, Giraud S, Avril S, Coqueret O. Transcriptional regulation by a DNA-associated form of cyclin D1. Mol Biol Cell. 2005;16:1850-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sikorski TW, Buratowski S. The basal initiation machinery: beyond the general transcription factors. Curr Opin Cell Biol. 2009;21:344-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kornberg RD. Mediator and the mechanism of transcriptional activation. Trends Biochem Sci. 2005;30:235-9 [DOI] [PubMed] [Google Scholar]
- 30. Malik S, Roeder RG. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat Rev Genet. 2010;11:761-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holstege FC, Jennings EG, Wyrick JJ, et al. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717-28 [DOI] [PubMed] [Google Scholar]
- 32. Phatnani HP, Greenleaf AL. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 2006;20:2922-36 [DOI] [PubMed] [Google Scholar]
- 33. Chapman RD, Heidemann M, Hintermair C, Eick D. Molecular evolution of the RNA polymerase II CTD. Trends Genet. 2008;24:289-96 [DOI] [PubMed] [Google Scholar]
- 34. Egloff S, Murphy S. Cracking the RNA polymerase II CTD code. Trends Genet. 2008;24:280-8 [DOI] [PubMed] [Google Scholar]
- 35. Yankulov KY, Bentley DL. Regulation of CDK7 substrate specificity by MAT1 and TFIIH. EMBO J. 1997;16:1638-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roy R, Adamczewski JP, Seroz T, et al. The MO15 cell cycle kinase is associated with the TFIIH transcription-DNA repair factor. Cell. 1994;79:1093-101 [DOI] [PubMed] [Google Scholar]
- 37. Devault A, Martinez AM, Fesquet D, et al. MAT1 (‘menage a trois’) a new RING finger protein subunit stabilizing cyclin H-cdk7 complexes in starfish and Xenopus CAK. EMBO J. 1995;14:5027-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shiekhattar R, Mermelstein F, Fisher RP, et al. Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature. 1995;374:283-7 [DOI] [PubMed] [Google Scholar]
- 39. Adamczewski JP, Rossignol M, Tassan JP, Nigg EA, Moncollin V, Egly JM. MAT1, cdk7 and cyclin H form a kinase complex which is UV light-sensitive upon association with TFIIH. EMBO J. 1996;15:1877-84 [PMC free article] [PubMed] [Google Scholar]
- 40. Helenius K, Yang Y, Alasaari J, Makela TP. Mat1 inhibits peroxisome proliferator-activated receptor gamma-mediated adipocyte differentiation. Mol Cell Biol. 2009;29:315-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Price DH. P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol Cell Biol. 2000;20:2629-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peng J, Zhu Y, Milton JT, Price DH. Identification of multiple cyclin subunits of human P-TEFb. Genes Dev. 1998;12:755-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gegonne A, Weissman JD, Lu H, et al. TFIID component TAF7 functionally interacts with both TFIIH and P-TEFb. Proc Natl Acad Sci U S A. 2008;105:5367-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moiola C, De Luca P, Gardner K, Vazquez E, De Siervi A. Cyclin T1 overexpression induces malignant transformation and tumor growth. Cell Cycle. 2010;9:3119-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Poon RY, Yamashita K, Howell M, Ershler MA, Belyavsky A, Hunt T. Cell cycle regulation of the p34cdc2/p33cdk2-activating kinase p40MO15. J Cell Sci. 1994;107:2789-99 [DOI] [PubMed] [Google Scholar]
- 46. Tassan JP, Schultz SJ, Bartek J, Nigg EA. Cell cycle analysis of the activity, subcellular localization, and subunit composition of human CAK (CDK-activating kinase). J Cell Biol. 1994;127:467-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brown AJ, Jones T, Shuttleworth J. Expression and activity of p40MO15, the catalytic subunit of cdk-activating kinase, during Xenopus oogenesis and embryogenesis. Mol Biol Cell. 1994;5:921-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garriga J, Peng J, Parreno, Price DH, Henderson EE, Grana X. Upregulation of cyclin T1/CDK9 complexes during T cell activation. Oncogene. 1998;17:3093-102 [DOI] [PubMed] [Google Scholar]
- 49. Ghose R, Liou LY, Herrmann CH, Rice AP. Induction of TAK (cyclin T1/P-TEFb) in purified resting CD4(+) T lymphocytes by combination of cytokines. J Virol. 2001;75:11336-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Herrmann CH, Carroll RG, Wei P, Jones KA, Rice AP. Tat-associated kinase, TAK, activity is regulated by distinct mechanisms in peripheral blood lymphocytes and promonocytic cell lines. J Virol. 1998;72:9881-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marshall RM, Salerno D, Garriga J, Grana X. Cyclin T1 expression is regulated by multiple signaling pathways and mechanisms during activation of human peripheral blood lymphocytes. J Immunol. 2005;175:6402-11 [DOI] [PubMed] [Google Scholar]
- 52. Bellan C, De Falco G, Lazzi S, et al. CDK9/CYCLIN T1 expression during normal lymphoid differentiation and malignant transformation. J Pathol. 2004;203:946-52 [DOI] [PubMed] [Google Scholar]
- 53. Blazek D, Kohoutek J, Bartholomeeusen K, et al. The Cyclin K/Cdk12 complex maintains genomic stability via regulation of expression of DNA damage response genes. Genes Dev. 2011;25:2158-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bartkowiak B, Liu P, Phatnani HP, et al. CDK12 is a transcription elongation-associated CTD kinase, the metazoan ortholog of yeast Ctk1. Genes Dev. 2010;24:2303-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang L, Li N, Wang C, et al. Cyclin L2, a novel RNA polymerase II-associated cyclin, is involved in pre-mRNA splicing and induces apoptosis of human hepatocellular carcinoma cells. J Biol Chem. 2004;279:11639-48 [DOI] [PubMed] [Google Scholar]
- 56. Dickinson LA, Edgar AJ, Ehley J, Gottesfeld JM. Cyclin L is an RS domain protein involved in pre-mRNA splicing. J Biol Chem. 2002;277:25465-73 [DOI] [PubMed] [Google Scholar]
- 57. Loyer P, Trembley JH, Grenet JA, et al. Characterization of cyclin L1 and L2 interactions with CDK11 and splicing factors: influence of cyclin L isoforms on splice site selection. J Biol Chem. 2008;283:7721-32 [DOI] [PubMed] [Google Scholar]
- 58. Redon R, Hussenet T, Bour G, et al. Amplicon mapping and transcriptional analysis pinpoint cyclin L as a candidate oncogene in head and neck cancer. Cancer Res. 2002;62:6211-7 [PubMed] [Google Scholar]
- 59. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sicinski P, Donaher JL, Parker SB, et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621-30 [DOI] [PubMed] [Google Scholar]
- 62. Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364-72 [DOI] [PubMed] [Google Scholar]
- 63. Ma C, Papermaster D, Cepko CL. A unique pattern of photoreceptor degeneration in cyclin D1 mutant mice. Proc Natl Acad Sci U S A. 1998;95:9938-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Das G, Choi Y, Sicinski P, Levine EM. Cyclin D1 fine-tunes the neurogenic output of embryonic retinal progenitor cells. Neural Dev. 2009;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017-21 [DOI] [PubMed] [Google Scholar]
- 66. Rowlands TM, Pechenkina IV, Hatsell SJ, Pestell RG, Cowin P. Dissecting the roles of beta-catenin and cyclin D1 during mammary development and neoplasia. Proc Natl Acad Sci U S A. 2003;100:11400-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reddy HK, Mettus RV, Rane SG, Grana X, Litvin J, Reddy EP. Cyclin-dependent kinase 4 expression is essential for neu-induced breast tumorigenesis. Cancer Res. 2005;65:10174-8 [DOI] [PubMed] [Google Scholar]
- 68. Jeselsohn R, Brown NE, Arendt L, et al. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell. 2010;17:65-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Landis MW, Pawlyk BS, Li T, Sicinski P, Hinds PW. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell. 2006;9:13-22 [DOI] [PubMed] [Google Scholar]
- 70. Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84-8 [DOI] [PubMed] [Google Scholar]
- 71. Mack PD, Lester VK, Promislow DE. Age-specific effects of novel mutations in Drosophila melanogaster II. Fecundity and male mating ability. Genetica. 2000;110:31-41 [DOI] [PubMed] [Google Scholar]
- 72. Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2005;24:552-60 [DOI] [PubMed] [Google Scholar]
- 73. Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene. 2004;23:6980-5 [DOI] [PubMed] [Google Scholar]
- 74. Smith GH, Medina D. Re-evaluation of mammary stem cell biology based on in vivo transplantation. Breast Cancer Res. 2008;10:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wagner KU, Boulanger CA, Henry MD, et al. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377-86 [DOI] [PubMed] [Google Scholar]
- 76. Choi YJ, Li X, Hydbring P, et al. The requirement for cyclin d function in tumor maintenance. Cancer Cell. 2012;22:438-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lew DJ, Dulic V, Reed SI. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991;66:1197-206 [DOI] [PubMed] [Google Scholar]
- 78. Leopold P, O’Farrell PH. An evolutionarily conserved cyclin homolog from Drosophila rescues yeast deficient in G1 cyclins. Cell. 1991;66: 1207-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ren S, Rollins BJ. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell. 2004;117:239-51 [DOI] [PubMed] [Google Scholar]
- 80. Miyata Y, Liu Y, Jankovic V, et al. Cyclin C regulates human hematopoietic stem/progenitor cell quiescence. Stem Cells. 2010;28:308-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Baldini E, Camerini A, Sgambato A, et al. Cyclin A and E2F1 overexpression correlate with reduced disease-free survival in node-negative breast cancer patients. Anticancer Res. 2006;26:4415-21 [PubMed] [Google Scholar]
- 82. Wegiel B, Bjartell A, Tuomela J, et al. Multiple cellular mechanisms related to cyclin A1 in prostate cancer invasion and metastasis. J Natl Cancer Inst. 2008;100:1022-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li JQ, Miki H, Wu F, et al. Cyclin A correlates with carcinogenesis and metastasis, and p27(kip1) correlates with lymphatic invasion, in colorectal neoplasms. Hum Pathol. 2002;33:1006-15 [DOI] [PubMed] [Google Scholar]
- 84. Rosenberg AR, Zindy F, Le Deist F, et al. Overexpression of human cyclin A advances entry into S phase. Oncogene. 1995;10:1501-9 [PubMed] [Google Scholar]
- 85. Chibazakura T, Kamachi K, Ohara M, Tane S, Yoshikawa H, Roberts JM. Cyclin A promotes S-phase entry via interaction with the replication licensing factor Mcm7. Mol Cell Biol. 2011;31:248-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Furuno N, den Elzen N, Pines J. Human cyclin A is required for mitosis until mid prophase. J Cell Biol. 1999;147:295-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pagano M, Draetta G. Cyclin A, cell cycle control and oncogenesis. Prog Growth Factor Res. 1991;3:267-77 [DOI] [PubMed] [Google Scholar]
- 88. Gong D, Pomerening JR, Myers JW, et al. Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of cyclin B1. Curr Biol. 2007;17:85-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kalaszczynska I, Geng Y, Iino T, et al. Cyclin A is redundant in fibroblasts but essential in hematopoietic and embryonic stem cells. Cell. 2009;138:352-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dorn J, Spatz H, Schmieder M, et al. Cyclin H expression is increased in GIST with very-high risk of malignancy. BMC Cancer. 2010;10:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bavi P, Abubaker J, Hussain A, et al. Reduced or absent cyclin H expression is an independent prognostic marker for poor outcome in diffuse large B-cell lymphoma. Hum Pathol. 2008;39:885-94 [DOI] [PubMed] [Google Scholar]
- 92. Solomon MJ, Harper JW, Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993;12:3133-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Poon RY, Yamashita K, Adamczewski JP, Hunt T, Shuttleworth J. The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J. 1993;12:3123-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fesquet D, Labbe JC, Derancourt J, et al. The MO15 gene encodes the catalytic subunit of a protein kinase that activates cdc2 and other cyclin-dependent kinases (CDKs) through phosphorylation of Thr161 and its homologues. EMBO J. 1993;12:3111-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Patel SA, Simon MC. Functional analysis of the Cdk7.cyclin H.Mat1 complex in mouse embryonic stem cells and embryos. J Biol Chem. 2010;285:15587-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Larochelle S, Batliner J, Gamble MJ, et al. Dichotomous but stringent substrate selection by the dual-function Cdk7 complex revealed by chemical genetics. Nat Struct Mol Biol. 2006;13:55-62 [DOI] [PubMed] [Google Scholar]
- 97. Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481:287-94 [DOI] [PubMed] [Google Scholar]
- 98. Giglia-Mari G, Zotter A, Vermeulen W. DNA damage response. Cold Spring Harb Perspect Biol. 2011;3:a000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Johnson N, Shapiro GI. Cyclin-dependent kinases (cdks) and the DNA damage response: rationale for cdk inhibitor-chemotherapy combinations as an anticancer strategy for solid tumors. Expert Opin Ther Targets. 2010; 14:1199-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wang C, Fan S, Li Z, et al. Cyclin D1 antagonizes BRCA1 repression of estrogen receptor alpha activity. Cancer Res. 2005;65:6557-67 [DOI] [PubMed] [Google Scholar]
- 101. Bai C, Sen P, Hofmann K, et al. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263-74 [DOI] [PubMed] [Google Scholar]
- 102. Fung TK, Siu WY, Yam CH, Lau A, Poon RY. Cyclin F is degraded during G2-M by mechanisms fundamentally different from other cyclins. J Biol Chem. 2002;277:35140-9 [DOI] [PubMed] [Google Scholar]
- 103. Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739-51 [DOI] [PubMed] [Google Scholar]
- 104. D’Angiolella V, Donato V, Vijayakumar S, et al. SCF(Cyclin F) controls centrosome homeostasis and mitotic fidelity through CP110 degradation. Nature. 2010;466:138-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chen Z, Indjeian VB, McManus M, Wang L, Dynlacht BD. CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev Cell. 2002;3:339-50 [DOI] [PubMed] [Google Scholar]
- 106. D’Angiolella V, Donato V, Forrester FM, et al. Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell. 2012;149:1023-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Emanuele MJ, Elia AE, Xu Q, et al. Global identification of modular cullin-RING ligase substrates. Cell. 2011;147:459-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Xu X, Page JL, Surtees JA, et al. Broad overexpression of ribonucleotide reductase genes in mice specifically induces lung neoplasms. Cancer Res. 2008;68:2652-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ferrandina G, Mey V, Nannizzi S, et al. Expression of nucleoside transporters, deoxycitidine kinase, ribonucleotide reductase regulatory subunits, and gemcitabine catabolic enzymes in primary ovarian cancer. Cancer Chemother Pharmacol. 2010;65:679-86 [DOI] [PubMed] [Google Scholar]
- 110. Grade M, Hummon AB, Camps J, et al. A genomic strategy for the functional validation of colorectal cancer genes identifies potential therapeutic targets. Int J Cancer. 2011;128:1069-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Satow R, Shitashige M, Kanai Y, et al. Combined functional genome survey of therapeutic targets for hepatocellular carcinoma. Clin Cancer Res. 2010;16:2518-28 [DOI] [PubMed] [Google Scholar]
- 112. Kretschmer C, Sterner-Kock A, Siedentopf F, Schoenegg W, Schlag PM, Kemmner W. Identification of early molecular markers for breast cancer. Mol Cancer. 2011;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ito Y, Yoshida H, Uruno T, et al. Decreased expression of cyclin G2 is significantly linked to the malignant transformation of papillary carcinoma of the thyroid. Anticancer Res. 2003;23:2335-8 [PubMed] [Google Scholar]
- 114. Kim Y, Shintani S, Kohno Y, Zhang R, Wong DT. Cyclin G2 dysregulation in human oral cancer. Cancer Res. 2004;64:8980-6 [DOI] [PubMed] [Google Scholar]
- 115. Horne MC, Goolsby GL, Donaldson KL, Tran D, Neubauer M, Wahl AF. Cyclin G1 and cyclin G2 comprise a new family of cyclins with contrasting tissue-specific and cell cycle-regulated expression. J Biol Chem. 1996;271:6050-61 [DOI] [PubMed] [Google Scholar]
- 116. Okamoto K, Beach D. Cyclin G is a transcriptional target of the p53 tumor suppressor protein. EMBO J. 1994;13:4816-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Adorno M, Cordenonsi M, Montagner M, et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87-98 [DOI] [PubMed] [Google Scholar]
- 118. Bennin DA, Don AS, Brake T, et al. Cyclin G2 associates with protein phosphatase 2A catalytic and regulatory B’ subunits in active complexes and induces nuclear aberrations and a G1/S hase cell cycle arrest. J Biol Chem. 2002;277:27449-67 [DOI] [PubMed] [Google Scholar]
- 119. Arachchige-Don AS, Dallapiazza RF, Bennin DA, Brake T, Cowan CE, Horne MC. Cyclin G2 is a centrosome-associated nucleocytoplasmic shuttling protein that influences microtubule stability and induces a p53-dependent cell cycle arrest. Exp Cell Res. 2006;312:4181-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Shimizu A, Nishida J, Ueoka Y, et al. CyclinG contributes to G2/M arrest of cells in response to DNA damage. Biochem Biophys Res Commun. 1998;242:529-33 [DOI] [PubMed] [Google Scholar]
- 121. Kimura SH, Ikawa M, Ito A, Okabe M, Nojima H. Cyclin G1 is involved in G2/M arrest in response to DNA damage and in growth control after damage recovery. Oncogene. 2001;20:3290-300 [DOI] [PubMed] [Google Scholar]
- 122. Okamoto K, Prives C. A role of cyclin G in the process of apoptosis. Oncogene. 1999;18:4606-15 [DOI] [PubMed] [Google Scholar]
- 123. Seo HR, Lee DH, Lee HJ, et al. Cyclin G1 overcomes radiation-induced G2 arrest and increases cell death through transcriptional activation of cyclin B1. Cell Death Differ. 2006;13:1475-84 [DOI] [PubMed] [Google Scholar]
- 124. Zimmermann M, Arachchige-Don AS, Donaldson MS, Dallapiazza RF, Cowan CE, Horne MC. Elevated cyclin G2 expression intersects with DNA damage checkpoint signaling and is required for a potent G2/M checkpoint arrest response to doxorubicin. J Biol Chem. 2012;287:22838-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bakhoum SF, Compton DA. Chromosomal instability and cancer: a complex relationship with therapeutic potential. J Clin Invest. 2012;122:1138-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Holland AJ, Cleveland DW. Losing balance: the origin and impact of aneuploidy in cancer. EMBO Rep. 2012;13:501-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pfau SJ, Amon A. Chromosomal instability and aneuploidy in cancer: from yeast to man. EMBO Rep. 2012;13:515-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Oyama T, Kashiwabara K, Yoshimoto K, Arnold A, Koerner F. Frequent overexpression of the cyclin D1 oncogene in invasive lobular carcinoma of the breast. Cancer Res. 1998;58:2876-80 [PubMed] [Google Scholar]
- 129. van Diest PJ, Michalides RJ, Jannink L, et al. Cyclin D1 expression in invasive breast cancer. Correlations and prognostic value. Am J Pathol. 1997;150:705-11 [PMC free article] [PubMed] [Google Scholar]
- 130. Shoker BS, Jarvis C, Davies MP, Iqbal M, Sibson DR, Sloane JP. Immunodetectable cyclin D(1)is associated with oestrogen receptor but not Ki67 in normal, cancerous and precancerous breast lesions. Br J Cancer. 2001;84:1064-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Weinstat-Saslow D, Merin MJ, Manrow RE, et al. Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nat Med. 1995;1:1257-60 [DOI] [PubMed] [Google Scholar]
- 132. Montanaro L, Vici M, Donati G, et al. Controversial relationship between the expression of the RB pathway components and RB protein phosphorylation in human breast cancer. Histol Histopathol. 2007;22:769-75 [DOI] [PubMed] [Google Scholar]
- 133. Casimiro MC, Pestell RG. Cyclin d1 induces chromosomal instability. Oncotarget. 2012;3:224-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Spruck CH, Won KA, Reed SI. Deregulated cyclin E induces chromosome instability. Nature. 1999;401:297-300 [DOI] [PubMed] [Google Scholar]
- 135. Nelsen CJ, Kuriyama R, Hirsc B, et al. Short term cyclin D1 overexpression induces centrosome amplification, mitotic spindle abnormalities, and aneuploidy. J Biol Chem. 2005;280:768-76 [DOI] [PubMed] [Google Scholar]
- 136. Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet. 2006;38:1043-8 [DOI] [PubMed] [Google Scholar]
- 137. Wang A, Yoshimi N, Ino N, Tanaka T, Mori H. Overexpression of cyclin B1 in human colorectal cancers. J Cancer Res Clin Oncol. 1997;123:124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Soria JC, Jang SJ, Khuri FR, et al. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 2000;60:4000-4 [PubMed] [Google Scholar]
- 139. Hassan KA, Ang K, El-Naggar AK, et al. Cyclin B1 overexpression and resistance to radiotherapy in head and neck squamous cell carcinoma. Cancer Res. 2002;62:6414-7 [PubMed] [Google Scholar]
- 140. Kawamoto H, Koizumi H, Uchikoshi T. Expression of the G2-M checkpoint regulators cyclin B1 and cdc2 in nonmalignant and malignant human breast lesions: immunocytochemical and quantitative image analyses. Am J Pathol. 1997;150:15-23 [PMC free article] [PubMed] [Google Scholar]
- 141. Suzuki T, Urano T, Miki Y, et al. Nuclear cyclin B1 in human breast carcinoma as a potent prognostic factor. Cancer Sci. 2007;98:644-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sarafan-Vasseur N, Lamy A, Bourguignon J, et al. Overexpression of B-type cyclins alters chromosomal segregation. Oncogene. 2002;21:2051-7 [DOI] [PubMed] [Google Scholar]
- 143. Hunt T. Maturation promoting factor, cyclin and the control of M-phase. Curr Opin Cell Biol. 1989;1:268-74 [DOI] [PubMed] [Google Scholar]
- 144. Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503-8 [DOI] [PubMed] [Google Scholar]
- 145. Lohka MJ, Hayes MK, Maller JL. Purification of maturation-promoting factor, an intracellular regulator of early mitotic events. Proc Natl Acad Sci U S A. 1988;85:3009-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gautier J, Norbury C, Lohka M, Nurse P, Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988;54:433-9 [DOI] [PubMed] [Google Scholar]
- 147. Labbe JC, Capon JP, Caput D, et al. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989;8:3053-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990;60:487-94 [DOI] [PubMed] [Google Scholar]
- 149. Castilho PV, Williams BC, Mochida S, Zhao Y, Goldberg ML. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol Biol Cell. 2009;20:4777-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Mochida S, Ikeo S, Gannon J, Hunt T. Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 2009;28:2777-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Vigneron S, Brioudes E, Burgess A, Labbe JC, Lorca T, Castro A. Greatwall maintains mitosis through regulation of PP2A. EMBO J. 2009;28:2786-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hara M, Abe Y, Tanaka T, Yamamoto T, Okumura E, Kishimoto T. Greatwall kinase and cyclin B-Cdk1 are both critical constituents of M-phase-promoting factor. Nat Commun. 2012;3:1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wingate H, Puskas A, Duong M, et al. Low molecular weight cyclin E is specific in breast cancer and is associated with mechanisms of tumor progression. Cell Cycle. 2009;8:1062-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Schraml P, Bucher C, Bissig H, et al. Cyclin E overexpression and amplification in human tumours. J Pathol. 2003;200:375-82 [DOI] [PubMed] [Google Scholar]
- 155. Fukuse T, Hirata T, Naiki H, Hitomi S, Wada H. Prognostic significance of cyclin E overexpression in resected non-small cell lung cancer. Cancer Res. 2000;60:242-4 [PubMed] [Google Scholar]
- 156. Yasui W, Akama Y, Kuniyasu H, et al. Expression of cyclin E in human gastric adenomas and adenocarcinomas: correlation with proliferative activity and p53 status. J Exp Ther Oncol. 1996;1:88-94 [PubMed] [Google Scholar]
- 157. Erlanson M, Landberg G. Prognostic implications of p27 and cyclin E protein contents in malignant lymphomas. Leuk Lymphoma. 2001;40:461-70 [DOI] [PubMed] [Google Scholar]
- 158. Wolowiec D, Mekki Y, Ffrench P, et al. Differential expression of cell proliferation regulatory proteins in B- and T-lineage acute lymphoblastic leukaemias. Br J Haematol. 1996;95: 518-23 [DOI] [PubMed] [Google Scholar]
- 159. Iida H, Towatari M, Tanimoto M, Morishita Y, Kodera Y, Saito H. Overexpression of cyclin E in acute myelogenous leukemia. Blood. 1997;90:3707-13 [PubMed] [Google Scholar]
- 160. Keyomars K, O’Leary N, Molnar G, Lees E, Fingert HJ, Pardee AB. Cyclin E, a potential prognostic marker for breast cancer. Cancer Res. 1994;54:380-5 [PubMed] [Google Scholar]
- 161. Porter DC, Zhang N, Danes C, et al. Tumor-specific proteolytic processing of cyclin E generates hyperactive lower-molecular-weight forms. Mol Cell Biol. 2001;21:6254-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Keyomarsi K, Tucker SL, Buchholz TA, et al. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347:1566-75 [DOI] [PubMed] [Google Scholar]
- 163. Hwang HC, Clurman BE. Cyclin E in normal and neoplastic cell cycles. Oncogene. 2005;24:2776-86 [DOI] [PubMed] [Google Scholar]
- 164. Geng Y, Yu Q, Sicinska E, et al. Cyclin E ablation in the mouse. Cell. 2003;114:431-43 [DOI] [PubMed] [Google Scholar]
- 165. Kollner HJ, Lutterberg C. [The value of functional analysis for orthodontic diagnosis]. Zahn Mund Kieferheilkd Zentralbl. 1988;76:404-8 [PubMed] [Google Scholar]
- 166. Keyomarsi K, Pardee AB. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci U S A. 1993;90:1112-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Akama Y, Yasui W, Yokozaki H, et al. Frequent amplification of the cyclin E gene in human gastric carcinomas. Jpn J Cancer Res. 1995;86:617-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kitahara K, Yasui W, Kuniyasu H, et al. Concurrent amplification of cyclin E and CDK2 genes in colorectal carcinomas. Int J Cancer. 1995;62:25-8 [DOI] [PubMed] [Google Scholar]
- 169. Rudolph P, Kuhling H, Alm P, et al. Differential prognostic impact of the cyclins E and B in premenopausal and postmenopausal women with lymph node-negative breast cancer. Int J Cancer. 2003;105:674-80 [DOI] [PubMed] [Google Scholar]
- 170. Ma Y, Fiering S, Black C, et al. Transgenic cyclin E triggers dysplasia and multiple pulmonary adenocarcinomas. Proc Natl Acad Sci U S A. 2007;104:4089-94 [DOI] [PMC free article] [PubMed] [Google Scholar]


