Abstract
Kidney transplant recipients usually have low vitamin D levels, especially in the early posttransplantation period, but the association between vitamin D status with renal outcomes is not well described in this population. Here, we studied a prospective cohort of 634 kidney recipients who underwent transplantation at a single institution between January 2005 and June 2010. In this cohort, low 25-hydroxyvitamin D concentrations 3 months after transplantation did not predict early death or graft loss but were independently associated with lower measured GFR at 12 months (P=0.001) and higher risk for interstitial fibrosis and tubular atrophy (P=0.01). In contrast, levels of calcium, phosphorus, calcitriol, parathyroid hormone, or fibroblast growth factor-23 were not consistently associated with any of the studied outcomes. In conclusion, low 25-hydroxyvitamin D concentration measured 3 months after transplantation is an independent risk factor for interstitial fibrosis progression and is associated with a lower GFR 1 year after transplantation.
Kidney transplantation is the preferred treatment for the increasing number of patients with ESRD. Transplantation allows patients to avoid the heavy constraints associated with dialysis therapy and is associated with a lower mortality and morbidity than is hemodialysis.1 Evolution of GFR in transplant recipients appears to be imprinted at an early stage after the surgery. Indeed, the estimated GFR (eGFR) reached 1 year after transplantation is critically associated with allograft outcome.2,3 This observation prompts research on modifiable factors affecting GFR in the first year after transplantation.
Vitamin D is a critical hormone controlling mineral homeostasis. It promotes phosphate and calcium absorption by the gut and increases calcium reabsorption by the renal distal tubule, thereby providing the positive calcium and phosphorus flux required for bone mineralization. The formation of fully active vitamin D requires two-step hydroxylation of its precursors, either cholecalciferol or ergocalciferol. Hepatocytes mediate the first hydroxylation on carbon 25 to produce 25-hydroxyvitamin D (25[OH]D),4 which binds the vitamin D receptor (VDR) with only a modest affinity. The complete activation of vitamin D requires further hydroxylation on carbon 1 by the enzyme CYP27B1, resulting in the formation of calcitriol or 1,25 dihydroxyvitamin D (1,25[OH]D). This last step takes place mainly in the proximal tubular cells of the kidney and is tightly regulated.5 Parathyroid hormone (PTH) and hypophosphatemia increase CYP27B1 expression in the proximal tubular cells, whereas the phosphatonin fibroblast growth factor-23 (FGF-23) decreases it.6
Recent research has expanded the spectrum of vitamin D actions beyond mineral metabolism. Concordant evidence sustains a wide range of unconventional vitamin D action on the immune, cardiovascular, and renal systems. Of note, vitamin D signaling protects against progression of chronic renal lesions in different models of CKD.7–15 More recently, calcitriol and paricalcitol, two agonists of VDR signaling, have been shown to lessen albuminuria in patients with IgA nephropathy16 and diabetic nephropathy,17 respectively. In addition, two community-based studies and a small observational study in patients with CKD who have not had transplantation found an association between low 25(OH)D levels and the risk of ESRD18 or eGFR decline.19 Transplant recipients frequently have low 25(OH)D and 1,25(OH)D levels, especially early after transplantation. Whether these modifiable variables influence kidney function or transplant outcome is unknown.
Since 2005, the standard care of kidney transplant recipients at our institution includes a measurement of GFR by iohexol clearance, an assessment of mineral metabolism hormones, and screening kidney transplant biopsies performed 3 and 12 months after transplantation (Supplemental Figure 1). We took advantage of this close monitoring to assess the link between vitamin D status at 3 months and transplantation outcome. We approached this question with three complementary analyses. First, we explored the association of 3-month vitamin D status with early mortality and allograft loss. Second, we evaluated the link between vitamin D status at 3 months and measured GFR (mGFR) reached 12 months after transplantation. Finally, we analyzed the association between vitamin D status 3 months after transplantation and interstitial fibrosis and tubular atrophy progression between 3 and 12 months.
Results
Study Population
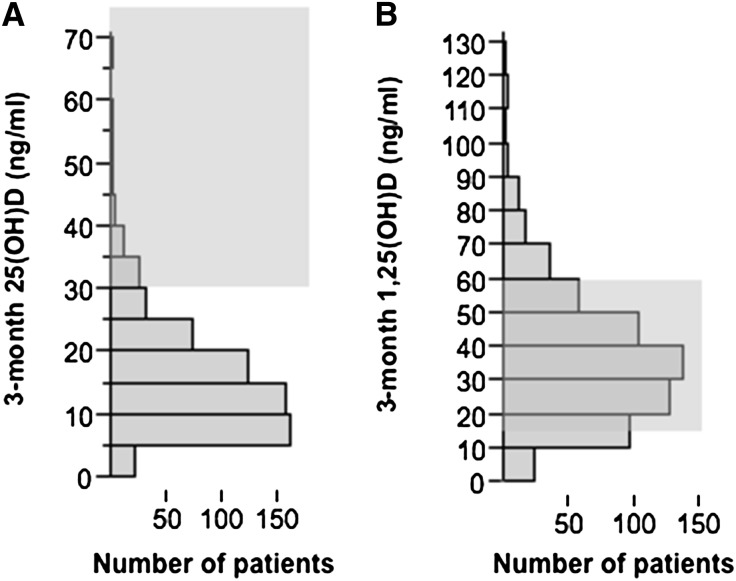
During a 5-year period, 634 patients had renal transplantation and were assessed 2–4 months after transplantation. Table 1 shows the patients’ characteristics. Overall, 25(OH)D concentrations were low (median concentration, 13 ng/ml; interquartile range, 9–20 mg/nl). The 25(OH)D level was <30 ng/ml in 91.7% of patients (Figure 1A). In contrast, 1,25(OH)D concentrations were within the normal range in most patients (Figure 1B). At 3 months, 25(OH)D correlated significantly with 1,25(OH)D (r=0.29; P<0.001) and PTH (r=−0.28; P=0.001) levels but not with FGF-23, ionized blood calcium, phosphate, or mGFR. The characteristics of the patients with or without vitamin D deficiency (defined as 25[OH]D < 15 ng/ml) 3 months after transplantation are presented in Table 1. It is worth noting that longer time on hemodialysis was associated with 25(OH)D deficiency. In addition, 3 months after transplantation, 1,25(OH)D correlated significantly with PTH (r=0.18; P=0.002), mGFR (r=0.33; P=0.001), and FGF-23 (r=−0.17; P=0.002); correlated weakly with serum phosphate concentration (r=−0.09; P=0.02); and was not correlated with ionized blood calcium concentration.
Table 1.
Patient characteristics 3 months after transplantation
| Characteristic | All Patients (n=634) | 3-Month 25(OH)D Level < 15 ng/ml (n=348) | 3-Month 25(OH)D Level ≥ 15 ng/ml (n=286) | P Valuea |
|---|---|---|---|---|
| Age at transplantation (yr) | 48.3±13.4 | 48±14 | 50±13 | 0.41 |
| Male sex | 58.7 (372) | 58.0 (202) | 59.4 (170) | 0.72 |
| Initial nephropathy | 0.16 | |||
| Primary GN | 23.2 (147) | 24.1 (84) | 22.0 (63) | |
| Cystic nephropathy | 15.3 (97) | 12.9 (45) | 18.2 (52) | |
| Uropathy | 8.2 (52) | 6.9 (24) | 9.8 (28) | |
| Diabetes | 7.6 (48) | 9.2 (32) | 5.6 (16) | |
| Vascular nephropathy | 6.2 (39) | 6.3 (22) | 5.9 (17) | |
| Interstitial nephropathy | 3.6 (23) | 4.6 (16) | 2.4 (7) | |
| Other | 15.9 (101) | 17.0 (59) | 14.7 (42) | |
| Unknown | 20.0 (127) | 19.0 (66) | 21.3 (61) | |
| Preemptive transplantation | 13.7 (86) | 10.7 (37) | 17.3 (49) | 0.02 |
| Treated by hemodialysis before transplantation | 81.2 (510) | 84.6 (292) | 77.0 (218) | 0.02 |
| Treated by peritoneal dialysis before transplantation | 3.7 (23) | 3.2 (11) | 4.2 (12) | 0.49 |
| Time on dialysis before transplantation (mo) | 35.4 (12.0–73.2) | 38.8 (15.2–76.3) | 30.0 (7.95–69.4) | 0.01 |
| Parathyroidectomy before transplantation | 12.6 (80) | 12.1 (42) | 13.3 (38) | 0.65 |
| Repeat transplantation (for first transplantations) | 77.7 (491) | 77.2 (267) | 78.3 (224) | 0.73 |
| Living donor | 20.8 (131) | 17.7 (61) | 24.6 (70) | 0.04 |
| Donor age (yr) | 53±16 | 53±16 | 53±16 | 0.87 |
| Extended donor criteria | 51.4 (314) | 52.3 (176) | 50.2 (138) | 0.59 |
| Cold ischemia time (min) | 1122 (753–1492) | 1151 (851–1476) | 1090 (492–1500) | 0.15 |
| Delayed graft function | 32.3 (205) | 35.3 (123) | 28.7 (82) | 0.07 |
| Induction treatment | 95.6 (593) | 96.2 (328) | 95.0 (265) | 0.46 |
| Type of induction treatment | 0.78 | |||
| Basiliximab | 69.6 (413) | 68.6 (225) | 70.9 (188) | |
| Antilymphocytic serum | 29.0 (172) | 29.9 (98) | 27.9 (74) | |
| Others | 1.3 (8) | 1.52 (5) | 1.1 (3) | |
| Calcineurin inhibitor–based regimen | 98.5 (605) | 98.8 (333) | 98.2 (272) | 0.65 |
| Cellular acute rejection before 3 mo | 8.2 (52) | 8.62 (30) | 7.69 (22) | 0.67 |
| Humoral acute rejection before 3 mo | 4.6 (29) | 4.02 (14) | 5.24 (15) | 0.46 |
| Body mass index (kg/m2) | 24.1±4.3 | 24.2±4.53 | 24.0±4.09 | 0.55 |
| Systolic BP (mmHg) | 136.2±17.9 | 136.7±17.8 | 135.7±18.0 | 0.48 |
| Diastolic BP (mmHg) | 77.9±10.7 | 77.8±11.1 | 78.1±10.1 | 0.71 |
| mGFR (ml/min) | 57±17 | 57±17 | 58±17 | 0.28 |
| Ionized calcium (mmol/L) | 1.27±0.09 | 1.27±0.09 | 1.27±0.09 | 0.21 |
| Phosphate (mmol/L) | 0.77±0.21 | 0.77±0.22 | 0.77±0.20 | 0.80 |
| 1,25(OH)D (pg/L) | 35 (23–48) | 31 (19–41) | 40 (27–53) | P<0.001 |
| PTH (ng/L) | 98 (64–159) | 111 (78–198) | 81 (55–137) | P<0.001 |
| FGF-23 (RU/ml) | 70 (47–116) | 67 (44–115) | 73 (52–119) | 0.23 |
| Cinacalcet treatment | 3.6 (23) | 3.2 (11) | 4.2 (12) | 0.49 |
Values are reported as percentage (number of patients), mean ± SD, or median (interquartile range), as appropriate.
P value represents tests of significance from t test, Wilcoxon test, chi-squared test, or Fisher exact test, as appropriate.
Figure 1.
Kidney tranplant recipients have low 25(OH)D 3 months after transplantation. Distribution of (A) 25-hydroxy vitamin D (25[OH]D) and (B) 1-25-dihydroxy vitamin D (1,25[OH]D) in kidney recipients 3 months after transplantation. Gray areas indicate target range for 25(OH)D and normal range for 1,25(OH)D in patients with CKD who have not undergone transplantation.
Association of 3-Month 25(OH)D Concentration with Survival and Graft Loss
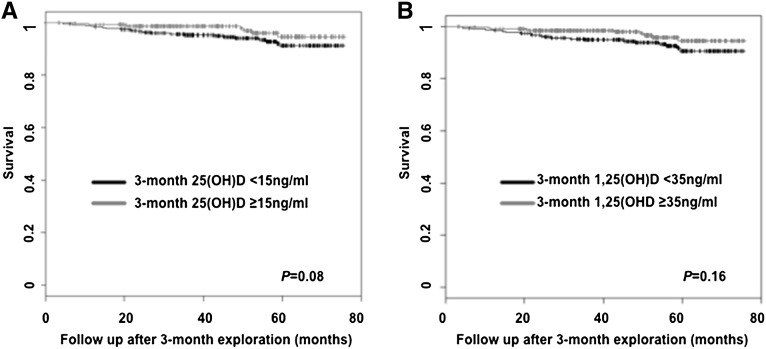
The median follow-up time was 48.6 months (interquartile range, 3.2–75.6 months). At the end of the study, 19 patients were lost to follow-up, 30 patients had lost their allograft, 28 patients had died with a functioning graft, and 3 patients had died after losing their allograft. The principal causes of death were infections in 12 patients, malignancies in 9 patients, and cardiovascular accidents in 5 patients. Kaplan-Meier survival curves revealed similar risk of death in patients with low or high 3-month 25(OH)D and 3-month 1,25(OH)D (Figure 2). Similarly, Cox regression models showed no significant association between continuous 3-month 25(OH)D or 3-month 1,25(OH)D levels and mortality (hazard ratio [HR], 0.97 [95% confidence interval (CI), 0.93–1.02], P=0.27 for increase of 1 unit of 25[OH]D; HR, 0.86 [95% CI, 0.70–1.06], P=0.16 for increase of 10 units of 1,25[OH]D, respectively). In addition, competitive risk analysis revealed no association between graft loss and 3-month 25(OH)D or 3-month 1,25(OH)D levels (HR, 0.99 [95% CI, 0.95–1.04], P=0.65 for increase of 1 unit of 25[OH]D; HR, 0.98 [95% CI, 0.77–1.25], P=0.86 for increase of 10 units of 1,25[OH]D, respectively).
Figure 2.
Three-month vitamin D levels do not associate with patient survival. Patient survival curves according to (A) 25(OH)D and (B) 1,25(OH)D status, 3 months after transplantation.
Association of 3-Month 25(OH)D with 12-Month mGFR
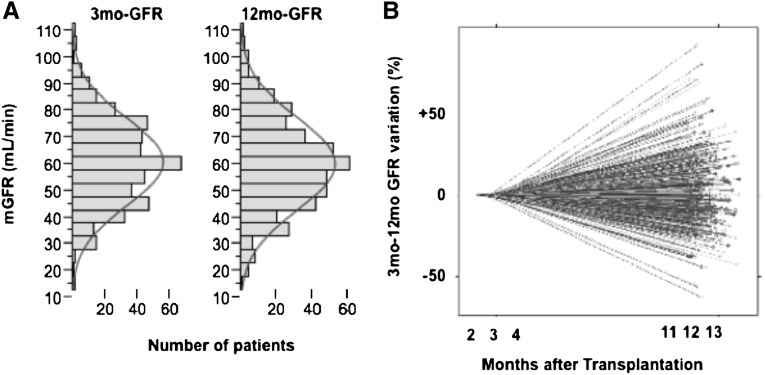
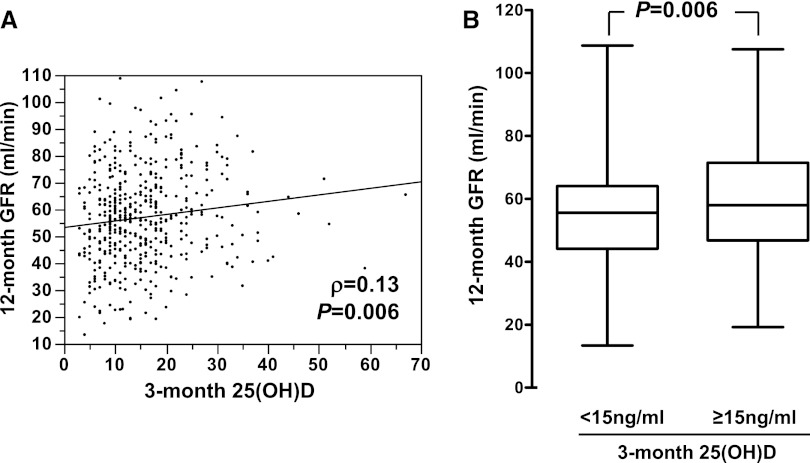
Among the 634 patients tested 3 months after transplantation, 465 had a second measurement of mGFR at 12 months. The characteristics of the subgroup of patients who were reassessed at 12 months did not differ from those of the whole-study population. Although the overall distribution of mGFR was similar at 3 months and 12 months (Figure 3A), the analysis of individual mGFR variation revealed significant heterogeneity (Figure 3B). Overall, between 3 and 12 months, mGFR worsened (mGFR variation < −0.6 ml/min per month) in 157 (33.7%) patients, improved (mGFR variation > 0.6 ml/min per month) in 132 (28.3%) patients, and remained stable in 176 (37.8%). The results of the univariate analyses of the factors linked to 12-month mGFR are listed in Table 2. Unsurprisingly, 3-month mGFR was a strong predictor of 12-month mGFR (r=0.78; P<0.001). Of note, 12-month mGFR correlated with 3-month 25(OH)D (r=0.13; P=0.006), 3-month 1,25(OH)D (ρ=0.31; P<0.001), and 3-month FGF-23 (ρ=−0.30; P<0.001) (Table 2 and Figure 4). Patients with 3-month 25(OH)D levels <15 ng/ml had a mean 12-month mGFR ± SD of 55±17 ml/min, whereas patients with 3-month 25(OH)D levels >15 ng/ml had a 3-month mGFR of 59±17 ml/min (Figure 4B). Multivariate analyses revealed that patient sex, donor age, 3-month mGFR, 3-month body mass index, and 3-month 25(OH)D (considered as a continuous variable), but not 1,25(OH)D or FGF-23, were independent predictors of 12-month mGFR (Table 3 for 25[OH]D and 1,25[OH]D; data not shown for FGF-23). Consideration of a cutoff value of 15 ng/ml for 3-month 25(OH)D did not modify the significance of this association (Supplemental Table 1). A second measurement of 25(OH)D was also performed at 12 months (Figure 5), along with GFR measurement. Overall, 25(OH)D concentration increased between 3 months and 12 months. However, 25(OH)D concentration remained below the recommended threshold for CKD (30 ng/ml) in 65.7% of patients. In contrast with 3-month 25(OH)D concentration, 12-month 25(OH)D concentration was not correlated with 12-month mGFR (r=0.04; P=0.43).
Figure 3.
Transplanted patients show significant variations in mGFR between 3 and 12 months after transplantation. (A) Distribution of mGFR in kidney recipients 3 months and 12 months after transplantation. (B) Individual variation in mGFR between 3 and 12 months after transplantation.
Table 2.
Association of the baseline variables with mGFR at 12 months in univariate analysis
| Characteristic | 12-Month mGFR or Correlation Coefficient | P Value |
|---|---|---|
| Sex | <0.001 | |
| Male | 59.6±16.9 | |
| Female | 53.6±17.1 | |
| Age at transplantation | r=−0.26 | <0.001 |
| Donor type | <0.001 | |
| Deceased donor | 55.1±17.3 | |
| Living donor | 63.6±15.2 | |
| Donor age | r=−0.48 | <0.001 |
| Preemptive transplantation | 0.065 | |
| Yes | 60.1±16.4 | |
| No | 56.3±17.3 | |
| Cold ischemia time (min) | <0.001 | |
| ≤250 | 63.4±15.2 | |
| 250–1200 | 57.9±16.9 | |
| >1200 | 52.3±16.8 | |
| Delayed graft function | 0.07 | |
| Yes | 54.8±19.2 | |
| No | 57.9±16.3 | |
| Acute cellular rejection before 3 months | 0.46 | |
| Yes | 53.6±13.1 | |
| No | 60.0±21.7 | |
| Acute humoral rejection before 3 months | 0.97 | |
| Yes | 56.8±15.2 | |
| No | 58.5±16.1 | |
| 3-month body mass index | r=0.09 | 0.046 |
| 3-month mGFR | r=0.78 | <0.001 |
| 3-month 25(OH)D | r=0.13 | 0.006 |
| 3-month PTH | ρ=0.07 | 0.15 |
| 3-month FGF-23 | ρ=0.30 | <0.001 |
| 3-month 1,25(OH)D | ρ=0.31 | <0.001 |
Values of 12-month mGFR are expressed in ml/min and reported as mean ± SD.
P values represent tests of significance from t test, Wilcoxon test, or Pearson or Spearman correlation tests, as appropriate.
Figure 4.
Low 25(OH)D 3 months after transplantation correlates with lower GFR at 12 months. (A) Correlation between 25(OH)D 3 months after transplantation (3-month 25[OH]D) and measured GFR at 12 months (12-month mGFR) (B) 12-month mGFR according to 3-month 25(OH)D status.
Table 3.
Association of baseline variables with mGFR at 12 months using multiple linear regressions.
| Model | Coefficient ± SD | P Value |
|---|---|---|
| Full modela plus 3-month 25(OH)D | ||
| Intercept | 17.1±4.02 | |
| Male sex | 2.86±1.00 | 0.004 |
| Donor ageb | −0.22±0.03 | <0.001 |
| 3-month body mass indexb | 0.27±0.12 | 0.02 |
| 3-month mGFRb | 0.70±0.03 | <0.001 |
| 3-month 25(OH)Db | 0.17±0.05 | 0.001 |
| Full modela plus 3-month 1, 25(OH)D | ||
| Intercept | 19.6±3.95 | |
| Male sex | 3.34±1.01 | 0.001 |
| Donor ageb | −0.22±0.04 | <0.001 |
| Preemptive transplantation | 3.38±1.40 | 0.02 |
| 3-month body mass indexb | 0.28±0.12 | 0.02 |
| 3-month mGFRb | 0.70±0.03 | <0.001 |
Only the variables independently associated with 12-mo mGFR are presented (final models after backward selection procedure).
The full model includes recipient sex, age at transplantation, donor type, donor age, preemptive transplantation, delayed graft function, 3-month body mass index, and 3-month mGFR.
For an increase of 1 unit of the variable.
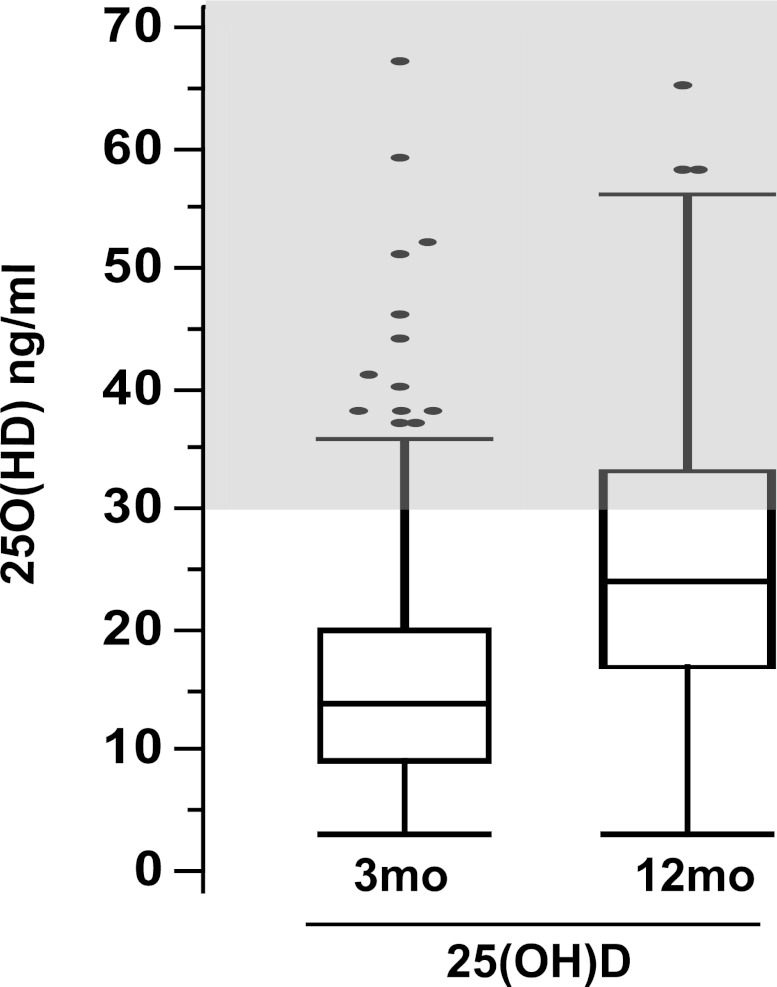
Figure 5.
Although 25(OH)D concentrations tend to increase between 3 and 12 months after transplantation, they usually remain below the recommended threshold for CKD patients. Distribution of 25(OH)D 3 and 12 months after transplantation. Gray area indicates target range for 25(OH)D in patients with CKD who have not undergone kidney transplantation.
Vitamin D Status and Interstitial Fibrosis and Tubular Atrophy Score Progression
GFR may be influenced by hemodynamic factors that are not related to the progression of renal lesions. Thus, we sought to determine whether the apparent effect of 3-month 25(OH)D on 12-month mGFR was associated with deterioration of the renal allograft parenchyma. On the complex spectrum of allograft renal lesions, progressive interstitial fibrosis and tubular atrophy (IF/TA) is a major factor associated with GFR decline and graft loss.20 Therefore, we tested the association between 3-month 25(OH)D and IF/TA progression. Among the 464 patients, 306 had usable screening biopsy samples obtained at 3 and 12 months. Among them, 37 samples were not analyzed because severe IF/TA was already present on biopsy at 3 months (interstitial fibrosis score + tubular atrophy score > 4), precluding further progression. The factors associated with IF/TA progression were studied in the remaining 265 patients. The characteristics of this subpopulation are presented in Supplemental Table 2. Between 3 months and 12 months, the composite IF/TA score progressed in 114 patients (43%). Factors associated with IF/TA progression are shown in Table 4. The multivariate analysis showed that donor age (P=0.01) and 3-month 25(OH)D concentration (P=0.01) were independently associated with IF/TA progression (Table 5). The increased risk of IF/TA progression in patients with low 25(OH)D was not associated with an increased incidence of biopsy-proven acute rejection in the same period (Supplemental Table 3).
Table 4.
Association of baseline variables with interstitial fibrosis/tubular atrophy score progression in univariate analysis
| Characteristic | Nonprogressors (n=151) | Progressors (n=114) | P Valuea |
|---|---|---|---|
| Male sex | 54.3 (82) | 58.8 (67) | 0.47 |
| Age at transplantation (yr) | 45.4±12.5 | 47.7±13.3 | 0.16 |
| Living donor | 27.3 (41) | 18.4 (21) | 0.09 |
| Donor age (yr) | 48.6±16.1 | 54.4±14.0 | 0.002 |
| Preemptive transplantation | 20.5(31) | 11.4(13) | 0.048 |
| Cold ischemia time | 0.19 | ||
| <250 min | 26.7 (39) | 18.0 (20) | |
| 250–1200 min | 35.6 (52) | 35.1 (39) | |
| >1200 min | 37.7 (55) | 46.8 (52) | |
| Delayed graft function | 20.5 (31) | 32.5 (37) | 0.03 |
| Acute cellular rejection before 3 months | 9.30 (14) | 11.4 (13) | 0.57 |
| Acute humoral rejection before 3 months | 7.90 (12) | 4.40 (5) | 0.24 |
| 3-month body mass index (kg/m2) | 23.7±3.92 | 23.6±3.84 | 0.83 |
| 3-month mGFR (ml/min per 1.73 m2) | 61.7±13.5 | 56.3±14.8 | 0.002 |
| 3-month 25(OH)D (ng/ml) | 16.8±10.1 | 13.2±7.00 | 0.001 |
| 3-month 1,25(OH)D (pg/ml) | 41.45±20.8 | 33.82±16.7 | 0.002 |
| 3-month PTH (pg/ml) | 84.0 (59.0–118.5) | 102.5 (64.0–180.0) | 0.01 |
| 3-month FGF-23 (RU/ml) | 62.0 (44.0–100.0) | 64.5 (44.8–102.8) | 0.62 |
Values are reported as percentage (number of patients), mean ± SD, or median (interquartile range), as appropriate.
P value represents tests of significance from t test, Wilcoxon test, or Pearson or Spearman correlation tests as appropriate.
Table 5.
Association of baseline variables with interstitial fibrosis/tubular atrophy score progression between 3 months and 12 months in multivariate analysis using multiple logistic regression
| Variable | Odds Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Donor agea | 1.26 (1.07–1.50) | 0.007 |
| 3-month 25(OH)Db | 0.96 (0.92–0.98) | 0.003 |
Only the variables independently associated with interstitial fibrosis/tubular atrophy score progression are presented (final model after backward selection procedure). The full model includes donor type, donor age, delayed graft function, preemptive transplantation, 3-month GFR, 3-month PTH, and 3-month 25(OH)D.
For an increase of 10 units of the variable.
For an increase of 1 unit of the variable.
Discussion
In this large prospective observational study of patients who had recently received a kidney transplant, low 25(OH)D levels 3 months after transplantation were independently associated with reduced mGFR 9 months later. Although the variation of mGFR was of low magnitude, this effect was robust to different statistical analyses and was consistent with an adverse evolution of chronic tubular and interstitial lesions in patients with low 3-month 25(OH)D concentrations. In contrast, other hormones implicated in mineral metabolism, including 1,25(OH)D, PTH, and FGF-23, were not independent predictors of 12-month mGFR or IF/TA progression. This study is, to our knowledge, the first linking vitamin D status with functional and histologic renal outcome in kidney transplant recipients. These results extend the findings of recent community-based studies linking 25(OH)D with the risk of ESRD and estimated GFR decline.18,19 Finally, by showing that mGFR decline and IF/TA progression correlated with 25(OH)D rather than 1,25(OH)D levels, this work raises the question of the therapeutic usefulness of vitamin D supplementation in preserving kidney allograft function.
An important point of this study is the use of a direct measurement of GFR rather than a creatinine-based GFR estimation. Creatinine-based equations to estimate GFR are an invaluable tool for clinician. However, creatinine concentration may be affected by factors other than GFR, such as variation in creatinine tubular secretion or creatinine production (i.e., increase in muscle mass). This latter point may be of crucial importance in assessing the effect of vitamin D on GFR. Concordant evidence suggests that vitamin D affects striated muscle cell biology.21 Indeed, invalidation of VDR in mice reduced muscle diameters,22 and consistent studies found an association between low 25(OH)D and reduced muscle strength in elderly people.21 In addition, two studies reported an association between low 25(OH)D levels and fatty degeneration of thigh and rotator cuff muscles.23,24 This potential effect of vitamin D on muscle biology is likely to affect creatinine production. Indeed, an increase in creatininemia due to enhanced creatinine production without variation in GFR has been reported under calcitriol therapy.25 Thus, the effect of vitamin D on creatinine production represents a potential important bias in studies using creatinine-based equations to estimate GFR or the albumin-to-creatinine ratio to evaluate variation in albuminuria. In this study, the direct measurement of GFR avoids this bias and strengthens the association between poor vitamin D status and adverse evolution of GFR.
This study also has limitations. First, because of the low incidence of death and allograft loss, this analysis is underpowered regarding renal survival. Also of note, the relatively short follow-up achieved so far precludes conclusions regarding the effects of 25(OH)D level on GFR variation after the first year of transplantation. Extended patient follow-up is mandatory to answer this important issue. Finally, plasma 25(OH)D concentrations vary depending on sun exposure, dietary load, and supplementation. Therefore, 3-month 25(OH)D may not adequately reflect the overall exposure to 25(OH)D during this 9-month period. However, our results indicating that 3-month 25(OH)D but not 12-month 25(OH)D correlated with kidney function at 12 months point at a determinant role of 25(OH)D exposure in the early posttransplantation period. To determine whether the repletion of 25(OH)D stocks at 12 months affects the later evolution of kidney function requires a longer follow-up. Keeping in mind those limitations, these results strongly suggest that vitamin D deficiency has a deleterious effect on the renal allograft in the early posttransplantation period.
We can speculate on the mechanisms underlining the effect of vitamin D on kidney function and IF/TA progression. In the context of renal transplantation, it is well established that the recipient immune response against the kidney allograft is an important contributor to renal deterioration. Vitamin D modulates both innate and adaptive immune response,26 and few observations have suggested that vitamin D could modulate allogeneic response. Calcitriol administration was shown to reduce class II major histocompatibility complex and co-stimulatory molecule expression in peripheral blood mononuclear cells from kidney transplant recipients.27 Additionally, alloreactive T cells are more frequently observed in vitamin D–deficient patients awaiting kidney transplantation.28 In contrast to those studies, our results indicate that low 25(OH)D concentration is not linked to an increased occurrence of acute rejection. Thus, the apparent effect of vitamin D deficiency on allograft function is unlikely to be the consequence of a stronger alloimmune response.
Aside from modulating immune response, VDR signaling reduces the progression of renal lesions in different nonimmune models of CKD. Active vitamin D analogues ameliorate renal function in a rat model of chronic allograft nephropathy,29 in subtotally nephrectomized rats,7,30–32 and in other models of CKD.8,10,33 Although VDR-independent actions of vitamin D analogues have been demonstrated, recent experiments applying unilateral ureteral obstruction on VDR−/− mice have revealed that VDR signaling played an instrumental role in preventing CKD progression.13,15 The molecular mechanisms responsible for the positive effect of vitamin D in CKD remain unclear because various consequences of VDR signaling have been reported, including preservation of podocyte function,34 reduction of glomerular inflammation, and reduction of tubular cell proliferation.7 VDR signaling also interferes with important pathways of CKD progression, including the renin-angiotensin system (RAS),35 epidermal growth factor receptor,36 and TGF-β signaling.29,32 Increased expression of renin and RAS activation appear to be major contributors to the increased sensitivity of VDR−/− mice to unilateral ureteral obstruction.13 However, the additive effects of VDR agonists on RAS blockage in both rodents37 and human CKD16,38 also argue for RAS-independent mechanisms. The exact contribution of the different cell populations targeted by vitamin D remains unclear so far, and further study using lineage-specific VDR invalidation is mandatory to clarify VDR function in CKD progression.
A striking finding in our study is the unique association between 25(OH)D as opposed to 1,25(OH)D and allograft function at 12 months and IF/TA progression from 3 months to 1 year after transplantation. 1,25(OH)D is less commonly measured than is 25(OH)D. Indeed, 1,25(OH)D status has not been assessed in most studies demonstrating a link between vitamin D deficiency and important clinical endpoints, including cardiovascular disease,39 mortality,40 certain types of cancer,41 and, of note, ESRD18 and GFR decline.19 Because 25(OH)D and 1,25(OH)D were systematically measured in this study, we could discriminate the relative association of these two measures with renal outcome. Our finding that low concentration of 25(OH)D but not of 1,25(OH)D predicted GFR decline and IF/TA progression in the first year after kidney transplantation raises important questions. Because of its 500-fold lower affinity for VDR, 25(OH)D is usually considered an inactive compound that reflects vitamin D stocks, whereas circulating 1,25(OH)D is thought to represent biologically active vitamin D.42 Thus, the predominant association of 25(OH)D with the evolution of kidney function retrieved in our study may appear paradoxical. However, a significant role for 25(OH)D concentration in regulating important cellular processes in the context of normal 1,25(OH)D concentrations is well established for PTH secretion.43 In parathyroid cells, VDR stimulation downregulates PTH gene transcription,44 and specific VDR ablation in parathyroid cells in mice results in an increase in PTH level.45 Strikingly, in most conditions in humans, and in this study, PTH levels correlate negatively with 25(OH)D but not with 1,25(OH)D concentration,46–49 suggesting that circulating 25(OH)D is the main determinant of VDR signaling in parathyroid cells. The molecular mechanisms potentially accounting for this effect of 25(OH)D concentration on VDR signaling include direct binding of 25(OH)D to VDR and its local conversion to 1,25(OH)D. Although of low affinity, the direct binding of 25(OH)D to VDR has been shown to result in VDR signaling. Indeed, the administration of high-dose cholecalciferol partially rescued the phenotype of 1α-hydroxylase–deficient mice and patients.50 However, supraphysiologic concentrations of 25(OH)D are needed to achieve efficient VDR signaling in this context. Thus, as the majority of the patients in our study had normal or even high calcitriol concentration, it is unlikely that direct binding of VDR by 25(OH)D significantly contributes to VDR signaling in this population. Local conversion of 25(OH)D to calcitriol is allowed by the local expression of the 1α-hydroxylase enzyme in various tissues including parathyroid;51–53 immune cells;54 and, in the kidney, tubular cells and possibly podocytes.55 This locally produced calcitriol plays an important role as an intracrine or paracrine activator of the VDR in monocytes,56 cancer cell lines,57 and parathyroid cells.43 Whether the same mechanism is involved in the effect of vitamin D on CKD progression has not been assessed but represents an interesting explanation of the predominant association of 25(OH)D concentration with 12-month mGFR.
The potential therapeutic effects of nutritional vitamin D supplementation represent an important corollary of our study. The potential interest of cholecalciferol administration was recently documented in patients with ESRD in whom a significant effect on hyperparathyroidism and left ventricular hypertrophy was recorded.58 In contrast, a recent randomized controlled trial showed no effect of the active vitamin D compound paricalcitol on cardiac structure or function in patients with CKD.59 Whether these discrepancies reflect a stronger effect of cholecalciferol supplementation on cardiac dysfunction in CKD remains to be tested. Of note, cardiomyocytes in rats express CYP27B1 and respond to cholecalciferol in vitro.60
Studies from our group and other have already shown that cholecalciferol supplementation is a safe way to increase 25(OH)D concentrations in kidney transplant recipients, but the small number of treated patients included in these studies and their short follow-up do not allow any conclusion on the effect of these treatments on kidney function.61–63 Our results should encourage randomized controlled trials evaluating the effect of early cholecalciferol supplementation on renal allograft function and provide valuable information on the magnitude of the expected effects of such treatments on renal function and histologic features.
Concise Methods
Study Population
All patients underwent transplantation and were followed in the Department of Renal Transplantation at Necker Hospital, Paris, France. Since September 2005, the standard longitudinal care of kidney recipients includes the measurement of GFR and mineral metabolism variables 3 and 12 months after transplantation. Between June 6, 2005, and June 7, 2010, all patients who were assessed 3 months (from 2 to 4 months) after transplantation were included in this study. Patients in whom the first GFR measurement was performed >4 months after transplantation (generally because of ongoing complications necessitating patient hospitalization) were excluded. The characteristics of the recipient, the donor, the transplantation procedure, and the outcome were prospectively collected and computerized in the Données Informatisées et Validées en Transplantation cross-validated French database (http://www.divat.fr/).64 The collected data included information on the recipient (age, sex, primary cause of CKD, previous time spent on dialysis), the donor (age, sex, deceased or living donor, cold ischemia time), the immunosuppressive regimen, and the occurrence of acute rejection or delayed graft function, defined as the need for hemodialysis during the first week after transplantation. In addition, height, body weight, and BP were systematically recorded at the time of GFR measurements.
Biochemistry
GFR was measured by iohexol clearance or inulin clearance when allergy to iohexol was suspected on the basis of patient history. Patients were infused with iohexol (Omnipaque 360) or inulin (Inutest 25%) as previously described.65 Blood and urine samples were collected every hour for 5 hours, and iohexol (or inulin), as sodium, potassium, calcium, phosphorus, creatinine, urea, glucose, and uric acid concentrations were measured. Iohexol and inulin concentrations were determined by HPLC and an enzymatic method, respectively, as previously described.61
25(OH)D and 1,25(OH)D concentrations were measured by radioimmunoassay (DiaSorin, Stillwater, MN) and radioimmunoassay after immunoextraction (IDS Ltd, Boldon, United Kingdom), respectively. Serum PTH concentration was measured with an immunochemiluminescent assay performed on the Elecsys analyzer (Roche Diagnostics, Meylan, France). Total calcium, ionized calcium, phosphate, and creatinine concentrations were measured using standard methods. FGF-23 plasma concentration was measured with an ELISA (Immutopics, Inc., San Clemente, CA) that recognizes the C-terminal part of the peptide.
Analysis of Renal Graft Biopsy Specimens
In our department, the standard care of kidney recipients includes renal graft screening biopsies that are routinely performed at months 3 and 12 after transplantation, with results scored according to the Banff classification.66 Briefly, cortical interstitial fibrosis and tubular atrophy areas were evaluated semiquantitatively, and the corresponding IF and TA scores were determined according to Banff classification (0, <5% of the cortical area; 1, 6%–25%; 2, 26%–50%; and 3, >50%). Progression of IF/TA between 3 and 12 months was defined as an increase in score of at least 1 point. Inadequate biopsy samples (with fewer than seven glomeruli and one artery) were excluded from analysis.
Statistical Analyses
Results are expressed as numbers and percentages for categorical variables and as mean ± SD or median (range) for continuous variables. Correlations between biologic measures were assessed using Pearson (r) or Spearman (ρ) correlation coefficients.
Survival curves were computed by the Kaplan-Meier method and compared by the log-rank test to select prognostic factors of overall survival. Overall survival was defined as time from transplantation to death or date of last follow-up. Cox regression models were used for multivariate analyses. Allograft loss was analyzed with a Gray model that considered death as a competing event.
Effect of biologic and clinical variables on 12-month mGFR was assessed using Pearson or Spearman correlation coefficients for quantitative variables and t test (or Wilcoxon test) for categorical variables. Independent predictive factors of 12-month mGFR were assessed using multivariate linear regression model.
Finally, effect of biologic and clinical variables on interstitial fibrosis and tubular atrophy progression was assessed using a chi-squared test for qualitative variables and a t test for quantitative variables. Independent predictive factors of progression were assessed using a multivariate logistic regression model.
For all multivariate regression models, P<0.10 was used for the variable entry criteria. Then, a backward selection procedure was applied. Only the factors associated with the considered outcome with P<0.05 were kept in the final model. A forward selection procedure was used to control the robustness of the final model.
Statistical analysis was performed using the R package, version 2.10.
Disclosures
None.
Acknowledgments
The authors thank Dr. Mordi Muorah and Dr. Khalil El Karoui for their advice; Juliane Posson for her help with the DIVAT database; and Dr. Marie Courbebaisse, Dr. Dominique Eladari, Dr. Ghania Daoud, Marie Louise Siber, and Véronique Renaud for their help in collecting patient data and determining mGFR. The renal transplant unit from Necker Hospital is part of the Centaure network.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012060614/-/DCSupplemental.
References
- 1.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP: Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int 62: 311–318, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Kasiske BL, Andany MA, Danielson B: A thirty percent chronic decline in inverse serum creatinine is an excellent predictor of late renal allograft failure. Am J Kidney Dis 39: 762–768, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW: Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A 101: 7711–7715, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanhooke JL, Prahl JM, Kimmel-Jehan C, Mendelsohn M, Danielson EW, Healy KD, DeLuca HF: CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3-1alpha-hydroxylase promoter activity in the skin. Proc Natl Acad Sci U S A 103: 75–80, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T: Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, Ritz E, Amann K: 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol Renal Physiol 286: F526–F533, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Migliori M, Giovannini L, Panichi V, Filippi C, Taccola D, Origlia N, Mannari C, Camussi G: Treatment with 1,25-dihydroxyvitamin D3 preserves glomerular slit diaphragm-associated protein expression in experimental glomerulonephritis. Int J Immunopathol Pharmacol 18: 779–790, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Xiao H, Shi W, Liu S, Wang W, Zhang B, Zhang Y, Xu L, Liang X, Liang Y: 1,25-Dihydroxyvitamin D(3) prevents puromycin aminonucleoside-induced apoptosis of glomerular podocytes by activating the phosphatidylinositol 3-kinase/Akt-signaling pathway. Am J Nephrol 30: 34–43, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Zou MS, Yu J, Zhou JH, Nie GM, Ding DS, Luo LM, Xu HT, He WS: 1,25-Dihydroxyvitamin D3 ameliorates podocytopenia in rats with adriamycin-induced nephropathy. Intern Med 49: 2677–2686, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Zou MS, Yu J, Nie GM, He WS, Luo LM, Xu HT: 1, 25-dihydroxyvitamin D3 decreases adriamycin-induced podocyte apoptosis and loss. Int J Med Sci 7: 290–299, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang XX, Jiang T, Shen Y, Santamaria H, Solis N, Arbeeny C, Levi M: Vitamin D receptor agonist doxercalciferol modulates dietary fat-induced renal disease and renal lipid metabolism. Am J Physiol Renal Physiol 300: F801–F810, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Kong J, Deb DK, Chang A, Li YC: Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol 21: 966–973, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He W, Kang YS, Dai C, Liu Y: Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22: 90–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García IM, Altamirano L, Mazzei LJ, Fornés MW, Molina MN, Ferder L, Manucha W: Role of mitochondria in paricalcitol-mediated cytoprotection during obstructive nephropathy. Am J Physiol Renal Physiol 302: F1595–F1605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu LJ, Lv JC, Shi SF, Chen YQ, Zhang H, Wang HY: Oral calcitriol for reduction of proteinuria in patients with IgA nephropathy: A randomized controlled trial. Am J Kidney Dis 59: 67–74, 2012 [DOI] [PubMed] [Google Scholar]
- 17.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D: Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): A randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Melamed ML, Astor B, Michos ED, Hostetter TH, Powe NR, Muntner P: 25-hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol 20: 2631–2639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Boer IH, Katz R, Chonchol M, Ix JH, Sarnak MJ, Shlipak MG, Siscovick DS, Kestenbaum B: Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol 6: 2141–2149, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serón D, Moreso F: Protocol biopsies in renal transplantation: Prognostic value of structural monitoring. Kidney Int 72: 690–697, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Dirks-Naylor AJ, Lennon-Edwards S: The effects of vitamin D on skeletal muscle function and cellular signaling. J Steroid Biochem Mol Biol 125: 159–168, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, Kato S, Matsumoto T: Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 144: 5138–5144, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Oh JH, Kim SH, Kim JH, Shin YH, Yoon JP, Oh CH: The level of vitamin D in the serum correlates with fatty degeneration of the muscles of the rotator cuff. J Bone Joint Surg Br 91: 1587–1593, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Tagliafico AS, Ameri P, Bovio M, Puntoni M, Capaccio E, Murialdo G, Martinoli C: Relationship between fatty degeneration of thigh muscles and vitamin D status in the elderly: A preliminary MRI study. AJR Am J Roentgenol 194: 728–734, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Agarwal R, Hynson JE, Hecht TJ, Light RP, Sinha AD: Short-term vitamin D receptor activation increases serum creatinine due to increased production with no effect on the glomerular filtration rate. Kidney Int 80: 1073–1079, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Hewison M: Vitamin D and innate and adaptive immunity. Vitam Horm 86: 23–62, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Ahmadpoor P, Ilkhanizadeh B, Ghasemmahdi L, Makhdoomi K, Ghafari A: Effect of active vitamin D on expression of co-stimulatory molecules and HLA-DR in renal transplant recipients. Exp Clin Transplant 7: 99–103, 2009 [PubMed] [Google Scholar]
- 28.Sawinski D, Uribarri J, Peace D, Yao T, Wauhop P, Trzcinka P, Ostrow K, Poggio ED, Heeger PS: 25-OH-vitamin D deficiency and cellular alloimmunity as measured by panel of reactive T cell testing in dialysis patients. Am J Transplant 10: 2287–2295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hullett DA, Laeseke PF, Malin G, Nessel R, Sollinger HW, Becker BN: Prevention of chronic allograft nephropathy with vitamin D. Transpl Int 18: 1175–1186, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Schwarz U, Amann K, Orth SR, Simonaviciene A, Wessels S, Ritz E: Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int 53: 1696–1705, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Hirata M, Makibayashi K, Katsumata K, Kusano K, Watanabe T, Fukushima N, Doi T: 22-Oxacalcitriol prevents progressive glomerulosclerosis without adversely affecting calcium and phosphorus metabolism in subtotally nephrectomized rats. Nephrol Dial Transplant 17: 2132–2137, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Mizobuchi M, Morrissey J, Finch JL, Martin DR, Liapis H, Akizawa T, Slatopolsky E: Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol 18: 1796–1806, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Panichi V, Migliori M, Taccola D, Filippi C, De Nisco L, Giovannini L, Palla R, Tetta C, Camussi G: Effects of 1,25(OH)2D3 in experimental mesangial proliferative nephritis in rats. Kidney Int 60: 87–95, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Li YC: Podocytes as target of vitamin D. Curr Diabetes Rev 7: 35–40, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP: 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dusso A, Arcidiacono MV, Yang J, Tokumoto M: Vitamin D inhibition of TACE and prevention of renal osteodystrophy and cardiovascular mortality. J Steroid Biochem Mol Biol 121: 193–198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finch JL, Suarez EB, Husain K, Ferder L, Cardema MC, Glenn DJ, Gardner DG, Liapis H, Slatopolsky E: Effect of combining an ACE inhibitor and a VDR activator on glomerulosclerosis, proteinuria, and renal oxidative stress in uremic rats. Am J Physiol Renal Physiol 302: F141–F149, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Fishbane S, Chittineni H, Packman M, Dutka P, Ali N, Durie N: Oral paricalcitol in the treatment of patients with CKD and proteinuria: A randomized trial. Am J Kidney Dis 54: 647–652, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC: Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 49: 1063–1069, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Melamed ML, Michos ED, Post W, Astor B: 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 168: 1629–1637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenab M, Bueno-de-Mesquita HB, Ferrari P, van Duijnhoven FJ, Norat T, Pischon T, Jansen EH, Slimani N, Byrnes G, Rinaldi S, Tjønneland A, Olsen A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Kaaks R, Linseisen J, Boeing H, Bergmann MM, Trichopoulou A, Misirli G, Trichopoulos D, Berrino F, Vineis P, Panico S, Palli D, Tumino R, Ros MM, van Gils CH, Peeters PH, Brustad M, Lund E, Tormo MJ, Ardanaz E, Rodríguez L, Sánchez MJ, Dorronsoro M, Gonzalez CA, Hallmans G, Palmqvist R, Roddam A, Key TJ, Khaw KT, Autier P, Hainaut P, Riboli E: Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: A nested case-control study. BMJ 340: b5500, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisman JA, DeLuca HF: Intestinal 1,25-dihydroxyvitamin D3 binding protein: specificity of binding. Steroids 30: 245–257, 1977 [DOI] [PubMed] [Google Scholar]
- 43.Bienaimé F, Prié D, Friedlander G, Souberbielle JC: Vitamin D metabolism and activity in the parathyroid gland. Mol Cell Endocrinol 347: 30–41, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Demay MB, Kiernan MS, DeLuca HF, Kronenberg HM: Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A 89: 8097–8101, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meir T, Levi R, Lieben L, Libutti S, Carmeliet G, Bouillon R, Silver J, Naveh-Many T: Deletion of the vitamin D receptor specifically in the parathyroid demonstrates a limited role for the receptor in parathyroid physiology. Am J Physiol Renal Physiol 297: F1192–F1198, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Vieth R, Ladak Y, Walfish PG: Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab 88: 185–191, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Guillemant J, Cabrol S, Allemandou A, Peres G, Guillemant S: Vitamin D-dependent seasonal variation of PTH in growing male adolescents. Bone 17: 513–516, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Christensen MH, Lien EA, Hustad S, Almås B: Seasonal and age-related differences in serum 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and parathyroid hormone in patients from Western Norway. Scand J Clin Lab Invest 70: 281–286, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Tomida K, Hamano T, Mikami S, Fujii N, Okada N, Matsui I, Nagasawa Y, Moriyama T, Ito T, Imai E, Isaka Y, Rakugi H: Serum 25-hydroxyvitamin D as an independent determinant of 1-84 PTH and bone mineral density in non-diabetic predialysis CKD patients. Bone 44: 678–683, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Zhang ZL, Ding XF, Tong J, Li BY: Partial rescue of the phenotype in 1α-hydroxylase gene knockout mice by vitamin D3 injection. Endocr Res 36: 101–108, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Ritter CS, Armbrecht HJ, Slatopolsky E, Brown AJ: 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int 70: 654–659, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Segersten U, Correa P, Hewison M, Hellman P, Dralle H, Carling T, Akerström G, Westin G: 25-hydroxyvitamin D(3)-1alpha-hydroxylase expression in normal and pathological parathyroid glands. J Clin Endocrinol Metab 87: 2967–2972, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Kawahara M, Iwasaki Y, Sakaguchi K, Taguchi T, Nishiyama M, Nigawara T, Tsugita M, Kambayashi M, Suda T, Hashimoto K: Predominant role of 25OHD in the negative regulation of PTH expression: clinical relevance for hypovitaminosis D. Life Sci 82: 677–683, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Cross HS: Extrarenal vitamin D hydroxylase expression and activity in normal and malignant cells: Modification of expression by epigenetic mechanisms and dietary substances. Nutr Rev 65: S108–S112, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Zhou J, Minto AW, Hack BK, Alexander JJ, Haas M, Li YC, Heilig CW, Quigg RJ: Altered vitamin D metabolism in type II diabetic mouse glomeruli may provide protection from diabetic nephropathy. Kidney Int 70: 882–891, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL: Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Schwartz GG, Eads D, Rao A, Cramer SD, Willingham MC, Chen TC, Jamieson DP, Wang L, Burnstein KL, Holick MF, Koumenis C: Pancreatic cancer cells express 25-hydroxyvitamin D-1 alpha-hydroxylase and their proliferation is inhibited by the prohormone 25-hydroxyvitamin D3. Carcinogenesis 25: 1015–1026, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, Gil C, Cortez J, Ferreira A: Cholecalciferol supplementation in hemodialysis patients: Effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol 5: 905–911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD: Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: The PRIMO randomized controlled trial. JAMA 307: 674–684, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Chen S, Glenn DJ, Ni W, Grigsby CL, Olsen K, Nishimoto M, Law CS, Gardner DG: Expression of the vitamin D receptor is increased in the hypertrophic heart. Hypertension 52: 1106–1112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Courbebaisse M, Thervet E, Souberbielle JC, Zuber J, Eladari D, Martinez F, Mamzer-Bruneel MF, Urena P, Legendre C, Friedlander G, Prié D: Effects of vitamin D supplementation on the calcium-phosphate balance in renal transplant patients. Kidney Int 75: 646–651, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Wissing KM, Broeders N, Moreno-Reyes R, Gervy C, Stallenberg B, Abramowicz D: A controlled study of vitamin D3 to prevent bone loss in renal-transplant patients receiving low doses of steroids. Transplantation 79: 108–115, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Kanter Berga J, Crespo Albiach J, Beltran Catalan S, Gavela Martinez E, Sancho Calabuig A, Avila Bernabeu A, Pallardo Mateu LM: Vitamin D deficiency in a renal transplant population: Safe repletion with moderate doses of calcidiol. Transplant Proc 42: 2917–2920, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Foucher Y, Daguin P, Akl A, Kessler M, Ladrière M, Legendre C, Kreis H, Rostaing L, Kamar N, Mourad G, Garrigue V, Bayle F, H de Ligny B, Büchler M, Meier C, Daurès JP, Soulillou JP, Giral M: A clinical scoring system highly predictive of long-term kidney graft survival. Kidney Int 78: 1288–1294, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Courbebaisse M, Diet C, Timsit MO, Mamzer MF, Thervet E, Noel LH, Legendre C, Friedlander G, Martinez F, Prié D: Effects of cinacalcet in renal transplant patients with hyperparathyroidism. Am J Nephrol 35: 341–348, 2012 [DOI] [PubMed] [Google Scholar]
- 66.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ: Banff ‘05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 7: 518–526, 2007 [DOI] [PubMed] [Google Scholar]