Abstract
Background. Cerebrospinal fluid (CSF) and neuroimaging abnormalities demonstrate neuronal injury during chronic AIDS, but data on these biomarkers during primary human immunodeficiency virus (HIV) infection is limited.
Methods. We compared CSF concentrations of neurofilament light chain, t-tau, p-tau, amyloid precursor proteins, and amyloid-beta 42 in 92 subjects with primary HIV infection and 25 controls. We examined relationships with disease progression and neuroinflammation, neuropsychological testing, and proton-magnetic resonance spectroscopy (MRS)–based metabolites.
Results. Neurofilament light chain was elevated in primary HIV infection compared with controls (P = .0004) and correlated with CSF neopterin (r = 0.38; P = .0005), interferon gamma-induced protein 10 (r = 0.39; P = .002), white blood cells (r = 0.32; P = .004), protein (r = 0.59; P < .0001), and CSF/plasma albumin ratio (r = 0.60; P < .0001). Neurofilament light chain correlated with decreased N-acteylaspartate/creatine and glutamate/creatine in the anterior cingulate (r = −0.35, P = .02; r = −0.40, P = .009, respectively), frontal white matter (r = −0.43, P = .003; r = −0.30, P = .048, respectively), and parietal gray matter (r = −0.43, P = .003; r = −0.47, P = .001, respectively). Beta-amyloid was elevated in the primary infection group (P = .0005) and correlated with time infected (r = 0.34; P = .003). Neither marker correlated with neuropsychological abnormalities. T-tau and soluble amyloid precursor proteins did not differ between groups.
Conclusions. Elevated neurofilament light chain and its correlation with MRS-based metabolites suggest early neuronal injury in a subset of participants with primary HIV infection through mechanisms involving central nervous system inflammation.
Keywords: HIV/AIDS, primary HIV infection, biomarkers, neurological damage, neurofilament light chain, magnetic resonance spectroscopy
Although the incidence of human immunodeficiency virus (HIV)–associated dementia has decreased with the widespread use of combination antiretroviral therapy (cART), patients continue to experience varying degrees of HIV-associated neurological disorder [1]. Because even mild impairment affects quality of life [2], the neurological consequences of HIV infection have gained increased attention, as has the need to distinguish active processes from static abnormalities in the central nervous system (CNS) of these patients [3].
Cerebrospinal fluid (CSF) biomarkers, the altered concentrations of which have been associated with neurodegenerative processes, include neurofilament light chain, a structural component of myelinated axons; t-tau, a microtubule-associated protein promoting axon stability; its hyperphosphorylated component, p-tau; soluble amyloid precursor proteins-α and -β, products of amyloid processing pathways; and soluble amyloid-beta 42. These markers are abnormal in HIV-associated dementia [4–7] and neuroasymptomatic advanced AIDS [4, 8–10]. However, the extent of neurological injury during presymptomatic HIV infection, especially early infection, is not completely understood. Limited data suggest that neurological injury occurs in some individuals during primary HIV infection [4], defined as the first year after viral transmission. During primary infection, up to 10% of individuals develop neurological signs and symptoms, and the virus can be detected in CSF and brain tissue [11]. HIV infiltrates the CSF as early as 8 days posttransmission [12], and immune activation occurs throughout primary infection [13], suggesting the potential for CNS injury in the earliest stages of infection.
Proton-magnetic resonance spectroscopy (MRS) is a noninvasive imaging modality that has been used to monitor neuronal injury through analysis of metabolite abnormalities. N-acetylaspartate and glutamate, often expressed relative to creatine levels, are markers of neuronal health that deplete with injury [14, 15]. Work in macaques suggests that the neuronal manifestations of HIV occur rapidly but are reversible with cART [16]. Human studies have shown that N-acetylaspartate/creatine ratios decline in chronic untreated HIV infection (eg, [17]) and improve [18], but may not normalize, with therapy [19]. Recently, we have shown with MRS that metabolite levels do not differ significantly between controls and patients with primary HIV infection at baseline but that abnormalities in the latter worsen over time and improve with early cART [20].
In this study, we quantified CSF biomarkers as a proxy for neuronal injury in individuals with primary HIV infection and compared them with those in HIV-uninfected controls. We hypothesized that primary HIV infection is characterized by increased concentrations of CSF neurofilament light chain and that this increase correlates with elevated concentrations of CSF markers of neuroinflammation and decreased concentrations of N-acetylaspartate and glutamate measured by MRS. We expected tau and amyloid levels in primary HIV infection to not differ from controls, as has been previously reported [7] in chronically HIV-infected, neuroasymptomatic individuals.
METHODS
Study Design and Participants
This cross-sectional study enrolled 92 cART-naive participants with primary HIV infection between 1987 and 2011 in San Francisco, California; Gothenburg, Sweden; and Sydney, Australia.
Participants were referred from physicians or counseling and testing centers based on known or suspected recent HIV infection. Participants were eligible if they met criteria for primary infection based on nucleic acid testing and less-sensitive enzyme-linked immunosorbent assay according to the standard serologic testing algorithm for recent HIV seroconversion (STARHS) [21]. Estimated number of days posttransmission was calculated assuming exposure occurred 14 days before the acute retroviral syndrome [22] or, in the absence of symptoms, as halfway between the last negative and the first positive test result.
A neurologist screened for exclusion criteria including active unrelated neurological disorders. Subjects were screened with standardized inventories for mental illness and substance abuse, and presence of these was recorded. Data was excluded from the analysis if participants demonstrated signs of intoxication and/or reported substance use on the visit day.
Data about CSF HIV RNA and neurological symptoms have been previously reported in a subset of participants [13, 23], as have neurofilament light chain results in 16 subjects using a different, less-sensitive assay [4]. Twenty-five HIV-uninfected volunteers provided comparison samples. All participants provided written informed consent in studies approved by the institutional review board or equivalent entity at each institution.
Specimen Sampling, Processing, and Laboratory Analysis
Participants underwent detailed neurological history and physical examination, as well as collection of blood and CSF specimens between 7:30 am and 12:00 pm. HIV RNA levels in cell-free CSF and plasma were measured by the ultrasensitive Roche Amplicor HIV-1 Monitor PCR, Cobas TaqMan RealTime HIV-1, or the Abbott RealTime HIV-1 assays at local sites. CSF total white blood cells (WBCs) and protein and CD4+ and CD8+ T-lymphocyte counts were measured on fresh samples by flow cytometry.
Cell-free CSF and blood plasma were aliquoted and stored within 6 hours of collection in −70°C or −80°C freezers monitored by National Institutes of Standards and Technology–certified thermometers. Neurofilament light chain concentration was measured using a new, highly sensitive, 2-site enzymatic quantitative immunoassay with a lower limit of detection of 50 ng/L in the laboratory of Dr Zetterberg. The upper-normal CSF neurofilament light chain levels at the laboratory were <380 (in subjects aged <30 years), <560 (in subjects aged 30–39 years), <890 (in subjects aged 40–59 years), and <1850 ng/L (in subjects aged >59 years) [24]. Reference values were obtained in the Zetterberg laboratory based on the analysis of 108 neurologically healthy HIV-uninfected individuals. Detection of t-tau, beta-amyloid, and soluble amyloid precursor proteins-α and -β used standard enzyme-linked immunosorbent assays in the Zetterberg laboratory. Blood and CSF neopterin measurements were performed in the laboratory of Dr Fuchs using commercially available immunoassays; interferon gamma-induced protein 10 (IP-10) and monocyte chemotactic protein 1 (MCP-1) measurements were performed locally using commercially available assays.
Proton-Magnetic Resonance Spectroscopy
After standard clinical MR imaging, proton (1H)-MRS was performed on a 4-Tesla Siemens/Bruker scanner in 53 primary HIV infection participants at baseline in the neuroimaging laboratory of Dr Meyerhoff. Water-suppressed short echo-time (TE) single-volume STEAM spectra (TR/TE/TM = 2000/12/10 ms; spectral width = 2000 Hz; spectral data size = 2048 points; 128 scans; total acquisition time = 4:16 min) were acquired from 4 volumes selected on sagittal T1-weighted and axial T2-weighted images. Different tissue types were targeted: anterior cingulate (20 × 20 × 20 mm3), frontal white matter (15 × 25 × 20 mm3), basal ganglia (17 × 35 × 15 mm3), and parietal gray matter (25 × 20 × 20 mm3). We chose these areas based on acute simian immunodeficiency virus studies in macaques that had shown changes in these regions [16, 25, 26]. Meyerhoff and others have previously described most MRS processing methods [27]. Briefly, all MR imaging and single-volume 1H MRS data were stored in an SQL database and were processed and analyzed with updated software tools developed in-house [28] or in routine use for many years. The spectral fitting software SITOOLS used a parametric model of known metabolite resonances and modeled spectral components, including those of macromolecules, to fit all spectral resonances and nonparametric parameters to the baseline. A priori spectral information used the frequencies, phases, and approximate relative amplitudes of all major metabolites at 4-Tesla and also included resonances for macromolecules. The obtained raw metabolite signal integrals were corrected for MR imaging–derived tissue and CSF contributions to the MRS volumes, corrected for different receiver and transmitter settings when necessary, and then normalized to the cerebral water signal obtained from the corresponding volumes. For each metabolite, these adjusted signal integrals (“peak areas”) were converted to metabolite ratios: glutamate/creatine, myo-inositol/creatine, N-acetylaspartate/creatine, and choline-containing metabolites/creatine. We excluded spectra from analyses if they exhibited poor signal-to-noise ratios, excessive water signal, or other significant artifacts. High-field proton MRS at 4-Tesla has highly sensitive signal-to-noise detection and thus allowed us to determine individual peaks of glutamate and glutamine instead of the combined “Glx” peak.
Neuropsychological Testing
Neuropsychological testing was performed only at the San Francisco site, and all participants were fluent in English. At baseline, a trained psychometrist administered a neuropsychological testing battery composed of motor (timed gait, finger tap nondominant hand, grooved pegboard), processing speed (trail making A, digit symbol), executive function (trail making B, verbal fluency), learning (Rey Auditory Verbal Learning Test, figural memory), and memory (Rey Auditory Verbal Learning Test delay, figural memory delay) domains. To control for the social and demographic variability in the group, z scores for neuropsychological testing were used for all analyses, which were derived from comparing raw scores to age-, sex-, ethnicity-, and level-of-education–matched norms. We calculated a z domain score by averaging all z scores within that domain and calculated an NPZ-4 score by summing grooved pegboard, digit symbol, finger tapping, and timed gait. A total z score was calculated as a composite of all tests, and a global deficit score was calculated in the standard manner [29].
Statistical Analyses
Nonparametric descriptive statistics used the Mann–Whitney U test and the Kruskal–Wallis test with post hoc testing corrected with Dunn's multiple comparison, all performed with SPSS version 19.0 and GraphPad Prism version 5.0d. Correlations between measured parameters employed Spearman's rank correlation coefficient; parametric correlations and linear regression were also conducted for illustrative purposes. A multivariable regression model to investigate independent predictors of CSF neurofilament light chain included age, CSF neopterin, WBC, protein, IP-10, and CSF/plasma albumin ratio; these parameters had been identified as significant predictors in both the parametric and nonparametric univariable models.
RESULTS
Study Participant and HIV Disease Characteristics
Table 1 shows background clinical and demographic information for primary HIV infection participants (n = 92) and HIV-uninfected controls (n = 25). HIV-infected participants were a median of 3.1 months posttransmission and were younger and more likely to be male than the controls. Eight of 92 (8.7%) participants in the primary HIV infection group had previously experienced ≥1 neurological symptoms during seroconversion, and the majority harbored infection with HIV subtype B [13].
Table 1.
Demographic and Descriptive Characteristics of Primary HIV Infection Participants and HIV-Uninfected Controls
| PHI (n = 92) | HIV-uninfected (n = 25) | P Value | |
|---|---|---|---|
| % Male | 95.2% | 80% | .03 |
| Age, years | 36 (28–46) | 43 (40–49) | .001 |
| Estimated days of infection | 92 (52–152) | … | … |
| CD4 count, cells/uL | 536 (392–682) | 836 (703–1056) | <.0001 |
| Log10 plasma VL | 4.6 (4.0–5.2) | … | … |
| Log10 CSF VL | 2.9 (2.0–3.6) | … | … |
| CSF protein, mg/dL | 41 (32–53) | 47 (33–56) | .71 |
| CSF WBCs, cells/uL | 6 (2–11) | 1 (0–2.5) | <.0001 |
| % ARS neuro symptoms | 8.7% | … | … |
Values are shown as median (IQR) except where noted.
Abbreviations: ARS, acute retroviral syndrome; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; PHI, primary HIV infection; VL, viral load; WBC, white blood cells.
CSF Markers of Neuronal Injury During Primary HIV Infection
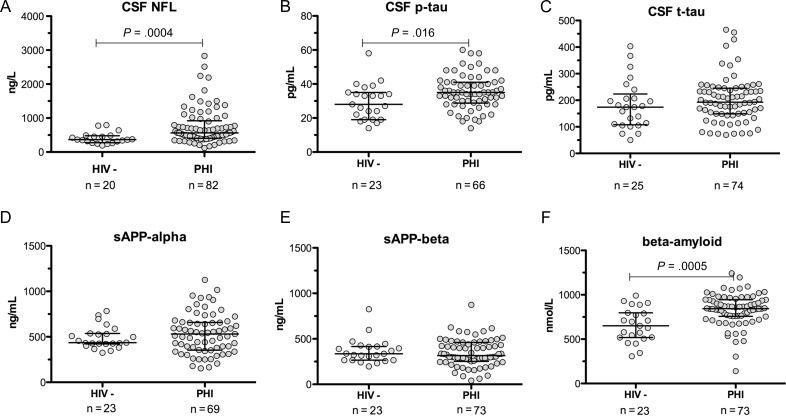
Figure 1 shows comparisons of 6 CSF biomarkers between the 2 groups. Median neurofilament light chain in 82 primary HIV infection participants was elevated compared with 20 controls (P = .0004; Figure 1A). When stratified by age, 36 of 82 (44%) participants had neurofilament light chain elevations above the upper limit of normal for their age group: 13 of 24 (54%) aged <30 years, 12 of 24 (50%) aged 30–39 years, 10 of 31 (32%) aged 40–59 years, and 1 of 3 (33%) aged >59 years.
Figure 1.
A–F, Cerebrospinal fluid biomarkers of neuronal injury in the primary human immunodeficiency virus (HIV) infection group and HIV-uninfected control group. Abbreviations: CSF, cerebrospinal fluid; HIV-, human immunodeficiency virus–uninfected control group; NFL, neurofilament light chain; PHI, primary human immunodeficiency virus infection group; p-tau, hyperphosphorylated tau; sAPP, soluble amyloid precursor protein; t-tau, total tau.
P-tau was elevated in 66 primary HIV infection participants compared with 23 controls (P = .016, Figure 1B). There were no significant differences between groups in t-tau or soluble amyloid precursor proteins-α and -β (Figure 1C–E). Amyloid-beta 42 was elevated in 73 primary HIV infection participants compared with 23 controls (P = .0005; Figure 1F).
Participants who had experienced neurologically symptomatic seroconversion did not have higher neurofilament light chain, p-tau, or amyloid-beta 42 than those who had not. Even when previously symptomatic participants were excluded, these biomarkers remained elevated in the primary infection group compared with controls.
Determinants of Elevated Neurofilament Light Chain During Primary HIV Infection
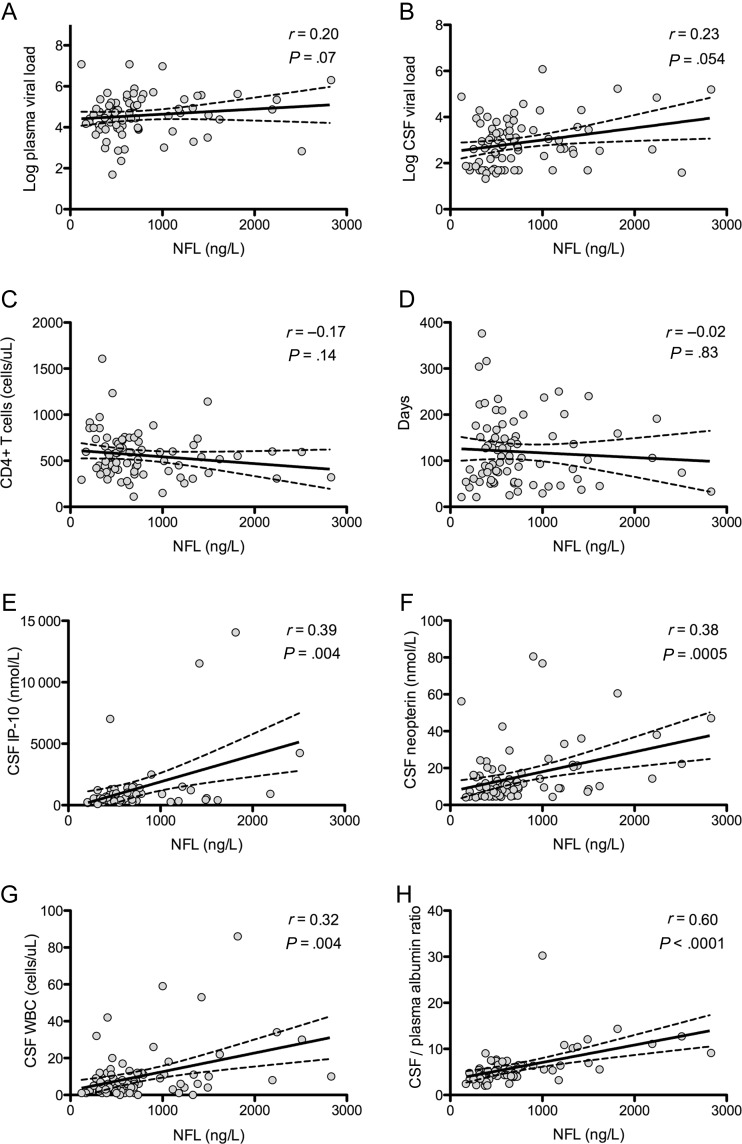
CSF neurofilament light chain correlated with concentrations of inflammatory markers including CSF neopterin (r = 0.38; P = .0005) and IP-10 (r = 0.39; P = .002), WBC count (r = 0.32; P = .004), protein (P = .59; P < .0001), and CSF/plasma albumin ratio (r = 0.60; P < .0001). Significant correlations were not found between neurofilament light chain and CD4+ T-cell count, estimated days postinfection at sampling, plasma and CSF HIV RNA levels (Figure 2), or CSF MCP-1 (not shown).
Figure 2.
A–H, Correlates of neurofilament light chain levels in primary human immunodeficiency virus infection. r represents the Spearman correlation coefficient and corresponding P value. Solid lines represent best-fit regression line, and dashed lines represent 95% confidence intervals. Abbreviations: CSF, cerebrospinal fluid; IP-10, interferon gamma-induced protein 10; NFL, neurofilament light chain; WBC, white blood cell.
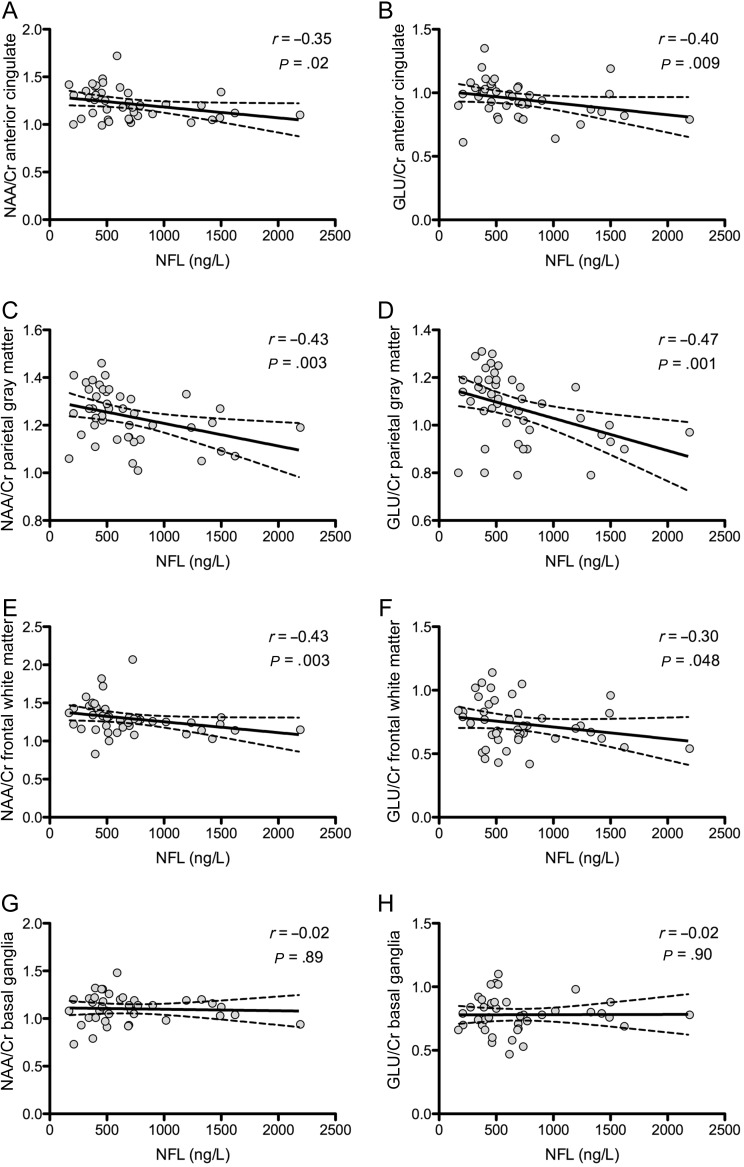
High neurofilament light chain levels correlated with low N-acetylaspartate/creatine and glutamate/creatine ratios in the anterior cingulate (r = −0.35, P = .02; r = −0.40, P = .009, respectively), frontal white matter (r = −0.43, P = .003; r = −0.30, P = .048, respectively), and more strongly in the parietal gray matter (r = −0.43, P = .003; r = −0.47, P = .001, respectively; Figure 3). N-acetylaspartate/creatine and glutamate/creatine ratios were correlated across these 3 regions (r > 0.50; P < .001). No significant correlations were present between neurofilament light chain and glutamate/creatine or N-acetylaspartate/creatine in the basal ganglia or with myo-inositol/creatine or choline/creatine from any region.
Figure 3.
A–H, Correlations of neurofilament light chain levels with regional proton-magnetic resonance spectroscopy–derived metabolite ratios. r represents the Spearman correlation coefficient and corresponding P value. Solid lines represent best-fit regression line, and dashed lines represent 95% confidence intervals. Abbreviations: Glu/Cr, glutamate/creatine; NAA/Cr, N-acetylaspartate/creatine; NFL, neurofilament light chain.
Multivariable linear regression modeling was used to identify independent predictors of CSF neurofilament light chain in primary infection participants and revealed independent correlations with age, CSF WBC, and CSF/plasma albumin ratio (adjusted r2 = 0.624).
Determinants of Elevated Amyloid-Beta 42 During Primary HIV Infection
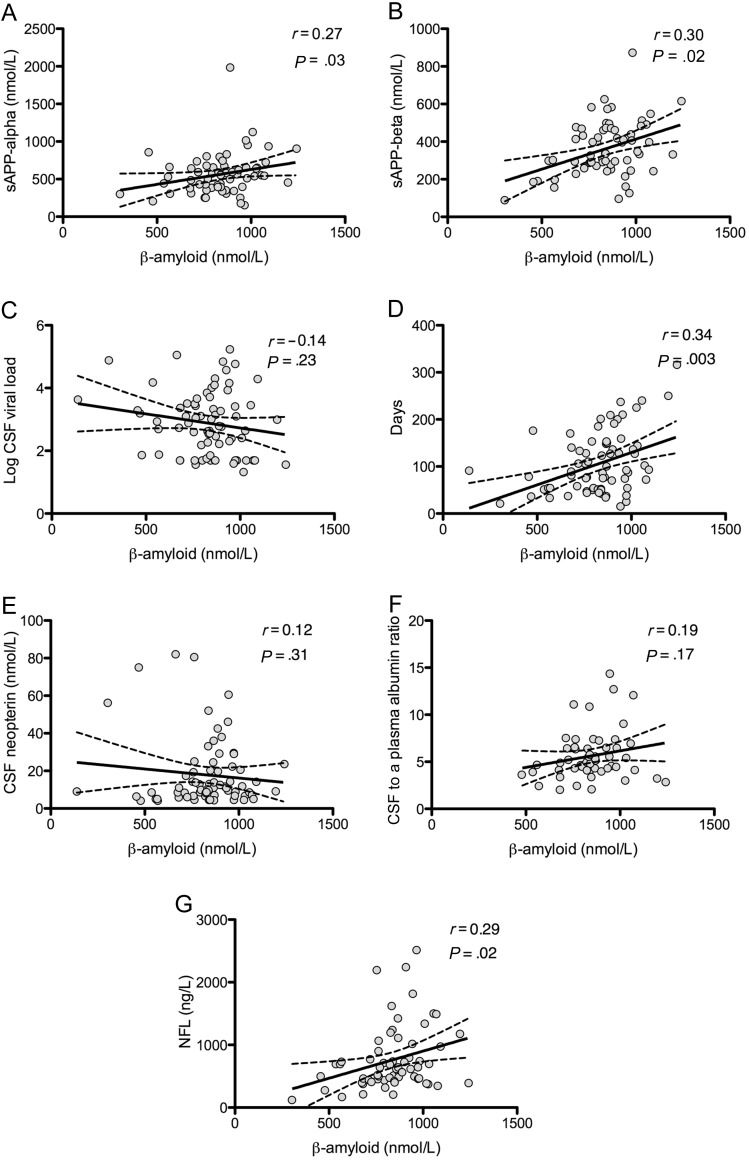
Amyloid-beta 42 did not correlate with age in the primary HIV infection group but did correlate with levels of soluble amyloid precursor proteins-α and -β (r = 0.27, P = .03; r = 0.30, P = .02, respectively) and with days of infection (r = 0.34; P = .003). There were no significant correlations between amyloid-beta 42 and plasma or CSF viral load, CSF/plasma albumin ratio, or CSF protein, neopterin, MCP-1, or IP-10 (Figure 4). Notably, neurofilament light chain and amyloid-beta 42 did show a modest correlation (r = 0.29; P = .02).
Figure 4.
A–G, Correlates of beta-amyloid in primary human immunodeficiency virus infection. Solid lines represent best-fit regression line, and dashed lines represent 95% confidence intervals. Abbreviations: CSF, cerebrospinal fluid; NFL, neurofilament light chain; sAPP, soluble amyloid precursor protein.
Neuropsychological Testing
At baseline, there were no significant correlations between CSF neurofilament light chain and composite z scores for motor function, processing speed, memory, or learning. Neurofilament light chain and the composite z score for executive function tended to be correlated (r = 0.27; P = .049).
DISCUSSION
Our findings demonstrate that biomarkers of neuronal injury, including neurofilament light chain and amyloid-beta 42, are abnormal in the CSF of a subset of individuals with primary HIV infection and that neurofilament light chain concentration correlates with established MRS markers of neuronal injury. This suggests that neuronal injury, in addition to viral replication [11], immune activation [5, 30], and blood–brain barrier breakdown [11] occurs early in the course of HIV infection in some individuals. The association of neurofilament light chain with markers of inflammation suggests a relationship between injury and immune activation. The finding that MRS measures of cortical and white matter N-acetylaspartate/creatine and glutamate/creatine demonstrate associations with CSF neurofilament light chain concentrations is the first data that we know of that relate noninvasive neuroimaging markers of neuronal injury to CSF neural marker abnormalities in HIV infection. Neuronal health and stability appears compromised in some brain regions in the earliest stages of HIV infection. The lack of correlation between neurofilament light chain levels and common neuropsychological test performance may suggest that neurofilament light chain elevation reflects subclinical injury during this stage of infection.
Neurofilament Light Chain
Neurofilament light chain concentration serves as a sensitive indicator of CNS axonal injury in neurodegenerative disorders including Alzheimer disease, atypical Parkinsonian syndromes, and amyotrophic lateral sclerosis [31, 32], as well as in multiple sclerosis [33] and traumatic brain injury [34]. CSF neurofilament light chain concentrations increase in untreated individuals with HIV-associated dementia and neurological opportunistic infections [4]. Neurofilament light chain decreases with cART initiation, increases with cART discontinuation, and may serve as a predictor for which individuals go on to develop neurological disease [8, 35, 36].
Previously, neurologically asymptomatic HIV-1–infected individuals with chronic infection and CD4+ T-cell counts >200 cells/µL were thought not to have elevated CSF neurofilament light chain, and this threshold was thought to be sufficient to prevent CNS disease [36]. However, further work in chronically infected subjects indicated that HIV-infected individuals with CD4+ T-cell counts above 200 cells/µL can have elevations in neurofilament light chain upon the cessation of cART [8], that lower CD4+ T-cell counts tend to be associated with higher neurofilament light chain [4], and that very low CD4+ T-cell counts are associated with increased neurofilament light chain concentrations [10].
Previous work using a less-sensitive assay identified elevated neurofilament light chain in 4 of 16 (25%) subjects with primary HIV infection but showed no significant difference compared with controls [4]. Our findings using a more-sensitive assay demonstrate increased neurofilament light chain in the group comparison, as well as in 44% of participants who had elevations in this marker compared with the age-appropriate upper limit of normal. This suggests that, in at least a subset of participants, neurologic injury occurs during primary HIV infection even in subjects without neurological symptoms during seroconversion. Along with CSF HIV RNA and inflammatory markers, elevated neurofilament light chain levels may help identify individuals with an active disease process. Because it has been suggested that neurofilament light chain can predict neurologic disease progression [35], it is possible that neurofilament light chain may identify individuals that might benefit from early pharmacologic intervention aimed at protecting the brain from neuronal injury.
A significant proportion of these participants did not have elevated neurofilament light chain, suggesting possibly that an unknown viral or host factor increases the susceptibility of certain individuals to neurological injury during this period.
Correlates of Elevated Neurofilament Light Chain During Primary HIV Infection
Overall, the results suggest an association between neurofilament light chain and inflammatory processes. It is notable that such an association did not exist with amyloid-beta 42, implying that neurofilament light chain might be a more specific marker for inflammatory injury. During primary infection, neurofilament light chain was not associated with CD4+ T-lymphocyte count, which may reflect the fact that CD4 count during this period is a correlate of acute systemic immune response to HIV acquisition rather than duration and progression of infection. Neurofilament light chain also did not strongly associate with markers of infection, including plasma and CSF HIV RNA. However, because the N-acetylaspartate/creatine ratio is a putative marker of neuronal health, our results suggest that declining neuronal health is associated with increased neuronal injury as identified through elevated CSF neurofilament light chain. This is particularly true in the parietal gray matter and frontal white matter, consistent with studies in animal models [25, 26].
We also found a negative association between the glutamate/creatine ratio and neurofilament light chain. Elevated glutamate/creatine is a putative marker for excitotoxicity, but it is also considered a marker of neuronal integrity (eg, [15]). Previous studies have shown that HIV-infected individuals with cognitive deficits have lower glutamate/creatine levels, particularly in the parietal gray matter but not the frontal white matter [15]. Here, we found a similar regional specificity in that high neurofilament light chain levels correlate strongly with low glutamate/creatine in the parietal gray matter but not in the frontal white matter. The strong correlations between glutamate/creatine and N-acetylaspartate/creatine ratios across all brain regions emphasize the value of these metabolites in the assessment of neuronal health and suggest that the consistent regional metabolite correlations with neurofilament light chain are meaningful.
We found no convincing correlation between CSF neurofilament light chain and performance on most cognitive domains assessed by a circumscribed battery of neuropsychological tests. However, an modest association between higher neurofilament light chain and poorer performance in tests of executive function may reflect a relationship between neural injury and impairment of this cognitive domain during early infection.
Tau and Amyloid Proteins
Tau and amyloid proteins are valuable in the identification of neurodegenerative disorders [37–39], but their use in HIV infection is less clear. T-tau and p-tau patterns in HIV-associated neurologic disorder and HIV-associated dementia are inconsistent [7, 40, 41]. In this study, elevated p-tau occurred in the context of unchanged t-tau, a pattern different from chronic AIDS [41], Alzheimer disease [39], and Creutzfeldt–Jakob disease [42]. The difference is weaker than that for neurofilament light chain or amyloid-beta 42, and we are therefore unable to conclude whether t-tau and p-tau could be useful measures of neuronal injury in primary HIV infection.
Amyloid precursor proteins are cleaved by secretases into soluble amyloid precursor proteins-α and -β; cleavage to the -β form generates a molecule leading to amyloid-beta 42 [7]. Amyloid-beta 42 decreases in Alzheimer disease [38] and in HIV-associated neurologic disorder [43], HIV-associated dementia [41], and CNS opportunistic infections [7]. Pathological studies show amyloid deposition in brain tissue of HIV-infected individuals [44], but this marker has not been explored in primary HIV infection.
The elevation in amyloid-beta 42 could be accounted for by a number of mechanisms. It is unlikely due to the age of the participants in each group, as median levels for controls is concordant with those for young controls in other studies [7] and the median value in primary HIV infection participants is higher than in neuroasymptomatic HIV-infected individuals [7].
Elevated CSF amyloid-beta 42 has recently been associated with cerebral inflammation [45]. Here, amyloid-beta 42 did not correlate with markers of neuroinflammation in primary HIV infection participants, but the markers we measured differed from those for which correlations have been identified (tumor necrosis factor α, interleukin 6, or interleukin 8) in other studies [45].
Limitations
This cross-sectional study captured participants at a single timepoint; future work with longitudinal data will help determine how these biomarkers change with time. Our study participants were almost exclusively men, which poses a problem for generalizing these results to HIV-infected women. Because the median duration of infection was 3.1 months, it is unclear when during primary HIV infection these biomarker abnormalities begin to occur. We defined our hypotheses before conducting our analyses, but still made many statistical comparisons; because of this, we attempted to exercise restraint in statistical interpretation and to correct for multiple comparisons. Nevertheless, we find the data, particularly in the contrasts in correlated variables between neurofilament light chain and amyloid-beta 42, to be a convincing starting point for further exploration of the mechanisms of neurologic injury during primary HIV infection.
Implications
Our finding of neuronal injury during primary HIV infection has important implications for the understanding of HIV pathogenesis and management. Previously, neuronal injury was thought to be a product of prolonged infection. The presence of abnormally elevated CSF neurofilament light chain levels in 44% of our participants adds to a growing body of evidence suggesting that neurological injury begins in primary HIV infection. Thus, although initiation of cART often occurs once an immunological threshold is crossed after several years of infection, CNS injury might begin soon after seroconversion in the setting of early neuroinvasion and immune activation. If this were the case, it would provide additional rationale for early pharmacological intervention in HIV infection aimed at mitigating CNS injury.
Notes
Acknowledgments. The authors thank the research participants.
M. J. P. organized and analyzed the data and wrote the manuscript; D. J. M. contributed in design, implementation, acquisition, processing, and interpretation of MRS studies and edited the manuscript; R. W. P. contributed in study design, implementation, data collection, interpretation of data and edited the manuscript; J. P. contributed in study coordination, data collection, and analysis of neuropsychological data; E. L. contributed in study coordination and data collection; A. Y. organized and analyzed the MRS data; R. W. acquired and processed MRS data; D. F. contributed in laboratory analysis of inflammatory markers, interpretation of data, and edited the manuscript; B. B. contributed in subject recruitment, data interpretation, and edited the manuscript; P. C. contributed in subject recruitment, data interpretation, and edited the manuscript; K. R. contributed in design, scoring, and interpretation of neuropsychological studies; L. H. and M. G. contributed in subject recruitment, laboratory analysis of biomarkers, interpretation of data, and edited the manuscript; H. Z. contributed in laboratory analysis of biomarkers, interpretation of data, and edited the manuscript; and S. S. contributed in study design, implementation, data collection, interpretation of data, edited the manuscript, and led the research.
Financial support. This work was supported by National Institutes of Health (grants R01MH081772, K23MH074466, R01 NS043103, P01A1071713, M01RR00083), UCSF AIDS Research Institute, UCSF Academic Senate, and UCSF REAC, the Sahlgrenska Academy at University of Gothenburg (project ALFGBG-11067), Swedish Research Council (project 2007-7092), the Wolfson Foundation, the Italian Ministry of Health, AIDS Program 2009-2010, and a grant from the Doris Duke Charitable Foundation to Yale School of Medicine to support Clinical Research Fellow Michael Peluso. The MRS material resulted from resources and the use of facilities at the San Francisco Veterans Administration Medical Center.
Potential conflicts of interest. R. W. P. reports grant funding from Merck for an investigator-initiated research study and reports receiving lecture honoraria and travel fees from Abbott. B. J. B. reports board membership with ViiV, receiving lecture honoraria from ViiV, MerckSharpeDohme, Boehringer Ingelheim, and Abbot, and payment for the development of educational presentations from Gilead. P. C. serves on the board for Abbott, Biogen, and Janssen and reports consultancy for Biogen and Johnson & Johnson and support for lectures and educational materials from Abbott, Boehringer BMS, Gilead, Janssen, and Merck. K. R. reports receiving consultancy fees from ViiV and Abbott. MG reports serving on scientific advisory boards from Abbott, Boehringer, Ingelheim, BMS, Gilead, and Janssen/Tibotec and payment for lectures and/or travel fees from Abbott, BMS, Gilead, GlaxoSmithKline/ViiV, MSD, and Janseen/Tibotec. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadek JR, Vigil O, Grant I, Heaton RK HNRC Group. The impact of neuropsychological functioning and depressed mood on functional complaints in HIV-1 infection and methamphetamine dependence. J Clin Exp Neuropsychol. 2007;29:266–7. doi: 10.1080/13803390600659384. [DOI] [PubMed] [Google Scholar]
- 3.Price RW, Epstein LG, Becker JT, et al. Biomarkers of HIV-1 CNS infection and injury. Neurology. 2007;69:1781–8. doi: 10.1212/01.wnl.0000278457.55877.eb. [DOI] [PubMed] [Google Scholar]
- 4.Abdulle S, Mellgren A, Brew BJ, et al. CSF neurofilament protein (NFL)—a marker of active HIV-related neurodegeneration. J Neurol. 2007;254:1026–32. doi: 10.1007/s00415-006-0481-8. [DOI] [PubMed] [Google Scholar]
- 5.Hagberg L, Fuchs D, Rosengren L, Gisslen M. Intrathecal immune activation is associated with cerebrospinal fluid markers of neuronal destruction in AIDS patients. J Neuroimmunol. 2000;102:51–5. doi: 10.1016/s0165-5728(99)00150-2. [DOI] [PubMed] [Google Scholar]
- 6.Andersson L, Blennow K, Fuchs D, Svennerholm B, Gisslen M. Increased cerebrospinal fluid protein tau concentration in neuro-AIDS. J Neurol Sci. 1999;171:92–6. doi: 10.1016/s0022-510x(99)00253-1. [DOI] [PubMed] [Google Scholar]
- 7.Gisslen M, Krut J, Andreasson U, et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009;9:63. doi: 10.1186/1471-2377-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gisslen M, Rosengren L, Hagberg L, Deeks SG, Price RW. Cerebrospinal fluid signs of neuronal damage after antiretroviral treatment interruption in HIV-1 infection. AIDS Res Ther. 2005;2:6. doi: 10.1186/1742-6405-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nebuloni M, Pellegrinelli A, Ferri A, et al. Beta amyloid precursor protein and patterns of HIV p24 immunohistochemistry in different brain areas of AIDS patients. AIDS. 2001;15:571–5. doi: 10.1097/00002030-200103300-00005. [DOI] [PubMed] [Google Scholar]
- 10.Krut J, Zetterberg H, Fuchs D, et al. Signs of neural injury in asymptomatic HIV infection is mainly found in subjects with very low CD4 cell counts [paper 392]. Program and abstracts of the 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011. p. 88. CROI. [Google Scholar]
- 11.Pilcher CD, Shugars DC, Fiscus SA, et al. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS. 2001;15:837–45. doi: 10.1097/00002030-200105040-00004. [DOI] [PubMed] [Google Scholar]
- 12.Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–82. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spudich S, Gisslen M, Hagberg L, et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204:753–60. doi: 10.1093/infdis/jir387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lentz MR, Kim JP, Westmoreland SV, et al. Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology. 2005;235:461–8. doi: 10.1148/radiol.2352040003. [DOI] [PubMed] [Google Scholar]
- 15.Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging. 2010;32:1045–53. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez RG, Greco JB, He J, et al. New insights into the neuroimmunity of SIV infection by magnetic resonance spectroscopy. J Neuroimmune Pharmacol. 2006;1:152–9. doi: 10.1007/s11481-006-9016-4. [DOI] [PubMed] [Google Scholar]
- 17.Meyerhoff DJ, MacKay S, Bachman L, et al. Reduced brain N-acetylaspartate suggests neuronal loss in cognitively impaired human immunodeficiency virus-seropositive individuals: in vivo 1H magnetic resonance spectroscopic imaging. Neurology. 1993;43:509–15. doi: 10.1212/wnl.43.3_part_1.509. [DOI] [PubMed] [Google Scholar]
- 18.Stankoff B, Tourbah A, Suarez S, et al. Clinical and spectroscopic improvement in HIV-associated cognitive impairment. Neurology. 2001;56:112–5. doi: 10.1212/wnl.56.1.112. [DOI] [PubMed] [Google Scholar]
- 19.Yiannoutsos CT, Ernst T, Chang L, et al. Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage. 2004;23:928–35. doi: 10.1016/j.neuroimage.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 20.Young A, Yiannoutsos C, Lee E, et al. Progressive changes in cerebral metabolites and effect of ART in primary HIV-1 infection: a magnetic resonance spectroscopy study [paper 79]. Program and abstracts of the 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA: CROI. 2012. p. 23. [Google Scholar]
- 21.Zetola NM, Pilcher CD. Diagnosis and management of acute HIV infection. Infect Dis Clin North Am. 2007;21:19–48. doi: 10.1016/j.idc.2007.01.008. vii. [DOI] [PubMed] [Google Scholar]
- 22.Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–64. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 23.Tambussi G, Gori A, Capiluppi B, et al. Neurological symptoms during primary human immunodeficiency virus (HIV) infection correlate with high levels of HIV RNA in cerebrospinal fluid. Clin Infect Dis. 2000;30:962–5. doi: 10.1086/313810. [DOI] [PubMed] [Google Scholar]
- 24.Krut J, Zetterberg H, Fuchs D, et al. Signs of neural injury in asymptomatic HIV infection is mainly found in subjects with very low CD4 cell counts [paper 392]. In: Program and abstracts of the 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA: CROI.2011. [Google Scholar]
- 25.Ratai EM, Pilkenton SJ, Greco JB, et al. In vivo proton magnetic resonance spectroscopy reveals region specific metabolic responses to SIV infection in the macaque brain. BMC Neurosci. 2009;10:63. doi: 10.1186/1471-2202-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratai EM, Annamalai L, Burdo T, et al. Brain creatine elevation and N-Acetylaspartate reduction indicates neuronal dysfunction in the setting of enhanced glial energy metabolism in a macaque model of neuroAIDS. Magn Reson Med. 2011;66:625–34. doi: 10.1002/mrm.22821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend. 2012;125:27–36. doi: 10.1016/j.drugalcdep.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med. 1998;40:822–31. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- 29.Heaton RK, Grant I, Butters N, et al. The HNRC 500—neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1995;1:231–5. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- 30.Spudich SS, Nilsson AC, Lollo ND, et al. Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis. 2005;5:98. doi: 10.1186/1471-2334-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–44. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 32.Norgren N, Rosengren L, Stigbrand T. Elevated neurofilament levels in neurological diseases. Brain Res. 2003;987:25–31. doi: 10.1016/s0006-8993(03)03219-0. [DOI] [PubMed] [Google Scholar]
- 33.Norgren N, Sundstrom P, Svenningsson A, Rosengren L, Stigbrand T, Gunnarsson M. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology. 2004;63:1586–90. doi: 10.1212/01.wnl.0000142988.49341.d1. [DOI] [PubMed] [Google Scholar]
- 34.Zetterberg H, Hietala MA, Jonsson M, et al. Neurochemical aftermath of amateur boxing. Arch Neurol. 2006;63:1277–80. doi: 10.1001/archneur.63.9.1277. [DOI] [PubMed] [Google Scholar]
- 35.Gisslen M, Hagberg L, Brew BJ, Cinque P, Price RW, Rosengren L. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis. 2007;195:1774–8. doi: 10.1086/518043. [DOI] [PubMed] [Google Scholar]
- 36.Mellgren A, Price RW, Hagberg L, Rosengren L, Brew BJ, Gisslen M. Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology. 2007;69:1536–41. doi: 10.1212/01.wnl.0000277635.05973.55. [DOI] [PubMed] [Google Scholar]
- 37.Sjogren M, Davidsson P, Tullberg M, et al. Both total and phosphorylated tau are increased in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;70:624–30. doi: 10.1136/jnnp.70.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herukka SK, Hallikainen M, Soininen H, Pirttila T. CSF Abeta42 and tau or phosphorylated tau and prediction of progressive mild cognitive impairment. Neurology. 2005;64:1294–7. doi: 10.1212/01.WNL.0000156914.16988.56. [DOI] [PubMed] [Google Scholar]
- 40.Ellis RJ, Seubert P, Motter R, et al. Cerebrospinal fluid tau protein is not elevated in HIV-associated neurologic disease in humans. HIV Neurobehavioral Research Center Group (HNRC) Neurosci Lett. 1998;254:1–4. doi: 10.1016/s0304-3940(98)00549-7. [DOI] [PubMed] [Google Scholar]
- 41.Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65:1490–2. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- 42.Riemenschneider M, Wagenpfeil S, Vanderstichele H, et al. Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt-Jakob disease from other dementias. Mol Psychiatry. 2003;8:343–7. doi: 10.1038/sj.mp.4001220. [DOI] [PubMed] [Google Scholar]
- 43.Clifford DB, Fagan AM, Holtzman DM, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73:1982–7. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–11. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- 45.Reinsfelt B, Westerlind A, Blennow K, Zetterberg H, Ricksten SE. Open-heart surgery increases cerebrospinal fluid levels of Alzheimer-associated amyloid beta. Acta Anaesthesiol Scand. 2012;57(1):82–8. doi: 10.1111/j.1399-6576.2012.02769.x. [DOI] [PubMed] [Google Scholar]