Abstract
Objective
We sought to determine hospital variation in the use of positive inotropic agents in patients with heart failure.
Background
Clinical guidelines recommend targeted use of positive inotropic agents in highly selected patients, but data are limited and the recommendations are not specific.
Methods
We analyzed data from 376 hospitals including 189,948 hospitalizations for heart failure during 2009–10. We used hierarchical logistic regression models to estimate hospital-level risk-standardized rates of inotrope use and risk-standardized in-hospital mortality rates.
Results
The risk-standardized rates of inotrope use ranged across hospitals from 0.9% to 44.6% (median: 6.3%, inter-quartile range: 4.3% to 9.2%). We identified various hospital patterns based on the type of agents: dobutamine-predominant (29% of hospitals), dopamine-predominant (25%), milrinone-predominant (1%), mixed dobutamine/dopamine pattern (32%), and mixed pattern including all 3 agents (13%). When studying the factors associated with inter-hospital variation, the best model performance was with the HGLM models that adjusted for patient case mix and an individual hospital effect (ROCs from 0.77 to 0.88). The intra-class correlation coefficients of the HGLMs (0.113 for any inotrope) indicated that a noteworthy proportion of the observed variation was related to an “individual institutional effect.” Hospital rates or patterns of use were not associated with differences in length of stay or risk-standardized mortality rates.
Conclusions
We found marked differences in the use of inotropic agents for heart failure patients among a diverse group of hospitals. This variability, occurring in the context of little clinical evidence, indicates an urgent need to define the appropriate use of these medications.
Keywords: heart failure, inotrope utilization, in-hospital mortality, variation in care
INTRODUCTION
Heart failure is a leading cause of hospital admission, accounting for almost 1 million hospitalizations in the United States annually (1). In the absence of major advances in the treatment of this condition, early mortality has declined only modestly over the past 2 decades (2). Outcome measures have revealed that hospitals vary in their 30-day risk-standardized mortality rates (RSMRs), indicating that hospital-level differences in treatment patterns may affect patient outcomes (3,4).
Positive inotropic agents are used in the treatment of the highest risk patients hospitalized with heart failure. Dopamine and dobutamine have been available for decades and were approved by the Food and Drug Administration before the mandate to evaluate the benefits and risks of new drugs in large trials. A third positive inotropic agent, milrinone, was approved in 1988 for the treatment of acute decompensated heart failure based on its hemodynamic effects rather than on clinical endpoints. Data on the comparative effectiveness of these agents on the outcomes of patients with heart failure are lacking (5). The only large clinical trial of milrinone compared its effect with placebo in hospitalized patients without end-organ hypoperfusion and found an increased risk of adverse events (6). Other positive inotropic agents that were tested in trials, such as amrinone and vesnarinone, were shown to increase mortality (7,8).
The most recent Guidelines for the Diagnosis and Management of Heart Failure from the American College of Cardiology (ACC)/American Heart Association (AHA) recommend limited use of these agents, stating that “intravenous inotropic drugs such as dopamine, dobutamine or milrinone might be reasonable for those patients presenting with documented severe systolic dysfunction, low blood pressure and evidence of low cardiac output, with or without congestion, to maintain systemic perfusion and preserve end-organ performance” (9). The guidelines explicitly state that intravenous positive inotropic agents are not recommended for hospitalized patients with heart failure who do not have evidence of decreased organ perfusion. The clinical practice guidelines of the Heart Failure Society of America (10) and the European Society of Cardiology (11) mirror the AHA/ACC recommendation. The recommendations are based on expert opinion.
Little information is available about how utilization of positive inotropic agents varies among hospitals. Scarce evidence and relatively weak guideline recommendations indicate the potential for marked variation. Accordingly, we investigated treatment patterns of inotrope use among patients hospitalized for heart failure in a large network of hospitals in the United States. We also report the relationship between inotrope use and in-hospital RSMRs and length of stay, including comparisons of hospitals with high and low utilization patterns.
METHODS
Data Source
We conducted a cross-sectional study using data from Perspective™, a voluntary, fee-supported database developed by Premier, Inc. for measuring quality and healthcare utilization. As of 2010, Perspective™ contained data from more than 500 hospitals in the United States, including more than 130 million cumulative hospital discharges. Inpatient discharges represent about 20% of all acute care inpatient hospitalizations nationwide. In addition to the information available in the standard hospital discharge file, Perspective™ contains a date-stamped log of all billed items at the patient level including medications and laboratory, diagnostic, and therapeutic services. For this study, patient data were de-identified in accordance with the Health Insurance Portability and Accountability Act and hospitals were identified by a random identifier assigned by Premier. The Yale University Human Investigation Committee reviewed the protocol for this study and determined that it is not considered to be Human Subjects Research as defined by the Office of Human Research Protections.
Patients and Hospitals
Our analysis included the first episode of hospitalization per patient between January 1, 2009 and December 31, 2010 that had a principal diagnosis of heart failure (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, 428.9) or a principal diagnosis of respiratory failure (ICD-9-CM code 518.81) combined with a secondary diagnosis of heart failure. We excluded patients <18 years of age or those whose physicians were pediatricians, since our focus was not on congenital disease. We excluded hospitalizations with a duration of 1 day, as well as transfers to or from another acute care facility because we could not accurately assess treatment with inotropic therapy during an entire hospitalization.
In addition to patient age, sex, race/ethnicity, and insurance status, we used software (version 3.4, 3.5 and 3.6 for federal fiscal years 2009, 2010 and 2011, respectively) provided by the Healthcare Costs and Utilization Project of the Agency for Healthcare Research and Quality to classify comorbidities from the standard hospital discharge file based on methods described by Elixhauser and Steiner (12). This tool provides a Diagnosis Related Group screen of ICD-9-CM secondary diagnoses.
For each hospital, Perspective™ contains information, collected from the American Hospital Association database, on bed count, teaching status, geographic location (by Census division), and whether it serves an urban or rural population. Participating hospitals were geographically diverse, with a composition similar to that of acute care hospitals nationwide. They were predominantly small to midsize nonteaching facilities that serve a largely urban population.
Hospital Utilization and Clinical Outcomes
We assessed the use of dopamine, dobutamine and milrinone, which are the 3 positive inotropic agents that were noted in the ACC/AHA Heart Failure Guidelines. We also evaluated the hospitals’ utilization of a number of diagnostic or therapeutic procedures including pulmonary artery catheterization, ventricular assist device, heart transplant, mechanical ventilation, and implantable cardioverter defibrillator, with and without cardiac resynchronization therapy. We also assessed median length of stay per hospital and in-hospital RSMRs.
Statistical Analysis
We constructed summary statistics using frequencies and proportions for categorical data and means, medians and inter-quartile ranges (IQRs) for continuous variables.
To determine the patient and hospital characteristics that were associated with the use of inotropic agents, we constructed 4 logistic regression models: 1 for overall inotropic use and 1 for each of the 3 inotropic agents. Patient characteristics including age group, sex and comorbidities were considered as candidate covariates. We selected the variables for the final model using a stepwise algorithm. After controlling for selected patient characteristics, we fit logistic regression models to further evaluate the effects of hospital characteristics (hospital size, heart failure volume, urban or rural setting, geographic location by Census division, and teaching status). We report Odds Ratios (ORs) and corresponding 95% Confidence Intervals (CIs) for each significant factor.
We used hierarchical generalized linear models (HGLMs) to calculate risk-standardized utilization rates for use of any inotropic agent and for use of each inotropic agent (4,13). We selected patient characteristics used as covariates for risk adjustment by stepwise algorithm using logistic regression models. We performed additional scaling to ensure that the unadjusted and adjusted rates were comparable such that the slopes of the weighted linear regression of the unadjusted rate and the adjusted rate were equal to 1. We also used hierarchical logistic regression to estimate the in-hospital RSMR, adjusting for patient characteristics including age, gender and all comorbidities as well as a hospital individual effect as a random effect. We employed a modified version of a previously published 30-day mortality model with data elements restricted to those available during the index admission (4). We used weighted linear regression models to assess the relationship between hospital inotrope use and RSMRs.
To further assess the contribution of “institutional effect” to the variation in use of positive inotropic agents, we compared the receiver operating characteristic (ROC) curves of the logistic regression models that adjust for only patient case mix to the ROC curves of the HGLM models that also take into account institutional factors. We calculated the intra-class correlation coefficient for the HGLM models as described elsewhere (14).
Next, we categorized hospitals as either a “predominant” user for 1 of the 3 agents or as a “mixed” user based on their pattern of inotrope utilization. For these classifications, we included only hospitals with at least 15 hospitalizations over 2 years with the use of any positive inotropic agent. We calculated the total usage by adding the number of hospitalizations using dobutamine, dopamine or milrinone. Since patients can receive more than 1 agent during a single hospitalization, the total usage may exceed the number of hospitalizations. We then calculated the percentage of hospitalizations receiving each inotropic agent by hospital, with the total usage as the denominator. If a hospital had at least 55% of hospitalizations receiving any single agent (55% for dobutamine and dopamine, 50% for milrinone), it was deemed a “predominant” user for this agent. The cutoffs were chosen empirically based on hospital distribution of percent use. If none of the agents exceeded 55% of total use and total dobutamine and dopamine use was ≥80%, the hospital was categorized as a “dobutamine/dopamine-mixed” user. The rest of the hospitals were characterized as “dobutamine/dopamine/milrinone-mixed” users. We used Kruskal-Wallis and chi-square tests to assess the association between utilization pattern and different hospital characteristics.
We conducted analyses with SAS version 9.2 (SAS Institute Inc., Cary, NC), estimated the hierarchical logistic models using the GLIMMIX macro in SAS, and created the figures with R (version 2.11.1) (15).
RESULTS
Hospital and Patient Characteristics
We identified 189,948 hospitalizations from 376 hospitals that met our enrollment criteria. Of these hospitals, 53% had >250 beds, 73% were non-teaching, and 78% were located in urban settings. The median volume of patients with heart failure per hospital over the 2 years was 394 (IQR: 161–770; range 1–2076).
We assessed patient characteristics by hospital. Median patient age was 76 years (IQR by hospital: 64–84), median percent of women was 52.7% (IQR: 49.1–55.7) and median percent of white patients was 78.4% (IQR: 49.5–92.3). The most common comorbidities included hypertension (median among hospitals: 70.0%), coronary atherosclerosis (55.1%), cardiac dysrhythmias (47.9%), disorders of lipid metabolism (43.5%), renal failure (37.9%), and diabetes without complications (33.7%). The majority of admissions (median among hospitals 65.3%, IQR: 52.8–75.7) were through the Emergency Department. Medicare was the most common form of health insurance, accounting for approximately two-thirds (median among hospitals 65.7%) of patients. The most frequent procedures performed were renal dialysis and mechanical ventilation with median utilization among hospitals of 5.3% (IQR: 2.4–7.6) and 5.4% (IQR: 3.6–7.4), respectively. Cardiac procedures were rare, with median use among hospitals of 1.2% (IQR: 0.0–4.4) for automatic implantable cardioverter defibrillators with or without cardiac resynchronization therapy and 0.2% (IQR: 0.0–0.8) for pulmonary artery catheterization.
Hospital Use of Positive Inotropic Agents
Of all hospitalizations, 13,676 (7.2%) included a treatment with a positive inotropic agent. Among hospitals, the unadjusted treatment rate ranged from a minimum of 0% to a maximum of 38.0%. The hospital risk-standardized treatment rate ranged from a minimum of 0.9 to a maximum of 44.6% (IQR: 4.3–9.2%, median: 6.3%) (Figure 1). Dobutamine was most common (range: 0.4–47.4%, IQR: 2.2–6.8%, median: 3.7%), followed by dopamine (range: 0.6–16.3%, IQR: 2.5–4.7%, median: 3.3%). Milrinone use was much less on average and highly variable (range: 0.02–67.5%, IQR: 0.5–2.0%, median: 0.78%).
Figure 1.
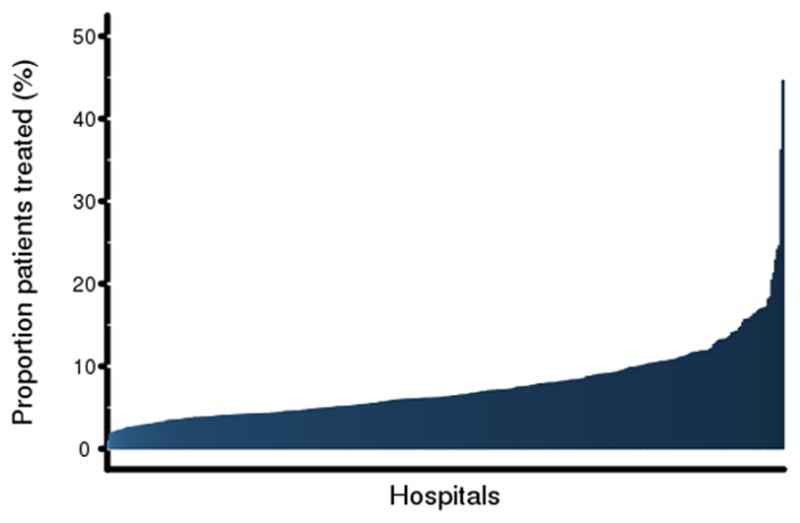
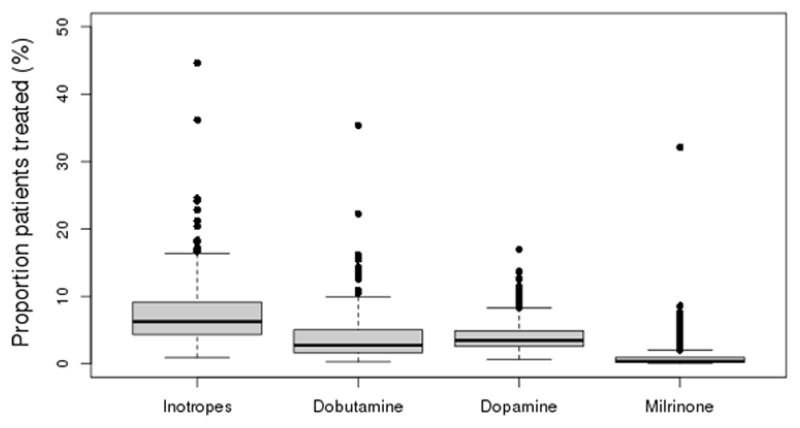
Risk-standardized rates of inotrope use across hospitals.
Panel A shows the proportion of patients who received any positive inotropic agent at each hospital ranked from lowest to highest use.
Panel B shows the distribution of hospital risk-standardized rates for each inotropic agent as well as for any inotrope.
The pattern of agents used varied widely across hospitals. Of the 225 hospitals with at least 15 hospitalizations involving inotrope treatment, 65 (29%) were dobutamine-predominant, 56 (25%) were dopamine-predominant, 3 (1%) were milrinone-predominant, 71 (32%) were dobutamine/dopamine-mixed pattern, and 30 (13%) were dobutamine/dopamine/milrinone-mixed (Table 1). After adjustment for patient case mix, the likelihood of treatment with a positive inotropic agent varied by hospital use patterns (p<0.0001 for the association of use pattern with percent use). There was also a significant association between hospital pattern of inotrope use and hospital size (p<0.01). We did not find a significant relationship between pattern of inotrope use and teaching status, heart failure volume, median length of stay, or hospital percent of heart transplant or implantation of ventricular assist devices. Figure 2 illustrates various hospital patterns based on overall percentage of inotrope use as well as mix of agents used.
Table 1.
Hospital patterns of inotrope use.
| Pattern* | N (%) (N=225) | Median (IQR) (N=225)
|
Teaching hospitals (%) (N=225) | Hospitals performing LVAD or heart transplant (%) (N=225) | ||||
|---|---|---|---|---|---|---|---|---|
| Risk-standardized overall inotropic agent use (%) | Beds (No.) | 2-year heart failure volume | RSMR | Length of Stay† (day) | ||||
| Dobutamine-predominant | 65 (29) | 9.4 (6.6–14.0) | 325 (219–409) | 817 (542–1160) | 0.045 (0.038–0.054) | 4 (4–5) | 25 | 12.3 |
| Dopamine-predominant | 56 (25) | 6.1 (4.6–8.5) | 316 (240–448) | 805 (547–1181) | 0.047 (0.040–0.053) | 4 (4–5) | 32 | 7.1 |
| Milrinone-predominant | 3 (1) | 11.8 (7.0–36.2) | 308 (137–351) | 352 (126–1848) | 0.036 (0.035–0.048) | 4 (4–5) | 33 | 33.3 |
| D/D-mixed | 71 (32) | 7.8 (5.4–10.3) | 355 (258–457) | 991 (573–1271) | 0.050 (0.039–0.056) | 4 (4–5) | 45 | 8.5 |
| D/D/M-mixed | 30 (13) | 8.5 (6.4–11.8) | 458 (312–683) | 1179 (649–2024) | 0.043 (0.039–0.049) | 4 (4–5) | 37 | 20.0 |
| p-value | <0.0001 | 0.013 | 0.125 | 0.260 | 0.611 | 0.165 | 0.164 | |
Patterns were determined only in hospitals with at least 15 hospitalizations over 2 years using any positive inotropic agent; 151 hospitals were excluded.
Median of hospital median of length of stay (day)
D/D, dobutamine/dopamine; D/D/M, dobutamine/dopamine/milrinone; HF, heart failure; IQR, inter-quartile range; LVAD, left ventricular assist device; RSMR, risk-standardized mortality rate
Figure 2.
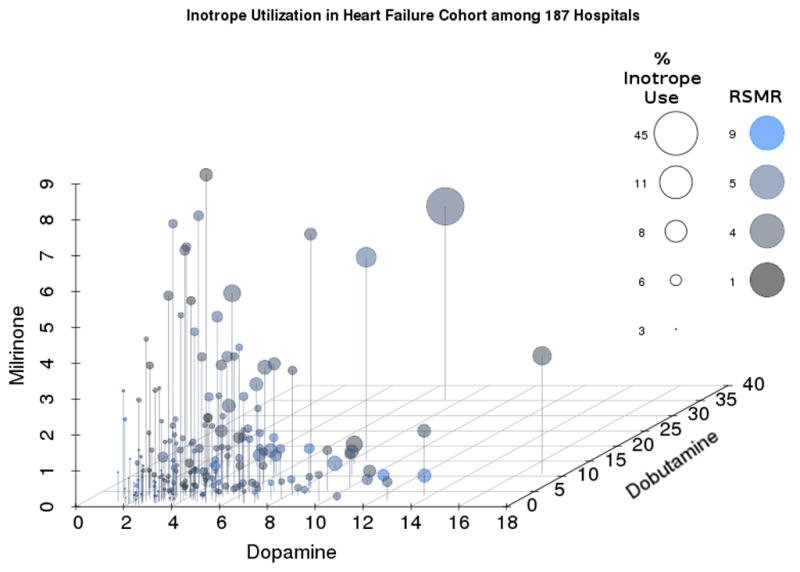
Hospital patterns based on overall percentage of inotrope use in 187 hospitals as well as mix of agents used, and association with hospital risk-standardized mortality rates. X, y, and z values were defined as raw percentages of hospital-level use of dopamine, dobutamine and milrinone, respectively, among the selected cohort. We plotted values through the Scatterplot3d library.(22) We scaled size and color values relative to their minimum and maximum to fit visual limits of the graphical display. Mapping circle sizes to percentage of overall inotrope use and setting hue saturation value colors to risk-standardized mortality rates illustrates the positive association between overall inotrope use and mix of agents used, and the lack of association with hospital risk-standardized mortality rate.
There were 151 hospitals with <15 cases of inotrope use over 2 years that were not included in the pattern classifications. Their risk standardized median percent of inotrope use was 4.7 (IQR: 3.8–6.2%). These hospitals were small-sized (median number of beds: 121, IQR: 71–216), had a low volume of patients with heart failure (median 155, IQR: 87–275), and were mainly non-teaching (85.4%).
Patient and Hospital Characteristics Associated with the Use of Positive Inotropic Agents
We assessed the association between patient characteristics and inotrope use, including overall use and use of individual inotropic agent (Appendix). Patients with cardiac arrest and ventricular fibrillation had the highest likelihood of receiving inotropes, both for combined inotrope use and use of each individual agent. The likelihood of receiving inotropes was also higher in younger patients, most notably for milrinone. Other comorbidities associated with higher likelihood of receiving inotropic treatment included acute myocardial infarction, valvular disease, fluid and electrolyte disorders, coagulopathy, cardiac dysrhythmias, coronary atherosclerosis and other heart disease, renal failure, and aortic and peripheral arterial embolism or thrombosis. Female patients had a lower likelihood of being treated with positive inotropic agents. The ROCs of these logistic regression models ranged from 0.69 for dobutamine to 0.75 for dopamine and milrinone.
After adjusting for patient characteristics, we assessed the association between inotrope utilization and hospital characteristics by adding the following hospital characteristics to the model: size, heart failure volume, urban vs. rural setting, teaching vs. non-teaching status, and geographic location by Census division (Table 2). These logistic regression models showed the likelihood of receiving dopamine (OR: 1.13, 95% CI: 1.07–1.21) or milrinone (OR: 1.23, 95% CI: 1.10–1.36) to be higher in teaching hospitals and the odds of receiving dobutamine (OR: 1.31, 95% CI: 1.20–1.44) or milrinone (OR: 1.65, 95% CI: 1.33–2.05) to be higher in urban hospitals. Hospitals with the highest odds of using milrinone had lower volume of patients with heart failure (between 26 and 200 hospitalizations over 2 years). The odds of using positive inotropic agents were highest in the East South Central (OR: 1.52, 95% CI: 1.41–1.65) and lowest in the New England (OR: 0.43, 95% CI: 0.37–0.50) regions. The odds of being treated with any positive inotropic agent were highest in hospitals having between 251 and 400 beds.
Table 2.
Hospital factors associated with the use of positive inotropic agents.
| Characteristic | OR (95% CI)
|
|||
|---|---|---|---|---|
| Inotrope | Dobutamine | Dopamine | Milrinone | |
| Annual heart failure volume | ||||
| <25 | 1.90 (0.92–3.92) | 1.49 (0.53–4.16) | 2.09 (0.85–5.14) | <0.01 (<0.01–>9999.99) |
| 26 – 200 | 0.96 (0.85–1.08) | 0.90 (0.77–1.06) | 0.89 (0.75–1.06) | 1.69 (1.25–2.29) |
| 201 – 500 | 0.87 (0.80–0.93) | 0.76 (0.69–0.84) | 1.06 (0.95–1.18) | 0.76 (0.60–0.96) |
| 501 – 1000 | 1.00 (0.95–1.05) | 1.00 (0.94–1.07) | 1.04 (0.96–1.12) | 1.01 (0.88–1.15) |
| 1001 – 1500 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| >1500 | 0.94 (0.90–0.99) | 0.94 (0.88–1.00) | 0.91 (0.84–0.98) | 1.09 (0.97–1.23) |
| Hospital size (no. of beds) | ||||
| 1 – 100 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 101 – 250 | 1.34 (1.18–1.52) | 1.29 (1.09–1.53) | 1.28 (1.08–1.51) | 2.56 (1.46–4.50) |
| 251 – 400 | 1.69 (1.49–1.92) | 1.63 (1.37–1.93) | 1.45 (1.22–1.72) | 5.28 (3.02–9.22) |
| >400 | 1.55 (1.36–1.77) | 1.51 (1.27–1.80) | 1.35 (1.13–1.61) | 4.77 (2.72–8.39) |
| Urban (vs. rural) | 1.20 (1.12–1.28) | 1.31 (1.20–1.44) | 1.00 (0.91–1.10) | 1.65 (1.33–2.05) |
| Teaching (vs. non-teaching) | 0.99 (0.94–1.03) | 0.87 (0.83–0.93) | 1.13 (1.07–1.21) | 1.23 (1.10–1.36) |
| Location by Census division | ||||
| East South Central | 1.52 (1.41–1.65) | 1.72 (1.57–1.89) | 1.32 (1.16–1.49) | 1.08 (0.87–1.33) |
| Middle Atlantic | 0.60 (0.56–0.65) | 0.52 (0.47–0.56) | 0.72 (0.65–0.80) | 1.06 (0.91–1.24) |
| Mountain | 0.84 (0.75–0.94) | 0.75 (0.65–0.87) | 1.16 (0.99–1.36) | 1.43 (1.11–1.85) |
| New England | 0.43 (0.37–0.50) | 0.28 (0.23–0.35) | 0.75 (0.62–0.92) | 0.42 (0.28–0.63) |
| Pacific | 0.75 (0.70–0.81) | 0.62 (0.56–0.68) | 1.33 (1.20–1.48) | 0.43 (0.34–0.54) |
| South Atlantic | 0.78 (0.73–0.83) | 0.61 (0.56–0.66) | 1.19 (1.09–1.30) | 0.90 (0.77–1.04) |
| West North Central | 0.80 (0.73–0.87) | 0.75 (0.67–0.84) | 1.05 (0.92–1.19) | 0.56 (0.42–0.73) |
| West South Central | 1.00 (0.93–1.08) | 0.84 (0.77–0.93) | 1.41 (1.27–1.58) | 1.07 (0.90–1.29) |
| East North Central | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
CI, Confidence Interval; OR, Odds Ratio
The addition of hospital characteristics to the logistic regression models was associated with improvement in the performance of all 5 models (Table 3). The ROCs ranged from 0.71 for dobutamine to 0.76 for dopamine and 0.77 for milrinone. However, the best performance was obtained with the HGLM models that adjusted for patient case mix and an individual hospital effect as random effects. The ROCs for HGLMs ranged from 0.77 to 0.88. Furthermore, the intra-class correlation coefficients of the HGLMs indicated that a noteworthy proportion of the variance in inotrope utilization could be explained by the individual institutional effect after accounting for differences in patient case mix. Individual institutional effect could explain 34% of variability in milrinone use, 19% of variability in dobutamine use, and 10% of variability in dopamine use.
Table 3.
Importance of “institutional factor” in variation of inotrope utilization.
| n (%) of patients (N=189,948) | ROC without institutional effect* | ROC with general institutional characteristics† | ROC with institutional effect‡ | ICC | |
|---|---|---|---|---|---|
| Any inotrope | 13,728 (7.2) | 0.706 | 0.721 | 0.769 | 0.113 |
| Dobutamine | 7562 (4.0) | 0.686 | 0.714 | 0.789 | 0.189 |
| Dopamine | 6482 (3.4) | 0.752 | 0.760 | 0.802 | 0.095 |
| Milrinone | 2025 (1.1) | 0.745 | 0.774 | 0.877 | 0.340 |
Logistic regression models including patient characteristics as covariates
Logistic regression models including patient characteristics and hospital characteristics as covariates
Hierarchical logistic regression models including patient characteristics and hospital random effects
ROC, receiver operating characteristic; ICC, intra-class correlation coefficient
Use of Positive Inotropic Agents and Overall Hospital Mortality
When all patients were included, the median of the unadjusted in-hospital mortality rates was 4.4% (IQR: 3.2–5.7%). The median of the in-hospital RSMRs was 4.7% (IQR: 3.9–5.5%). There was no significant relationship between RSMR and hospital percent of inotrope use or hospital pattern of use (Tables 1 and 4, Figure 2). When we stratified hospitals by the crude percent of inotrope use weighted for heart failure volume, we found no difference in RSMRs between hospitals in the top and bottom 10th percentiles. When we stratified hospitals by use of heart transplant/ventricular assist device, we found no association between RSMRs and percent of inotrope use.
Table 4.
Association between hospital use of inotropes and in-hospital risk-standardized mortality rate.
| % Median (IQR)
|
p-value | ||
|---|---|---|---|
| Inotrope use | RSMR | ||
| All hospitals (283) | 6.1 (3.9–9.0) | 4.7 (3.9–5.5) | 0.358 |
| All hospitals stratified by use of inotropic agents* (N=283) | |||
| Top 10th percentile | 14.9 (13.9–17.6) | 4.6 (3.9–5.6) | |
| 11–24th percentile | 10.4 (9.7–11.1) | 4.6 (3.9–5.5) | 0.445 |
| 25–75th percentile | 6.1 (5.0–7.5) | 4.7 (4.0–5.5) | |
| 76–89th percentile | 3.3 (2.8–3.6) | 4.3 (3.6–5.7) | |
| Bottom 10th percentile | 1.8 (1.4–2.1) | 4.7 (4.1–5.9) | |
| VAD/heart transplant hospitals (N=25) | 8.7 (6.8–11.7) | 4.5 (3.9–5.3) | 0.916 |
| Non-VAD/heart transplant hospitals (N=258) | |||
| Top 10th percentile | 14.5 (13.8–17.5) | 4.6 (4.1–5.4) | 0.484 |
| 11–24th percentile | 10.1 (9.5–10.5) | 4.8 (3.9–5.8) | |
| 25–75th percentile | 5.7 (4.8–7.0) | 4.8 (4.0–5.4) | |
| 76–89th percentile | 3.2 (2.8–3.5) | 4.0 (3.6–5.7) | |
| Bottom 10th percentile | 1.8 (1.5–2.1) | 4.8 (4.1–6.0) | |
Unadjusted rates of inotropic agent use and only hospitals with at least 200 heart failure cases over the 2 years
IQR, inter-quartile range; RSMR, risk-standardized mortality rate; VAD, ventricular assist device
DISCUSSION
In this large observational study, we found marked differences in the pattern of use of positive inotropic agents among a diverse group of hospitals in the United States. Variation in rates and types of medication reflected differences in hospitals as well as patient characteristics. We did not find an association between patterns of use and in-hospital RSMRs or length of stay.
Despite the potential harm associated with positive inotropic agents (16,17) and the lack of strong endorsement by clinical practice guidelines, they are commonly used. Guidelines state that inotropes should be confined to carefully selected patients with low blood pressure and reduced cardiac output who can have blood pressure and heart rhythm monitored closely (9). Registries suggest that this group represents about 3% of all patients hospitalized with heart failure (18). Our study and others suggest that many more (7% to 12%) patients are being treated with these agents (19,20). In the Acute Decompensated Heart Failure National Registry (ADHERE), the mean systolic blood pressure for patients treated with dobutamine was 124.0 ± 29.3 mmHg and 121.3 ± 27.4 mmHg for those treated with milrinone. Of the 6198 patients (9% of the total cohort) who were treated with these agents, only 507 (8%) had a systolic blood pressure <90 mmHg (19). The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) reported that the use of vasoactive therapy among its participants was not significantly influenced by blood pressure or cardiac index (21).
The pattern of agents used was also quite variable between hospitals. Overall rates of use were related to the mix of agents, with the highest percentage of overall inotropic use found in milrinone-predominant hospitals. In the absence of evidence about the comparative effectiveness of these drugs in patients with heart failure, the likelihood of a patient being treated with a specific agent seems mostly dependent on the institution to which the patient is admitted. Despite concerns about the safety of inotrope use, we failed to find differences in mortality or length of stay. Nevertheless, given that hospitalizations for heart failure are common and that inotropic agents have potential for harm in at least some patient populations, the observed variation in patterns of practice highlights the urgent need for greater evidence to guide these care decisions.
There are several limitations to consider. First, hospitals in the Premier network may not be a representative sample of all hospitals in the United States. However, preliminary comparisons between patient and hospital characteristics for the hospitals that submit data to Premier and those of the probability sample of hospitals and patients selected for the National Hospital Discharge Survey suggest that the patient populations are similar with regard to age, gender, length of stay, mortality, primary discharge diagnosis, and primary procedure groups. In addition, the patients included in our study had very similar characteristics to those of the heart failure patients described in registries such as ADHERE or Get With The Guidelines-Heart Failure (18,21). Second, this database does not include clinical data such as left ventricular ejection fraction, vital signs (e.g., heart rate and blood pressure) or laboratory test results (serum creatinine) that are important determinants of inotrope use and may contribute to improving the risk adjustment for patient case mix across hospitals. However, the differences we observed are larger than would be expected based on differences in case mix. Despite the lack of these clinical and biological data, the performance of the models (predictive ability) showed that inotrope utilization at the hospital level can be adequately modeled when accounting for both patient case mix and institutional clustering effects. Third, we included only the first admission rather than all hospitalizations per patient. This was because analyses showed that percentage of inotrope utilization was higher in patients with multiple hospitalizations. Therefore, including all hospitalizations would have overestimated the relative importance of institution-related factors (vs. patient-related factors) in explaining the variation in inotrope use. Indeed when all hospitalizations per patient were included, the ICCs were slightly higher (0.131 for any inotrope use, 0.216 for dobutamine, 0.102 for dopamine and 0.372 for milrinone).
Our analyses demonstrate that a noteworthy proportion of the variation observed in inotrope use was related to an individual institutional effect. This finding is in agreement with ESCAPE, in which the most important predictor of use was the study site (hospital) to which the patient was admitted despite the inclusion of more patient-level clinical and biological data in the multivariable analysis (21).
Conclusion
The marked differences that we observed in the rates and patterns of inotrope use in the treatment of patients hospitalized with heart failure in the United States are largely attributed to unmeasured institutional factors, making the likelihood and type of treatment with an inotropic agent for any given patient highly dependent on the hospital to which the patient is admitted. This study heralds an urgent need for further investigation to define the proper role of inotropic agents in the treatment of patients with decompensated heart failure.
Acknowledgments
Funding: This work was supported by grant DF10-301 from the Patrick and Catherine Weldon Donaghue Medical Research Foundation in West Hartford, Connecticut and by grant UL1 RR024139-06S1 from the National Center for Advancing Translational Sciences in Bethesda, Maryland. Dr. Krumholz is supported by grant U01 HL105270-02 (Center for Cardiovascular Outcomes Research at Yale University) and Dr. Allen is supported by grant K23 HL105896-01A1, both from the National Heart, Lung, and Blood Institute in Bethesda, Maryland. Dr. Gleim is supported by grant 11 POST-77100000 from the American Heart Association Founders Affiliate in Dallas, Texas.
ABBREVIATIONS AND ACRONYMS
- ADHERE
Acute Decompensated Heart Failure National Registry
- ACC
American College of Cardiology
- AHA
American Heart Association
- CI
Confidence Interval
- ESCAPE
Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness
- HGLM
Hierarchical Generalized Linear Model
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IQR
Inter-quartile Range
- OR
Odds Ratio
- ROC
Receiver Operating Characteristic
- RSMR
Risk-standardized Mortality Rate
APPENDIX. Patient Factors Associated with the Use of Positive Inotropic Agents
| Characteristic | OR (95% CI)
|
|||
|---|---|---|---|---|
| Inotrope | Dobutamine | Dopamine | Milrinone | |
| Age group (years) | ||||
| 18–54 | 2.32 (2.16–2.49) | 2.59 (2.35–2.84) | 1.55 (1.39–1.71) | 5.90 (4.89–7.10) |
| 55–64 | 2.28 (2.13–2.43) | 2.43 (2.23–2.65) | 1.80 (1.64–1.97) | 4.60 (3.84–5.50) |
| 65–74 | 2.00 (1.88–2.12) | 2.16 (1.99–2.34) | 1.65 (1.52–1.79) | 3.56 (3.01–4.23) |
| 75–84 | 1.63 (1.54–1.72) | 1.78 (1.65–1.92) | 1.42 (1.31–1.53) | 2.27 (1.92–2.68) |
| >85 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Female (vs. male) | 0.74 (0.71–0.77) | 0.67 (0.64–0.71) | 0.85 (0.80–0.89) | 0.68 (0.62–0.75) |
| AHRQ software comorbidity | ||||
| Valvular disease | 2.07 (1.88–2.29) | 1.44 (1.26–1.65) | 2.51 (2.23–2.82) | 1.41 (1.11–1.80) |
| Pulmonary circulation disease | 1.26(1.13–1.41) | 1.64 (1.44–1.87) | ||
| Hypertension | 0.71 (0.69–0.74) | 0.72 (0.68–0.76) | 0.73 (0.69–0.77) | 0.65 (0.59–0.71) |
| Paralysis | 0.83 (0.70–0.99) | 1.18 (1.00–1.38) | ||
| Other neurological disorders | 0.93 (0.87–1.00) | 0.79 (0.72–0.86) | 1.09 (1.00–1.19) | 0.64 (0.52–0.78) |
| Chronic pulmonary disease | 0.71 (0.64–0.78) | |||
| Diabetes, no chronic complications | 0.89 (0.81–0.99) | |||
| Diabetes with chronic complications | 0.89 (0.84–0.94) | 0.87 (0.81–0.94) | 0.91 (0.84–0.99) | 0.73 (0.63–0.85) |
| Hypothyroidism | 1.15 (1.09–1.21) | 1.18 (1.11–1.26) | 1.14 (1.06–1.22) | |
| Renal failure | 1.41 (1.35–1.46) | 1.41 (1.35–1.49) | 1.35 (1.28–1.43) | 1.42 (1.29–1.56) |
| Liver disease | 1.18 (1.07–1.31) | 1.28 (1.13–1.44) | 1.23 (1.07–1.41) | |
| Acquired immune deficiency syndrome | 0.54 (0.35–0.83) | 0.24 (0.06–0.98) | ||
| Lymphoma | 0.73 (0.56–0.95) | |||
| Metastatic cancer | 0.84 (0.71–0.99) | 0.53 (0.40–0.69) | 0.50 (0.29–0.85) | |
| Chronic blood loss anemia | 1.19 (1.02–1.38) | 1.41 (1.01–1.98) | ||
| Rheumatoid arthritis/collagen vascular disease | 0.66 (0.48–0.91) | |||
| Coagulopathy | 1.74 (1.63–1.85) | 1.69 (1.57–1.83) | 1.79 (1.65–1.95) | 2.22 (1.95–2.53) |
| Obesity | 0.91 (0.87–0.96) | 0.88 (0.82–0.93) | 0.80 (0.70–0.90) | |
| Weight loss | 1.65 (1.54–1.76) | 1.46 (1.34–1.60) | 1.85 (1.70–2.01) | 1.74 (1.49–2.03) |
| Fluid and electrolyte disorders | 1.88 (1.81–1.95) | 1.75 (1.67–1.84) | 2.15 (2.04–2.27) | 1.71 (1.56–1.88) |
| Deficiency anemias | 0.94 (0.90–0.98) | 0.93 (0.88–0.98) | 0.94 (0.89–1.00) | |
| Alcohol abuse | 0.73 (0.58–0.93) | |||
| Drug abuse | 0.86 (0.75–0.98) | |||
| Psychoses | 1.16 (1.01–1.34) | 0.73 (0.55–0.98) | ||
| Depression | 0.92 (0.86–0.98) | 0.90 (0.82–0.98) | ||
| Disorders of lipid metabolism | 0.91 (0.88–0.95) | 0.93 (0.89–0.98) | 0.85 (0.81–0.90) | |
| Peripheral Vascular Disease | 1.11 (1.04–1.20) | |||
| Additional cardiovascular comorbidities | ||||
| Coronary atherosclerosis and other heart disease | 1.46 (1.40–1.52) | 1.57 (1.49–1.65) | 1.25 (1.18–1.32) | 1.77 (1.60–1.96) |
| Acute myocardial infarction | 2.33 (2.18–2.49) | 1.70 (1.56–1.86) | 3.21 (2.97–3.47) | 1.70 (1.45–2.00) |
| Aortic and peripheral arterial embolism or thrombosis | 1.71 (1.24–2.35) | 1.74 (1.18–2.58) | 1.97 (1.06–3.69) | |
| Cardiac dysrhythmias | 1.42 (1.37–1.47) | 1.33 (1.27–1.40) | 1.47 (1.39–1.55) | 1.82 (1.66–2.00) |
| Cardiac arrest and ventricular fibrillation | 5.85 (5.36–6.38) | 2.83 (2.52–3.17) | 8.53 (7.76–9.38) | 2.43 (1.99–2.98) |
| Insurance | ||||
| Medicare managed care | 0.93 (0.85–1.02) | 0.94 (0.84–1.06) | 0.90 (0.79–1.03) | 0.87 (0.70–1.08) |
| Medicare traditional | 0.98 (0.90–1.06) | 0.97 (0.87–1.08) | 0.99 (0.88–1.12) | 0.90 (0.74–1.08) |
| Other | 0.82 (0.70–0.96) | 0.75 (0.60–0.92) | 0.83 (0.66–1.04) | 0.94 (0.68–1.32) |
| Private | 0.97 (0.89–1.05) | 0.97 (0.87–1.08) | 0.98 (0.86–1.11) | 1.12 (0.93–1.34) |
| Uninsured | 0.85 (0.76–0.96) | 0.92 (0.80–1.07) | 0.80 (0.67–0.96) | 0.76 (0.59–1.00) |
| Medicaid | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
AHRQ, Agency for Healthcare Research and Quality;
CI, Confidence Interval; OR, Odds Ratio
Footnotes
Relationships with Industry: Dr. Krumholz reports that he is the recipient of a research grant from Medtronic, Inc. through Yale University and is chair of a cardiac scientific advisory board for UnitedHealth. Dr. Allen reports that he is a consultant for Amgen, Inc. The other authors report no relationships with industry.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ. Heart disease and stroke statistics-2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993–2006. JAMA. 2010;303:2141–7. doi: 10.1001/jama.2010.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure. Update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3:459–67. doi: 10.1161/CIRCOUTCOMES.110.957613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 5.De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–89. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 6.Cuffe MS, Califf RM, Adams KF, Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–7. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 7.Cohn JN, Goldstein SO, Greenberg BH, et al. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure. Vesnarinone Trial Investigators. N Engl J Med. 1998;339:1810–6. doi: 10.1056/NEJM199812173392503. [DOI] [PubMed] [Google Scholar]
- 8.Moran JF, Rad N, Scanlon PJ. Long term survival of class IV heart failure patients treated with oral amrinone. J Clin Pharmacol. 1989;29:494–9. doi: 10.1002/j.1552-4604.1989.tb03370.x. [DOI] [PubMed] [Google Scholar]
- 9.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–89. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Elixhauser A, Steiner C. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Christiansen CL, Morris CN. Improving the statistical approach to health care provider profiling. Ann Intern Med. 1997;127:764–8. doi: 10.7326/0003-4819-127-8_part_2-199710151-00065. [DOI] [PubMed] [Google Scholar]
- 14.Moineddin R, Matheson FI, Glazier RH. A simulation study of sample size for multilevel logistic regression models. BMC Med Res Methodol. 2007;7:34. doi: 10.1186/1471-2288-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Team R. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. [Google Scholar]
- 16.Adams KF, DeMarco T, Berkowitz R. Inotrope use and negative outcomes in treatment of acute heart failure in patients with preserved systolic function: data from the ADHERE database. Circulation. 2003;108 (Suppl IV):695. [Google Scholar]
- 17.O’Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST) Am Heart J. 1999;138:78–86. doi: 10.1016/s0002-8703(99)70250-4. [DOI] [PubMed] [Google Scholar]
- 18.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) J Am Coll Cardiol. 2005;46:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 20.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 21.Elkayam U, Tasissa G, Binanay C, et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J. 2007;153:98–104. doi: 10.1016/j.ahj.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Ligges U, Mächler M. Scatterplot3d. An R package for visualizing multivariate data. J Stat Software. 2003;8:1–20. [Google Scholar]


