Abstract
Traditional risk factors fail to explain the increased risk for cardiovascular morbidity and mortality in ESRD. Cyanate, a reactive electrophilic species in equilibrium with urea, posttranslationally modifies proteins through a process called carbamylation, which promotes atherosclerosis. The plasma level of protein-bound homocitrulline (PBHCit), which results from carbamylation, predicts major adverse cardiac events in patients with normal renal function, but whether this relationship is similar in ESRD is unknown. We quantified serum PBHCit in a cohort of 347 patients undergoing maintenance hemodialysis with 5 years of follow-up. Kaplan-Meier analyses revealed a significant association between elevated PBHCit and death (log-rank P<0.01). After adjustment for patient characteristics, laboratory values, and comorbid conditions, the risk for death among patients with PBHCit values in the highest tertile was more than double the risk among patients with values in the middle tertile (adjusted hazard ratio [HR], 2.4; 95% confidence interval [CI], 1.5–3.9) or the lowest tertile (adjusted HR, 2.3; 95% CI, 1.5–3.7). Including PBHCit significantly improved the multivariable model, with a net reclassification index of 14% (P<0.01). In summary, seurm PBHCit, a footprint of protein carbamylation, predicts increased cardiovascular risk in patients with ESRD, supporting a mechanistic link among uremia, inflammation, and atherosclerosis.
ESRD is a risk factor for cardiovascular disease (CVD). In fact, cardiovascular mortality in patients with ESRD is approximately 9% per year among those undergoing dialysis and accounts for >50% of all-cause mortality in patients with ESRD.1–4 This increase in disease burden cannot be solely explained by traditional cardiovascular risk factors and has prompted the search for specific CKD biomarkers for CVD.5
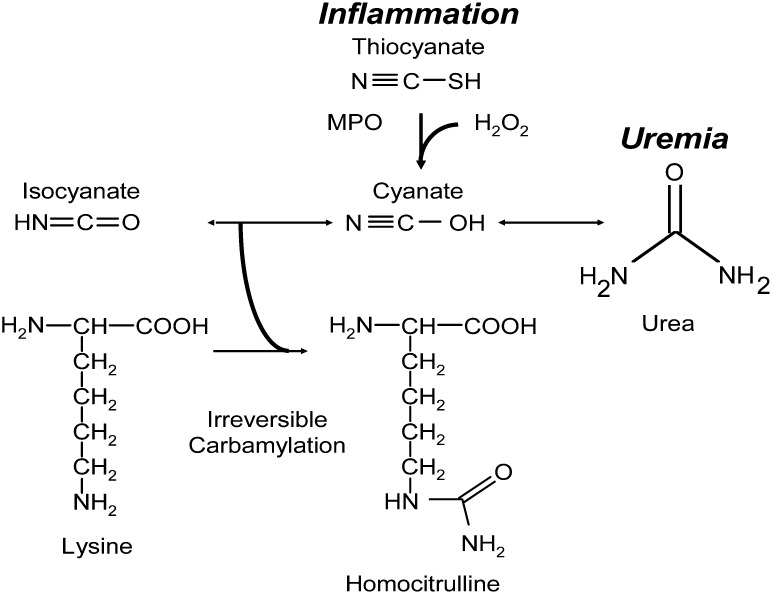
The complex pathophysiology associated with ESRD and chronic uremia has been hypothesized to be mediated, in part, through posttranslational modification of proteins by carbamylation.6–13 Urea, a byproduct of protein metabolism, is in equilibrium with an electrophilic pair of species, the cyanate ↔ isocyanate couple, which can react with nucleophilic amino acid side groups, such as the ε-amino moiety of lysine, resulting in posttranslational modification of proteins13–15 (Figure 1). Proteins purified from patients with chronic uremia have demonstrated dysfunction associated with the degree of carbamylation.12,13,15 Despite these observations, a direct relationship between systemic measures of protein carbamylation and incident cardiovascular risks has not yet been shown. Of note, researchers recently reported a novel pathway linking inflammation, another promoter of atherosclerosis, and protein carbamylation.13 Myeloperoxidase (MPO), a heme peroxidase enzyme with established multiple proatherogenic associations (including enrichment in culprit lesions from patients suffering from sudden cardiac death), catalyzed the formation of cyanate by co-reactants hydrogen peroxide and thiocyanate, leading to protein carbamylation (Figure 1).13,16 LDL carbamylation by MPO rendered the lipoprotein atherogenic, and systemic levels of protein-bound carbamyllysine (also known as homocitrulline [PBHCit]) predicted incident risks of major adverse cardiac events (MACE, the composite of myocardial infarction, stroke, revascularization or death) among persons with normal renal function.13 Further evidence supporting a potential mechanistic link between protein carbamylation and the pathogenesis of atherosclerosis comes from reports that uremic or carbamylated LDL induces vascular endothelial cell apoptosis, has decreased recognition by the LDL receptor, and is recognized by macrophage scavenger receptor class A.7,13
Figure 1.
Scheme of protein carbamylation. Thiocyanate (SCN−) is a naturally occurring pseudohalide found in dietary sources. MPO uses SCN− as cosubstrate with hydrogen peroxide (H2O2) to form cyanate. Urea is elevated in patients with kidney dysfunction and is in equilibrium with cyanate and isocyanate. Carbamylation of nucleophilic amino groups (lysine, for example) modifies protein structure and ultimately causes dysfunction.
On the basis of the preceding observations, we hypothesized that PBHCit may have potential clinical utility in risk-stratifying patients with kidney disease, a high-risk cohort in whom traditional risk factors do not adequately identify incident cardiovascular risks. We now report results examining the relationship between systemic levels of PBHCit and incident mortality risks in patients undergoing maintenance hemodialysis (MHD).
Results
Systemic Levels of PBHCit Are Higher in MHD Patients versus Controls and Are Associated with Uremia, Markers of Inflammation, and Mortality in MHD Patients
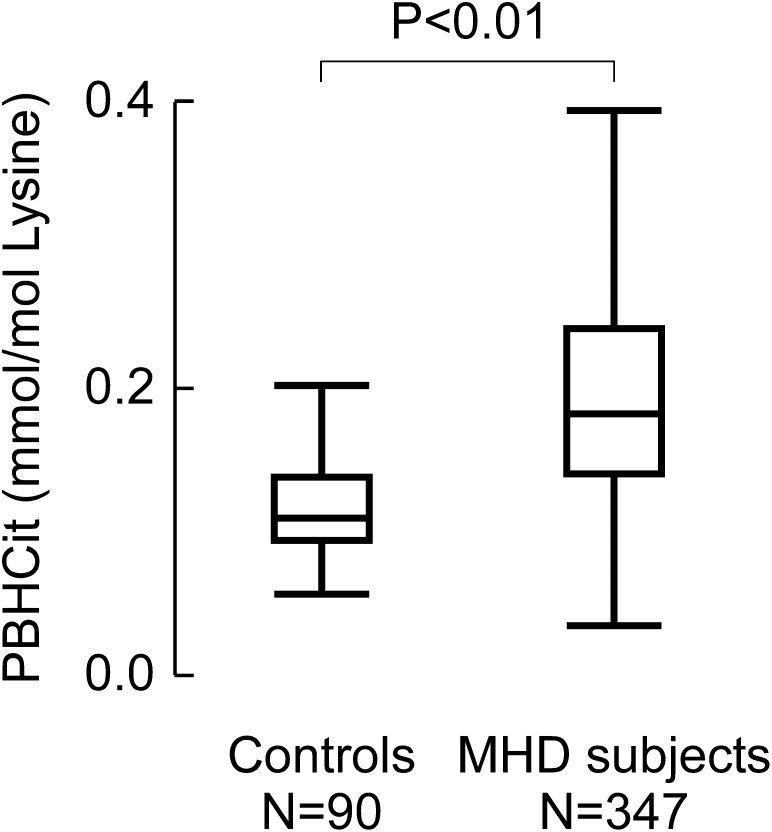
Patient demographic characteristics, clinical characteristics, and laboratory measurements are noted in Table 1. The patients (n=347) had a median age of 56 years (interquartile range [IQR], 45–65 years); 46% of the patients were women, and 53% had diabetes. The median predialysis systolic BP was 151 mmHg (IQR, 134–166 mmHg), and the median serum total cholesterol level was 141 mg/dl (IQR, 117–173 mg/dl). These demographic data are similar to those in the remaining parent cohort of 546 patients (age, 55 years [IQR, 42–65 years; P=0.18]; 47% women [P=0.70], 53% patients with diabetes [P=0.98], median predialysis systolic BP of 148 mmHg [IQR, 132–166 mmHg; P=0.26], and serum cholesterol level of 143 mg/dl [IQR, 118–172 mg/dl; P=0.67). In our overall MHD study cohort, the median PBHCit level was 0.183 mmol PBHCit/mol Lys (IQR, 0.139–0.242 mmol PBHCit/mol Lys) and was significantly higher than values in age-matched controls with no history of CVD and normal renal function (median, 0.109 mmol PBHCit/mol Lys [IQR, 0.093–0.138 mmol PBHCit/mol Lys]) (P<0.01) (Figure 2). PBHCit was also significantly higher among patients who died during the 5-year follow-up period (n=142; median, 0.200 mmol PBHCit/mol Lys [IQR, 0.142–0.252 mmol PBHCit/mol Lys]) than among patients who survived (n=205; median, 0.179 mmol PBHCit/mol Lys [IQR, 0.136–0.219 mmol PBHCit/mol Lys]) (P=0.02) (Table 1).
Table 1.
Baseline patient demographic, clinical, laboratory, and inflammatory measurements in a cohort of 347 patients receiving MHD
| Variable | Alive (n=205) | Dead (n=142) | P Value |
|---|---|---|---|
| Demographic | |||
| Age (yr) | 51 (40–60) | 64 (55–71) | <0.01 |
| Women (%) | 54 | 40 | 0.01 |
| African American (%) | 25 | 30 | 0.33 |
| Hispanic (%) | 57 | 49 | 0.15 |
| Clinical | |||
| History of CVD (%) | 43 | 51 | 0.18 |
| Diabetes mellitus (%) | 44 | 67 | <0.01 |
| Charlson comorbidity index | 2 (0–3) | 3 (1–4) | <0.01 |
| Near-infrared body fat (%) | 25 (17–34) | 29 (21–36) | 0.02 |
| BMI (kg/m2) | 25.4 (22.0–29.1) | 25.1 (22.1–30.1) | 0.97 |
| Dialysis vintage (mo) | 27 (13–52) | 26 (13–53) | 0.98 |
| Dialysis dose in Kt/V (single pool) | 1.5 (1.4–1.7) | 1.5 (1.4–1.7) | 0.26 |
| NPCR (g/kg per day) | 1.1 (0.93–1.2) | 1.0 (0.85–1.2) | 0.03 |
| Administered erythropoietin (U/wk) | 11,150 (6030–16,970) | 14,630 (7846–21,810) | <0.01 |
| Predialysis systolic BP (mmHg) | 147 (133–166) | 152 (134–168) | 0.54 |
| Laboratory | |||
| BUN (mg/dl) | 66 (54–77) | 64 (51–78) | 0.31 |
| Creatinine (mg/dl) | 11 (9.3–14) | 9.4 (7.5–11.2) | <0.01 |
| Total cholesterol (mg/dl) | 144 (123–175) | 135 (108–172) | 0.08 |
| Albumin (g/dl) | 3.9 (3.7–4.2) | 3.8 (3.5–3.9) | <0.01 |
| Calcium (mg/dl) | 9.3 (8.9–9.8) | 9.2 (8.8–9.6) | 0.23 |
| Phosphorous (mg/dl) | 5.8 (4.8–6.9) | 5.7 (4.6–6.7) | 0.70 |
| Intact parathyroid hormone (pg/ml) | 215 (133–416) | 256 (144–405) | 0.63 |
| Blood hemoglobin (g/dl) | 12 (11–12.6) | 12 (11–12.6) | 0.68 |
| Bicarbonate (HCO3−) (mEq/L) | 22.0 (20–24) | 22 (20–24) | 0.59 |
| Inflammatory markers | |||
| PBHcit (mmol/mol Lys) | 0.179 (0.136–0.220) | 0.200 (0.252–0.142) | 0.02 |
| Total homocysteine (µmol/L) | 22 (18–27) | 22 (18–28) | 0.74 |
| MPO (pmol/L) | 735 (422–1351) | 837 (428–1401) | 0.37 |
| CRP (mg/L) | 3.5 (1.6–7.3) | 5.2 (2.7–8.9) | <0.01 |
| IL-6 (ng/L) | 6.5 (4.0–13) | 12.4 (7.1–24) | <0.01 |
| TNF-α (ng/L) | 7.1 (5.0–9.1) | 6.6 (4.7–9.2) | 0.53 |
| Lactate dehydrogenase (U/L) | 155 (137–182) | 164 (138–189) | 0.11 |
| WBC count (×1000) | 6.9 (5.6–8.2) | 7.0 (5.9–8.7) | 0.19 |
Unless otherwise noted, data are expressed as medians with interquartile ranges. BMI, body mass index; NPCR, normalized protein catabolic rate; WBC, white blood cell.
Figure 2.
PBHCit concentrations in healthy persons with normal renal function (n=90) and patients undergoing MHD (n=347). Shown are box whisker plots for each group. The box represents the interquartile range, the line within the box is the median level, and the whiskers represent a length equal to 1.5 times the interquartile range (the difference between the 25th and 75th percentiles) or the lowest value per the method of Tukey. The P value represents the Wilcoxon rank sum comparison between groups.
Correlation studies on relevant clinical characteristics and laboratory measurements in the serum of these patients are shown in Table 2. Of note, PBHCit showed the strongest association with BUN levels among the biomarkers examined. Of note, whereas PBHCit is associated with mortality in MHD patients, BUN is not (Supplemental Table 1). In contrast, we observed no significant association between systemic concentrations of PBHCit and MPO concentration, a known catalytic source of PBHCit in persons with normal renal function, among the MHD patients.
Table 2.
Spearman correlations of serum PBHCit with demographic, laboratory, and inflammatory characteristics of patients undergoing MHD
| Variable | R Value | P Value |
|---|---|---|
| Clinical | ||
| NPCR (g/kg per day) | 0.20 | <0.01 |
| Near-infrared body fat (%) | −0.12 | 0.04 |
| Dialysis vintage | 0.13 | 0.02 |
| Administered erythropoietin (U/wk) | 0.12 | 0.04 |
| Laboratory | ||
| BUN (mg/dl) | 0.38 | <0.01 |
| Creatinine (mg/dl) | 0.17 | <0.01 |
| Total cholesterol (mg/dl) | −0.21 | <0.01 |
| Bicarbonate (HCO3−) (mEq/L) | −0.14 | <0.01 |
| Intact parathyroid hormone (pg/ml) | 0.13 | 0.02 |
| MPO (pmol/L) | −0.05 | 0.34 |
NPCR, normalized protein catabolic rate.
Systemic Levels of PBHCit Have Prognostic Utility Among MHD Patients
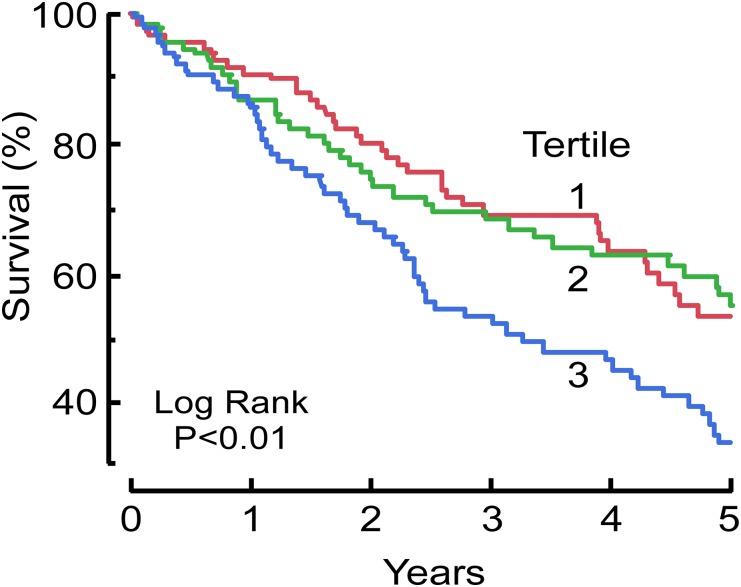
We next stratified patients into three tertiles and examined the relationship of PBHCit and death in time-to-event analyses. Kaplan-Meier survival plots of increasing tertiles of PBHCit demonstrate a significant association with mortality during the ensuing 5 years of follow-up (Figure 3). MHD patients with the highest levels of PBHCit demonstrated a substantially increased risk of death compared with patients in the middle and bottom tertiles, which showed equivalent mortality risk. We further examined the prognostic utility of tertiles of PBHCit to predict death in MHD patients (Table 3). Relative to the lowest tertile of PBHCit, those with the highest level of PBHCit were at significantly increased mortality risk. The prognostic utility of PBHCit remained an independent predictor of survival even after several multivariate adjustments. Of note, the association remained significant even after we factored in comorbid conditions, measures of kidney disease (including BUN), nutritional status, duration of MHD, and multiple inflammatory markers (C-reactive protein [CRP], TNF-α, and MPO). We also examined a subset of MHD patients for whom cardiovascular mortality had been reported. PBHCit remained an independent predictor of cardiovascular mortality (Supplemental Table 2).
Figure 3.
Kaplan-Meier survival analysis of tertiles of PBHCit in patients with ESRD receiving MHD during a 5-year period. PBHCit tertiles 1, 2, and 3 consisted of 116, 116, and 115 patients, respectively. Tertile cut points (mmol PBHCit/mol Lys): T1, <0.1546; T2, 0.1546–0.2165; T3, >0.2165.
Table 3.
Hazard ratios of tertiles of PBHCit versus death during a 5-year period
| Tertiles | Unadjusted HR (95% CI) | P Value | Minimally Adjusted HR (95% CI)a | P Value | Case-Mix–Adjusted HR (95% CI)b | P Value | Fully Adjusted HR (95% CI)c | P Value |
|---|---|---|---|---|---|---|---|---|
| Middle versus lowest | 1.0 (0.7–1.6) | 0.98 | 1.0 (0.7–1.6) | 0.87 | 1.0 (0.7–1.6) | 0.91 | 1.0 (07–1.7) | 0.88 |
| Highest versus middle | 1.7 (1.1–2.6) | <0.01 | 1.8 (1.2–2.7) | <0.01 | 2.0 (1.3–3.1) | <0.01 | 2.4 (1.5–3.9) | <0.01 |
| Highest versus lowest | 1.7 (1.1–2.6) | <0.01 | 1.8 (1.2–2.8) | <0.01 | 2.0 (1.4–3.1) | <0.01 | 2.3 (1.5–3.7) | <0.01 |
HR, hazard ratio; CI, confidence interval.
Adjusted for age and sex.
Adjusted for age, sex, race (black, Hispanic), history of CVD, diabetes mellitus, Charlson comorbidity index, and dialysis vintage.
Adjusted for age, sex, race (black, Hispanic), history of CVD, diabetes mellitus, Charlson comorbidity index, dialysis vintage, MPO, IL-6, BUN, creatinine, albumin, CRP, TNF-α, body mass index, erythropoietin, and normalized protein catabolic rate.
Systemic Levels of PBHCit Reclassifies Additional MHD Patients at Risk For Death
The preceding data suggest that PBHCit may help to identify patients with ESRD who are at increased (and decreased) near-term mortality risk independent of traditional risk factors. In a final set of studies, we examined the effect of PBHCit on predicting mortality in MHD patients during a 5-year period using net reclassification improvement and integrated discrimination improvement analyses (Table 4). In the fully adjusted model, addition of PBHCit significantly reclassified 14% of MHD patients, indicating that addition of PBHCit can help to identify MHD patients at risk not recognized by current methods.
Table 4.
Net reclassification improvement and integrated discrimination improvement for PBHCit and mortality risk during a 5-year period
| Variable | Integrated Discrimination Improvement (%) | P Value | Event-Specific Reclassification (%) | P Value |
|---|---|---|---|---|
| Without PBHcit | – | – | – | – |
| With PBHcit | 14.0 | <0.01 | 13.6 | <0.01 |
Adjusted for age, sex, race (black, Hispanic), history of CVD, diabetes mellitus, Charlson comorbidity index, dialysis vintage, MPO, IL-6, BUN, creatinine, albumin, CRP, TNF-α, body mass index, erythropoietin, and normalized protein catabolic rate.
Discussion
Increased cardiovascular disease risk is a prominent clinical feature among patients with severe kidney disease.1–4 Greater than 50% of these patients die of CVD, and almost all patients with CKD or ESRD experience accelerated CVD.1–4 Although most traditional cardiovascular risk factors have some prognostic value in patients with CKD, their utility in patients with ESRD becomes more limited: A 10- to 20-fold increased risk of cardiovascular death exists even after adjustment for traditional cardiovascular risk factors.2,17 These observations, coupled with the fact that patients with CKD experience a significantly greater proportion of CVD than Framingham risk models would predict, have served as stimulus for the need to identify other measures of CVD risk within the unique setting of kidney disease.5 The exploration of the relationship of various inflammatory markers and protein-bound compounds with morbidity and mortality in patients with CKD has yielded some potential candidate biomarkers, such as oxidized HDL, markers of platelet reactivity, and glycated hemoglobin.18–28 However, much of the pathology and physiologic dysfunction associated with ESRD has been hypothesized to be the result of both increased inflammation and “uremic-specific” compounds; it is remarkable that the biochemical process of protein carbamylation sits at the nexus of both of these pathways.8,10,15,17,29–32 The present studies demonstrate that quantification of systemic levels of PBHCit, a quantitative index of global systemic protein carbmaylation, may serve as a novel way to risk-stratify patients with ESRD who are undergoing MHD.
Protein carbamylation is a posttranslational modification widely known to occur in patients with chronic uremia.8,10,13,15 Despite these well known historical observations, to our knowledge, no studies have directly demonstrated that measures of protein carbamylation in the vasculature can predict incident mortality risks independent of other known risk factors and comorbid conditions in patients with ESRD. We previously reported that systemic PBHCit levels could be used to predict incident MACE risks in patients with normal kidney function.13 The present studies expand on these initial observations and now demonstrate potential clinical utility in PBHCit levels for risk-stratifying patients with ESRD, with patients who have the highest levels of PBHCit being at the highest risk. Notably, these associations are independent of other factors, including nutrition, cardiovascular risk factors, and inflammation markers (e.g., MPO).
This study has several limitations. First, the patients studied in this cohort are from the United States, and the applicability of the study findings across nationalities remains unclear. Second, cardiovascular mortality data were available only for the first 3 years of the study, so we examined cardiovascular mortality only for 3-year outcomes. We did, however, have all-cause mortality information for all patients and examined all-cause mortality (5-year) as our primary outcome because it is the most robust and ultimate outcome measure. Nonetheless, our studies reveal that PBHCit remained an independent predictor of incident cardiovascular mortality (3-year) and all-cause mortality (5-year). Third, we did not have residual renal function data; however, most of the study participants were undergoing prevalent, thrice-weekly hemodialysis. It is thus highly unlikely, although not impossible, to have significant residual renal function, in sharp contrast to patients receiving peritoneal dialysis.33 Finally, as an observational study, this study cannot exclude the inherent possibility of residual confounding.
The mechanisms at play for increased protein carbamylation within patients with ESRD are not known, but to some extent they are due to the chronic uremia associated with kidney dysfunction.34 However, it is notable that the correlation between PBHCit and urea levels within the cohort, although significant, were relatively modest in magnitude (R=0.38) (Table 2), indicating that only about 14% of the variation in PBHCit level could be explained by changes in urea level. The contribution of MPO to generation of PBHCit in this cohort is unclear. Historically, the toxemia of uremia has in part been attributed to protein carbamylation, perhaps because incubation of proteins with urea results in loss of enzymatic activity and changes in protein isoelectric point and molecular weight attributed to posttranslational modification via carbamylation.13,14,35 For example, erythropoietin isolated from patients with chronic uremia has dysfunction associated with increasing protein carbamylation.31 In addition, LDL isolated from uremic patients and uremic mouse aortic plaque have an enhanced degree of carbamylation and possess multiple proatherogenic biologic properties on in vitro testing.7,10,13,34 However, ESRD, like CVD, has become increasingly recognized as a systemic inflammatory disease.17,29,30,36,37 MPO, an abundant granulocyte protein, is a known mediator of vascular inflammation and plaque vulnerability and has been mechanistically linked to almost all stages of CVD, including culprit lesions (i.e., at sites of atherosclerotic plaque rupture and intracoronary thrombus).15,16,38–44 MPO has prognostic ability to help risk-stratify these same MHD patients,37 and thiocyanate, a preferred substrate for MPO and obligate cosubstrate with hydrogen peroxide in MPO-catalyzed protein carbamylation, is elevated in patients undergoing hemodialysis.13,45 However, MPO mass concentrations in the present cohort had a very weak correlation with PBHCit (Table 2), and its inclusion in multilogistic regression analyses failed to attenuate the prognostic utility of PBHCit (Table 3). It is worth noting that current MPO assays that have been cleared by the U.S. Food and Drug Administration for cardiovascular risk stratification (used in this study) are based on MPO mass, not activity; an assay of the latter is perhaps more likely to be correlated to PBHCit levels. Thus, the exact role of MPO in PBHCit formation within MHD patients remains unclear. Other mechanisms not yet identified might contribute to PBHCit formation and are linked to inflammation. It is tempting to speculate that one possibility is the generation of autoantibodies to PBHCit. Anticitrulline antibodies have recently become recognized in patients with rheumatoid arthritis, and these have been shown to cross-react with PBHCit.46 Whether similar autoantibodies to carbamylation develop, an abundant posttranslational modification within the MHD patient, warrants further investigation. However, given the weak correlations with the inflammatory markers monitored, it is possible that inflammation does not play a significant role in protein carbamylation in dialysis patients. Finally, it should also be noted, however, that measures of MPO mass as opposed to activity poorly correlated with the production of MPO-dependent oxidation products in multiple animal models, where use of MPO-knockout mice confirm a major role for MPO in the formation of the oxidation products.47,48
Our findings have several potential clinical implications beyond risk stratification. First, the ability to identify patients with heightened propensity for protein carbamylation due to the presence of urea provides a more relevant functional measure of adequacy of hemodialysis. Second, the presence of elevated PBHCit may identify an underlying process that can be targeted for therapeutic intervention. It is also interesting that urea levels were only modestly correlated with PBHCit levels (R=0.38; P<0.01) and thus could account for only a fraction of the variance in systemic protein carbamylation. This fact, coupled with the prior recognition of MPO as an enzymatic catalyst for protein carbamylation within sites of inflammation (such as atherosclerotic plaque), suggests that inhibition of MPO may be a potential therapeutic target of interest in patients with ESRD. Further studies are warranted to determine whether strategies to reduce the production or increase the clearance of PBHCit provide therapeutic benefit in the setting of ESRD.
In conclusion, we show that a marker of systemic protein carbamylation, PBHCit, is associated with increased mortality risk among MHD patients independent of existing CVD, renal disease, and nutritional status risk factors. Together, these data suggest that carbamylation serves as a common mechanistic link among chronic uremia, inflammation, and atherosclerosis in patients with kidney disease.
Concise Methods
Research Participants
Serum samples (n=347) were selected randomly from 1300 outpatients in the Nutritional and Inflammatory Evaluation in Dialysis (NIED) study undergoing maintenance hemodialysis (MHD) in 8 DaVita Inc. dialysis facilities in the Los Angeles, California, area. Inclusion criteria included age at least 18 years of age, MHD for at least 8 weeks, and a signed internal review board consent form. All patients received uniform hemodialysis treatments via high-flux membranes with reuse and standard water purification and processing techniques.49 This study adheres to principles described in the Declaration of Helsinki. Exclusion criteria included a life expectancy of <6 months.50 In the first phase of the NIED study, consented patients from the eight dialysis sites had blood drawn and subsequent serum laboratory analysis (see below) was performed. Residual serum was frozen for future investigations.
The medical charts from each patient were reviewed for pertinent histories of comorbid conditions and determination of cardiovascular mortality; a modified version of the Charlson comorbidity index was used for analysis (no age or kidney disease components).51,52 Healthy volunteers were recruited at the Cleveland Clinic, Cleveland, Ohio, to determine PBHCit levels in apparently healthy persons. All healthy controls gave written informed consent, and the Institutional Review Board of the Cleveland Clinic approved the study protocol. Plasma from 90 age-matched controls with no history of cardiovascular or kidney disease were identified for stable isotope dilution liquid chromatography-tandem mass spectrometry analyses and PBHCit quantified as described below.
Laboratory Tests
Routine laboratory tests were performed by DaVita Laboratories (Deland, FL).
Serum BUN concentrations were obtained midweek and coincide with quarterly blood tests at DaVita Laboratories. Serum CRP and cytokine were measured at the General Clinical Research Center Laboratories of Harbor-UCLA Medical Center. CRP was measured by a turbidimetric immunoassay (WPCI, Osaka, Japan).53,54 IL-6 and TNF-α were measured using a solid-phase sandwich ELISA with recombinant human IL-6 and TNF-α as standards (R&D Systems, Minneapolis, MN). Serum total homocysteine levels in MHD patients were determined using HPLC at the General Clinical Research Center Laboratories of Harbor-UCLA Medical Center. Near-Infrared Interactance technology (National Institutes of Health, Bethesda, MD) was used to measure fat and lean body mass, as previously described.37,55,56 HCO3− levels were measured at DaVita Laboratories as previously described.57 MPO levels were performed using the Food and Drug Administration–cleared CardioMPO assay (Cleveland Heart Labs, Cleveland, OH).
PBHCit Quantification
Analysis was performed as previously described.13 In brief, serum PBHCit from MHD patients and controls was quantified after delipidation and HCl hydrolysis using HPLC with online electrospray ionization tandem mass spectrometry on an AB SCIEX QTRAP 5500. Stable isotope-labeled internal standards ([13C6,15N2 ] l-lysine:2HCl [Cambridge Isotopes, Andover, MA] and ε-[13C,15N] carbamyl-l-lysine) were added before acid hydrolysis. ε-[13C,15N] carbamyl-l-lysine was synthesized by a reaction of poly-l-lysine (Sigma, St. Louis, MO) with potassium [13C, 15N] thiocyanate (Sigma) and H2O2 in the presence of bovine MPO. The reaction was followed by acid hydrolysis, HPLC purification, and analysis by mass spectrometry (>99% purity). Results are expressed as a ratio of protein-bound homocitrulline (mmol) to lysine (mol). Quality-control plasma samples were routinely prepared and analyzed in tandem with experimental samples. The coefficient of variation for all intra- and interday analyses for PBHCit remained <10%. These analyses were performed independently of the NIED study and were blinded from the study population, characteristics, and outcomes.
Statistical Analyses
Data are presented as median (IQR) for continuous measures and as number (percentage) for categorical measures in patients who were alive and had died during a 5-year period and over tertiles of PBHCit (Table 1 and Supplemental Table 3). PBHCit was classified as nonnormally distributed by performing a distribution plot of PBHCit and a Sharpe-Wilk normal distribution goodness-of-fit test (P<0.01). Continuous measures were compared between two independent groups using two-tailed Wilcoxon rank-sum tests (Mann-Whitney test) owing to the nonsymmetric distribution of many of the measures considered. Chi-squared tests were used to compare categorical measures. Spearman correlations were used as deemed appropriate for association studies. MHD patients were stratified by increasing concentrations of PBHCit into tertiles (lowest tertile [n=116]: median, 0.124 [IQR, 0.095–0.140 mmol PBHCit/mol Lys]; middle tertile [n=116]: median, 0.183 [IQR, 0.168–0.197 mmol PBHCit/mol Lys]; highest tertile [n=115]: median, 0.272 [IQR, 0.242–0.322 mmol PBHCit/mol Lys]) for survival analysis. Kaplan-Meier plots for death and tertiles of PBHCit were used for initial analysis of MHD patient survival across tertiles. Hazard ratios for death were generated using Cox proportional hazards modeling for tertiles of PBHCit with unadjusted and minimally adjusted (for age and sex), case-mix–adjusted (for age, sex, race [black, Hispanic], history of CVD, diabetes mellitus, Charlson comorbidity index, and dialysis vintage) and fully adjusted (for age, sex, race [black, Hispanic], history of CVD, diabetes mellitus, Charlson comorbidity index, dialysis vintage, MPO, IL-6, BUN, creatinine, albumin, body mass index, CRP, TNF-α, erythropoietin, and normalized protein catabolic rate) models. Univariate associations with mortality for factors included in modeling can be found in Supplemental Table 4. The association of risk ratios was considered significant if P<0.05. We evaluated the improvement in model performance introduced by the inclusion of PBHCit using net reclassification improvement and integrated discrimination improvement as described by Pencina et al.58 P values compare models with and without PBHCit. Both models were adjusted for the variables noted for the full adjustment model.58 The predicted probabilities of death were estimated from the Cox model. All statistical analyses were performed using JMP, version 9, or SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
Disclosures
Z.W. and S.L.H. are named as coinventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Z.W. has the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Liposciences, Inc. W.H.W.T. received research grant support from Abbott Laboratories and served as consultant for Medtronic Inc. and St. Jude Medical. S.L.H. has been paid as a consultant by Cleveland Heart Lab Inc., Esperion, Liposciences Inc., Merck & Co. Inc., and Pfizer Inc.; has received research funds from Abbott, Cleveland Heart Lab, Esperion, and Liposciences, Inc.; and has the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Abbott Laboratories, Cleveland Heart Lab Inc., Frantz Biomarkers, Liposciences Inc., and Siemens.
Acknowledgments
We thank Dr. Marie Louise Brennan for her insight and ideas in support of this project. An abstract reporting preliminary results related to this work was presented at the American Heart Association (Circulation. 2008;118:S337).
This research was supported by National Institutes of Health grants P01 HL076491, P01 HL098055, P01 HL103453, P20HL113452, R01 HL103866, and R01 HL103931. S.L.H. is supported in part by the Leducq Fondation and a gift from the Leonard Krieger Fund. Mass Spectrometry instrumentation used was housed within the Cleveland Clinic Mass Spectrometry Facility with partial support through a Center of Innovation by AB SCIEX.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012030254/-/DCSupplemental.
References
- 1.Collins AJ: Cardiovascular mortality in end-stage renal disease. Am J Med Sci 325: 163–167, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ: Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9[Suppl]: S16–S23, 1998 [PubMed] [Google Scholar]
- 3.Parfrey PS, Foley RN: The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol 10: 1606–1615, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Foley RN, Parfrey PS: Cardiovascular disease and mortality in ESRD. J Nephrol 11: 239–245, 1998 [PubMed] [Google Scholar]
- 5.Wright J, Hutchison A: Cardiovascular disease in patients with chronic kidney disease. Vasc Health Risk Manag 5: 713–722, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baigent C, Burbury K, Wheeler D: Premature cardiovascular disease in chronic renal failure. Lancet 356: 147–152, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Hörkkö S, Huttunen K, Kervinen K, Kesäniemi YA: Decreased clearance of uraemic and mildly carbamylated low-density lipoprotein. Eur J Clin Invest 24: 105–113, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Kraus LM, Elberger AJ, Handorf CR, Pabst MJ, Kraus AP, Jr: Urea-derived cyanate forms epsilon-amino-carbamoyl-lysine (homocitrulline) in leukocyte proteins in patients with end-stage renal disease on peritoneal dialysis. J Lab Clin Med 123: 882–891, 1994 [PubMed] [Google Scholar]
- 9.Mun KC, Kim HC, Kwak CS: Cyanate as a hemolytic factor. Ren Fail 22: 809–814, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Ok E, Basnakian AG, Apostolov EO, Barri YM, Shah SV: Carbamylated low-density lipoprotein induces death of endothelial cells: A link to atherosclerosis in patients with kidney disease. Kidney Int 68: 173–178, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray D, Barre PE: Outcome and risk factors of ischemic heart disease in chronic uremia. Kidney Int 49: 1428–1434, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Stim J, Shaykh M, Anwar F, Ansari A, Arruda JA, Dunea G: Factors determining hemoglobin carbamylation in renal failure. Kidney Int 48: 1605–1610, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Hörkkö S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL: Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med 13: 1176–1184, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Stark G, Stein WH, Moore S: Reactions of the cyanate present in aqueous urea with amino acids and proteins. J Biol Chem 235: 3177–3181, 1960 [Google Scholar]
- 15.Kraus LM, Kraus AP, Jr: Carbamoylation of amino acids and proteins in uremia. Kidney Int Suppl 78: S102–S107, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P: Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol 158: 879–891, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Depner TA: Uremic toxicity: Urea and beyond. Semin Dial 14: 246–251, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Honda H, Ueda M, Kojima S, Mashiba S, Michihata T, Takahashi K, Shishido K, Akizawa T: Oxidized high-density lipoprotein as a risk factor for cardiovascular events in prevalent hemodialysis patients. Atherosclerosis 220: 493–501, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Chmielewski M, Bragfors-Helin AC, Stenvinkel P, Lindholm B, Anderstam B: Serum soluble CD36, assessed by a novel monoclonal antibody-based sandwich ELISA, predicts cardiovascular mortality in dialysis patients. Clin Chim Acta 411: 2079–2082, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Scialla JJ, Plantinga LC, Kao WH, Jaar B, Powe NR, Parekh RS: Soluble P-selectin levels are associated with cardiovascular mortality and sudden cardiac death in male dialysis patients. Am J Nephrol 33: 224–230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovesdy CP, Kalantar-Zadeh K: Review article: Biomarkers of clinical outcomes in advanced chronic kidney disease. Nephrology (Carlton) 14: 408–415, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verduijn M, Maréchal C, Coester AM, Sampimon DE, Boeschoten EW, Dekker FW, Goffin E, Krediet RT, Devuyst O: The -174G/C variant of IL6 as risk factor for mortality and technique failure in a large cohort of peritoneal dialysis patients. Nephrol Dial Transplant 27: 3516–3523, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Verduijn M, Prein RA, Stenvinkel P, Carrero JJ, le Cessie S, Witasp A, Nordfors L, Krediet RT, Boeschoten EW, Dekker FW: Is fetuin-A a mortality risk factor in dialysis patients or a mere risk marker? A Mendelian randomization approach. Nephrol Dial Transplant 26: 239–245, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Amabile N, Guérin AP, Tedgui A, Boulanger CM, London GM: Predictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: A pilot study. Nephrol Dial Transplant 27: 1873–1880, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Chen KH, Lin JL, Lin-Tan DT, Hsu CW, Huang WH, Yen TH, Weng SM, Hung CC: Glycated hemoglobin predicts mortality in nondiabetic patients receiving chronic peritoneal dialysis. Am J Nephrol 32: 567–574, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Sebeková K, Klenovicsová K, Ferenczová J, Hedvig J, Podracká L, Heidland A: Advanced oxidation protein products and advanced glycation end products in children and adolescents with chronic renal insufficiency. J Ren Nutr 22: 143–148, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, Chonchol M, HOST Investigators : FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD: Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 63: 793–808, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ: Association between cholesterol level and mortality in dialysis patients: Role of inflammation and malnutrition. JAMA 291: 451–459, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Mun KC, Golper TA: Impaired biological activity of erythropoietin by cyanate carbamylation. Blood Purif 18: 13–17, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P: “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 54: 561–569, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Horinek A, Misra M: Does residual renal function decline more rapidly in hemodialysis than in peritoneal dialysis? How good is the evidence? Adv Perit Dial 20: 137–140, 2004 [PubMed] [Google Scholar]
- 34.Apostolov EO, Ray D, Savenka AV, Shah SV, Basnakian AG: Chronic uremia stimulates LDL carbamylation and atherosclerosis. J Am Soc Nephrol 21: 1852–1857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bobb D, Hofstee BH: Gel isoelectric focusing for following the successive carbamylations of amino groups in chymotrypsinogen A. Anal Biochem 40: 209–217, 1971 [DOI] [PubMed] [Google Scholar]
- 36.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM: The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62: 1524–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Kalantar-Zadeh K, Brennan ML, Hazen SL: Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis 48: 59–68, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Hazen SL: Myeloperoxidase and plaque vulnerability. Arterioscler Thromb Vasc Biol 24: 1143–1146, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P: Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol 24: 1309–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Abu-Soud HM, Hazen SL: Nitric oxide modulates the catalytic activity of myeloperoxidase. J Biol Chem 275: 5425–5430, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Podrez EA, Febbraio M, Sheibani N, Schmitt D, Silverstein RL, Hajjar DP, Cohen PA, Frazier WA, Hoff HF, Hazen SL: Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest 105: 1095–1108, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudolph TK, Wipper S, Reiter B, Rudolph V, Coym A, Detter C, Lau D, Klinke A, Friedrichs K, Rau T, Pekarova M, Russ D, Knöll K, Kolk M, Schroeder B, Wegscheider K, Andresen H, Schwedhelm E, Boeger R, Ehmke H, Baldus S: Myeloperoxidase deficiency preserves vasomotor function in humans. Eur Heart J 33: 1625–1634, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vita JA, Brennan ML, Gokce N, Mann SA, Goormastic M, Shishehbor MH, Penn MS, Keaney JF, Jr, Hazen SL: Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation 110: 1134–1139, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL: Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest 114: 529–541, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasuike Y, Nakanishi T, Moriguchi R, Otaki Y, Nanami M, Hama Y, Naka M, Miyagawa K, Izumi M, Takamitsu Y: Accumulation of cyanide and thiocyanate in haemodialysis patients. Nephrol Dial Transplant 19: 1474–1479, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Turunen S, Koivula MK, Risteli L, Risteli J: Anticitrulline antibodies can be caused by homocitrulline-containing proteins in rabbits. Arthritis Rheum 62: 3345–3352, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Brennan ML, Wu W, Fu X, Shen Z, Song W, Frost H, Vadseth C, Narine L, Lenkiewicz E, Borchers MT, Lusis AJ, Lee JJ, Lee NA, Abu-Soud HM, Ischiropoulos H, Hazen SL: A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem 277: 17415–17427, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Zhang R, Brennan ML, Shen Z, MacPherson JC, Schmitt D, Molenda CE, Hazen SL: Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem 277: 46116–46122, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Colman S, Bross R, Benner D, Chow J, Braglia A, Arzaghi J, Dennis J, Martinez L, Baldo DB, Agarwal V, Trundnowski T, Zitterkoph J, Martinez B, Khawar OS, Kalantar-Zadeh K: The Nutritional and Inflammatory Evaluation in Dialysis patients (NIED) study: Overview of the NIED study and the role of dietitians. J Ren Nutr 15: 231–243, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Rambod M, Bross R, Zitterkoph J, Benner D, Pithia J, Colman S, Kovesdy CP, Kopple JD, Kalantar-Zadeh K: Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am J Kidney Dis 53: 298–309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML: A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med 108: 609–613, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Fried L, Bernardini J, Piraino B: Charlson comorbidity index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis 37: 337–342, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Erbağci AB, Tarakçioğlu M, Aksoy M, Kocabaş R, Nacak M, Aynacioğlu AS, Sivrikoz C: Diagnostic value of CRP and Lp(a) in coronary heart disease. Acta Cardiol 57: 197–204, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR: Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 347: 1557–1565, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Kalantar-Zadeh K, Block G, Kelly MP, Schroepfer C, Rodriguez RA, Humphreys MH: Near infra-red interactance for longitudinal assessment of nutrition in dialysis patients. J Ren Nutr 11: 23–31, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Kalantar-Zadeh K, Dunne E, Nixon K, Kahn K, Lee GH, Kleiner M, Luft FC: Near infra-red interactance for nutritional assessment of dialysis patients. Nephrol Dial Transplant 14: 169–175, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K: Association between serum bicarbonate and death in hemodialysis patients: Is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol 1: 70–78, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS: Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 27: 157–172, 2008 [DOI] [PubMed] [Google Scholar]