Abstract
Rosuvastatin is one of the most potent statins available for reducing circulating low-density lipoprotein cholesterol (LDL-C) levels, which enables more high-risk patients to achieve their lipid goals. Its favorable balance of effects on atherogenic and protective lipoproteins and its pleiotropic effects, including anti-inflammatory and antioxidant effects and improvement in endothelial dysfunction, are associated with slowing of progression of atherosclerosis within the artery wall and have been translated into clinical benefits for cardiovascular outcomes. This review provides an update on the safety and the efficacy of rosuvastatin in recent large clinical trials. It appears that rosuvastatin has a beneficial effect on the progression of atherosclerosis across the clinical dosage range of 2.5–40 mg. It reduced cardiovascular events in relatively low-risk subjects with elevated high-sensitivity C-reactive protein and normal low-density lipoprotein cholesterol. As with other statins, rosuvastatin did not show overall benefit in terms of survival in patients with heart failure, but certain clinical or biochemical markers reflecting underlying disease characteristics may help to identify subgroups of patients that benefit from statin therapy. In patients with end-stage renal disease undergoing chronic hemodialysis, rosuvastatin had no effect on reducing cardiovascular events. Although there is a slightly increased risk of incident diabetes with this class of agents, the absolute benefits of statin therapy on cardiovascular events overweigh the risk in patients with moderate or high cardiovascular risk or with documented cardiovascular disease. As with other statins, rosuvastatin is an appropriate therapy in addition to antihypertensive treatment to reduce cardiovascular risk in hypertensive patients.
Keywords: atherosclerosis, cardiovascular disease, lipids, rosuvastatin
Introduction
Rosuvastatin is one of the most potent widely available statins, and is approved for reducing circulating low-density lipoprotein cholesterol (LDL-C) levels. Since it was first introduced in the market about a decade ago, the safety and the efficacy of rosuvastatin have been extensively evaluated in a wide variety of patients in clinical trials and in postmarketing surveillance.1–4 Large-scale clinical studies demonstrate that rosuvastatin provides greater reductions in LDL-C than most other statins, enabling more patients to achieve their LDL-C goals.5 In addition, rosuvastatin has beneficial effects on other components of the atherogenic lipid profile, such as decreasing plasma levels of triglycerides and apolipoprotein B, modifying LDL particle size and LDL subfraction distributions towards a less atherogenic phenotype, and raising plasma high-density lipoprotein cholesterol (HDL-C) concentrations.6–9 As observed with other statins, rosuvastatin has lipid-independent pleiotropic effects, eg, anti-inflammatory, antioxidant, and antithrombotic effects, and can improve endothelial function.10,11 These lipid-modifying and pleiotropic effects of rosuvastatin and other statins have been translated into beneficial effects on atherosclerosis and result in significant reductions in cardiovascular events, as observed in clinical studies.1,3,12,13 This review aims to provide an update on the safety and the efficacy of rosuvastatin in recent large clinical trials.
The materials reviewed were identified by searching PubMed for publications between 2001 to February 2013, using “rosuvastatin” as the search term. The search was limited to clinical studies, and in vitro and animal studies were generally excluded. Articles written in languages other than English were not included. This review focused on larger randomized studies, but referenced smaller-scale studies, observational studies, and other reviews where appropriate. We also searched reference lists of articles identified by this search strategy and selected those judged relevant. The manufacturer’s published information about rosuvastatin was also used.
Pharmacokinetic profiles
Rosuvastatin is a fully synthetic 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor and has dose-linear pharmacokinetics.14 The absolute bioavailability of rosuvastatin is approximately 20%, which is comparable to atorvastatin, fluvastatin, and pravastatin, but higher than that of lovastatin and simvastatin, which are extensively metabolized in the gut and liver (Table 1).15 After a single oral dose, the maximum plasma concentrations of rosuvastatin are reached at 3–5 hours. Rosuvastatin is 88% bound to plasma proteins, mostly albumin, and this binding is reversible and independent of plasma concentrations. The mean volume of distribution of rosuvastatin is approximately 134 L in a steady state.16–18 Rosuvastatin undergoes little metabolism, and only a small proportion of rosuvastatin (about 10%) is recovered as metabolites, mainly N-desmethyl rosuvastatin, which has approximately one-sixth to one-half the HMG-CoA reductase inhibitory activity of rosuvastatin.18 Cytochrome P450 (CYP) 2C9 is the principal isoenzyme responsible for the metabolism of rosuvastatin, with a minimal effect from CYP2C19. Rosuvastatin and its metabolites are primarily excreted in the feces (90%), and the elimination half-life of rosuvastatin is approximately 19 hours after oral administration.16–18
Table 1.
Pharmacokinetic properties of statins
| Parameters | Atorvastatin | Fluvastatin | Lovastatin | Pitavastatin | Pravastatin | Rosuvastatin | Simvastatin |
|---|---|---|---|---|---|---|---|
| Bioavailability, % | 12 | 24–30 | 5 | 60–80 | 18 | 20 | <5 |
| Protein binding, % | >98 | >98 | >98 | >99 | 50 | 88 | >95 |
| Half-life, hours | 7–20 | 1–3 | 2–5 | 10–13 | 1–3 | 19 | 2–5 |
| Hepatic extraction, % | 70 | ≥70 | ≥70 | 80 | 45 | 63 | ≥80 |
| Renal excretion, % | <5 | 6 | 10 | 15 | 20 | 10 | 13 |
| Metabolism | +++ | +++ | +++ | ++ | + | + | +++ |
| CYP | 3A4, 2C8 | 2C9 | 3A4/5, 2C8 | 2C9, 2C8 | 3A4 | 2C9, 2C19 | 3A4/5, 2C8 |
| Influx transporters | SLCO1B1 | SLCO1B1 | SLCO1B1, SLC16A4 | SLCO1B1/1B3/2B1/1A2 | SLCO1B1/2B1 SLC22A8, SLC16A1 | SLCO1B1/1B3/2B1/1A2 SLC10A1 | SLCO1B1 |
| Efflux transporters | ABCB1/G2 | ABCG2 | ABCB1 | ABCB1/C2/G2 | ABCB1/B11/C2/G2 | ABCB1/C2/G2 | ABCB1/G2 |
Abbreviations: ABC, ATP-binding cassette; CYP, cytochrome P450 enzymes; SLC, solute carrier; SLCO, solute carrier organic anion transporter.
Notes: “+” indicates the drug undergoes metabolism; the number of “+” indicates the extent of metabolism.
The plasma concentrations of rosuvastatin in Japanese and in Chinese are approximately double those in Caucasians,18 and this has led to regulatory authorities, including the US Food and Drug Administration (FDA), recommending lower starting doses (5 mg) in Asian patients since 2005.19 In Japan, the recommended starting dose of rosuvastatin is only 2.5 mg.20 Although the contribution of environmental and genetic factors to the observed increases in rosuvastatin drug levels in East Asians have not been fully determined, a functional polymorphism, 421C > A, in the drug-efflux transporter ATP-binding cassette G2 gene (ABCG2) significantly influenced the pharmacokinetics of rosuvastatin in Chinese and Caucasians.21,22 Subjects with one or two copies of the variant allele had plasma rosuvastatin levels twice as high as those with the wild-type genotype.21,22 This variant is more common in Chinese and other East Asians (allele frequency about 35%) than in Caucasians (14%),23 and probably contributes to the ethnic difference in the pharmacokinetics of rosuvastatin.
There were no differences in plasma concentrations of rosuvastatin between men and women or between nonelderly and elderly subjects (age ≥ 65 years).18,24 In patients with severe renal impairment (creatinine clearance < 30 mL/minute/1.73 m2) but not mild to moderate renal impairment (creatinine clearance ≥ 30 mL/minute/1.73 m2), the plasma concentrations of rosuvastatin were significantly increased (to about threefold) compared with healthy subjects.18 Patients on chronic hemodialysis had approximately 50% greater steady-state plasma concentrations of rosuvastatin compared with healthy volunteers with normal renal function.18 The plasma concentrations of rosuvastatin were modestly increased in patients with chronic alcoholic liver disease.18
Since rosuvastatin is minimally metabolized by CYP enzymes, it has a lower risk of drug–drug interactions and related adverse drug reactions than other statins for which the disposition is dependent on CYP enzymes. As a hydrophilic statin, the active transport of rosuvastatin into hepatocytes is largely dependent on the hepatic influx transporter organic anion transporter protein (OATP) 1B1. A recent review summarizing drug–drug interactions with rosuvastatin indicates that drugs that antagonize OATP1B1-mediated hepatic uptake of rosuvastatin are more likely to interact with rosuvastatin.25 A potential pharmacokinetic interaction may occur when rosuvastatin is coadministered with vitamin K antagonists, cyclosporine, gemfibrozil, antiretroviral agents and some oral antidiabetic agents (glyburide, glimepiride, troglitazone, pioglitazone, glipizide, gliclazide, and tolbutamide).25,26
Therapeutic efficacy
Lipids
The lipid-lowering efficacy of rosuvastatin has been extensively studied in clinical studies in patients with a wide range of lipid disorders.1,6,27 The Statin Therapies for Elevated Lipid Levels Compared Across Doses to Rosuvastatin (STELLAR) study, which was a 6-week, parallel-group, open-label, randomized, multicenter trial in over 2400 patients with hypercholesterolemia, compared the lipid-lowering effect of rosuvastatin with other commonly used statins.28 It showed that rosuvastatin (10–40 mg daily) reduced LDL-C by 46%–55% versus (vs) 37%–51% with atorvastatin (10–80 mg), 28%–46% with simvastatin (10–80 mg), and 20%–30% with pravastatin (10–40 mg).6,28 In addition, rosuvastatin also decreased triglycerides more significantly than simvastatin and pravastatin, but was comparable to atorvastatin in this effect. In addition, rosuvastatin increased HDL-C by a mean of 8%–10% compared with 2%–7% in all other statin groups.
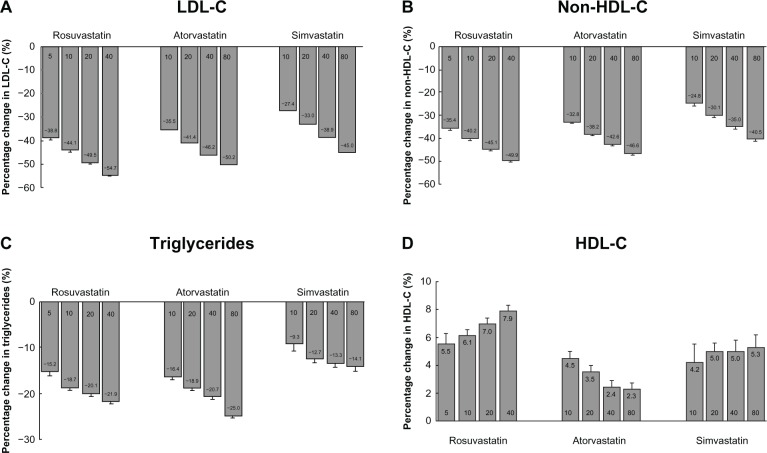
A recent meta-analysis that pooled individual data from 32,258 patients who participated in studies comparing the efficacy of rosuvastatin with that of atorvastatin or simvastatin showed that doubling the statin doses was associated with greater lowering of LDL-C by 4%–6% and non-HDL-C by 3%–6%, with the greatest effect observed in rosuvastatin-treated patients (Figure 1).5 A subsequent analysis on changes in HDL-C showed that the HDL-C-raising ability of rosuvastatin and simvastatin appeared to be comparable, with both being superior to atorvastatin.29 Increases in HDL-C were positively related to statin dose with rosuvastatin and simvastatin but inversely related to dose with atorvastatin. The change in HDL-C with statins was independent of LDL-C change, but was influenced by baseline HDL-C and triglyceride levels and the presence of diabetes.29
Figure 1.
Percentage changes from baseline in (A) LDL-C, (B) non-HDL-C, (C) triglycerides, and (D) HDL-C across dose range for each statin in the VOYAGER database.
Note: Copyright © 2010. Elsevier. Data reproduced from Nicholls SJ, Brandrup-Wognsen G, Palmer M, Barter PJ. Meta-analysis of comparative efficacy of increasing dose of atorvastatin versus rosuvastatin versus simvastatin on lowering levels of atherogenic lipids (from VOYAGER). Am J Cardiol. 2010;105:69–76.5
Copyright © 2010. The American Society for Biochemistry and Molecular Biology. Data reproduced from Barter PJ, Brandrup-Wognsen G, Palmer MK, Nicholls SJ. Effect of statins on HDL-C: a complex process unrelated to changes in LDL-C: analysis of the VOYAGER database. J Lipid Res. 2010;51:1546–1553.29
Abbreviations: LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
It has been reported that rosuvastatin at different doses significantly reduced plasma concentrations of apolipoprotein B, the most readily available measure of LDL particle number, in patients with hypercholesterolemia, hypertriglyceridemia, low HDL-C, mixed dyslipidemia, and heterozygous familial hypercholesterolemia.6 Few studies have directly investigated the effect of rosuvastatin on modifying LDL particle size and LDL subfraction distributions.8,30–34 It appears that the increase in LDL particle size and the favorable effects on LDL subclass distribution with rosuvastatin were more obvious in patients with hypertriglyceridemia.
Blood pressure
Endothelial dysfunction is an early marker of vascular damage and is a common finding in hypertension.35 Current evidence suggests that statins have pleiotropic effects on vascular function, with mechanisms that include an increase in the synthesis of vascular nitric oxide, inhibition of vascular smooth-muscle cell proliferation and migration, anti-inflammatory actions, downregulation of angiotensin II type 1 receptor expression, and antioxidative effects.36–38 It has been suggested that most patients with hypertension should take statin treatment to reduce cardiovascular risk in addition to their antihypertensive treatment.39 Furthermore, it has been reported that statins themselves have a modest blood pressure-lowering effect, particularly in patients with poorly controlled hypertension.40 These effects are considered to be mediated by the beneficial effects of statins on endothelial function, their interactions with the renin–angiotensin system, and their influence on large artery compliance.36,37 Some animal studies showed that rosuvastatin reduced blood pressure in spontaneously hypertensive rats and in rats with C-reactive protein (CRP)-induced endothelial dysfunction and hypertension, and these effects appeared to be related to its endothelial protection and antioxidant effects.41,42 Future randomized controlled trials are needed to investigate the blood pressure-lowering effect of rosuvastatin in patients with increased cardiovascular risk.
Surrogate biomarkers for atherosclerosis
A number of imaging trials have assessed the impact of rosuvastatin on atherosclerosis progression. In these surrogate end-point trials, imaging techniques such as intravascular ultrasound (IVUS) and measurement of carotid intima-media thickness (CIMT) using B-mode ultrasound were used to measure statin-induced changes in CIMT or plaque volume/burden, which were linked to the cardiovascular events.
In the ASTEROID (A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound Derived Coronary Atheroma Burden) study, rosuvastatin 40 mg daily for 24 months produced a significant regression of coronary atherosclerosis in 349 patients with coronary heart disease who had evaluable serial IVUS examinations at the end of the study.43 In this noncomparative and open-label study, rosuvastatin treatment was significantly (P < 0.001) associated with a mean reduction of 53% in LDL-C (from 130.4 mg/dL at baseline to 61 mg/dL) and an increase of 14.7% in HDL-C (from 43.1 mg/dL to 49.0 mg/dL). These lipid changes were associated with a median reduction of −6.8% or −12.5 mm3 in atheroma volume (P < 0.001 vs baseline). Regression of atheroma volume in the most diseased coronary arteries was observed in 78% of participants. There was a direct relationship between the on-treatment LDL-C level and the rate of disease progression, with regression typically occurring in subjects with LDL-C levels below 70 mg/dL.43 A subsequent analysis in 292 patients who had one or more segments with ≥25% diameter stenosis in the coronary angiogram performed at baseline showed there was a significant regression by decreasing percent-diameter stenosis and improving minimum lumen diameter, as measured by quantitative coronary angiography.44 A total of 7.5% of patients showed ≥ 10% change in percent-diameter stenosis for regression, whereas 89.4% had <10% change and 3.1% had progression.44 Another open-label study examined the effect of rosuvastatin (2.5–20 mg for 76 weeks) on plaque volume in Japanese subjects with coronary artery disease, including those receiving prior lipid-lowering therapy. There were significant (P < 0.0001) mean (± SD) reductions in LDL-C by −38.6% ± 16.9% and increases in HDL-C by 19.8% ± 22.9%, and these changes were associated with a −5.1% ± 14.1% reduction in coronary plaque volume assessed by IVUS.45 However, these studies in patients with advanced coronary disease were limited by the uncontrolled design.
The Outcome of Rosuvastatin Treatment on Carotid Artery Atheroma: A Magnetic Resonance Imaging Observation (ORION) trial assessed the effects of rosuvastatin on carotid plaque volume and composition by using magnetic resonance imaging.46 In this randomized, double-blind study, 33 patients with fasting LDL-C ≥ 100 and <250 mg/dL and 16%–79% carotid stenosis by duplex ultrasound were treated with either a low (5 mg) or high (40/80 mg) dose of rosuvastatin for 24 months.46 During the study, the LDL-C was reduced by 38.2% and 59.9% in the low- and high-dose groups, respectively (both P < 0.001). There were no significant changes in carotid plaque volume for either dosage group. However, in patients with a lipid-rich necrotic core at baseline, the mean proportion of the vessel wall composed of lipid-rich necrotic core decreased by 41.4% (P = 0.005). These findings suggested that statin therapy had a beneficial effect on plaque volume and composition.
The Measuring Effects on Intima-Media Thickness: An Evaluation of Rosuvastatin (METEOR) study used CIMT assessment to investigate the impact of rosuvastatin therapy in patients with a low cardiovascular risk (10-year Framingham risk of <10%), mild hypercholesterolemia, and subclinical atherosclerosis, with a maximum CIMT of 1.2–3.5 mm.47 In this randomized, double-blind, placebo-controlled study, treatment with rosuvastatin 40 mg daily was associated with lowering LDL-C by 49% to 78 mg/dL and less CIMT progression over 2 years compared with placebo. The change in maximum CIMT for the carotid sites was −0.0014 mm/year for the rosuvastatin group compared with a progression of 0.0131 mm/year for the placebo group (P < 0.0001).47 These results revealed a positive effect of high dose of rosuvastatin on the progression of atherosclerosis in subjects with early signs of carotid artery disease and at low cardiovascular risk who would not routinely be treated with statin therapy.
However, lower doses of rosuvastatin are usually administered in asymptomatic patients with hypercholesterolemia in clinical practice. Riccioni et al performed an open-label, noncontrolled study to evaluate the effect of rosuvastatin 10 mg daily for 2 years on CIMT in 45 patients with hypercholesterolemia and asymptomatic carotid atherosclerosis (CIMT ≥ 0.8 mm at baseline).48 Rosuvastatin treatment was significantly associated with a 26.6% reduction in left CIMT (1.20 vs 0.90 mm, P < 0.001) and a 22.2% reduction in right CIMT (1.22 vs 0.95 mm, P < 0.001).48 Another small prospective randomized study performed with 38 Japanese patients with chronic kidney disease showed that rosuvastatin 2.5 mg daily for 12 months significantly reduced maximal CIMT (1.89 vs 1.75 mm, P = 0.02) and modified the inflammatory state of these patients.49 A very recent study examined the effect of rosuvastatin on progression of atherosclerosis in 36 HIV-infected patients with asymptomatic carotid atherosclerosis and hypercholesterolemia who were on stable antiretroviral therapy.50 Rosuvastatin 10 mg daily for 24 months led to a significant reduction in IMT in all extracranial carotid arteries, with the greatest magnitude observed in carotid bifurcations (a mean decrease of 18.7% in the right artery and 21.4% in the left artery) and in internal carotid arteries (a mean decrease of 23.7% in the right artery and 25.6% in the left artery).50 The results of these studies suggested a beneficial effect of lower doses of rosuvastatin on atherosclerosis progression.
The Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin Versus Atorvastatin (SATURN) compared the effects of maximal doses of rosuvastatin (40 mg daily) and atorvastatin (80 mg daily) on progression of coronary atherosclerosis in 1039 patients with angiographic coronary artery disease.51 After the 2-year treatment, the two statin regimens resulted in significant and comparable regression of coronary atherosclerosis, with the percentage atheroma volume decreasing by 0.99% with atorvastatin and by 1.22% with rosuvastatin (P = 0.17), although a lower level of LDL-C and a higher level of HDL-C were achieved with rosuvastatin treatment compared with atorvastatin.51
Cardiovascular events
Several large randomized controlled clinical trials have evaluated the effect of rosuvastatin in reducing major adverse cardiovascular events in various clinical settings (Table 2).
Table 2.
Effect of rosuvastatin (R) in the primary and secondary prevention of cardiovascular disease in randomized controlled trials
| Clinical trial | Patients | Duration | Treatment groups | End point | Results |
|---|---|---|---|---|---|
| JUPITER52 | 17,802 apparently healthy individuals with normal levels of LDL-C and increased hsCRP | 1.9 years (median) | R 20 mg vs placebo | Occurrence of first major cardiovascular events, including the combined incidence of nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, arterial revascularization procedures, and deaths due to cardiovascular causes | A 44% reduction in primary end point |
| CORONA58 | 5011 patients ≥ 60 years of age with New York Heart Association class II–IV ischemic, systolic heart failure | 33 months (median) | R 10 mg vs placebo | The primary outcome was death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. Secondary outcomes included death from any cause, any coronary event, death from cardiovascular causes, and the number of hospitalizations | No improvement in the primary outcome or the number of deaths from any cause, but reduced the number of cardiovascular hospitalizations |
| GISSI-HF59 | 4574 adult patients with chronic heart failure of New York Heart Association class II–IV, irrespective of cause and left ventricular ejection fraction | 3.9 years (median) | R 10 mg vs placebo | Time to death, and time to death or admission to hospital for cardiovascular reasons | No effect on the clinical outcomes |
| AURORA65 | 2776 patients, 50–80 years of age, undergoing hemodialysis | 3.8 years (median) | R 10 mg vs placebo | The primary end point was death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. Secondary end points included death from all causes and individual cardiac and vascular events | No effect on the primary or the secondary end point |
Abbreviations: LDL-C, low-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; vs, versus.
Primary prevention
The Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial is the largest clinical study so far to assess the beneficial effect of statin therapy on the primary prevention of cardiovascular disease (CVD).52 A total of 17,802 apparently healthy individuals with relatively normal levels of LDL-C (<130 mg/dL or 3.4 mmol/L) and increased high-sensitivity C-reactive protein (hsCRP) (>2 mg/L) were randomized 1:1 to receive either rosuvastatin 20 mg or matched placebo once daily and were followed up every 6 months. The primary efficacy end point was the occurrence of first major cardiovascular events, including the combined incidence of nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, arterial revascularization procedures, and deaths due to cardiovascular causes. The study was stopped prematurely after a median follow-up of 1.9 years (maximum follow-up of 5 years) because rosuvastatin therapy demonstrated a highly significant 44% reduction in cardiovascular events.52 There were significant differences between rosuvastatin and placebo in the incidence of the individual components of the primary efficacy end point, including nonfatal myocardial infarction (hazard ratio [HR] 0.35, 95% confidence interval [CI] 0.22–0.58), nonfatal stroke (HR 0.52, 95% CI 0.33–0.80), and arterial revascularization (HR 0.54, 95% CI 0.41–0.72).52,53 Participants who achieved the lowest concentrations of both LDL-C and hsCRP had the best outcome.54 This study demonstrated that the use of rosuvastatin is associated with a favorable outcome in patients with evidence of elevated systemic inflammatory markers and one additional risk factor. The result of the JUPITER trial prompted the FDA to approve the use of rosuvastatin – in older subjects (>50 years in men, >60 years in women) with elevated hsCRP levels (>2 mg/L) and at least one additional cardiovascular risk factor – in early 2010.55 In the JUPITER study, rosuvastatin was also found to be effective in reducing the occurrence of symptomatic venous thromboembolism, with 34 events in the rosuvastatin group compared to 60 events in the placebo group (HR with rosuvastatin 0.57, 95% CI 0.37–0.86; P = 0.007).56 However, the JUPITER trial was stopped early after a median follow-up of less than 2 years, and critics of the trial have suggested that the early termination possibly overestimated the treatment effect.57 The effect of longer-term therapy with rosuvastatin in patients with increased risk of CVD remains to be determined.
Patients with heart failure
There are two randomized, double-blind, placebo-controlled trails to evaluate the effect of rosuvastatin on cardiovascular outcome in patients with heart failure: the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) study58 and the Gruppo Italiano per lo Studio della Supravvivenza nell’Insufficienza Cardiac (GISSI HF) study.59 In the CORONA study, rosuvastatin 10 mg daily for 33 months did not reduce the primary outcome, including incidence of cardiovascular death, myocardial infarction and stroke, or total mortality, in older patients with systolic heart failure from ischemic heart disease, although the number of cardiovascular hospitalizations was reduced with rosuvastatin treatment.58 Similarly, rosuvastatin 10 mg had no effect on the clinical outcomes in patients with chronic heart failure of any cause during a follow-up period of a median of 3.9 years in the GISSI HF study.59
Interestingly, subsequent analysis from these two trials suggested that certain clinical or biochemical markers reflecting underlying disease characteristics may identify subgroups of heart-failure patients that benefit from statin therapy. For example, the hsCRP levels decreased by 37.1% after 3 months of rosuvastatin treatment in the CORONA study, and a post hoc analysis showed that patients with an hsCRP concentration ≥ 2.0 mg/L at baseline had a higher rate of the primary outcome in the placebo group compared with patients with an hsCRP concentration < 2.0 mg/L and rosuvastatin improved outcomes in patients with a CRP level of ≥2.0 mg/L at baseline.60 Another analysis suggested that plasma amino-terminal pro-brain natriuretic peptide (NT-proBNP), a marker of cardiac dysfunction and prognosis, could also be used to identify a subgroup of heart-failure patients (NT-proBNP < 103 pmol/L) that benefit from statin therapy.61
It has been suggested that galectin-3, a member of the lectin family that has regulatory roles in fibrogenesis, inflammation, tissue repair, and cell proliferation, may play a role in the pathophysiology of heart failure through promotion of myocardial fibrosis and inflammation.62,63 A recent analysis in the CORONA participants showed that patients with lower galectin-3 levels (≤19.0 ng/mL), potentially reflecting lower and reversible levels of myocardial fibrosis, appeared to benefit from rosuvastatin therapy.63 Furthermore, patients with low levels of both galectin-3 and NT-proBNP demonstrated the lowest rate of adverse outcomes with rosuvastatin treatment.63
Latini et al further investigated whether pentraxin-3, a component of the humeral arm of the innate immune system produced at the site of inflammation, had prognostic values in chronic heart failure and whether rosuvastatin treatment affected the plasma pentraxin-3 levels in patients with heart failure in the CORONA and GISSI-HF trails.64 They found that baseline elevated pentraxin-3 was associated with a higher risk of all-cause mortality, cardiovascular mortality, or hospitalization for worsening heart failure. Unexpectedly, after 3 months of treatment, the pentraxin-3 levels in the rosuvastatin arm increased significantly compared with placebo. The 3-month changes in pentraxin-3 were associated with fatal events after adjustment for hsCRP or NT-proBNP. The mechanisms responsible for pentraxin-3 elevation in patients receiving rosuvastatin are not known and merit further investigation.
Patients undergoing hemodialysis
The effects of rosuvastatin on cardiovascular outcome in patients undergoing hemodialysis were assessed in the AURORA (A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events) trial.65 In this randomized, double-blind, prospective trial, 2776 patients aged 50–80 years were randomized to receive rosuvastatin, 10 mg daily, or placebo for a median follow-up period of 3.8 years. There was no significant effect of rosuvastatin on the combined primary end point of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, although the initiation of treatment with rosuvastatin lowered the LDL-C, triglyceride, and hsCRP levels. No relationship was found between the cardiovascular end points and baseline or on-treatment LDL-C levels. Rosuvastatin had no effect on individual components of the primary end point or all-cause mortality in this group of patients either.65 These results are similar to previous findings with atorvastatin in patients with diabetes undergoing hemodialysis.66 However, the AURORA study excluded patients who were already receiving statins before the study, and thus selection bias or the possibility that investigators excluded patients whom they believed warranted statin therapy cannot be ruled out. Furthermore, this study only recruited patients who were 50–80 years of age, and thus the possible benefits of rosuvastatin in younger patients were not explored.66
High dose before percutaneous coronary intervention
There is increasing evidence that a single high dose of statin pretreatment before percutaneous coronary intervention (PCI) in patients with acute coronary syndrome (ACS) is associated with a reduced incidence of short-term adverse events and periprocedural myocardial infarction.67,68 It has been reported that a single high dose of rosuvastatin (40 mg) loading is beneficial on the outcome of patients with ACS who underwent PCI. In this unblinded randomized trial in 445 patients with non-ST segment-elevation ACS, rosuvastatin loading approximately 16 hours before PCI resulted in a 53% reduction in the risk of periprocedural myocardial infarction and a 63% reduction in the risk of 30-day major adverse cardiac events (MACEs), including cardiac death, nonfatal myocardial infarction, nonfatal stroke, and any ischemia-driven revascularization, compared to no statin pretreatment.68 A 12-month clinical follow-up study in this group of patients showed that the incidence of MACEs occurred in 20.5% of patients in the control group and 9.8% of patients in the rosuvastatin group (P = 0.002).69 Multivariate analysis revealed that rosuvastatin loading was an independent predictor of a reduction in the risk of MACEs at 12 months (odds ratio 0.5, P = 0.006). In another randomized study in 67 Chinese patients with non-ST segment-elevation ACS who were randomly assigned to the group of no statin pretreatment or to the rosuvastatin group (20 mg 12 hours before PCI, and a further 20 mg 2 hours before PCI), high loading-dose rosuvastatin therapy before PCI was associated with the reduction of periprocedural myocardial infarction, MACEs, and inflammatory response.70 A very recent randomized, double-blind, placebo-controlled study showed that a single high dose (20 mg) of rosuvastatin prior to PCI reduced postprocedural myocardial injury in Chinese patients with ACS, with a concomitant attenuation of postprocedural increase in hsCRP and interleukin 6 levels,71 suggesting that these beneficial effects of statin pretreatment on clinical outcomes are possibly via inhibition of the periprocedural inflammatory response.
Safety
The extensive clinical experience with rosuvastatin in a broad range of patients in different ethnic groups has clearly established the safety of rosuvastatin in doses up to 40 mg.2,62–64 The higher dose of 80 mg was associated with increased risk of development of severe myopathy and rhabdomyolysis in phase III trials, and was discontinued from development.72 In general, rosuvastatin is well tolerated, and its safety profile at approved doses was similar to those of the other available statins.2,73–75 The muscle-toxicity risk for rosuvastatin was comparable to or slightly less than the risk with the other statins. In placebo-controlled trials, myopathy possibly related to treatment was reported in up to 0.1% of patients taking rosuvastatin doses of up to 40 mg, and higher doses appeared to be associated with increased risk of myopathy.76,77
Proteinuria has been identified as a consequence of potent statin therapy, but the development of proteinuria was not predictive of acute or progressive renal disease,76 and several studies have shown no adverse effect on renal function with rosuvastatin.78–80 Dipstick-positive proteinuria and microscopic hematuria were observed among rosuvastatin-treated patients, predominantly in patients dosed above the recommended dose range (ie, 80 mg), in the rosuvastatin clinical development program.18 However, this finding was more frequent in patients taking rosuvastatin 40 mg, when compared to lower doses of rosuvastatin or comparator statins.18 A recent retrospective analysis of renal adverse events in a large, diverse population of patients (n = 40,600) included in the rosuvastatin clinical development program showed that rosuvastatin treatment was not associated with an increased risk of developing renal impairment or renal failure among participants in studies designed to assess its effects on blood-lipid levels, progression of atherosclerosis, or the risk of sustaining major cardiovascular events.81 These findings suggest that rosuvastatin does not affect the risk of developing renal insufficiency or renal failure in patients who do not have advanced preexisting renal disease.
It is known that all statins may induce elevation of liver enzymes (in particular, alanine and aspartate transaminases) above normal values, and no particular statin appears to cause these elevations more frequently than others,2,77,82 although in some studies high-dose atorvastatin showed greater rates of transaminase elevation than simvastatin, especially in females.83 There was a small but noticeable increase in hepatic enzymes across the dose range of rosuvastatin, with an overall incidence of elevated enzymes seen on 0%–0.4% of the samples assayed.18 It has been questioned whether the effect of statins on transaminases indicates hepatotoxicity or just a hepatic reaction to a greater reduction in lipid levels, as all lipid-lowering drugs may increase liver enzymes.73,77 The risk of liver failure with rosuvastatin is extremely low and does not differ from the background rate of liver failure in the general population.2,73
However, the JUPITER trial identified a small but measurable risk of physician-reported incident diabetes mellitus with rosuvastatin treatment,52 and this reignited attention on the link between statin therapy and diabetes. Subsequent meta-analyses of 13 major placebo-controlled statin trials including the JUPITER trial demonstrated that this was a class effect for the statins, with a 9% increased risk of developing diabetes over 4 years observed.84 It was further reported that patients receiving intensive-dose statin therapy had increased risk of new-onset diabetes but a reduced risk for cardiovascular events compared with moderate-dose therapy over a weighted mean follow-up of 4.9 years, with odds ratios of 1.12 (95% CI 1.04–1.44) and 0.84 (95% CI 0.75–0.94), respectively.85 A more recent meta-analysis suggested that different types of statins had different potential to increase the incidence of diabetes, with pravastatin being numerically associated with the lowest risk, whereas rosuvastatin was associated with the highest risk of developing diabetes.86 Furthermore, the meta-regression analysis showed that the risk for developing diabetes was not influenced by the different abilities of statins to reduce cholesterol and suggested molecule-dependent mechanisms may be responsible for the new onset of diabetes with statins.86 It should be noted that the risk of developing diabetes with rosuvastatin in the JUPITER trial was limited to participants who had biochemical evidence of impaired fasting glucose or multiple components of the metabolic syndrome, and these patients were already at high risk of developing diabetes.87 Further investigations are needed to confirm whether rosuvastatin treatment is associated with a higher risk of incidence of diabetes than other statins.
Conclusion
Rosuvastatin is one of the most effective statins available for reducing LDL-C and improving HDL-C and enabling more high-risk patients to achieve their lipid goals. Its favorable balance of effects on atherogenic and protective lipoproteins and its pleiotropic actions are associated with slowing of progression of atherosclerosis within the artery wall and with the clinical benefits of improved cardiovascular outcomes. Although there is a slightly increased risk of incident diabetes with rosuvastatin, as with other statins, the absolute benefit of statin therapy on cardiovascular events overweighs this risk in patients with moderate or high cardiovascular risk, including many patients with hypertension or diabetes or those with documented CVD.
Acknowledgments
The work described in this paper was partly supported by a grant from the Food and Health Bureau (project HHSRF 09100321) of the Hong Kong Special Administrative Region, People’s Republic of China. The funding source had no role in the preparation of this manuscript. No writing assistance was utilized in the production of this manuscript. Professor Tomlinson has received research funding to perform clinical studies from Abbott Laboratories Ltd, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck Serono, Merck Sharp and Dohme, Novartis, Roche, and Takeda, and has acted as a consultant or speaker on occasions for Amgen, AstraZeneca, Boehringer Ingelheim, Genzyme, Janssen, Merck Serono, Merck Sharp and Dohme, Ranbaxy, and Servier.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Luvai A, Mbagaya W, Hall AS, Barth JH. Rosuvastatin: a review of the pharmacology and clinical effectiveness in cardiovascular disease. Clin Med Insights Cardiol. 2012;6:17–33. doi: 10.4137/CMC.S4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toth PP, Dayspring TD. Drug safety evaluation of rosuvastatin. Expert Opin Drug Saf. 2011;10:969–986. doi: 10.1517/14740338.2012.626764. [DOI] [PubMed] [Google Scholar]
- 3.Fabbri G, Maggioni AP. Cardiovascular risk reduction: what do recent trials with rosuvastatin tell us? Adv Ther. 2009;26:469–487. doi: 10.1007/s12325-009-0025-6. [DOI] [PubMed] [Google Scholar]
- 4.Garcia Rodriguez LA, Herings R, Johansson S. Use of multiple international healthcare databases for the detection of rare drug-associated outcomes: a pharmacoepidemiological programme comparing rosuvastatin with other marketed statins. Pharmacoepidemiol Drug Saf. 2010;19:1218–1224. doi: 10.1002/pds.2032. [DOI] [PubMed] [Google Scholar]
- 5.Nicholls SJ, Brandrup-Wognsen G, Palmer M, Barter PJ. Meta-analysis of comparative efficacy of increasing dose of atorvastatin versus rosuvastatin versus simvastatin on lowering levels of atherogenic lipids (from VOYAGER) Am J Cardiol. 2010;105:69–76. doi: 10.1016/j.amjcard.2009.08.651. [DOI] [PubMed] [Google Scholar]
- 6.Rizzo M, Berneis K, Spinas GA, Rini GB, Kapur NK. Quantitative and qualitative effects of rosuvastatin on LDL-cholesterol: what is the clinical significance? Int J Clin Pract. 2009;63:478–485. doi: 10.1111/j.1742-1241.2008.01979.x. [DOI] [PubMed] [Google Scholar]
- 7.Saito Y, Yamada N, Shirai K, et al. Effect of rosuvastatin 5–20 mg on triglycerides and other lipid parameters in Japanese patients with hypertriglyceridemia. Atherosclerosis. 2007;194:505–511. doi: 10.1016/j.atherosclerosis.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 8.Caslake MJ, Stewart G, Day SP, et al. Phenotype-dependent and -independent actions of rosuvastatin on atherogenic lipoprotein subfractions in hyperlipidaemia. Atherosclerosis. 2003;171:245–253. doi: 10.1016/j.atherosclerosis.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 9.McTaggart F, Jones P. Effects of statins on high-density lipoproteins: a potential contribution to cardiovascular benefit. Cardiovasc Drugs Ther. 2008;22:321–338. doi: 10.1007/s10557-008-6113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Athyros VG, Kakafika AI, Tziomalos K, Karagiannis A, Mikhailidis DP. Pleiotropic effects of statins – clinical evidence. Curr Pharm Des. 2009;15:479–489. doi: 10.2174/138161209787315729. [DOI] [PubMed] [Google Scholar]
- 11.Blum A, Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis. 2009;203:325–330. doi: 10.1016/j.atherosclerosis.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Nicholls SJ, Uno K, Kataoka Y. Clinical experience with rosuvastatin in the management of hyperlipidemia and the reduction of cardiovascular risk. Expert Rev Cardiovasc Ther. 2011;9:1383–1390. doi: 10.1586/erc.11.145. [DOI] [PubMed] [Google Scholar]
- 13.Keating GM, Robinson DM. Rosuvastatin: a review of its effect on atherosclerosis. Am J Cardiovasc Drugs. 2008;8:127–146. doi: 10.1007/BF03256589. [DOI] [PubMed] [Google Scholar]
- 14.Martin PD, Warwick MJ, Dane AL, Cantarini MV. A double-blind, randomized, incomplete crossover trial to assess the dose proportionality of rosuvastatin in healthy volunteers. Clin Ther. 2003;25:2215–2224. doi: 10.1016/s0149-2918(03)80214-x. [DOI] [PubMed] [Google Scholar]
- 15.Hu M, Mak VWL, Chu TTY, Waye MMY, Tomlinson B. Pharmacogenetics of HMG-CoA reductase inhibitors: optimizing the prevention of coronary heart disease. Curr Pharmacogenomics Person Med. 2009;7:1–26. [Google Scholar]
- 16.Martin PD, Warwick MJ, Dane AL, Brindley C, Short T. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther. 2003;25:2553–2563. doi: 10.1016/s0149-2918(03)80316-8. [DOI] [PubMed] [Google Scholar]
- 17.Martin PD, Warwick MJ, Dane AL, et al. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther. 2003;25:2822–2835. doi: 10.1016/s0149-2918(03)80336-3. [DOI] [PubMed] [Google Scholar]
- 18.AstraZeneca Pharmaceuticals . Crestor (rosuvastatin calcium) [package insert] Wilmington (DE): AstraZeneca Pharmaceuticals; 2007. [Google Scholar]
- 19.Brown WV. New therapies on the horizon. Am J Manag Care. 2001;7:S148–S151. [PubMed] [Google Scholar]
- 20.Malinowski HJ, Westelinck A, Sato J, Ong T. Same drug, different dosing: differences in dosing for drugs approved in the United States, Europe, and Japan. J Clin Pharmacol. 2008;48:900–908. doi: 10.1177/0091270008319794. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Yu BN, He YJ, et al. Role of BCRP 421C > A polymorphism on rosuvastatin pharmacokinetics in healthy Chinese males. Clin Chim Acta. 2006;373:99–103. doi: 10.1016/j.cca.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Keskitalo JE, Zolk O, Fromm MF, Kurkinen KJ, Neuvonen PJ, Niemi M. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2009;86:197–203. doi: 10.1038/clpt.2009.79. [DOI] [PubMed] [Google Scholar]
- 23.Zamber CP, Lamba JK, Yasuda K, et al. Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics. 2003;13:19–28. doi: 10.1097/00008571-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Martin PD, Dane AL, Nwose OM, Schneck DW, Warwick MJ. No effect of age or gender on the pharmacokinetics of rosuvastatin: a new HMG-CoA reductase inhibitor. J Clin Pharmacol. 2002;42:1116–1121. doi: 10.1177/009127002401382722. [DOI] [PubMed] [Google Scholar]
- 25.Kostapanos MS, Milionis HJ, Elisaf MS. Rosuvastatin-associated adverse effects and drug-drug interactions in the clinical setting of dyslipidemia. Am J Cardiovasc Drugs. 2010;10:11–28. doi: 10.2165/13168600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.van de Steeg E, Greupink R, Schreurs M, et al. Drug-drug interactions between rosuvastatin and oral antidiabetic drugs occurring at the level of OATP1B1. Drug Metab Dispos. 2013;41:592–601. doi: 10.1124/dmd.112.049023. [DOI] [PubMed] [Google Scholar]
- 27.Brewer HB., Jr Benefit-risk assessment of rosuvastatin 10 to 40 milligrams. Am J Cardiol. 2003;92:23K–29K. doi: 10.1016/s0002-9149(03)00779-3. [DOI] [PubMed] [Google Scholar]
- 28.Jones PH, Davidson MH, Stein EA, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial) Am J Cardiol. 2003;92:152–160. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 29.Barter PJ, Brandrup-Wognsen G, Palmer MK, Nicholls SJ. Effect of statins on HDL-C: a complex process unrelated to changes in LDL-C: analysis of the VOYAGER database. J Lipid Res. 2010;51:1546–1553. doi: 10.1194/jlr.P002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kostapanos MS, Milionis HJ, Filippatos TD, et al. A 12-week, prospective, open-label analysis of the effect of rosuvastatin on triglyceride-rich lipoprotein metabolism in patients with primary dyslipidemia. Clin Ther. 2007;29:1403–1414. doi: 10.1016/j.clinthera.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Ai M, Otokozawa S, Asztalos BF, et al. Effects of maximal doses of atorvastatin versus rosuvastatin on small dense low-density lipoprotein cholesterol levels. Am J Cardiol. 2008;101:315–318. doi: 10.1016/j.amjcard.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 32.Kostapanos MS, Milionis HJ, Lagos KG, Rizos CB, Tselepis AD, Elisaf MS. Baseline triglyceride levels and insulin sensitivity are major determinants of the increase of LDL particle size and buoyancy induced by rosuvastatin treatment in patients with primary hyperlipidemia. Eur J Pharmacol. 2008;590:327–332. doi: 10.1016/j.ejphar.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Rosenson RS, Otvos JD, Hsia J. Effects of rosuvastatin and atorvastatin on LDL and HDL particle concentrations in patients with metabolic syndrome: a randomized, double-blind, controlled study. Diabetes Care. 2009;32:1087–1091. doi: 10.2337/dc08-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bittar R, Giral P, Aslangul E, et al. Effects of rosuvastatin versus pravastatin on low-density lipoprotein diameter in HIV-1-infected patients receiving ritonavir-boosted protease inhibitor. AIDS. 2012;26:1801–1805. doi: 10.1097/QAD.0b013e328357063c. [DOI] [PubMed] [Google Scholar]
- 35.Schulz E, Gori T, Munzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34:665–673. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 36.Feldstein CA. Statins in hypertension: are they a new class of antihypertensive agents? Am J Ther. 2010;17:255–262. doi: 10.1097/MJT.0b013e3181c0695e. [DOI] [PubMed] [Google Scholar]
- 37.Feldstein CA. Statins as antihypertensives. Recent Pat Cardiovasc Drug Discov. 2008;3:92–97. doi: 10.2174/157489008784705331. [DOI] [PubMed] [Google Scholar]
- 38.Tsiara S, Elisaf M, Mikhailidis DP. Early vascular benefits of statin therapy. Curr Med Res Opin. 2003;19:540–556. doi: 10.1185/030079903125002225. [DOI] [PubMed] [Google Scholar]
- 39.Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370:591–603. doi: 10.1016/S0140-6736(07)61299-9. [DOI] [PubMed] [Google Scholar]
- 40.Strazzullo P, Kerry SM, Barbato A, Versiero M, D’Elia L, Cappuccio FP. Do statins reduce blood pressure? A meta-analysis of randomized, controlled trials. Hypertension. 2007;49:792–798. doi: 10.1161/01.HYP.0000259737.43916.42. [DOI] [PubMed] [Google Scholar]
- 41.Sicard P, Delemasure S, Korandji C, et al. Anti-hypertensive effects of rosuvastatin are associated with decreased inflammation and oxidative stress markers in hypertensive rats. Free Radic Res. 2008;42:226–236. doi: 10.1080/10715760701885380. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Yang G, Zhao G, et al. Rosuvastatin attenuates the elevation in blood pressure induced by overexpression of human C-reactive protein. Hypertens Res. 2011;34:869–875. doi: 10.1038/hr.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 44.Ballantyne CM, Raichlen JS, Nicholls SJ, et al. Effect of rosuvastatin therapy on coronary artery stenoses assessed by quantitative coronary angiography: a study to evaluate the effect of rosuvastatin on intravascular ultrasound-derived coronary atheroma burden. Circulation. 2008;117:2458–2466. doi: 10.1161/CIRCULATIONAHA.108.773747. [DOI] [PubMed] [Google Scholar]
- 45.Takayama T, Hiro T, Yamagishi M, et al. Effect of rosuvastatin on coronary atheroma in stable coronary artery disease: multicenter coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS) Circ J. 2009;73:2110–2117. doi: 10.1253/circj.cj-09-0358. [DOI] [PubMed] [Google Scholar]
- 46.Underhill HR, Yuan C, Zhao XQ, et al. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: a high-resolution magnetic resonance imaging trial. Am Heart J. 2008;155:584, e1–e8. doi: 10.1016/j.ahj.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Crouse JR, 3rd, Raichlen JS, Riley WA, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297:1344–1353. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 48.Riccioni G, Cipollone F, Santovito D, et al. Effect of 2-year treatment with low-dose rosuvastatin on intima-media thickness in hypercholesterolemic subjects with asymptomatic carotid artery disease. Expert Opin Pharmacother. 2011;12:2599–2604. doi: 10.1517/14656566.2011.618497. [DOI] [PubMed] [Google Scholar]
- 49.Sawara Y, Takei T, Uchida K, et al. Effects of lipid-lowering therapy with rosuvastatin on atherosclerotic burden in patients with chronic kidney disease. Intern Med. 2008;47:1505–1510. doi: 10.2169/internalmedicine.47.1159. [DOI] [PubMed] [Google Scholar]
- 50.Calza L, Manfredi R, Colangeli V, et al. Two-year treatment with rosuvastatin reduces carotid intima-media thickness in HIV type 1-infected patients receiving highly active antiretroviral therapy with asymptomatic atherosclerosis and moderate cardiovascular risk. AIDS Res Hum Retroviruses. 2013;29:547–556. doi: 10.1089/aid.2012.0015. [DOI] [PubMed] [Google Scholar]
- 51.Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078–2087. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 52.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 53.Carter NJ. Rosuvastatin: a review of its use in the prevention of cardiovascular disease in apparently healthy women or men with normal LDL-C levels and elevated hsCRP levels. Am J Cardiovasc Drugs. 2010;10:383–400. doi: 10.2165/11204600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 54.Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 55.Food and Drug Administration Questions and answers for healthcare professionals: CRESTOR and the JUPITER Trial 2010Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm199891.htmAccessed September 6, 2012
- 56.Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Lorgeril M, Salen P, Abramson J, et al. Cholesterol lowering, cardiovascular diseases, and the rosuvastatin-JUPITER controversy: a critical reappraisal. Arch Intern Med. 2010;170:1032–1036. doi: 10.1001/archinternmed.2010.184. [DOI] [PubMed] [Google Scholar]
- 58.Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 59.Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4. [DOI] [PubMed] [Google Scholar]
- 60.McMurray JJ, Kjekshus J, Gullestad L, et al. Effects of statin therapy according to plasma high-sensitivity C-reactive protein concentration in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA): a retrospective analysis. Circulation. 2009;120:2188–2196. doi: 10.1161/CIRCULATIONAHA.109.849117. [DOI] [PubMed] [Google Scholar]
- 61.Cleland JG, McMurray JJ, Kjekshus J, et al. Plasma concentration of amino-terminal pro-brain natriuretic peptide in chronic heart failure: prediction of cardiovascular events and interaction with the effects of rosuvastatin: a report from CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure) J Am Coll Cardiol. 2009;54:1850–1859. doi: 10.1016/j.jacc.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 62.Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 63.Gullestad L, Ueland T, Kjekshus J, et al. Galectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) Eur Heart J. 2012;33:2290–2296. doi: 10.1093/eurheartj/ehs077. [DOI] [PubMed] [Google Scholar]
- 64.Latini R, Gullestad L, Masson S, et al. Pentraxin-3 in chronic heart failure: the CORONA and GISSI-HF trials. Eur J Heart Fail. 2012;14:992–999. doi: 10.1093/eurjhf/hfs092. [DOI] [PubMed] [Google Scholar]
- 65.Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 66.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 67.Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007;49:1272–1278. doi: 10.1016/j.jacc.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 68.Yun KH, Jeong MH, Oh SK, et al. The beneficial effect of high loading dose of rosuvastatin before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol. 2009;137:246–251. doi: 10.1016/j.ijcard.2008.06.055. [DOI] [PubMed] [Google Scholar]
- 69.Yun KH, Oh SK, Rhee SJ, Yoo NJ, Kim NH, Jeong JW. 12-month follow-up results of high dose rosuvastatin loading before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol. 2011;146:68–72. doi: 10.1016/j.ijcard.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 70.Luo J, Li J, Shen X, et al. The effects and mechanisms of high loading dose rosuvastatin therapy before percutaneous coronary intervention in patients with acute coronary syndrome. Int J Cardiol. 2012 Nov 26; doi: 10.1016/j.ijcard.2012.11.032. Epub. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, Dai H, Xing M, et al. Effect of a single high loading dose of rosuvastatin on percutaneous coronary intervention for acute coronary syndromes. J Cardiovasc Pharmacol Ther. 2013 Jan 29; doi: 10.1177/1074248412474346. Epub. [DOI] [PubMed] [Google Scholar]
- 72.Wolfe SM. Dangers of rosuvastatin identified before and after FDA approval. Lancet. 2004;363:2189–2190. doi: 10.1016/S0140-6736(04)16513-6. [DOI] [PubMed] [Google Scholar]
- 73.Guthrie RM, Martin DR. The safety of rosuvastatin: effects on renal and hepatic function. Expert Opin Drug Saf. 2007;6:573–581. doi: 10.1517/14740338.6.5.573. [DOI] [PubMed] [Google Scholar]
- 74.Shepherd J, Hunninghake DB, Stein EA, et al. Safety of rosuvastatin. Am J Cardiol. 2004;94:882–888. doi: 10.1016/j.amjcard.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 75.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shepherd J, Vidt DG, Miller E, Harris S, Blasetto J. Safety of rosuvastatin: update on 16,876 rosuvastatin-treated patients in a multinational clinical trial program. Cardiology. 2007;107:433–443. doi: 10.1159/000100908. [DOI] [PubMed] [Google Scholar]
- 77.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–1790. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 78.Vidt DG, Cressman MD, Harris S, Pears JS, Hutchinson HG. Rosuvastatin-induced arrest in progression of renal disease. Cardiology. 2004;102:52–60. doi: 10.1159/000077704. [DOI] [PubMed] [Google Scholar]
- 79.Vidt DG, Harris S, McTaggart F, Ditmarsch M, Sager PT, Sorof JM. Effect of short-term rosuvastatin treatment on estimated glomerular filtration rate. Am J Cardiol. 2006;97:1602–1606. doi: 10.1016/j.amjcard.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 80.Abe M, Maruyama N, Okada K, Matsumoto S, Matsumoto K, Soma M. Effects of lipid-lowering therapy with rosuvastatin on kidney function and oxidative stress in patients with diabetic nephropathy. J Atheroscler Thromb. 2011;18:1018–1028. doi: 10.5551/jat.9084. [DOI] [PubMed] [Google Scholar]
- 81.Stein EA, Vidt DG, Shepherd J, Cain VA, Anzalone D, Cressman MD. Renal safety of intensive cholesterol-lowering treatment with rosuvastatin: a retrospective analysis of renal adverse events among 40,600 participants in the rosuvastatin clinical development program. Atherosclerosis. 2012;221:471–477. doi: 10.1016/j.atherosclerosis.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 82.Gillett RC, Jr, Norrell A. Considerations for safe use of statins: liver enzyme abnormalities and muscle toxicity. Am Fam Physician. 2011;83:711–716. [PubMed] [Google Scholar]
- 83.Illingworth DR, Crouse JR, 3rd, Hunninghake DB, et al. A comparison of simvastatin and atorvastatin up to maximal recommended doses in a large multicenter randomized clinical trial. Curr Med Res Opin. 2001;17:43–50. [PubMed] [Google Scholar]
- 84.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 85.Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 86.Navarese EP, Buffon A, Andreotti F, et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013 Jan 24; doi: 10.1016/j.amjcard.2012.12.037. Epub. [DOI] [PubMed] [Google Scholar]
- 87.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]