Abstract
Background. The long-term impact of allogeneic hematopoietic stem cell transplantation (HSCT) on human immunodeficiency virus type 1 (HIV-1) reservoirs in patients receiving combination antiretroviral therapy (cART) is largely unknown.
Methods. We studied the effects of a reduced-intensity conditioning allogeneic HSCT from donors with wild-type–CCR5+ cells on HIV-1 peripheral blood reservoirs in 2 patients heterozygous for the ccr5Δ32 mutation. In-depth analyses of the HIV-1 reservoir size in peripheral blood, coreceptor use, and specific antibody responses were performed on samples obtained before and up to 3.5 years after HSCT receipt.
Results. Although HIV-1 DNA was readily detected in peripheral blood mononuclear cells (PBMCs) before and 2–3 months after HSCT receipt, HIV-1 DNA and RNA were undetectable in PBMCs, CD4+ T cells, or plasma up to 21 and 42 months after HSCT. The loss of detectable HIV-1 correlated temporally with full donor chimerism, development of graft-versus-host disease, and decreases in HIV-specific antibody levels.
Conclusions. The ability of donor cells to engraft without evidence of ongoing HIV-1 infection suggests that HIV-1 replication may be fully suppressed during cART and does not contribute to maintenance of viral reservoirs in peripheral blood in our patients. HSCTs with wild-type–CCR5+ donor cells can lead to a sustained reduction in the size of the peripheral reservoir of HIV-1.
Keywords: HIV-1, viral reservoirs, hematopoietic stem cell transplantation, HIV-1 persistence, CCR5, reduced-intensity allogeneic stem cell transplantation
The main challenge in curing human immunodeficiency virus type 1 (HIV-1) infection is the persistence of latently infected cells, which are established by permanent integration of the viral genome into host cell chromosomes [1, 2]. Long-term remission of HIV-1 infection has been reported in a patient who received a myeloablative allogeneic hematopoietic stem cell transplant (HSCT) for acute myeloid leukemia, using cells from a donor homozygous for the Δ32 mutation in the gene encoding CCR5, a coreceptor for HIV-1 entry [3, 4]. No evidence of active HIV-1 replication has been demonstrated in this patient after 5 years of follow-up in the absence of combination antiretroviral therapy (cART) [5]. The lack of functional CCR5 on donor stem cells likely played an important role in achieving virologic control in this patient, but other factors, including graft-versus-host effects, may have contributed to reduction in the viral reservoir and to long-term HIV-1 control [3, 4]. Aside from this one example, few other longitudinal studies have explored the effect of allogeneic HSCT on viral reservoirs and HIV-1–specific immune responses.
Cytoreductive conditioning is unlikely to be sufficient to eliminate HIV-1 reservoirs alone, as HIV-1 DNA persists after receipt of chemotherapy and/or an autologous HSCT for hematologic malignancies [6–9]. Short-term reductions in HIV-1 reservoirs have been described in 4 patients who were not receiving cART after receipt of an allogeneic HSCT that involved myeloablative conditioning regimens with total body irradiation and/or anti-lymphocyte immunotherapy, but all 4 died ≤10 months after transplantation [10–14]. HIV-1 was not detected in various tissues obtained at autopsy for 2 patients [10, 11]. HIV-1 DNA became undetectable in peripheral blood mononuclear cells (PBMCs) after transplantation in 2 patients who received cART, and HIV-1 RNA and DNA became detectable during a brief treatment interruption 114 days after transplantation in one of these patients [12, 14]. However, the treatment interruption occurred close to the time of conditioning, and the patient died 5 months after transplantation [12]. The long-term effects of transplantation on the HIV-1 reservoir in patients who received an allogeneic HSCT with wild-type–CCR5+ cells and the effects of transplantation involving reduced-intensity conditioning (RIC) chemotherapy are unknown.
We examined the impact of allogeneic HSCT on the peripheral blood viral reservoir size in 2 HIV-1–infected patients and studied the protective effects of cART on donor cells. We used highly sensitive assays to detect and quantify the presence of HIV-1 before and up to 3.5 years after RIC HSCT, and we explored longitudinal changes in HIV-1 coreceptor use and HIV-specific antibody responses.
MATERIAL AND METHODS
Study Subjects, Tissue Processing, and Molecular Chimerism Testing
The Dana-Farber Cancer Institute/Harvard Cancer Center Institutional Review Board approved the study, and signed informed consent was obtained from subjects. Medical record information, stored plasma, and PBMCs were obtained at 1 pretransplantation and 3 posttransplantation time points. Fresh PBMCs and plasma specimens were collected for the final study time point. Molecular chimerism testing was performed by genotypic analysis of 10 genomic loci (Life Technologies, Carlsbad, CA).
Quantification of HIV-1 DNA and 2–Long Terminal Repeat (LTR) Circles
Purified CD4+ T cells were isolated from PBMCs, using MACS cell separation columns (Miltenyi Biotec, Auburn, CA). DNA was extracted from PBMCs or purified CD4+ T cells, using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA). Episomal DNA was extracted from 5 million PBMCs, using the QIAprep Miniprep Kit (Qiagen) in accordance with instructions for the isolation of plasmids with a low number of copies. Chromosomal DNA was subsequently recovered from the Miniprep sodium dodecyl sulfate precipitate as described elsewhere [15]. HIV-1 DNA from total PBMCs or isolated CD4+ T cells was quantified using a Taqman real-time polymerase chain reaction (PCR) method as described elsewhere [16]. The assay involves amplification of a conserved region in the LTR/gag that is specific to nearly all group M HIV-1 sequences. Assays were performed in triplicate wells, and experiments were repeated for all samples with negative results.
The 2-LTR circles were quantified from 5 million PBMCs by reverse transcription PCR (RT-PCR) as described elsewhere [17]. A 149-bp sequence of the highly conserved human mitochondrial gene ND4 was quantified from each sample to estimate the efficiency of episomal DNA extraction, using primers MitoND4F 5′-ACCACTGACATGACTTTCCA and MitoND4R 5′-GTTAGGGGGTCGGAGGAA and FAM-MGB labeled probe 5′-CAACCACCCACAGCCTAATT.
Plasma HIV-1 RNA Measurement by Single-Copy Assay
An ultrasensitive assay capable of detecting plasma HIV-1 RNA at a lower limit of detection of 0.5–1.8 copies/mL was performed as described elsewhere [18, 19]. To rule out primer/probe sequence mismatch, sequence homology between the assay primers/probe and the target sequence in HIV-1 gag was confirmed from pretransplantation proviral DNA. Recombinant avian retrovirus gag RNA was used as an internal standard; samples from elite controllers served as positive controls.
Viral Outgrowth Assay
To determine whether CD4+ T cells harbored replication-competent HIV-1, a viral coculture assay was performed as previously described [20, 21]. Briefly, 8 and 10 million purified CD4+ T cells (from patients A and B, respectively) were activated with anti-CD3 antibody (Miltenyi Biotech) and irradiated PBMCs from HIV-negative donors. Cells were incubated in the presence of recombinant interleukin 2, and CD8-depleted PBMC blasts were added [21]. HIV-1 p24 antigen was assayed in culture supernatant by an enzyme-lined immunosorbent assay (Alliance HIV-1, PerkinElmer). CD4+ T cells from HIV-negative donors served as negative controls. CD4+ T cells from an HIV-1–infected patient receiving cART served as a positive control.
Determination of HIV-1 Coreceptor Expression and Use and Demonstration of PBMC Susceptibility to HIV-1
Surface expression of CCR5 was assessed in cryopreserved PBMCs by multicolor flow cytometry, using monoclonal antibodies α-CD3-Pacific Blue and α-CCR5-APC (BD Pharmingen). Full-length env was amplified by nested PCR from proviral DNA at each time point as described elsewhere [22]. An in-house, validated phenotypic assay to determine coreceptor use was performed using pseudoviruses incorporating full-length env from PBMC proviral DNA obtained at the pretransplantation and first posttransplantation time points [23]. To demonstrate that the engrafted donor cells could be infected with HIV-1 ex vivo, patient PBMCs from the last posttransplantation time point were inoculated with a laboratory-derived CCR5-using pseudovirus.
HIV-1 Specific Antibody Quantitation
A limiting-antigen enzyme immunoassay was used to measure the avidity of HIV-1 antibodies from serial plasma samples as described elsewhere [24]. This assay incorporates a multisubtype recombinant protein covering the immunodominant region of group M HIV-1 gp41 that allows binding of only high-avidity antibodies by limiting the amount of available antigen. In addition, the same samples underwent analysis by standard and less sensitive (LS) versions of the VITROS Anti-HIV-1 + 2 assay, using 1:400 dilutions of plasma.
RESULTS
Clinical Course and Donor Cell Chimerism
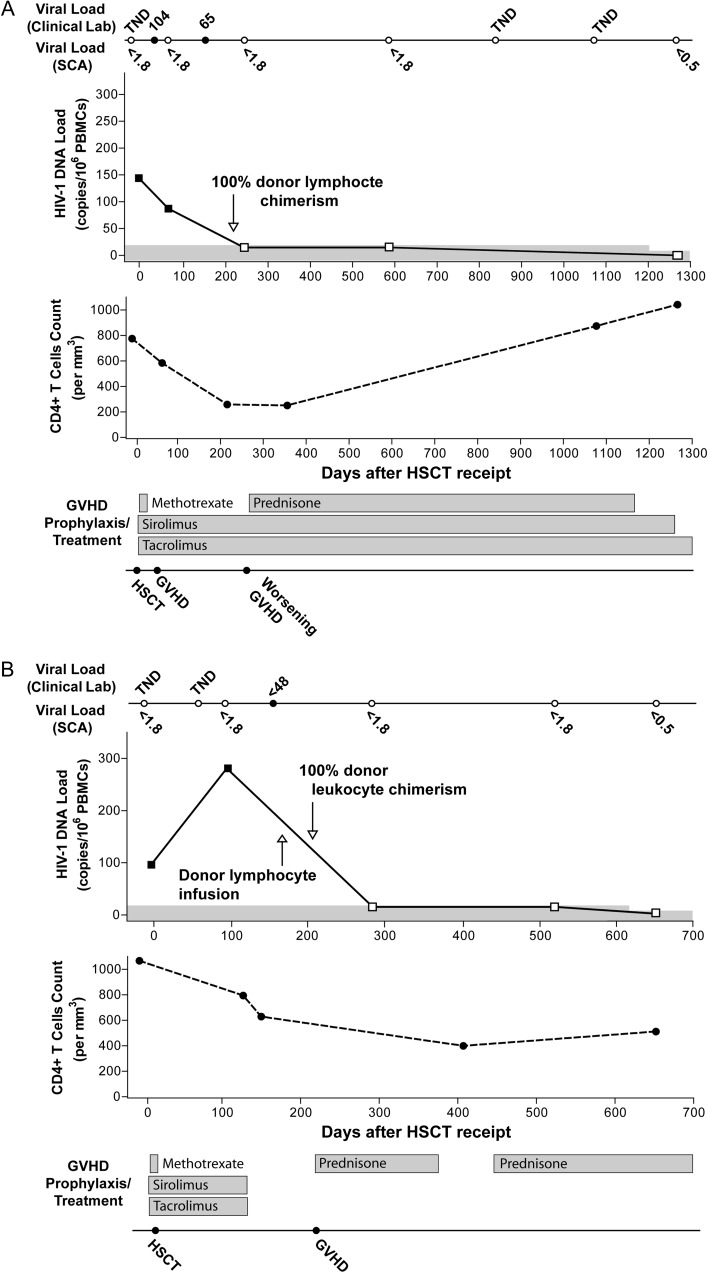
Patient A is a male with perinatally acquired infection who received a diagnosis of stage IV, nodular sclerosing Hodgkin lymphoma in 2006. After therapy with adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD), he experienced disease relapse and underwent autologous stem cell transplantation after ifosfamide, carboplatin, and etoposide (ICE) salvage chemotherapy 1 year after initial presentation. Lymphoma recurred again, and 1 year after autologous HSCT he received salvage therapy with gemcitabine, navelbine, and doxorubicin. After the patient achieved complete remission, he received a single HLA-C–mismatched (7/8 match) RIC (intravenous busulfan 3.2 mg/kg and fludarabine 120 mg/m2) HSCT from an unrelated donor. Stem cells were mobilized from peripheral blood by granulocyte colony-stimulating factor without further manipulation. Subsequently, he received short-course mini-methotrexate (on days 1, 3, and 6 after transplantation), sirolimus, and tacrolimus to prevent graft-versus-host disease (GVHD), as shown in Figure 1A. Tissue typing showed that 100% of peripheral blood granulocytes were donor derived by day 143 after transplantation and that 100% of CD3+ lymphocytes were donor derived by day 216 after transplantation (donor lymphocyte chimerism on day 167 after transplantation was 73%); full donor chimerism persisted through the most recent study time point, on day 1266 after transplantation. Nine months after HSCT receipt, patient A developed chronic GVHD with skin, eye, and liver involvement and was treated with prednisone, with good initial clinical response (Figure 1A). Two years after HSCT receipt, he required extracorporeal photopheresis for sclerodermatous chronic GVHD and had an excellent response. The patient received cART with tenofovir, emtricitabine, and efavirenz for 3–4 years prior to transplantation and continued to receive the same regimen throughout the peritransplantation and posttransplantation period. He received various cART regimens prior to the current regimen.
Figure 1.
Clinical course after allogeneic hematopoietic stem cell transplant (HSCT) receipt, including longitudinal changes in plasma levels of human immunodeficiency virus type 1 (HIV-1), cell-associated HIV-1 DNA, absolute CD4+ T-cell counts, and graft-versus-host disease therapy for patient A (A) and patient B (B). Viral load measurements are shown above and below the solid line on top of each figure. Results from clinical viral load testing, using the Cobas Taqman real-time polymerase chain reaction (PCR) assay (v. 1), are shown above the line (solid circles represent time points when RNA target was detected, whereas open circles denote time points when no target was detected; <48, detectable virus load below the assay limit of quantification of 48 copies/mL). Numbers below the line represent the limit of detection of a highly sensitive, single-copy real-time PCR assay (SCA) in copies per milliliter; no RNA was detected by SCA at any time point. HIV-1 peripheral blood mononuclear cell (PBMC) DNA became undetectable in both patients after full donor chimerism, and no DNA was detected in purified CD4+ T cells from the last assay time point (days 1266 and 562 after transplantation for patients A and B, respectively). The approximate relative sensitivity for HIV-1 DNA is represented by the top of the gray line, and open symbols represent the assay detection threshold for negative results. Viral coculture of highly stimulated, purified CD4+ T cells obtained from the last time point yielded no detectable HIV-1 p24 antigen from assay supernatants. Loss of detectable HIV-1 DNA and RNA was associated with recovery or plateau of absolute CD4+ T-cell counts. Abbreviations: TND, target not detected; GVHD, graft-versus-host disease; Lab, laboratory.
Patient B is a male with sexually acquired HIV-1 diagnosed in the mid-1980s and subsequent slow progression of HIV disease over the following 20 years. The plasma HIV-1 RNA levels from the late 1990s until treatment initiation in 2003 ranged from 13 000 to 60 000 copies/mL; the CD4+ T-cell count ranged from 481 to 668 cells/mm3. In 2003, he received a diagnosis of diffuse large B-cell lymphoma. Cyclophosphamide, doxorubicin, vincristine, and prednisone with rituximab (R-CHOP) were administered, along with efavirenz-based cART. In 2006, he received a diagnosis of new stage IV mixed-cellularity Hodgkin disease. After the patient received ABVD therapy in 2006, he received ICE salvage therapy for disease recurrence, followed by autologous HSCT receipt in 2007. Persistent thrombocytopenia and anemia developed, and myelodysplastic syndrome with multilineage dysplasia was diagnosed. In 2010, patient B received a matched, sibling-donor RIC allogeneic HSCT by means of the same mobilization and busulfan and fludarabine regimen described for patient A. Sirolimus, tacrolimus, and mini-methotrexate were administered following HSCT receipt, to prevent GHVD (Figure 1B). Because of poor initial engraftment, a donor lymphocyte infusion was given on day 171 after transplantation. Development of GVHD of the skin, liver, and oropharynx on day 220 required intermittent prednisone therapy (Figure 1B). Tissue typing on days 220 and 652 after transplantation showed 100% donor chimerism of all white blood cells, including lymphocytes (92% leukocyte chimerism on day 206 after transplantation). The initial efavirenz-based cART regimen had been changed to tenofovir, emtricitabine, nelfinavir, and abacavir approximately 3 years after initiation in 2003 (but before RIC HSCT) because of detectable viremia and the development of drug resistance mutations. Plasma HIV-1 RNA levels were known to be below the quantifiable range during the year prior to receipt of the allogeneic HSCT (Figure 1B), but cART was changed to tenofovir, emtricitabine, and raltegravir prior to and after HSCT, to minimize drug-drug interactions, with excellent adherence reported by the patient and his physician. HLA typing data for each patient are shown in Table 1.
Table 1.
HLAs of Patients A and B Corresponding to the Class I Major Histocompatibility Complex
| Patient | HLA-A |
HLA-B |
HLA-C |
|||
|---|---|---|---|---|---|---|
| A | A*0201 | A*2301 | B*4403 | B*5101 | Cw*0202 | Cw*0418a |
| B | A*02 | A*24 | B*08 | B*1517 (B63) | Cw*07 | Cw*07 |
a Unrelated stem cell donor was HLA C mismatched (Cw*0401).
Patient A experienced a significant reduction in absolute CD4+ T-cell counts following allogeneic HSCT receipt, with eventual return to levels similar to those of the pretransplantation sample (Figure 1A). In contrast, patient B had an initial decline in the absolute CD4+ T-cell count, followed by a plateau (Figure 1B).
HIV-1 DNA in PBMCs
Patient A had low levels of detectable HIV-1 DNA in PBMCs immediately prior to and 64 days after allogeneic HSCT (144 and 87 copies/106 PBMCs, respectively), but no DNA was detected 244, 587, and 1266 days after transplantation, despite repeated testing of each specimen (Figure 1). No HIV-1 DNA was detected from purified CD4+ T cells on day 1266 after transplantation. Likewise, patient B had detectable HIV-1 DNA in PBMCs prior to and immediately after allogeneic HSCT receipt (96 copies/106 PBMCs before transplantation and 281 copies/106 PBMCs on day 92 after transplantation), but no HIV-1 DNA could be detected in PBMCs on days 281, 519, and 652 after transplantation or in purified CD4+ T cells on day 652 after transplantation (Figure 1). The relative sensitivity of the HIV-1 DNA assay, estimated from the number of cells tested at each time point, ranged from approximately 6–18 copies/106 PBMCs in the banked samples to 2–2.5 copies/106 PBMCs in the most recently collected sample for both patients (>5 million cells were tested for each patient). The relative sensitivity for experiments that used purified CD4+ T cells at the last time point was approximately 4 copies/106 cells (2–3 copies/106 cells with repetitive sampling).
HIV-1 complementary DNA can enter the cell nucleus but fail to integrate, resulting in circularized molecules containing 1 or 2 copies of the LTR [17]. No 2-LTR circles were detected at any time point tested (through day 587 after transplantation) from both patients, with an assay sensitivity of approximately 1 copy/million PBMCs.
Viral Outgrowth
No HIV-1 p24 antigen was detected in CD4+ T cells obtained on posttransplantation days 1266 (for patient A) and 652 (for patient B) after 14–21 days of culture. The detection threshold was approximately 0.3–0.4 infectious units per million CD4+ lymphocytes. A sufficient number of cells was available for a single experiment for each study patient. By comparison, an exponential increase in HIV-1 p24 antigen was observed upon culture of purified CD4+ T cells from a control patient with hematologic malignancy who was receiving cART and had low levels of measurable HIV-1 DNA (45 copies/106 PBMCs).
Plasma HIV-1 RNA
Plasma HIV-1 RNA measurements were available from routine clinical testing, using the Roche Cobas Taqman RT-PCR assay (v.1), with an approximate lower limit of detection of 10–40 copies/mL. For patient A, the PCR target was not detected on pretransplantation day 6; was 104 and 65 copies/mL on posttransplantation days 29 and 143, respectively; and was not detected on posttransplantation days 827 and 1077 (Figure 1A). For patient B, HIV-1 RNA was not detected on pretransplantation day 5 and posttransplantation day 59 and was detected, but at a level of <48 copies/mL, on posttransplantation day 150 (Figure 1B). No low-level, residual HIV-1 RNA was detected using the ultrasensitive single-copy assay at any of the 5 study time points for either patient (Figure 1). The assay had lower limits of detection of 1.8 copies/mL for banked pretransplantation and posttransplantation samples and 0.5 copies/mL for samples from the final time point. Viral RNA was detected on the same assay plate in 3 of 4 samples from elite controllers with viral loads that were undetectable by clinical assays.
CCR5 Genotyping, HIV-1 Coreceptor Use, and PBMC Susceptibility to HIV-1 Infection
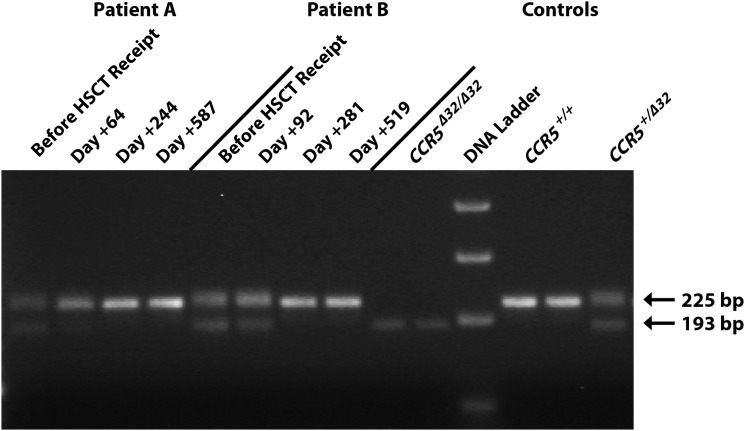
Figure 2 shows the electrophoretic pattern of wild-type and mutant (ccr5Δ32) CCR5 gene fragments amplified from PBMC DNA obtained at time points before and after transplantation. Both patients were heterozygous for the ccr5Δ32 mutation, but genotypic evidence of the mutation was lost in both patients after full donor chimerism. Phenotypic expression of CCR5 on CD3+ T cells obtained after HSCT receipt was confirmed by flow cytometry for both patients. Infectious pseudoviruses could be constructed from HIV-1 DNA in the pretransplantation and first posttransplantation samples from patient A and from the first posttransplantation sample from patient B; HIV-1 envelope DNA could not be amplified from samples obtained at later time points. Pseudoviruses from both patients exclusively used CCR5 for entry. Posttransplantation PBMCs from both patients could be infected by CCR5-using pseudoviruses, and viral entry was completely inhibited by maraviroc (data not shown).
Figure 2.
Agarose gel electrophoresis of peripheral blood mononuclear cell polymerase chain reaction DNA products from a 225-bp segment of the gene encoding human CCR5 before and after hematopoietic stem cell transplant (HSCT) receipt by patients A and B. Control patients with a known homozygous wild-type genotype (CCR5+/+), a heterozygous Δ32 genotype (CCR5+/Δ32), and a homozygous Δ32 genotype (CCR5Δ32/Δ32) are shown on the right for comparison. The 193-bp band represents the presence of a Δ32 mutant sequence. Both patients were CCR5+/Δ32 prior to HSCT receipt, but only the gene encoding wild-type CCR5 was detected by full-length CCR5 sequencing following donor cell chimerism. The image has been cropped and brightened, but the bands shown have not been individually modified or repositioned, and no nonlinear adjustments have been made.
HIV-1–Specific Antibody Quantification
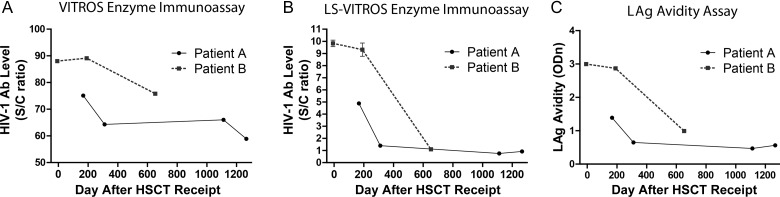
The level of HIV-1 antibodies decreased after transplantation, according to findings of standard and LS VITROS assays. A 5-fold decrease in the level HIV antibody, detected by the LS-VITROS assay, was observed in patient A (signal/cutoff [S/C] ratio, 4.84 on day 167 after transplantation and 0.88 on day 1266 after transplantation). Patient B had a relatively low level of antibody before transplantation (S/C ratio, 9.57, which is consistent with levels seen during early seroconversion to new infection), which decreased nearly 10-fold by day 652 after transplantation (S/C ratio, 1.08). Decreases in the titer and avidity of HIV-specific antibodies after transplantation were also observed for both patients, using a limiting-antigen avidity enzyme immunoassay (Figure 3).
Figure 3.
Human immunodeficiency virus type 1 (HIV-1)–specific antibody (Ab) levels and antigen avidity decreased following hematopoietic stem cell transplant receipt by patients A and B. A, Ab levels were measured using the VITROS Anti-HIV-1 + 2 enhanced chemiluminescence detection assay, as well as a less sensitive (LS) version with 1:400 diluted plasma (B). C, Avidity was measured using a limiting-antigen (LAg) avidity enzyme immunoassay. Abbreviations: ODn, normalized optical density; S/C, sign to cutoff.
DISCUSSION
Allogeneic HSCT by 2 HIV-1–infected patients during cART led to a substantial reduction in the peripheral blood reservoir of HIV-1 following full donor chimerism and recovery of CD4+ T-cell counts. The loss of detectable HIV-1 after full donor chimerism strongly suggests that latently infected host cells were replaced by donor cells that were protected from HIV-1 infection by cART. Although the initial decline in the proviral DNA load paralleled the decline in the CD4+ T-cell count in patient A, the CD4+ T-cell count declined only 4-fold, whereas the proviral DNA load declined by 2 log10. Moreover, proviral DNA remained undetectable despite the subsequent rebound in CD4+ T-cell counts to pretransplantation levels. Similarly in patient B, the 2-fold decline in CD4+ T-cell count could not account quantitatively for the 2-log10 decline in proviral DNA load.
Whether virus replication persists in lymphocytes in the setting of suppressive cART remains controversial, as does the question of whether such persistent replication is necessary to maintain the reservoir of latently infected CD4+ T cells [25–28]. The apparent ability of susceptible donor cells to engraft without becoming infected provides evidence that HIV-1 replication may be fully suppressed during receipt of cART and that residual viral replication during cART is not a major contributor to the maintenance of the HIV-1 reservoir in our patients' PBMCs. Transient low-level viremia observed immediately after transplantation in patient A may represent release of virus from existing reservoirs in the setting of immune activation from conditioning chemotherapy. No viral RNA was detected following reduction in the HIV-1 DNA load, but it is possible that circulating PBMCs are not in equilibrium with tissue compartments (eg, gut mucosa and brain) in which persistent viral replication may be taking place.
Long-term suppression of viral replication in the absence of cART has been reported in an HIV-infected patient heterozygous for ccr5Δ32 who remained without cART after receipt of an allogeneic HSCT with cells from a homozygous CCR5Δ32/Δ32 donor [4]. Our patients are also heterozygous for the ccr5Δ32 allele but received donor cells that expressed wild-type CCR5. Our results suggest that it is possible to achieve significant reductions in the reservoir of latently infected PBMCs following transplantation of wild-type CCR5+ stem cells so long as effective cART is continued. It is interesting to speculate whether being heterozygous for the ccr5Δ32 mutation prior to transplantation contributed to clearance of the PBMC HIV-1 DNA reservoir. Heterozygosity is associated with lower plasma HIV-1 RNA levels and slower disease progression in HIV-infected patients, but little is known about the effects of heterozygosity on latent reservoir size [29–32]. Patient B had been infected for 20 years with preserved CD4+ T-cell counts without cART until diagnosis of large B-cell lymphoma, suggesting that host factors may have contributed to slower disease progression. The contributions of pretransplantation host genetics to viral reservoir reduction after HSCT receipt merit further study.
Another important difference between our patients and those previously reported is that our subjects received a RIC regimen in preparation for their HSCT and did not receive total body irradiation or antithymocyte globulin. Memory T cells are activated by chemotherapy [33], and it is possible that the conditioning regimen played a role in reducing the proviral DNA level by nonselectively activating latently infected cells. Nevertheless, proviral HIV-1 DNA was detectable in PBMCs in the first posttransplantation samples from both patients, suggesting that chemotherapy did not have a durable effect on the size of the proviral DNA reservoir. Both patients experienced clinically significant episodes of GVHD that required treatment with immunosuppressive therapy. An antilymphopoietic effect of GVHD may have played a significant role in peripheral viral reservoir reduction by helping to clear infected host cells. This graft-versus-host effect may clear cells that are either latently infected or actively producing virus.
The significant decrease in HIV-1–specific antibody levels and avidity following full donor chimerism in both patients may be partly due to host B-cell depletion by the conditioning regimen, clearance of host B cells by the donor graft, and/or altered expansion of donor B cells during immune reconstitution [34–37]. HIV-specific antibody levels decline after long-term suppressive cART [38, 39]. Elite controllers have similar antibody responses as patients receiving cART, but elite controllers and viremic individuals have also been observed to have similar antibody levels [40–42]. Our patients had been receiving cART for several years before HSCT receipt, so the marked decline in HIV antibody levels and avidity after transplantation cannot be explained by cART alone. The decline in levels of anti-HIV antibodies after HSCT receipt suggests that there may be insufficient HIV-1 antigen presentation to induce a robust humoral response in the engrafted lymphocytes.
This study has a number of limitations. Insufficient material was available from previously banked samples to perform viral outgrowth assays from pretransplantation time points. Because of safety concerns, we have not yet had an opportunity to obtain samples from the gastrointestinal tract to determine whether proviral DNA or infectious virus persist in tissues outside of the peripheral blood. Similarly, we have not had an opportunity to collect larger quantities of plasma or cells through leukapheresis to probe specific reservoirs, such as the resting memory CD4+ T-cell population, in greater detail. Rare infected cells may be present at levels below the threshold of sensitivity of the HIV-1 DNA assay used in this study [43]. Of note, rebound of plasma viremia following cessation of cART has been observed in several individuals with very low levels of cellular HIV-1 [21], and drug-free remission has not been demonstrated in our patients, as they continue to receive cART.
In conclusion, we demonstrate sustained reduction of the HIV-1 proviral DNA level in PBMCs of 2 HIV-1–infected patients who received an allogeneic HSCT with CCR5+ donor cells during protective cART. The ability of susceptible donor cells to engraft without evidence of infection suggests that HIV-1 replication may be fully suppressed during cART and does not significantly contribute to maintenance of viral reservoirs in the peripheral blood of our patients. Tissue sampling and carefully monitored interruption of cART will be necessary to understand the extent and clinical significance of HIV-1 reservoir reduction after allogeneic HSCT. Although receipt of a HSCT is not a practical curative strategy for HIV infection in most individuals, our findings confirm that RIC allogeneic HSCT is a safe treatment in HIV-infected patients with hematologic malignancies whose infection is well controlled by cART. Detailed study of the effects of HSCT on viral reservoirs will provide a deeper understanding of viral persistence and aid the development of novel therapeutics aimed at eliminating HIV infection.
We subsequently studied the effects of a reduced-intensity conditioning allogeneic HSCT with wild-type–CCR5+ donor cells in a third male patient on suppressive cART who had previously undergone autologus HSCT for Hodgkin lymphoma. HIV-1 DNA levels in PBMCs were 545 copies/106 PBMC on pretransplantation day −16 and 186 copies/106 PBMC on posttransplantation day +178. A >50% decrease in HIV-1–specific antibody levels and antigen avidity were observed after transplantation. Bone marrow sampling on day +178 revealed 94% donor chimerism. The patient died from recurrent lymphoma on transplantation day +188.
Notes
Acknowledgments.We thank A. Tsibris and S. Deeks for helpful discussions and Mila Lebedeva for help with antibody quantitation.
T. J. H. designed the study, enrolled subjects, designed and performed experiments, performed statistical analyses, and wrote the manuscript. D. R. K. contributed to the study design and manuscript preparation and provided study oversight. Z. H., J. Z. L., G. S., M. P. B., S. M. K., F. F. G., and L. L. designed and performed experiments. S. H. and N. H. L. designed experiments. A. S. L. aided in study design and provided samples/clinical information. V. H., P. A., and R. J. S. provided samples/clinical information or helped with subject enrollment. M. S. contributed to the study design.
Disclaimer. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill and Melinda Gates Foundation.
Financial support. This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Disease (grants 1K23AI098480-01A1; UM1 AI068636 [to the AIDS Clinical Trials Group Virology Support Laboratory], P30 AI060354 [to the Harvard CFAR Program in Therapeutics], the Foundation for AIDS Research (amfAR Grant# 108466-52-RGRL), and U19 AI096109 [to the DARE Collaboratory]) and the Bill and Melinda Gates Foundation (global health grant OPP1017716).
Potential conflicts of interest. D. R. K. is a consultant to and has received honoraria from Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, InnoVirVax, Koronis, Merck, Roche, Roxane, Sangamo, and ViiV; grant support from Gilead and Merck; and speaking honoraria from Gilead. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Margolis DM. Histone deacetylase inhibitors and HIV latency. Curr Opin HIV AIDS. 2011;6:25–9. doi: 10.1097/COH.0b013e328341242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siliciano RF. What do we need to do to cure HIV infection. Top HIV Med. 2010;18:104–8. [PubMed] [Google Scholar]
- 3.Allers K, Hutter G, Hofmann J, et al. Evidence for the cure of HIV infection by CCR5{Delta}32/{Delta}32 stem cell transplantation. Blood. 2010;117:2791–9. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 4.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 5.Yukl SA, Chun T, Strain MC, et al. Challenges inherent in detecting HIV persistence during potentially curative interventions. Antiviral Therapy. 2012;17:A47. [Google Scholar]
- 6.Simonelli C, Zanussi S, Pratesi C, et al. Immune recovery after autologous stem cell transplantation is not different for HIV-infected versus HIV-uninfected patients with relapsed or refractory lymphoma. Clin Infect Dis. 2010;50:1672–9. doi: 10.1086/652866. [DOI] [PubMed] [Google Scholar]
- 7.Cillo A, Krishnan A, Mitsuyasu R, et al. 19th Conference on Retroviruses and Opportunistic Infections, Seattle, WA. Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for AIDS-related lymphoma [paper 154] 2012:5–8. March. [Google Scholar]
- 8.Cillo A, Krishnan A, Mitzuyasu R, et al. Fifth International Workshop of HIV Persistence During Therapy, St Maarten, West Indies December 6–9 2011. Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for AIDS-related lymphoma [Google Scholar]
- 9.Gabarre J, Marcelin AG, Azar N, et al. High-dose therapy plus autologous hematopoietic stem cell transplantation for human immunodeficiency virus (HIV)-related lymphoma: results and impact on HIV disease. Haematologica. 2004;89:1100–8. [PubMed] [Google Scholar]
- 10.Holland HK, Saral R, Rossi JJ, et al. Allogeneic bone marrow transplantation, zidovudine, and human immunodeficiency virus type 1 (HIV-1) infection. Studies in a patient with non-Hodgkin lymphoma. Ann Intern Med. 1989;111:973–81. doi: 10.7326/0003-4819-111-12-973. [DOI] [PubMed] [Google Scholar]
- 11.Contu L, La Nasa G, Arras M, et al. Allogeneic bone marrow transplantation combined with multiple anti-HIV-1 treatment in a case of AIDS. Bone Marrow Transplant. 1993;12:669–71. [PubMed] [Google Scholar]
- 12.Avettand-Fenoel V, Mahlaoui N, Chaix ML, et al. Failure of bone marrow transplantation to eradicate HIV reservoir despite efficient HAART. AIDS. 2007;21:776–7. doi: 10.1097/QAD.0b013e3280b01836. [DOI] [PubMed] [Google Scholar]
- 13.Huzicka I. Could bone marrow transplantation cure AIDS? Medical Hypotheses. 1999;52:247–57. doi: 10.1054/mehy.1997.0638. [DOI] [PubMed] [Google Scholar]
- 14.Woolfrey AE, Malhotra U, Harrington RD, et al. Generation of HIV-1-specific CD8+ cell responses following allogeneic hematopoietic cell transplantation. Blood. 2008;112:3484–7. doi: 10.1182/blood-2008-05-157511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharkey M, Babic DZ, Greenough T, Gulick R, Kuritzkes DR, Stevenson M. Episomal viral cDNAs identify a reservoir that fuels viral rebound after treatment interruption and that contributes to treatment failure. PLoS Pathog. 2011;7:e1001303. doi: 10.1371/journal.ppat.1001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malnati MS, Scarlatti G, Gatto F, et al. A universal real-time PCR assay for the quantification of group-M HIV-1 proviral load. Nat Protoc. 2008;3:1240–8. doi: 10.1038/nprot.2008.108. [DOI] [PubMed] [Google Scholar]
- 17.Butler SL, Johnson EP, Bushman FD. Human immunodeficiency virus cDNA metabolism: notable stability of two-long terminal repeat circles. J Virol. 2002;76:3739–47. doi: 10.1128/JVI.76.8.3739-3747.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer BE, Boritz E, Wilson CC. Effects of sustained HIV-1 plasma viremia on HIV-1 Gag-specific CD4(+) T cell maturation and function. J Immunol. 2004;172:3337–47. doi: 10.4049/jimmunol.172.5.3337. [DOI] [PubMed] [Google Scholar]
- 19.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105:3879–84. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chun TW, Justement JS, Moir S, et al. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. J Infect Dis. 2007;195:1762–4. doi: 10.1086/518250. [DOI] [PubMed] [Google Scholar]
- 21.Chun TW, Justement JS, Murray D, et al. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. AIDS. 2010;24:2803–8. doi: 10.1097/QAD.0b013e328340a239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henrich TJ, Tsibris AM, Lewine NR, et al. Evolution of CCR5 antagonist resistance in an HIV-1 subtype C clinical isolate. J Acquir Immune Defic Syndr. 2010;55:420–7. doi: 10.1097/QAI.0b013e3181f25574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin NH, Negusse DM, Beroukhim R, et al. The design and validation of a novel phenotypic assay to determine HIV-1 coreceptor usage of clinical isolates. J Virol Methods. 2010;169:39–46. doi: 10.1016/j.jviromet.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duong YT, Qiu M, De AK, et al. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One. 2012;7:e33328. doi: 10.1371/journal.pone.0033328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunthard HF, Frost SD, Leigh-Brown AJ, et al. Evolution of envelope sequences of human immunodeficiency virus type 1 in cellular reservoirs in the setting of potent antiviral therapy. J Virol. 1999;73:9404–12. doi: 10.1128/jvi.73.11.9404-9412.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez MA, Cabana M, Ibanez A, Clotet B, Arno A, Ruiz L. Human immunodeficiency virus type 1 genetic evolution in patients with prolonged suppression of plasma viremia. Virology. 1999;256:180–7. doi: 10.1006/viro.1999.9601. [DOI] [PubMed] [Google Scholar]
- 27.Gandhi RT, Bosch RJ, Aga E, et al. No evidence for decay of the latent reservoir in HIV-1-infected patients receiving intensive enfuvirtide-containing antiretroviral therapy. J Infect Dis. 2010;201:293–6. doi: 10.1086/649569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4(+) T cells. Nat Med. 2003;9:727–8. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y, Paxton WA, Wolinsky SM, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–3. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 30.Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–62. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 31.Stewart GJ, Ashton LJ, Biti RA, et al. Increased frequency of CCR-5 delta 32 heterozygotes among long-term non-progressors with HIV-1 infection. The Australian Long-Term Non-Progressor Study Group. Aids. 1997;11:1833–8. doi: 10.1097/00002030-199715000-00007. [DOI] [PubMed] [Google Scholar]
- 32.de Roda Husman AM, Koot M, Cornelissen M, et al. Association between CCR5 genotype and the clinical course of HIV-1 infection. Ann Intern Med. 1997;127:882–90. doi: 10.7326/0003-4819-127-10-199711150-00004. [DOI] [PubMed] [Google Scholar]
- 33.Irie E, Shirota Y, Suzuki C, et al. Severe hypogammaglobulinemia persisting for 6 years after treatment with rituximab combined chemotherapy due to arrest of B lymphocyte differentiation together with alteration of T lymphocyte homeostasis. Int J Hematol. 2010;91:501–8. doi: 10.1007/s12185-010-0528-6. [DOI] [PubMed] [Google Scholar]
- 34.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–74. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nordoy T, Husebekk A, Aaberge IS, et al. Humoral immunity to viral and bacterial antigens in lymphoma patients 4–10 years after high-dose therapy with ABMT. Serological responses to revaccinations according to EBMT guidelines. Bone Marrow Transplant. 2001;28:681–7. doi: 10.1038/sj.bmt.1703228. [DOI] [PubMed] [Google Scholar]
- 36.Ljungman P, Duraj V, Magnius L. Response to immunization against polio after allogeneic marrow transplantation. Bone Marrow Transplant. 1991;7:89–93. [PubMed] [Google Scholar]
- 37.Knoll A, Boehm S, Hahn J, Holler E, Jilg W. Reactivation of resolved hepatitis B virus infection after allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;33:925–9. doi: 10.1038/sj.bmt.1704457. [DOI] [PubMed] [Google Scholar]
- 38.Hatano H, Delwart EL, Norris PJ, et al. Evidence of persistent low-level viremia in long-term HAART-suppressed, HIV-infected individuals. AIDS. 2010;24:2535–9. doi: 10.1097/QAD.0b013e32833dba03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Killian MS, Norris PJ, Rawal BD, et al. The effects of early antiretroviral therapy and its discontinuation on the HIV-specific antibody response. AIDS Res Hum Retroviruses. 2006;22:640–7. doi: 10.1089/aid.2006.22.640. [DOI] [PubMed] [Google Scholar]
- 40.Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197:563–71. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 41.Lambotte O, Ferrari G, Moog C, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey JR, Lassen KG, Yang HC, et al. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J Virol. 2006;80:4758–70. doi: 10.1128/JVI.80.10.4758-4770.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304:3–15. doi: 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]