Abstract
Background. The association between human papillomavirus (HPV) infection and the risk of human immunodeficiency virus (HIV) seroconversion is unclear, and the genital cellular immunology has not been evaluated.
Methods. A case-control analysis nested within a male circumcision trial was conducted. Cases consisted of 44 male HIV seroconverters, and controls were 787 males who were persistently negative for HIV. The Roche HPV Linear Array Genotype Test detected high-risk HPV (HR-HPV) and low-risk HPV (LR-HPV) genotypes. Generalized estimating equations logistic regression was used to estimate adjusted odds ratios (aORs) of HIV seroconversion. In addition, densities of CD1a+ dendritic cells, CD4+ T cells, and CD8+ T cells were measured using immunohistochemistry analysis in foreskins of 79 males randomly selected from participants in the circumcision trial.
Results. HR-HPV or LR-HPV acquisition was not significantly associated with HIV seroconversion, after adjustment for sexual behaviors. However, HR-HPV and LR-HPV clearance was significantly associated with HIV seroconversion (aOR, 3.25 [95% confidence interval {CI}, 1.11–9.55] and 3.18 [95% CI, 1.14–8.90], respectively). The odds of HIV seroconversion increased with increasing number of HPV genotypes cleared (P < .001, by the test for trend). The median CD1a+ dendritic cell density in the foreskin epidermis was significantly higher among males who cleared HPV (72.0 cells/mm2 [interquartile range {IQR}, 29.4–138.3 cells/mm2]), compared with males who were persistently negative for HPV (32.1 cells/mm2 [IQR, 3.1–96.2 cells/mm2]; P = .047), and increased progressively with the number of HPV genotypes cleared (P = .05).
Conclusions. HPV clearance was associated with subsequent HIV seroconversion and also with increased epidermal dendritic cell density, which potentially mediates HIV seroconversion.
Keywords: human papillomavirus (HPV), male circumcision, HIV, AIDS, Uganda, penile cancer, cervical cancer, sexually transmitted infections
Human papillomavirus (HPV) infection is a common sexually transmitted infection (STI) associated with anogenital neoplasia and warts [1, 2]. Individuals with HIV infection, especially those who are immunocompromised, have higher rates of multiple infections with high-risk HPV (HR-HPV) types and more rapid progression to neoplasia [1, 3]. However, the relationship between HPV infection and the risk of HIV seroconversion is unclear.
Several studies have reported an association between prevalent or incident HPV infection and an increased risk of HIV seroconversion [4–8]. Although most of these studies adjusted for sexual risk behaviors, the association may still reflect bias from residual uncontrolled confounding. Additionally, HPV is more transmissible than human immunodeficiency virus (HIV) [9–12], and the concurrent transmission of both viruses cannot be excluded. Two studies involving women suggested that the highest risk of HIV seroconversion is associated with clearance of a preexisting HR-HPV infection [4, 8]. Since HPV clearance is not associated with high-risk sexual behaviors [12, 13], confounding by sexual behaviors is unlikely. No studies have evaluated whether HPV clearance in men is associated with HIV seroconversion. Recruitment of macrophages, dendritic cells, and T cells in the genital tract is thought to occur during HPV clearance [14], potentially providing target cells for subsequent HIV infection. However, there are no studies of the effects of HPV acquisition or clearance on the genital cellular immune milieu. We assessed the associations between HPV acquisition and clearance and HIV seroconversion in males, and we analyzed the cellular immune correlates of HPV acquisition and clearance in foreskin tissues obtained after male circumcision.
METHODS
Population and Clinical Samples
Uncircumcised males aged 15–49 years were enrolled into a trial of male circumcision for HIV and STI prevention in Rakai District, Uganda (clinical trials registration NCT00425984). The design and results of this study have been reported previously [15–17]. In brief, eligible males were informed of study procedures and risks and provided written informed consent for screening and enrollment. Males were randomized to receive either immediate circumcision (the intervention arm) or circumcision after 24 months of follow up (the nonintervention arm). At enrollment and the month 6, year 1, and year 2 follow-up visits, males provided swab specimens from the glans/coronal sulcus for HPV detection and venous blood for serologic testing for HIV, herpes simplex virus type 2 (HSV-2), and Treponema pallidum. Study participants were examined and interviewed to ascertain sociodemographic characteristics and sexual risk behaviors. Samples and interviews were collected by trained male staff, and the swabs and sera were stored at −80°C.
Of 5097 HIV-negative males enrolled in the trial who had ≥3 trial visits, 1297 (25.4%) were evaluated for HPV. These males were randomly selected from the trial population, except for married males, who were oversampled to permit a parallel study of HPV transmission to their female partners. A total of 1027 (79.2%) had ≥2 visits in which their HPV status was ascertained; 82 were HIV seroconverters, and 945 were persistently HIV negative.
To assess whether HPV acquisition and/or clearance were associated with incident HIV infection, we evaluated a subgroup of these 1027 males in a case-control study. The case group comprised 44 HIV seroconverters (53.7%) who had ≥1 HPV test performed in the interval prior to detection of HIV seroconversion, and the control group comprised 787 persistently HIV-negative males (83.3%) who had the same follow-up duration and number of follow-up visits as the cases. Cases could serve as seronegative controls during visits that occurred before HIV seroconversion.
The trial was approved by the Ugandan National Council for Research and Technology (Kampala, Uganda) and by 3 institutional review boards: the Science and Ethics Committee of the Uganda Virus Research Institute (Entebbe, Uganda), the Johns Hopkins University Bloomberg School of Public Health Institutional Review Board (Baltimore, MD), and the Western Institutional Review Board (Olympia, WA). The trial was overseen by an independent data safety monitoring board [15, 16, 18].
HPV, HSV-2, HIV, and T. pallidum Detection
Moistened polyester swabs containing specimens from the coronal sulcus and glans were stored in Digene specimen transport medium at −80°C until they were assayed. HPV detection and genotyping was conducted at Johns Hopkins University (Baltimore, MD). HPV genotyping was performed using the HPV Linear Array Genotyping Test (Roche Diagnostics, Indianapolis, IN), which detects 37 genotypes [19]. HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 were considered the primary HR-HPV genotypes [1]. Penile swab specimens with no detectable cellular β-globin and no detectable HPV were excluded from analysis because the adequacy of the sample could not be ensured.
HSV-2 infection was determined by HSV-2 enzyme-linked immunosorbent assay (ELISA; Kalon Biological, Guilford, United Kingdom), with an index value of 1.5 considered to be a positive result [16, 20, 21]. HIV status was determined using 2 separate ELISAs and confirmed by HIV-1 Western blotting, as previously described [15]. Active T. pallidum infection was determined on the basis of a positive rapid plasma reagin test result, followed by a positive T. pallidum particle agglutination assay [16].
Histopathologic Analysis, Immunohistochemistry Analysis, and Cell Density Quantification
Foreskin tissue samples were obtained from males in the nonintervention arm of the circumcision trial at the end of the trial and at the time of circumcision, and HPV acquisition and clearance status were defined by the difference in HPV status at time of circumcision (Figure 1). A total of 168 males who were HIV negative at the time of circumcision were randomly selected from the trial population for foreskin analysis; married males were oversampled to permit a parallel study to evaluate foreskin immune correlates with their female partners. Because it was demonstrated that HSV-2 infection alters the density of foreskin immune cells [22, 23], 87 males with a positive or indeterminate HSV-2 test result at the time of circumcision were not included. Of the 81 eligible males, 2 were persistently positive for HPV (ie, they did not acquire or clear HPV during the study) and were therefore excluded, yielding 79 males for evaluation.
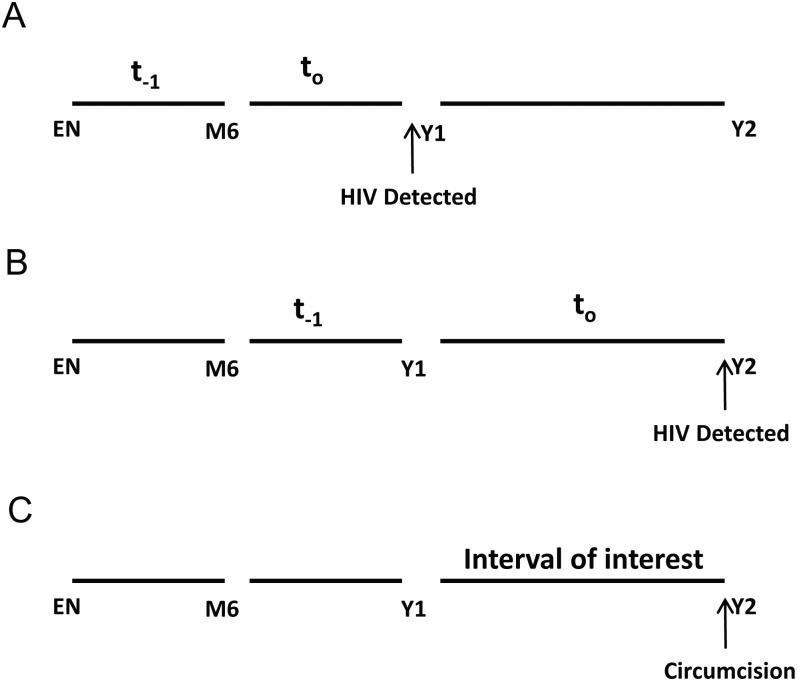
Figure 1.
A and B, Representative examples of how intervals were defined for males who were human immunodeficiency virus (HIV) negative at enrollment (EN) and month 6 (M6) and then had HIV detected at year 1 (Y1) or year 2 (Y2). For HIV seroconverters, to was defined as the interval during which HIV acquisition occurred. The last HIV-seronegative interval was defined as t−1. The t−1 intervals were the primary focus of this analysis because this interval clearly preceded the HIV seroconversion outcome and allowed unambiguous determination of temporality (ie, exposures during t−1 preceded case HIV seroconversion during to). C, The interval just prior to male circumcision was of primary interest for the association between foreskin cellular composition and HPV acquisition or clearance.
Foreskins underwent histopathologic and immunohistochemistry analyses, as reported previously [22, 23]. In brief, foreskins were fixed in 100% ethanol and stored at −80°C. Samples of foreskins were systematically obtained by unrolling the tissue and exposing the epithelial surface. Foreskins with visible scars or abnormal tissue, such as discoloration or nodules, were excluded. Cross-sections from the epithelial to subepithelial connective tissue, spaced roughly equally across the tissue surface, were cut with a razor blade into sections approximately 1 cm thick. This technique was used to obtain tissue from a variety of areas of the foreskin, including the internal and external surface. A minimum of 3 sections per subject specimen were taken and embedded in paraffin blocks. Paraffin sections 5 μm thick were cut using a microtome and mounted without pretreatment.
A standard immunohistochemistry protocol was used to identify CD1a (antibody clone 220, NovaCastra, Wetzler, Germany), CD4 (antibody clone IF6, NovaCastra, Wetzler, Germany), and CD8 (antibody clone C8-144B, Ventana, Tucson, AZ) for identification of CD1a+ dendritic cells, CD4+ T cells, and CD8+ T-cells, respectively, as previously reported [22, 23]. Briefly, all slides were digitally scanned at 20× magnification, using a ScanScope CS slide scanner (Aperio Technologies, Vista, CA). With use of custom software, a total of 10 fields of uniform size (area, 450 μm2) were selected from each subject across the 3 sections of foreskin in a semirandom compass strategy, using a low-power view of the entire slide image, which precluded visualization of any gross cell formations, such as inflammatory foci, when selecting fields [22]. The areas of tissue were selected at intact epidermal-dermal junctions, with use of the 4 compass points as a guide. Images of these fields were then evaluated by 2 independent technicians, who were masked to all subject data. The percentage area of the epidermis and dermis in each field was assessed, and cell counts were measured separately for the epidermal and dermal compartments. Positively staining cells were identified by the presence of a clearly visualized nucleus and, for CD4+ and CD8+ T cells, distinct cytoplasmic staining with red chromogen. Because of the larger size and arborized structure of the dendritic cells, the cells that lacked visible nuclei but displayed a distinct dendritic morphology were also counted. Cell density (defined as the number of cells per millimeter squared) was then calculated as described previously [22].
Statistical Analysis
The primary exposure of interest was HPV acquisition or clearance, and the unit of analysis was the interval between visits by individual participants. Each male was counted only once per interval for either HPV acquisition or clearance, irrespective of the number of HPV genotypes acquired or cleared. HPV acquisition was defined as detection of any new HPV genotype in a male who tested negative for that specific HPV genotype at the prior study visit. Clearance (ie, loss of detection) of HPV was estimated among males with HPV genotype–specific infections at the previous visit in whom the genotype was not detected at a subsequent study visit.
Among cases, the interval in which HIV was detected was defined as to, and the last HIV-seronegative interval preceding HIV seroconversion was defined as t−1 (Figure 1). The control intervals were similarly defined as to and t−1, corresponding to the case intervals. The t−1 intervals were the focus of this analysis because these intervals clearly preceded the HIV outcome and allowed unambiguous determination of temporality (ie, HPV exposures during t−1 preceded case HIV seroconversion during to). Interpretation of associations during to is ambiguous because one cannot determine whether HPV acquisition/clearance preceded or succeeded HIV infection and because HIV seroconversion is associated with increased acquisition and decreased clearance of HPV [24]. Additionally, HIV infection may lead to reactivation of latent HPV infection, leading to an overestimation of HPV acquisition [25].
Males who acquired or cleared HPV were compared to males who were persistently negative for HPV (ie, males with 2 consecutive HPV-negative samples). Dose response of HPV clearance and acquisition was evaluated using ordinal groupings of 1, 2, and ≥3 genotypes acquired or cleared. All analyses were stratified by HR-HPV and low-risk HPV (LR-HPV) such that risk of HIV seroconversion was assessed irrespective of coinfection, acquisition, or clearance of LR-HPV or HR-HPV. Since males could acquire or clear multiple HPV genotypes in a single interval, the associations of HPV acquisition and clearance with HIV seroconversion were also analyzed, using the following exposure categories: persistently negative for HPV during the interval, persistently positive for HPV during the interval (ie, 2 consecutive HPV-positive samples with the same genotype and no acquisition or clearance), acquired HPV during the interval, cleared HPV during the interval, and both acquired and cleared HPV during the interval.
Associations between fixed and time-varying potentially confounded variables with HIV seroconversion and HPV exposures were also analyzed. Fixed covariates included age, marital status, and education level at enrollment. Sexual risk behaviors (eg, number of sex partners, involvement in a nonmarital relationship, and condom use) and symptoms of STIs reported at follow-up visits for the interval of HIV seroconversion (to) and the corresponding interval for controls were included as time-varying covariates.
Crude and adjusted odds ratios (aORs) and 95% confidence intervals (CIs) of HIV seroconversion associated with HPV acquisition or clearance and potential confounding covariates were estimated using logistic regression with generalized estimating equations (GEE) and an exchangeable correlation structure to estimate robust variances [26]. Known HIV risk factors and factors that were associated with HIV seroconversion in univariate analyses at P < .2 were entered into a multivariable GEE logistic regression model with HPV exposure variables. The multivariate analysis adjusted for age, marital status, circumcision status, number of partners during the past interval, involvement in a nonmarital relationship, condom use, HSV-2 infection, and symptoms of STIs (ie, genital ulcer disease, dysuria, and urethral discharge). Trends in the risk of HIV seroconversion, by number of cleared genotypes, were assessed using the Cochran-Armitage test for trend.
For the foreskin tissue analysis, enrollment and follow-up characteristics, sexual risk behaviors, and STI symptoms were tabulated by HPV status, and differences were assessed using χ2 tests. The statistical significance of differences between HPV exposure groups in the median densities of CD4+ T cells, CD8+ T cells, and CD1a+ dendritic cells in the epidermis and dermis of the foreskin at the time of circumcision was assessed by the Mann–Whitney rank sum test. Trends in cell density, by number of genotypes cleared, were assessed using the Cuzick nonparametric test for trend and 1-tailed P values, because the study hypothesis was that HPV clearance increases the number of T cells and dendritic cells in the foreskin [27]. Analyses were performed using Stata software (version 11, StataCorp, College Station, TX) and R statistical software (version 2.14).
RESULTS
Study Population
Cases and controls were similar at enrollment with respect to education level, occupation, genital ulcer disease status, and serologic syphilis status (Table 1). However, cases were significantly more likely to be younger, to be unmarried, to have a higher number of sex partners, to be in a nonmarital relationship, to not use condoms, to report more STI symptoms (ie, urethral discharge), to be assigned to the nonintervention arm of the parent trial, to acquire HSV-2 during the study period, and be infected with HPV at enrollment.
Table 1.
Comparison of Baseline Demographic Characteristics and Sexual Risk Behaviors at Enrollment of the Cases (HIV Seroconverters) and Controls
| Variable | Cases, No. (%) (n = 44) | Controls, No. (%) (n = 787) | OR (95% CI) | P |
|---|---|---|---|---|
| Age, y | .01 | |||
| 15–19 | 8 (18.2) | 47 (6.0) | 1.00 | |
| 20–24 | 12 (27.3) | 176 (22.4) | 0.40 (.15–1.04) | |
| 25–29 | 10 (22.7) | 217 (27.6) | 0.27 (.10–.72) | |
| 30–49 | 14 (31.8) | 347 (44.1) | 0.24 (.09–.60) | |
| Education level | .58 | |||
| None | 4 (9.1) | 64 (8.1) | 1.00 | |
| Primary | 28 (63.6) | 557 (70.8) | 0.80 (.27–2.37) | |
| Secondary or higher | 12 (27.3) | 166 (21.1) | 1.16 (.36–3.72) | |
| Occupation | .56 | |||
| Non–wage earning | 12 (27.3) | 248 (31.5) | 1.00 | |
| Wage earning | 32 (72.7) | 539 (68.5) | 1.23 (.62–2.42) | |
| Marital status | <.001 | |||
| Never married | 20 (45.5) | 66 (8.4) | 1.00 | |
| Currently married | 21 (47.7) | 709 (90.1) | 0.10 (.05–.19) | |
| Previously married | 3 (6.8) | 12 (1.5) | 0.82 (.21–3.22) | |
| Nonmarital relationships | .001 | |||
| No | 27 (61.4) | 644 (81.8) | 1.00 | |
| Yes | 17 (38.6) | 143 (18.2) | 2.84 (1.51–5.34) | |
| Sex partners in past year, no. | .04 | |||
| None | 4 (9.1) | 26 (3.3) | 1.00 | |
| 1 | 18 (40.9) | 441 (56.0) | 0.27 (.08–.84) | |
| ≥2 | 22 (50.0) | 320 (40.7) | 0.45 (.14–1.39) | |
| Condom use in past year | .05 | |||
| No | 4 (9.1) | 26 (3.3) | 1.00 | |
| Yes | 40 (90.9) | 761 (96.7) | 0.34 (.11–1.03) | |
| Genital ulcer disease (self-reported) | .37 | |||
| No | 39 (88.6) | 727 (92.4) | 1.00 | |
| Yes | 5 (11.4) | 60 (7.6) | 1.55 (.59–4.09) | |
| Urethral discharge (self-reported) | .004 | |||
| No | 38 (86.4) | 754 (95.8) | 1.00 | |
| Yes | 6 (13.6) | 33 (4.2) | 3.61 (1.42–9.13) | |
| Dysuria (self-reported) | .13 | |||
| No | 39 (88.6) | 742 (94.3) | 1.00 | |
| Yes | 5 (11.4) | 45 (5.7) | 2.11 (.79–5.62) | |
| Circumcision trial arm | <.001 | |||
| Control | 35 (79.5) | 405 (51.5) | 1.00 | |
| Intervention | 9 (20.5) | 382 (48.5) | 0.27 (.13–.57) | |
| Syphilis statusa | .49 | |||
| Persistently negative | 41 (93.2) | 700 (90.0) | 1.00 | |
| Positive at enrollment | 3 (6.8) | 78 (10.0) | 0.66 (.20–2.17) | |
| HSV-2 statusb | <.001 | |||
| Persistently negative | 11 (25.0) | 373 (47.9) | 1.00 | |
| Indeterminate at enrollment | 10 (22.7) | 80 (10.3) | 4.24 (1.74–10.32) | |
| Persistently positive | 15 (34.1) | 292 (37.5) | 1.74 (.79–3.85) | |
| Seroconversion during study | 8 (18.2) | 33 (4.2) | 8.22 (3.09–21.85) | |
| HPV detected at enrollmentc | .001 | |||
| No | 7 (15.9) | 333 (42.3) | 1.00 | |
| Yes | 37 (84.1) | 454 (57.7) | 3.88 (1.71–8.80) |
The case group comprised males with HIV seroconversion, and the control group comprised males who were persistently HIV negative.
Abbreviations: CI, confidence interval; HPV, human papillomavirus; HSV-2, herpes simplex virus type 2; OR, odds ratio.
a Determined by a positive rapid plasma reagin test, followed by a Treponema pallidum particle agglutination assay. Data were missing for 9 controls.
b Based on testing at enrollment and the 24-month follow-up visit. Data were missing for 9 controls.
c Includes high-risk and low-risk HPV types.
Acquisition of HPV Associated With HIV Seroconversion
The association between cases of HIV seroconversion and HPV acquisition was assessed among males who acquired HPV during t−1. HR-HPV or LR-HPV acquisition were not significantly associated with HIV seroconversion, after adjustment for known HIV risk factors (aOR, 1.49 [95% CI, .44–5.01] and 2.21 [95% CI, .61–7.97], respectively; Table 2). The odds of HIV seroconversion also did not vary by the number of HPV genotypes acquired.
Table 2.
Odds of Human Immunodeficiency Virus (HIV) Seroconversion Among Males Who Acquired Human Papillomavirus (HPV) During the Interval Before HIV Seroconversion
| HPV Exposure Category, by Carcinogenic Risk | Intervals, No. (%) |
Odds Ratio (95% CI) |
|||
|---|---|---|---|---|---|
| Cases | Controls | Overall | Unadjusted | Adjusteda | |
| High risk | |||||
| Persistently negative | 6 (37.5) | 386 (61.6) | 392 | 1.00 | 1.00 |
| Acquiredb | 10 (62.5) | 241 (38.4) | 251 | 2.67 (.96–7.43) | 1.49 (.44–5.01) |
| Low risk | |||||
| Persistently negative | 6 (28.6) | 386 (55.7) | 392 | 1.00 | 1.00 |
| Acquiredb | 15 (71.4) | 307 (44.3) | 322 | 3.16 (1.21–8.24) | 2.21 (.61–7.97) |
| High risk or low risk | |||||
| Persistently negative | 6 (24.0) | 386 (46.9) | 392 | 1.00 | 1.00 |
| Acquired, no. of genotypesb | |||||
| 1 | 5 (20.0) | 274 (33.3) | 279 | 1.17 (.35–3.91) | 1.27 (.35–4.60) |
| 2 | 5 (20.0) | 80 (9.7) | 85 | 4.02 (1.19–13.57) | 2.90 (.74–11.40) |
| ≥3 | 9 (36.0) | 83 (10.1) | 92 | 7.05 (2.47–20.11) | 2.71 (.59–12.35) |
The case group comprised males with HIV seroconversion, and the control group comprised males who were persistently HIV negative.
Abbreviation: CI, confidence interval.
a The multivariate analysis adjusted for age, marital status, circumcision status, number of sex partners during past interval, nonmarital relationships, condom use, herpes simplex virus type 2 infection, and symptoms of sexually transmitted infections (ie, genital ulcer disease, dysuria, and urethral discharge).
b Six males acquired both high-risk and low-risk HPV genotypes.
HPV Clearance Associated With HIV Seroconversion
The unadjusted odds of HIV seroconversion were significantly increased among males who cleared either HR-HPV (OR, 4.12 [95% CI, 1.63–10.42]) or LR-HPV (OR, 4.22 [95% CI, 1.71–10.40]) during t−1, and these estimates remained statistically significant after adjustment for known HIV risk factors (aORs, 3.25 [95% CI, 1.11–9.55] for clearance of HR-HPV and 3.18 [95% CI, 1.14–8.90] for clearance of LR-HPV; Table 3). The odds of HIV seroconversion also increased with increasing number of HPV genotypes cleared (ORs, 1.73 [95% CI, .59–5.09] for clearance of 1 genotype, 5.44 [95% CI, 1.97–15.03] for clearance of 2 genotypes, and 6.34 [95% CI, 2.32–17.31] for clearance of ≥3 genotypes; P < .001, by the test for trend).
Table 3.
Odds of Human Immunodeficiency Virus (HIV) Seroconversion Among Males Who Cleared Human Papillomavirus (HPV) During the Interval Before HIV Seroconversion
| HPV Exposure Category, by Carcinogenic Risk | Intervals, No. (%) |
Odds Ratio (95% CI) |
|||
|---|---|---|---|---|---|
| Cases | Controls | Overall | Unadjusted | Adjusteda | |
| High risk | |||||
| Persistently negative | 6 (23.1) | 386 (55.0) | 392 | 1.00 | 1.00 |
| Clearedb | 20 (76.9) | 316 (45.0) | 336 | 4.12 (1.63–10.42) | 3.25 (1.11–9.55) |
| Low risk | |||||
| Persistently negative | 6 (18.8) | 386 (49.1) | 392 | 1.00 | 1.00 |
| Clearedb | 26 (81.2) | 400 (50.9) | 426 | 4.22 (1.71–10.40) | 3.18 (1.14–8.90) |
| Any risk | |||||
| Persistently negative | 6 (16.2) | 386 (41.1) | 392 | 1.00 | 1.00 |
| Cleared, no. of genotypesb | |||||
| 1 | 8 (21.6) | 298 (31.7) | 306 | 1.73 (.59–5.09) | 1.09 (.33–3.68) |
| 2 | 11 (29.7) | 131 (14.0) | 142 | 5.44 (1.97–15.03) | 5.66 (1.69–18.89) |
| ≥3 | 12 (32.4) | 124 (13.2) | 136 | 6.34 (2.32–17.31) | 3.85 (1.14–12.99) |
The case group comprised males with HIV seroconversion, and the control group comprised males who were persistently HIV negative.
Abbreviation: CI, confidence interval.
a The multivariate analysis adjusted for age, marital status, circumcision status, number of sex partners during past interval, nonmarital relationships, condom use, herpes simplex virus type 2 infection, and symptoms of sexually transmitted infections (ie, genital ulcer disease, dysuria, and urethral discharge).
b Fifteen males cleared both high-risk and low-risk HPV genotypes.
Males were also categorized on the basis of whether, during the interval preceding case HIV seroconversion(t-1 interval), they were persistently HPV negative, persistently HPV positive, acquired HPV, cleared HPV, or both acquired and cleared HPV (Table 4). HPV acquisition and persistent HPV infection were not significantly associated with the risk of HIV seroconversion in univariate analyses (ORs, 1.18 [95% CI, .33–4.24] and 2.17 [95% CI, .53–8.91], respectively). Males who cleared HPV (OR, 3.08 [95% CI, 1.19–8.01]) or cleared and acquired HPV (OR, 4.44 [95% CI, 1.70–11.62]) had an increased odds of HIV seroconversion. The odds of HIV seroconversion remained significant, after adjustment, for males who cleared and acquired HPV (aOR, 2.91 [95% CI, 1.01–8.37]).
Table 4.
Odds of Human Immunodeficiency Virus (HIV) Seroconversion, by Acquisition and Clearance of Human Papillomavirus (HPV), Regardless of Carcinogenic Risk, During the Interval Before HIV Seroconversion
| HPV Exposure Category | Intervals, No. (%) |
Odds Ratio (95% CI) |
|||
|---|---|---|---|---|---|
| Cases | Controls | Overall | Unadjusted | Adjusteda | |
| Persistently negative | 6 (13.6) | 386 (31.0) | 392 | 1.00 | 1.00 |
| Persistently positive | 3 (6.8) | 89 (7.1) | 92 | 2.17 (.53–8.91) | 2.52 (.57–11.12) |
| Acquired | 4 (9.1) | 219 (17.6) | 223 | 1.18 (.33–4.24) | 0.89 (.19–4.08) |
| Cleared | 16 (36.4) | 335 (26.9) | 351 | 3.08 (1.19–8.01) | 2.65 (.89–7.94) |
| Cleared and acquired | 15 (34.1) | 218 (17.5) | 233 | 4.44 (1.70–11.62) | 2.91 (1.01–8.37) |
The case group comprised males with HIV seroconversion, and the control group comprised males who were persistently HIV negative.
Abbreviation: CI, confidence interval.
a The multivariate analysis adjusted for age, marital status, circumcision status, number of sex partners during past interval, nonmarital relationships, condom use, herpes simplex virus type 2 infection, and symptoms of sexually transmitted infections (ie, genital ulcer disease, dysuria, and urethral discharge).
Dendritic Cell and T-Cell Densities in Foreskin Tissues
Foreskin tissue samples obtained from a random sample of 79 HIV-negative, HSV-2 negative males at time of circumcision were classified as persistently HPV negative, acquiring HPV, clearing HPV, or acquiring and clearing HPV during the interval prior to circumcision. Males in these groups did not differ by age, education, occupation, marital status, number of sexual partners, condom use, genital ulcer disease, urethral discharge, and dysuria at enrollment (Supplementary Table 1).
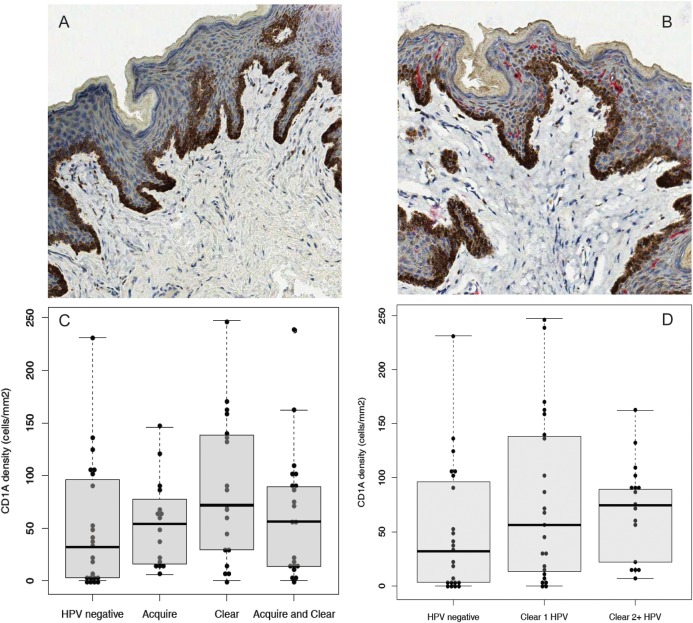
There was no significant difference in the density of foreskin epidermal CD1a+ dendritic cells between males who acquired HPV, compared with males who were persistently negative for HPV (P = .32; Figure 2 and Table 5). However, the median epidermal dendritic cell density was significantly higher among males who cleared HPV (72.0 cells/mm2 [interquartile range {IQR}, 29.4–138.3 cells/mm2]), compared with males who were persistently negative for HPV (32.1 cells/mm2 [IQR, 3.1–96.2 cells/mm2]; P = .047; Figure 2C and Table 5). The density of epidermal dendritic cells increased progressively with the higher number of HPV genotypes cleared (P = .05, by the test for trend; Figure 2D). The densities of epidermal CD4+ and CD8+ T cells were higher among males who acquired HPV, but this difference was not statistically significant (Table 5). There were no statistically significant differences in the density of dermal dendritic cells or the densities of epidermal or dermal CD4+ or CD8+ T cells among males who cleared or acquired HPV, relative to males who were persistently negative for HPV.
Figure 2.
Human papillomavirus (HPV) clearance (either HR-HPV or LR-HPV) is associated with a higher epidermal dendritic cell density. Representative immunohistochemical stains of foreskins for CD1a+ dendritic cells among HPV-negative males (A) and males who cleared HPV (B). Positively staining cells appear in red, with a final magnification of 200 × . The fixed area of each image is 450 μm2. Males were stratified by whether they were HPV negative, acquired HPV, cleared HPV, or both cleared and acquired HPV and were assessed for CD1a+ dendritic cell density (C). Males were also stratified by how many HPV genotypes they had cleared (D). Median cell densities with interquartile ranges are shown.
Table 5.
Density of CD1a+ Dendritic Cells, CD4+ T Cells, and CD8+ T Cells in the Foreskin Epidermis and Dermis Among 79 Males
| HPV Exposure Category,a by Cell Type | Density, Cells/mm2, Median (IQR) | P |
|---|---|---|
| Dendritic cells | ||
| Epidermal CD1a+ cells | ||
| Persistently negative | 32.1 (3.1–96.2) | Reference |
| Acquired | 54.2 (16.2–72.5) | .32 |
| Cleared | 72.0 (29.4–138.3) | .047 |
| Cleared and acquired | 56.3 (13.7–63.8) | .37 |
| Dermal CD1a+ cells | ||
| Persistently negative | 2.6 (0.0–9.7) | Reference |
| Acquired | 3.8 (1.4–7.4) | 1.00 |
| Cleared | 6.8 (1.6–12.0) | .28 |
| Cleared and acquired | 6.4 (1.8–10.0) | .34 |
| CD4+ T cells | ||
| Epidermal CD4+ T cells | ||
| Persistently negative | 3.2 (0.8–7.0) | Reference |
| Acquired | 8.6 (1.9–18.6) | .15 |
| Cleared | 4.3 (1.2–22.9) | .34 |
| Cleared and acquired | 3.9 (0.9–6.1) | .99 |
| Dermal CD4+ T cells | ||
| Persistently negative | 22.3 (10.2–53.3) | Reference |
| Acquired | 47.0 (12.1–71.7) | .70 |
| Cleared | 22.48 (12.0–88.0) | .76 |
| Cleared and acquired | 23.0 (4.1–63.3) | .47 |
| CD8+ T cells | ||
| Epidermal CD8+ T cells | ||
| Persistently negative | 4.5 (0.8–17.6) | Reference |
| Acquired | 11.0 (3.2–33.6) | .17 |
| Cleared | 6.8 (1.5–54.2) | .40 |
| Cleared and acquired | 6.8 (1.7–21.7) | .76 |
| Dermal CD8+ T cells | ||
| Persistently negative | 17.8 (7.5–49.2) | Reference |
| Acquired | 7.9 (5.4–30.7) | .43 |
| Cleared | 35.4 (2.1–55.9) | .74 |
| Cleared and acquired | 14.9 (4.7–34.2) | .45 |
Males were randomly selected from the parent trial evaluating male circumcision for human immunodeficiency virus infection and sexually transmitted infection prevention, as described in Methods. A total of 23 males were persistently negative for human papillomavirus (HPV), 16 acquired HPV, 19 cleared HPV, and 21 cleared and acquired HPV.
Abbreviation: IQR, interquartile range.
aHPV classified based on either HR-HPV or LR-HPV.
In a sensitivity analysis, when the 2 males who were persistently HPV positive and did not acquire or clear HPV were included with the controls who were persistently HPV negative, the epidermal dendritic cell density was higher among males who cleared HPV (P = .028), and the epidermal dendritic cell density increased progressively as the number of HPV genotypes cleared increased (P = .03, by the test for trend).
DISCUSSION
Both HPV acquisition and persistent HPV infection were not significantly associated with an increased risk of HIV seroconversion (Tables 2 and 4). Previous studies that reported an association between HPV acquisition and HIV seroconversion [5, 7] may have been confounded by sexual behaviors or concurrent transmission of both viruses from a dually infected sexual partner [28, 29]. However, clearance of penile HPV was associated with a 3.2-fold increased adjusted odds of HIV seroconversion, and the risk of HIV seroconversion increased progressively with the number of HPV genotypes cleared (Table 3). Clearance is unlikely to be confounded by high-risk sexual behaviors, since such behaviors are likely to be associated with repeat HPV exposures and because reinfection would be expected to reduce observed clearance of HPV. This is the first study of the association between HPV clearance and the risk of HIV seroconversion among males, and our findings are supported by 2 previous studies involving women, which also found that HPV clearance is associated with an increased risk of HIV seroconversion [4, 8].
The study of foreskins obtained during circumcision provides a model for evaluating cellular immune responses to HIV and HPV [30–32]. HIV infection occurs across the epidermal foreskin, where CD4+ T-lymphocytes and macrophages are relatively rare in the absence of inflammation [23, 33] but where 2 subtypes of epidermal dendritic cells (langerin-expressing Langerhans cells and DC-SIGN–expressing dendritic cells) are abundant [34]. Dendritic cells play an important role in HIV infection by delivering virions directly to primary CD4+ T cells [35]. Thus, the findings that males who cleared HPV had significantly higher dendritic cell densities in the foreskin epidermis and that this effect is dose dependent (Figure 2) suggest a plausible biologic mechanism whereby HPV clearance might increase the number of HIV target cells, potentially increasing susceptibility to HIV seroconversion.
Much of the infectious cycle of HPV is designed for immune evasion. HPV E6 and E7 oncoproteins interact and suppress the interferon signaling pathway [36]. Infected keratinocytes shed virus through natural cell death of terminally differentiating squamous epithelial cells without release of HPV-specific antigens that might induce a cellular inflammatory response [36]. Thus, HPV acquisition or persistent HPV infection are not typically associated with inflammation [14, 36]. Studies of regressing genital warts, however, have shown significantly increased numbers of CD4+ and CD8+ T cells and macrophages but a nonsignificant increase in the number of CD1a+ cells, compared with nonregressing lesions [14]. Wart regression is also associated with upregulation of lymphocytic adhesion molecules [14] and upregulation of proinflammatory cytokines [14, 36]. HPV clearance in this study was associated with an increased density of dendritic cells but not of CD4+ and CD8+ T cells, possibly because of the long interval between HPV clearance and circumcision. It takes approximately 6–18 months to clear HPV infection [12, 13, 37], and T cells are more transient than dendritic cells at the site of infection [38]. Additionally, some males who acquired HPV in the interval preceding male circumcision were likely in the process of HPV clearance, which, if true, would have resulted in the misclassification of these individuals.
This study has limitations. We were conservative and focused on the interval prior to HIV seroconversion (ie, t–1), when the temporal sequence of events is clear and not confounded by the effects of HIV infection on HPV acquisition and clearance (ie, reverse causality). Interpretation of associations during to is ambiguous because one cannot determine whether HPV acquisition or clearance preceded or succeeded HIV infection or whether HPV reactivation as a consequence of HIV infection confounded associations [24]. The 6- and 12-month intervals between sample collections are long and may have underestimated HPV incidence and clearance because individuals may have both acquired and cleared unrecognized HPV genotypes between study visits. In addition, the long intervals also prevented tight associations between the foreskin cellular milieu following HPV acquisition and clearance. We were conservative in our estimates of HPV acquisition and clearance, which were restricted to sequential samples with amplifiable cellular or viral DNA to ensure the adequacy of sample collection. Incident HPV detection probably represents a combination of true newly acquired infections and reactivation of prior latent infections [3, 25], and we were unable to distinguish between these 2 processes. The study was also unfortunately limited by a small sample size. Thus, it cannot be definitively determined that HPV clearance only, and not HPV acquisition or HPV persistence, was associated with the increased risk of HIV seroconversion.
If, as found in this study, HPV clearance is associated with HIV seroconversion and increased foreskin dendritic cell recruitment, this could be highly relevant to HIV susceptibility. These data might provide a basis for the development of future methods for HIV prevention, including new approaches through control of HPV or agents to modulate the genital inflammatory or cell-mediated immune response to HPV clearance.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the study participants and the Rakai Community Advisory Board, whose commitment and cooperation made this study possible.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID), NIH (grants U1AI5117I, 1K23AI093152-01A1, U01-AI-068613, and 3U01-AI075115-03S1); the Division of Intramural Research, NIAID, NIH (for laboratory support); and The Fogarty International Center (grants 5D43TW001508 and 2D43TW000010-19-AITRP for training staff to collect specimens and perform interviews). A. A. R. T. was supported by the NIH (grants 1K23AI093152-01A1 and R01AI087409-01A1), a Doris Duke Charitable Foundation Clinician Scientist Development Award (22006.02), and a Johns Hopkins University Clinician Scientist Award.
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 2.Partridge JM, Koutsky LA. Genital human papillomavirus infection in men. Lancet Infect Dis. 2006;6:21–31. doi: 10.1016/S1473-3099(05)70323-6. [DOI] [PubMed] [Google Scholar]
- 3.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–86. doi: 10.1093/jnci/dji073. [DOI] [PubMed] [Google Scholar]
- 4.Averbach SH, Gravitt PE, Nowak RG, et al. The association between cervical HPV infection and HIV acquisition among women in Zimbabwe. AIDS. 2010;24:1035–42. doi: 10.1097/qad.0b013e3283377973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Auvert B, Lissouba P, Cutler E, Zarca K, Puren A, Taljaard D. Association of oncogenic and nononcogenic human papillomavirus with HIV incidence. J Acquir Immune Defic Syndr. 2010;53:111–6. doi: 10.1097/QAI.0b013e3181b327e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbulawa ZZ, Coetzee D, Marais DJ, et al. Genital human papillomavirus prevalence and human papillomavirus concordance in heterosexual couples are positively associated with human immunodeficiency virus coinfection. J Infect Dis. 2009;199:1514–24. doi: 10.1086/598220. [DOI] [PubMed] [Google Scholar]
- 7.Smith JS, Moses S, Hudgens MG, et al. Increased risk of HIV acquisition among Kenyan men with human papillomavirus infection. J Infect Dis. 2010;201:1677–85. doi: 10.1086/652408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith-McCune KK, Shiboski S, Chirenje MZ, et al. Type-specific cervico-vaginal human papillomavirus infection increases risk of HIV acquisition independent of other sexually transmitted infections. PLoS One. 2010;5:e10094. doi: 10.1371/journal.pone.0010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobian AA, Gray RH. Male foreskin and oncogenic human papillomavirus infection in men and their female partners. Future Microbiol. 2011;6:739–45. doi: 10.2217/fmb.11.59. [DOI] [PubMed] [Google Scholar]
- 10.Gray RH, Serwadda D, Kong X, et al. Male circumcision decreases acquisition and increases clearance of high-risk human papillomavirus in HIV-negative men: a randomized trial in Rakai, Uganda. J Infect Dis. 2010;201:1455–62. doi: 10.1086/652184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serwadda D, Wawer MJ, Makumbi F, et al. Circumcision of HIV-infected men: effects on high-risk human papillomavirus infections in a randomized trial in Rakai, Uganda. J Infect Dis. 2010;201:1463–9. doi: 10.1086/652185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giuliano AR, Lee JH, Fulp W, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–40. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tobian AA, Kigozi G, Gravitt PE, et al. Human papillomavirus incidence and clearance among HIV-positive and HIV-negative men in Rakai, Uganda. AIDS. 2012;26:1555–65. doi: 10.1097/QAD.0b013e328353b83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman N, Birley HD, Renton AM, et al. Immunological events in regressing genital warts. Am J Clin Pathol. 1994;102:768–74. doi: 10.1093/ajcp/102.6.768. [DOI] [PubMed] [Google Scholar]
- 15.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 16.Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360:1298–309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobian AA, Ssempijja V, Kigozi G, et al. Incident HIV and herpes simplex virus type 2 infection among men in Rakai, Uganda. AIDS. 2009;23:1589–94. doi: 10.1097/QAD.0b013e32832d4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobian AA, Kong X, Wawer MJ, et al. Circumcision of HIV-infected men and transmission of human papillomavirus to female partners: analyses of data from a randomised trial in Rakai, Uganda. Lancet Infect Dis. 2011;11:604–12. doi: 10.1016/S1473-3099(11)70038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobian AAR, Charvat B, Ssempijja V, et al. Factors associated with the prevalence and incidence of herpes simplex virus type 2 infections among men in Rakai, Uganda. J Infect Dis. 2009;199:945–9. doi: 10.1086/597074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gamiel JL, Tobian AAR, Laeyendecker OB, et al. Improved performance of enzyme-linked immunosorbent assays and the effect of human immunodeficiency virus coinfection on the serologic detection of herpes simplex virus type 2 in Rakai, Uganda. Clin Vaccine Immunol. 2008;15:888–90. doi: 10.1128/CVI.00453-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson KE, Redd AD, Quinn TC, et al. Effects of HIV-1 and HSV-2 infection on lymphocyte and dendritic cell density in adult foreskins from Rakai, Uganda. J Infect Dis. 2011;203:602–9. doi: 10.1093/infdis/jiq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson KE, Sherman ME, Ssempiija V, et al. Foreskin inflammation is associated with HIV and herpes simplex virus type-2 infections in Rakai, Uganda. AIDS. 2009;23:1807–15. doi: 10.1097/QAD.0b013e32832efdf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowak RG, Gravitt P, Morrison CS, et al. Increases in human papillomavirus (HPV) detection during early HIV infection among women in Zimbabwe. J Infect Dis. 2011;203:1182–91. doi: 10.1093/infdis/jiq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gravitt PE. The known unknowns of HPV natural history. J Clin Invest. 2011;121:4593–9. doi: 10.1172/JCI57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang KY, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 27.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 28.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 29.Burchell AN, Coutlee F, Tellier PP, Hanley J, Franco EL. Genital transmission of human papillomavirus in recently formed heterosexual couples. J Infect Dis. 2011;204:1723–9. doi: 10.1093/infdis/jir644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tobian AA, Gray RH. The medical benefits of male circumcision. JAMA. 2011;306:1479–80. doi: 10.1001/jama.2011.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wawer MJ, Tobian AA, Kigozi G, et al. Effect of circumcision of HIV-negative men on transmission of human papillomavirus to HIV-negative women: a randomised trial in Rakai, Uganda. Lancet. 2011;277:209–18. doi: 10.1016/S0140-6736(10)61967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobian AA, Gray RH, Quinn TC. Male circumcision for the prevention of acquisition and transmission of sexually transmitted infections: the case for neonatal circumcision. Arch Pediatr Adolesc Med. 2010;164:78–84. doi: 10.1001/archpediatrics.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, Hladik F, Woodward A, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886–92. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. Aids. 2006;20:1491–5. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- 35.Felts RL, Narayan K, Estes JD, et al. 3D visualization of HIV transfer at the virological synapse between dendritic cells and T cells. Proc Natl Acad Sci U S A. 2010;107:13336–41. doi: 10.1073/pnas.1003040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24(Suppl 1):S16–22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Moscicki AB, Ellenberg JH, Farhat S, Xu J. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis. 2004;190:37–45. doi: 10.1086/421467. [DOI] [PubMed] [Google Scholar]
- 38.Ludewig B, Oehen S, Barchiesi F, Schwendener RA, Hengartner H, Zinkernagel RM. Protective antiviral cytotoxic T cell memory is most efficiently maintained by restimulation via dendritic cells. J Immunol. 1999;163:1839–44. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.