Abstract
There is little information on how the duration of overweight or obesity during life affects the risk for CKD. To investigate whether prolonged exposure to overweight during adult life increases the risk of later CKD in a cumulative manner, we analyzed data from the Medical Research Council National Survey of Health and Development, a socially stratified sample of 5362 singleton children born in 1 week in March 1946 in England, Scotland, and Wales. Multiple imputation expanded the analysis sample from the initial 1794 participants with complete data to 4584. This study collected self-reported body mass index (BMI) at ages 20 and 26 years and measured BMI at ages 36, 43, 53, and 60–64 years. The outcome of interest was CKD at age 60–64 years, suggested by estimated GFR (eGFR) <60 ml/min per 1.73 m2 and/or urine albumin-to-creatinine ratio (UACR) ≥3.5 mg/mmol. In analyses adjusted for childhood and adulthood social class, first becoming overweight at younger ages was associated with higher odds of developing CKD by age 60–64 years. Compared with those who first became overweight at age 60–64 years or never became overweight, those first overweight at age 26 or 36 years had approximately double the odds of developing CKD. The strength of this association decreased with increasing age when first overweight (P for trend <0.001). These associations were consistent for creatinine-based eGFR, cystatin C–based eGFR, and UACR. Taken together, these results suggest that preventing overweight in early adulthood may have a considerable effect on the prevalence of CKD in the population.
Over recent decades, the prevalence of obesity has increased dramatically in many parts of the world.1 The increasing recognition of kidney disease as a cardiovascular risk factor,2 together with globally increasing cardiovascular mortality,3 has led to a growing interest in the relationship between obesity and kidney disease, although the number of published analyses remains limited. A recent systematic review and meta-analysis4 concluded that adult obesity increases the risk for kidney disease—defined by the authors as CKD, ESRD, kidney stones, kidney cancer, or renal cell cancer—in the general adult population. Obesity was found to increase the risk of kidney disease by 40% (95% confidence interval [CI], 30%–50%) for adult overweight (25 ≤ body mass index (BMI) < 30 kg/m2) and 83% (95% CI, 57%–113%) for adult obesity (BMI ≥ 30 kg/m2) relative to normal weight (18.5 < BMI < 25 kg/m2). However, information on the prospective association between adult BMI and CKD is very limited; among the reviewed studies, only three cohort studies investigated the role of prior BMI in CKD incidence. Previous cohort studies of obesity and kidney disease have restricted their analysis to a single observation of obesity, with the exception of Hsu et al.,5 who conducted a subanalysis on people with more than one BMI measurement. There is thus little information on how life course exposure to obesity affects kidney disease risk.
The Medical Research Council National Survey of Health and Development (NSHD) provides a unique opportunity to explore how obesity throughout adult life affects kidney function in a prospective cohort representative of the general United Kingdom population. In addition to measurements of urine albumin and creatinine and serum creatinine, this study also measured serum cystatin C, which has been suggested as a superior marker of kidney function.6
The aim of this paper is to investigate whether the data from this study support the hypothesis that prolonged exposure to overweight in adulthood, defined in terms of BMI, increases the risk of later CKD in a cumulative manner. Furthermore, we aim to investigate the extent to which any association between adulthood overweight and CKD is mediated by diabetes and hypertension and whether data support a similar association with central adiposity.
Results
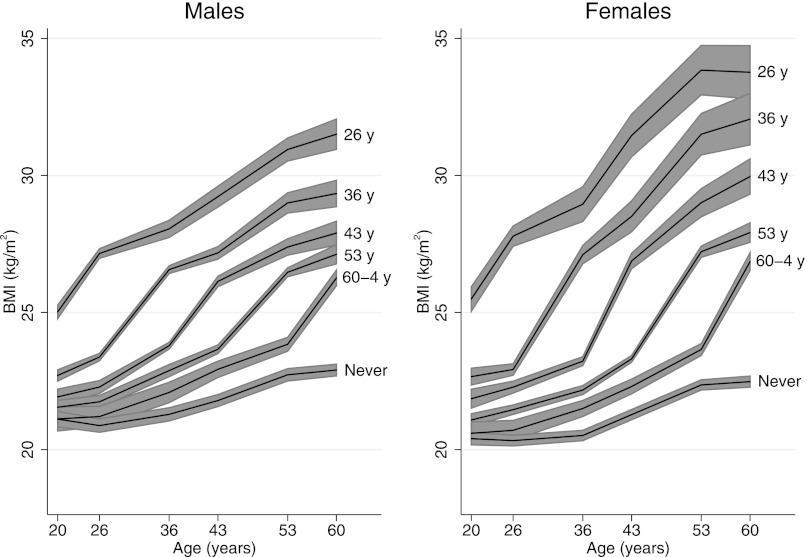
BMI data were available at one or more time points between age 20 and 60–64 years for 4463 study participants (83.2% of the initial NSHD sample). BMI and the prevalence of overweight increased during adulthood so that by age 60–64 years, 74.6% of men and 67.3% of women were overweight (Supplemental Figure 1). The mean BMI at age 60–64 years was higher among men and women who became overweight earlier (Figure 1). An individual’s BMI at age 60–64 years thus reflected the length of time spent overweight. Age at first overweight was also strongly associated with waist-to-hip ratio at age 60–64 years (P<0.001 for trend). The mean sex-adjusted waist-to-hip ratio was 7.6% (95% CI, 6.7 to 8.5) greater in study members who became overweight at age 26 years than in those who had not become overweight by age 60–64 years. Approximately 40% of men and women were from a non–manual-labor social class during childhood, and approximately 80% of men and women were in such a class in adulthood. BMI at ages 26, 36, 43, 53, and 60–64 years was inversely associated with both childhood and adulthood social class (all P<0.001). Sex-adjusted BMI at age 60–64 years was 1.3 kg/m2 (95% CI, 0.8 to 1.9 kg/m2) greater in study members of manual-labor social class in adulthood relative to those of non–manual-labor class. More than 6% of study members were in the increased or high midlife systolic BP latent trajectory. By age 60–64 years, 7% of study members reported being diabetic, 5% were receiving diabetes medication, and 30% were taking hypertension medication. Mean hemoglobin A1c ± SD was 5.8%±0.7%, and mean systolic BP was 134±18 mmHg.
Figure 1.
Mean body mass index (BMI) was higher throughout adulthood in men and women who became overweight at earlier ages. Shaded areas are 95% confidence intervals. Modified from reference 32, with permission.
Serum creatinine data were available for 1855 study participants: 20 (2.2%) men and 22 (2.3%) women had creatinine-based eGFR < 60 ml/min per 1.73 m2 (P=0.83 for sex difference). Serum cystatin C data were available for 2052 study participants, with 12 (1.2%) men and 35 (3.3%) women having cystatin C–based eGFR < 60 ml/min per 1.73 m2 (P=0.001). Urinary albumin-to-creatinine ratio (UACR) could be calculated for 2173 study participants: 40 (3.8%) men and 22 (1.9%) women had UACR ≥ 3.5 mg/mmol (P=0.01). The composite CKD measure was available for 1827 study participants, of whom 57 (6.3%) men and 51 (5.5%) women had CKD (P=0.44).
The subset of study participants with complete data are likely to be a more health-conscious subgroup who have adhered to follow-up for >60 years and thus are potentially unrepresentative of the cohort as a whole. An analysis of only complete cases may therefore provide biased results. We used multiple imputation7,8 to explore the robustness of the complete case results to this potential bias. The prevalence of reduced kidney function in the imputed data sets was generally increased slightly relative to that in the original data. For example, the prevalence of CKD by the composite measure was increased to 7.9% in men and 8.4% in women.
Main Analyses
Because few study participants with reduced kidney function had never become overweight, we combined the “first overweight at age 60–64 years” and “never overweight” categories for the purpose of the analysis.
For all four measures of kidney function, becoming overweight earlier was associated with increased odds of reduced kidney function in the multiple imputation (MI) analysis (P for trends < 0.001 to 0.05) (Tables 1–3). These trends remained after adjustment for confounding by childhood and adulthood socioeconomic position (P for trends < 0.001 to 0.06). Becoming overweight at age 26 or 36 years was, relative to becoming overweight at age 60–64 years or never becoming overweight, associated with an approximately 2.1-fold increase in the odds of reduced kidney function when creatinine-based eGFR was used (Table 1), a 2.8-fold increase when cystatin C–based eGFR was used (Table 1), and a 2.1-fold increase when the composite CKD measure was used (Table 3). Evidence of an association between becoming overweight at age 26 or 36 years and UACR was somewhat weaker (Table 2). These associations showed only minor attenuation after adjustment for childhood and adulthood socioeconomic position.
Table 1.
ORs for eGFR < 60 ml/min per 1.73 m2 by age when first overweight in the MI analysis (n=4584)
| Age when First Overweight (yr) | Percentagea among Total First Overweight at This Age | Percentagea of Those First Overweight at This Age with eGFR < 60 ml/min per 1.73 m2 | Model 1 | Model 2 | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value for Trend | OR (95% CI) | P Value for Trend | |||
| Creatinine-based eGFR | ||||||
| 26 | 21.0 | 4.7 | 2.08 (1.10–3.94) | 0.008 | 1.99 (1.04–3.80) | 0.01 |
| 36 | 18.8 | 5.0 | 2.16 (1.12–4.17) | 2.10 (1.09–4.07) | ||
| 43 | 14.3 | 3.6 | 1.51 (0.71–3.21) | 1.51 (0.71–3.19) | ||
| 53 | 16.9 | 3.0 | 1.27 (0.59–2.73) | 1.27 (0.59–2.73) | ||
| 60–64 or never | 29.0 | 2.4 | 1.00 | 1.00 | ||
| Cystatin-based eGFR | ||||||
| 26 | 21.0 | 3.7 | 2.78 (1.30–5.93) | 0.002 | 2.48 (1.16–5.29) | 0.005 |
| 36 | 18.8 | 3.6 | 2.74 (1.30–5.78) | 2.54 (1.21–5.34) | ||
| 43 | 14.3 | 3.0 | 1.86 (0.81–4.26) | 1.81 (0.79–4.16) | ||
| 53 | 16.9 | 2.2 | 1.28 (0.53–3.07) | 1.27 (0.53–3.02) | ||
| 60–64 or never | 29.0 | 1.8 | 1.00 | 1.00 | ||
Model 1: Adjusted for age at examination and sex. Model 2: Additionally adjusted for childhood and adulthood socioeconomic position.
Average across all 50 imputed data sets.
Table 3.
ORs for composite CKD measure by age when first overweight in the MI analysis (n=4584)
| Age When First Overweight (yr) | Percentagea of Total First Overweight at This Age | Percentagea of Those First Overweight at This Age with Composite CKD Measure | Model 1 | Model 2 | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value for Trend | OR (95% CI) | P Value for Trend | |||
| 26 | 21.0 | 10.6 | 2.05 (1.37–3.07) | <0.001 | 1.94 (1.28–2.93) | <0.001 |
| 36 | 18.8 | 10.9 | 2.09 (1.32–3.28) | 2.01 (1.27–3.17) | ||
| 43 | 14.3 | 7.6 | 1.35 (0.80–2.27) | 1.33 (0.79–2.24) | ||
| 53 | 16.9 | 6.6 | 1.15 (0.71–1.87) | 1.14 (0.71–1.86) | ||
| 60–64 or never | 29.0 | 5.8 | 1.00 | 1.00 | ||
Model 1: Adjusted for age at examination and sex. Model 2: Additionally adjusted for childhood and adulthood socioeconomic position.
Average across all 50 imputed data sets.
Table 2.
ORs for UACR ≥ 3.5 mg/mmol by age when first overweight in the MI analysis (n=4584)
| Age When First Overweight (yr) | Percentagea of Total First Overweight at This Age | Percentagea of Those First Overweight at This Age with UACR ≥ 3.5 mg/mmol | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value for Trend | OR (95% CI) | P Value for Trend | ||||
| 26 | 21.0 | 3.8 | 1.75 (0.93–3.28) | 0.05 | 1.72 (0.91–3.27) | 0.06 | |
| 36 | 18.8 | 3.7 | 1.71 (0.85–3.42) | 1.68 (0.83–3.36) | |||
| 43 | 14.3 | 2.3 | 1.05 (0.44–2.50) | 1.04 (0.44–2.46) | |||
| 53 | 16.9 | 2.5 | 1.18 (0.57–2.45) | 1.17 (0.56–2.42) | |||
| 60–64or never | 29.0 | 2.1 | 1.00 | 1.00 | |||
Model 1: Adjusted for age at examination and sex. Model 2: Additionally adjusted for childhood and adulthood socioeconomic position.
Average across all 50 imputed data sets.
Supplementary Analyses
In the corresponding complete case analyses, the evidence for an association between earlier overweight and reduced kidney function was similarly strong (P for trends after adjustment for childhood and adulthood socioeconomic position < 0.001 to 0.05) (Supplemental Tables 1–3). However, some of the individual odds ratios (ORs) associated with becoming overweight in early adulthood were greater in magnitude.
When we excluded individuals who did not remain overweight after they first became overweight, the evidence for associations between early overweight and reduced kidney function was strengthened slightly in the complete case analysis (it is not possible to perform this sensitivity analysis using MI). After adjustment for confounding by childhood and adulthood socioeconomic position, the P values for trends varied between <0.001 and 0.03 (results not shown).
The associations between overweight at each age and the four indicators of reduced kidney function in the MI analysis are shown in Supplemental Tables 4–6. Relative to not being overweight at that age, being overweight at all ages from 36 years onward was associated with at least a 1.6-fold increase in the odds of reduced kidney function, assessed using creatinine-based eGFR. When we considered cystatin C–based eGFR, overweight at all ages from 26 years onward was associated with reduced kidney function, peaking at an OR of 2.35 at age 36 years. Only overweight at age 36 years was found to be associated with reduced kidney function using UACR (OR, 1.67), with current overweight particularly weakly associated (OR, 1.07). When we considered the composite measure, overweight at all from age 26 years onward was associated with CKD, peaking at an OR of 1.92 at age 36 years. These associations were only slightly attenuated upon adjustment for childhood and adulthood socioeconomic position.
In the corresponding complete case analyses, the strongest associations were seen at the same ages but the strength of the associations was generally greater (Supplemental Tables 7–9).
The association between age at first overweight and kidney function at age 60–64 years was mediated to a similar extent by both diabetes and hypertension. In the MI analysis, the minimally adjusted ORs of 2.05 and 2.09 at ages 26 and 36 years for the composite CKD measure (P for overall trend < 0.001) were attenuated to 1.69 and 1.78 (P=0.003) after adjustment for diabetes alone, to 1.66 and 1.76 (P=0.003) after adjustment for hypertension alone, and to 1.46 and 1.59 (P=0.03) after adjustment for both diabetes and hypertension (results not shown). There was no evidence of statistical interaction between either diabetes or hypertension and age at first overweight.
Waist-to-hip ratio at all three ages (43, 53, and 60–64 years) was found to be associated with kidney function at age 60–64 years, although the cross-sectional association was markedly weaker. A 0.05-unit increase in waist-to-hip ratio corresponded to ORs of 1.24 (95% CI, 1.10 to 1.40), 1.21 (95% CI, 1.07 to 1.35), and 1.12 (95% CI, 1.01 to 1.25) at ages 43, 53, and 60–64 years, respectively, when we considered the composite CKD measure in the MI analysis (results not shown). Adding waist-to-hip ratio at age 43 or 53 years to the models for age at first overweight attenuated the associations to a moderate degree. In the MI analysis, the minimally adjusted ORs of 2.05 and 2.09 at ages 26 and 36 years for the composite CKD measure (P for overall trend < 0.001) were attenuated to 1.70 and 1.78 (P=0.004) after adjustment for waist-to-hip ratio at age 43 years and to 1.73 and 1.80 (P=0.003) after adjustment for waist-to-hip ratio at age 53 (results not shown).
The population attributable fraction for the composite CKD measure was calculated as 29.0% for the study sample and 35.9% for the current United States population. It should be noted that this comparison involves observed levels of overweight versus becoming overweight at age 60–64 years or never becoming overweight because these categories were combined in the analysis.
Finally, an additional sensitivity analysis looked at the association between age at first overweight and reduced kidney function using the original age at first overweight categories (i.e., not combining “first overweight at age 60–64 years” and “never overweight”). The effect of early overweight was somewhat stronger relative to “never overweight” than relative to “first overweight at age 60–64 years or never overweight,” but the precision was markedly reduced (results not shown). The overall tests for trend were very similar to those presented in the main analysis above.
Discussion
In a large, population-based, prospective study, we found overweight beginning early in adulthood (by ages 26 or 36 years) to be strongly associated with reduced renal function at age 60–64 years. To our knowledge we are the first to report how exposure to overweight across adulthood may affect kidney disease risk.
Waist-to-hip ratio in adulthood, particularly at ages 43 and 53 years, was found to be associated with kidney function at age 60–64 years. This reinforces the observed associations with overweight, implying they are not just due to a peculiarity of BMI (e.g., “overweight” in early adulthood really signifying high lean body mass that tracks through adulthood, leading to raised creatinine levels). In addition, we found diabetes and hypertension to both be moderate mediators of the association between age at overweight and kidney function.
We estimated that 36% of CKD cases at age 60–64 years in the current United States population could be avoided if nobody became overweight until at least age 60–64 years, assuming the same associations as in the analysis sample. This emphasizes the importance of obesity as a risk factor for CKD.
Because so few individuals returned to normal weight after becoming overweight, it was not possible to separate the effects of the timing of becoming overweight and duration of overweight. Alternative cohorts containing more individuals who return to normal weight would be required to disentangle these potential life course models for the effect of overweight.
Overweight and obesity have been shown in previous observational studies to be associated with various types of kidney disease. The associations observed in the present study are stronger than those in a similar meta-analysis of three cohort studies with CKD as an outcome4 and are closer to the estimates of the association between obesity and renal disease of all types, particularly ESRD.4 Fox et al.9 studied “new-onset kidney disease” (defined as GFR ≤64 ml/min per 1.73 m2 in men or ≤59 ml/min per 1.73 m2 in women) in 2585 men and women followed up for a mean of 18.5 years in the Framingham Offspring Study. Baseline BMI was found to increase the odds of kidney disease by 23% per SD unit (OR, 1.23; 95% CI, 1.08 to 1.41). Gelber et al.10 evaluated the association between BMI and CKD (defined as estimated GFR < 60 ml/min per 1.73 m2) in a cohort of 11,104 initially healthy men during an average follow-up of 14 years. They found higher baseline BMI to be consistently associated with CKD, with those in the highest BMI quintile having an OR of 1.45 (95% CI, 1.19 to 1.76) relative to those in the lowest quintile. However, in 9082 United States adults from the Second National Health and Nutrition Examination Survey(NHANES II), Stengel et al.11 found an increased risk of CKD (defined as receipt of treatment for ESRD or death related to CKD) only for those with baseline BMI ≥35 kg/m2 (morbid obesity). Later research has suggested that BMI is a suboptimal measure of risk and that other measures of central obesity, for example waist-to-hip ratio, should be used.12 Our study is in line with these earlier studies but adds important nuances: namely the importance of quantifying when the person became overweight or the duration of overweight.
The magnitude and size of the associations we found suggest that preventing overweight before age 40 years could reduce the risk of later risk of CKD considerably. Once CKD is present, no other known treatment target prevents progression of CKD to the same degree. Our findings suggest that interventions to reduce overweight at any age in adulthood may reduce the burden of later CKD, but to maximize the effect of any interventions they should focus on reducing the prevalence of overweight in early adulthood. Indeed, because of the acknowledged tracking of BMI from childhood into adulthood,13 it may be necessary to target interventions earlier still.
The mechanisms relating BMI to CKD are not well understood. Our findings may be due to a series of mechanisms, all initiated by high BMI, including (1) the influence of BMI on BP;14 (2) initial hyperfiltration, development of albuminuria, and subsequent decline in eGFR, as suggested by animal studies;15,16 (3) development of diabetes;17 and (4) influences on the immune system contributing to inflammation that are not, as yet, well understood.18 The data do not allow the sequence of events to be disentangled (i.e., whether albuminuria or inflammation preceded development of low eGFR). However, the size and direction of the associations, in particular the peaking of the associations at younger ages, suggest a “lag” between becoming overweight and subsequently developing CKD. In line with animal models, a series of cross-sectional studies have found increased eGFR and increased albuminuria to be positively associated with concurrent BMI.19–21
A limitation of this study is the low power due to the low prevalence of reduced kidney function in the cohort, although prevalence was increased slightly by the use of an MI approach. However, the patterns of associations within each measure of kidney function and the similarity in findings across the three different measures of kidney function (and a composite measure) suggest robustness to our findings. Results from the sensitivity analysis in which individuals who did not remain overweight once they had first become overweight were excluded strengthen the evidence for the association between early overweight and reduced kidney function at age 60–64 years.
In addition, the lack of study participants with reduced kidney function who had never become overweight meant that we had to combine the “first overweight at age 60–64 years” and “never overweight” categories for the purpose of the analysis. Using only “never overweight” study members as the baseline group increased the magnitude of all the associations at the expense of precision. Our results thus probably underestimated the real effects of interest.
We did not further divide overweight into overweight (25 ≤ BMI < 30 kg/m2) and obesity (BMI ≥ 30 kg/m2) because the prevalence of obesity was low in early adulthood in this cohort (3% at age 26 and 5% at age 36 years); this, coupled with the low prevalence of CKD, would have given us very low statistical power to detect any associations. In other settings, the overweight profile would probably be different from that of this cohort, so a focus on obesity may well be more appropriate.
The lack of repeated measures of kidney function meant that we were unable to ascertain CKD using the recommended definition of reduced kidney function for at least 3 months.22,23 This is likely to introduce nondifferential misclassification, again meaning that the true association is even stronger than described here.
Confounding by childhood and adulthood socioeconomic position did not explain the observed associations, although residual confounding is a possibility. Residual mediation by hypertension may also be possible. Several previous studies have reported a stronger association between obesity and kidney disease in women than in men.4,24 Although no evidence of sex differences was found in this study, our lack of statistical power to detect these interactions means we cannot rule out such differences.
A strength of this study is that it has repeated measures of BMI at regular intervals throughout adulthood, with heights and weights measured using standardized protocols at the majority of these ages, and sufficiently detailed information about socioeconomic position. Additionally, the availability of three different measures of kidney function allows a more thorough analysis. The cohort members remaining in the NSHD have been found to be broadly representative of native-born adults living in England, Scotland, and Wales at the time of data collection at age 5325 and at age 60–64 years.26 Hence, we are confident that our analysis sample retains the representatives of the study population as a whole. Use of MI allowed us to investigate the role of selection bias on the results, with the MI analyses giving results similar to those of the complete case analyses overall. The NSHD study population is, however, all white, and therefore our findings cannot be extrapolated to the nonwhite British population.
In conclusion, we found being overweight in early adulthood to be strongly associated with reduced kidney function in later life. Although we were unable to disentangle precisely whether it was the timing of overweight onset or the duration of overweight that was driving this association, either explanation suggests that preventing overweight in early adulthood may have a considerable effect on the prevalence of CKD in the population. Indeed, doing so appears to have a larger effect than any treatment for CKD known to date. The recent epidemic of obesity, resulting in overweight beginning at earlier ages (from early adult life and more recently from adolescence and childhood), is leading to a substantial increase in overweight/obesity of long duration, which could have important consequences for renal disease, as well as for cardiovascular disease.27
Concise Methods
Participants
The Medical Research Council NSHD is a socially stratified sample of 5362 singleton children born in 1 week in March 1946 in England, Scotland, and Wales. Medical and social data have been collected 23 times by home visits, medical examinations, and postal questionnaires.28
Measures
Adult heights and weights were measured at ages 36, 43, 53, and 60–64 years and were self-reported at ages 20 and 26 years. BMI, defined as weight (kg)/height (m2), was calculated at each age. A binary variable indicating overweight was calculated at each age, using the standard cut point of 25 kg/m2.
The main exposure of interest was the age at which a study participant first became overweight (26, 36, 43, 53, or 60–64 years or never). This was derived assuming that once an individual became overweight he or she remained overweight. Overweight at each separate age (20, 26, 36, 43, 53, and 60–64 years) was considered as a secondary analysis.
Four different indicators of reduced renal function at age 60–64 years were used: (1) creatinine-based eGFR < 60 ml/min per 1.73 m2; (2) cystatin C–based eGFR < 60 ml/min per 1.73 m2 (both calculated using the Chronic Kidney Disease–Epidemiology Collaboration formulae29,30); (3) UACR ≥ 3.5 mg/mmol; and (4) a composite CKD measure indicating whether any one or more of the previous three indicators were present.
Childhood socioeconomic position (manual labor/non–manual-labor) and adulthood socioeconomic position (manual labor/non–manual labor) were considered as potential confounders.
Diabetes (self-reported physician-diagnosed diabetes by age 60–64 years, receiving diabetes medication at age 60–64 years, hemoglobin A1c level at age 60–64 years) and hypertension (previously derived systolic BP latent trajectory between ages 36 and 53 years,31 receiving BP medication at age 60–64 years, measured systolic BP at age 60–64 years) were considered as mediating factors.
Some previous studies have indicated that central obesity is a stronger predictor of CKD than is BMI.4 A final analysis thus examined whether adulthood waist-to-hip ratio was associated with kidney function at age 60–64 years in the same way as adulthood overweight. Waist-to-hip ratios at ages 43, 53, and 60–64 years were derived using repeated measures of waist and hip circumferences.
Statistical Analyses
Main Analyses
Each kidney function outcome was related to age at first overweight using logistic regression. Models were minimally adjusted for sex and age (model 1), then additionally adjusted for childhood and adulthood SEP (model 2). A similar approach has previously been used to assess the relationship between patterns of overweight during adulthood and BP at age 53 years in the NSHD.32
To investigate the potential bias caused by missing data, we used an MI approach.7,8 See Supplemental Material for full details.
Supplementary Analyses
The analysis relating each kidney function outcome to age at first overweight outlined above was repeated using complete cases only as a comparison with the MI results. A sensitivity analysis was conducted in which any individuals who did not remain overweight once they had first become overweight were excluded.
We investigated the associations between each kidney function outcome and overweight status at each age with logistic regression, using both an MI approach and a complete case analysis for comparison.
The extent to which the association between age at first overweight and kidney function at age 60–64 years was mediated by diabetes and hypertension was examined by adding each of these to the models for age at first overweight and examining the extent to which the effect estimates were attenuated.
Associations between waist-to-hip ratio at each age and kidney function at age 60–64 years were examined in the same way as overweight at each age. Waist-to-hip ratio at ages 43 and 53 years were then added in turn to the models for age at first overweight to examine whether the association remained once waist-to-hip ratio was taken into account.
Finally, population attributable fractions for the composite CKD measure were calculated using the MI model adjusted for age, sex, and childhood and adulthood socioeconomic position.33 This represents the proportion of CKD cases occurring in the total population that would have been avoided if nobody had become overweight. Population attributable fractions were calculated using the study sample and for the current United States population. For the latter, overweight prevalences at the appropriate ages (26, 36, 43, 53, and 60–64 years) were derived by interpolating the NHANES 2009–2010 estimates of Flegal et al.,34 with the proportion of people becoming overweight in each interval calculated as the difference between the two consecutive prevalences (e.g., the proportion of people becoming overweight between ages 26 and 36 years was the difference between the overweight prevalence at age 36 and the overweight prevalence at age 26 years).
In none of the models was there any evidence of interactions with sex, so combined male and female models are presented throughout.
The analysis was performed using Stata software, version 12.35
Disclosures
None.
Acknowledgments
The authors are grateful to NSHD study members who took part in this latest data collection for their continuing support. We thank members of the NSHD scientific and data collection teams at the following centers: MRC Unit for Lifelong Health and Ageing; MRC Human Nutrition Research, Cambridge; Welcome Trust (WT) Clinical Research Facility (CRF) Manchester; WTCRF, Western General Hospital, Edinburgh; WTCRF, University Hospital Birmingham; WTCRF, University College London Hospital; CRF, University Hospital of Wales; CRF, St. Thomas’ Hospital London; and National Centre for Social Research. We also thank Dr. Ian Halsall, who provided expert advice on blood analyte levels.
This work was supported by Kidney Research United Kingdom (grant number RP34/2009) to R.J.S. and D.N. and the United Kingdom Medical Research Council to M.P., R.H., and D.K. Data collection was funded by the United Kingdom Medical Research Council (Unit Programme number U123092720), with additional analyses funded by the United Kingdom Medical Research Council (grant number G1001143).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.20120700675/-/DCSupplemental.
References
- 1.World Health Organisation : Obesity: Preventing and managing the global epidemic, Geneva, WHO, 2000 [PubMed] [Google Scholar]
- 2.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organisation : The Global Burden of Disease: 2004 Update, Geneva, WHO, 2008 [Google Scholar]
- 4.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ: Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int 73: 19–33, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS: Body mass index and risk for end-stage renal disease. Ann Intern Med 144: 21–28, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Dharnidharka VR, Kwon C, Stevens G: Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis 40: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR: Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 338: b2393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenward MG, Carpenter J: Multiple imputation: current perspectives. Stat Methods Med Res 16: 199–218, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, Gaziano JM: Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis 46: 871–880, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL: Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology 14: 479–487, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Elsayed EF, Sarnak MJ, Tighiouart H, Griffith JL, Kurth T, Salem DN, Levey AS, Weiner DE: Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis 52: 29–38, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy R, Wadsworth M, Kuh D: The influence of childhood weight and socioeconomic status on change in adult body mass index in a British national birth cohort. Int J Obes Relat Metab Disord 24: 725–734, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Silventoinen K, Magnusson PK, Neovius M, Sundström J, Batty GD, Tynelius P, Rasmussen F: Does obesity modify the effect of blood pressure on the risk of cardiovascular disease? A population-based cohort study of more than one million Swedish men. Circulation 118: 1637–1642, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE: Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol 12: 1211–1217, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Wolf G, Chen S, Han DC, Ziyadeh FN: Leptin and renal disease. Am J Kidney Dis 39: 1–11, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Dastani Z, Hivert MF, Timpson N, Perry JR, Yuan X, Scott RA, Henneman P, Heid IM, Kizer JR, Lyytikäinen LP, Fuchsberger C, Tanaka T, Morris AP, Small K, Isaacs A, Beekman M, Coassin S, Lohman K, Qi L, Kanoni S, Pankow JS, Uh HW, Wu Y, Bidulescu A, Rasmussen-Torvik LJ, Greenwood CM, Ladouceur M, Grimsby J, Manning AK, Liu CT, Kooner J, Mooser VE, Vollenweider P, Kapur KA, Chambers J, Wareham NJ, Langenberg C, Frants R, Willems-Vandijk K, Oostra BA, Willems SM, Lamina C, Winkler TW, Psaty BM, Tracy RP, Brody J, Chen I, Viikari J, Kähönen M, Pramstaller PP, Evans DM, St Pourcain B, Sattar N, Wood AR, Bandinelli S, Carlson OD, Egan JM, Böhringer S, van Heemst D, Kedenko L, Kristiansson K, Nuotio ML, Loo BM, Harris T, Garcia M, Kanaya A, Haun M, Klopp N, Wichmann HE, Deloukas P, Katsareli E, Couper DJ, Duncan BB, Kloppenburg M, Adair LS, Borja JB, Wilson JG, Musani S, Guo X, Johnson T, Semple R, Teslovich TM, Allison MA, Redline S, Buxbaum SG, Mohlke KL, Meulenbelt I, Ballantyne CM, Dedoussis GV, Hu FB, Liu Y, Paulweber B, Spector TD, Slagboom PE, Ferrucci L, Jula A, Perola M, Raitakari O, Florez JC, Salomaa V, Eriksson JG, Frayling TM, Hicks AA, Lehtimäki T, Smith GD, Siscovick DS, Kronenberg F, van Duijn C, Loos RJ, Waterworth DM, Meigs JB, Dupuis J, Richards JB, Voight BF, Scott LJ, Steinthorsdottir V, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, McCulloch LJ, Ferreira T, Grallert H, Amin N, Wu G, Willer CJ, Raychaudhuri S, McCarroll SA, Hofmann OM, Segrè AV, van Hoek M, Navarro P, Ardlie K, Balkau B, Benediktsson R, Bennett AJ, Blagieva R, Boerwinkle E, Bonnycastle LL, Boström KB, Bravenboer B, Bumpstead S, Burtt NP, Charpentier G, Chines PS, Cornelis M, Crawford G, Doney AS, Elliott KS, Elliott AL, Erdos MR, Fox CS, Franklin CS, Ganser M, Gieger C, Grarup N, Green T, Griffin S, Groves CJ, Guiducci C, Hadjadj S, Hassanali N, Herder C, Isomaa B, Jackson AU, Johnson PR, Jørgensen T, Kao WH, Kong A, Kraft P, Kuusisto J, Lauritzen T, Li M, Lieverse A, Lindgren CM, Lyssenko V, Marre M, Meitinger T, Midthjell K, Morken MA, Narisu N, Nilsson P, Owen KR, Payne F, Petersen AK, Platou C, Proença C, Prokopenko I, Rathmann W, Rayner NW, Robertson NR, Rocheleau G, Roden M, Sampson MJ, Saxena R, Shields BM, Shrader P, Sigurdsson G, Sparsø T, Strassburger K, Stringham HM, Sun Q, Swift AJ, Thorand B, Tichet J, Tuomi T, van Dam RM, van Haeften TW, van Herpt T, van Vliet-Ostaptchouk JV, Walters GB, Weedon MN, Wijmenga C, Witteman J, Bergman RN, Cauchi S, Collins FS, Gloyn AL, Gyllensten U, Hansen T, Hide WA, Hitman GA, Hofman A, Hunter DJ, Hveem K, Laakso M, Morris AD, Palmer CN, Rudan I, Sijbrands E, Stein LD, Tuomilehto J, Uitterlinden A, Walker M, Watanabe RM, Abecasis GR, Boehm BO, Campbell H, Daly MJ, Hattersley AT, Pedersen O, Barroso I, Groop L, Sladek R, Thorsteinsdottir U, Wilson JF, Illig T, Froguel P, van Duijn CM, Stefansson K, Altshuler D, Boehnke M, McCarthy MI, Soranzo N, Wheeler E, Glazer NL, Bouatia-Naji N, Mägi R, Randall J, Elliott P, Rybin D, Dehghan A, Hottenga JJ, Song K, Goel A, Lajunen T, Doney A, Cavalcanti-Proença C, Kumari M, Timpson NJ, Zabena C, Ingelsson E, An P, O’Connell J, Luan J, Elliott A, McCarroll SA, Roccasecca RM, Pattou F, Sethupathy P, Ariyurek Y, Barter P, Beilby JP, Ben-Shlomo Y, Bergmann S, Bochud M, Bonnefond A, Borch-Johnsen K, Böttcher Y, Brunner E, Bumpstead SJ, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Crisponi L, Day IN, de Geus EJ, Delplanque J, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Grundy S, Gwilliam R, Hallmans G, Hammond N, Han X, Hartikainen AL, Hayward C, Heath SC, Hercberg S, Hillman DR, Hingorani AD, Hui J, Hung J, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Mahley R, Mangino M, Martínez-Larrad MT, McAteer JB, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Mukherjee S, Naitza S, Neville MJ, Orrù M, Pakyz R, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Seedorf U, Sharp SJ, Shields B, Sigurðsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvänen AC, Tönjes A, Uitterlinden AG, van Dijk KW, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Ward KL, Watkins H, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Borecki IB, Meneton P, Magnusson PK, Nathan DM, Williams GH, Silander K, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Serrano-Ríos M, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pramstaller PP, Wright AF, Stumvoll M, Hamsten A, Buchanan TA, Valle TT, Rotter JI, Penninx BW, Boomsma DI, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Peltonen L, Mooser V, Sladek R, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Chasman DI, Johansen CT, Fouchier SW, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Feitosa MF, Orho-Melander M, Melander O, Li X, Li M, Cho YS, Go MJ, Kim YJ, Lee JY, Park T, Kim K, Sim X, Ong RT, Croteau-Chonka DC, Lange LA, Smith JD, Ziegler A, Zhang W, Zee RY, Whitfield JB, Thompson JR, Surakka I, Spector TD, Smit JH, Sinisalo J, Scott J, Saharinen J, Sabatti C, Rose LM, Roberts R, Rieder M, Parker AN, Pare G, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, McArdle W, Masson D, Martin NG, Marroni F, Lucas G, Luben R, Lokki ML, Lettre G, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, König IR, Khaw KT, Kaplan LM, Johansson Å, Janssens AC, Igl W, Hovingh GK, Hengstenberg C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Groop LC, Gonzalez E, Freimer NB, Erdmann J, Ejebe KG, Döring A, Dominiczak AF, Demissie S, Deloukas P, de Faire U, Crawford G, Chen YD, Caulfield MJ, Boekholdt SM, Assimes TL, Quertermous T, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Taylor HA, Jr, Gabriel SB, Holm H, Gudnason V, Krauss RM, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Strachan DP, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, Kathiresan S, DIAGRAM+ Consortium. MAGIC Consortium. GLGC Investigators. MuTHER Consortium. DIAGRAM Consortium. GIANT Consortium. Global B Pgen Consortium. Procardis Consortium. MAGIC investigators. GLGC Consortium : Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: A multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet 8: e1002607, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotamisligil GS: Inflammation and metabolic disorders. Nature 444: 860–867, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Nitsch D, Felber Dietrich D, von Eckardstein A, Gaspoz JM, Downs SH, Leuenberger P, Tschopp JM, Brändli O, Keller R, Gerbase MW, Probst-Hensch NM, Stutz EZ, Ackermann-Liebrich U, SAPALDIA team : Prevalence of renal impairment and its association with cardiovascular risk factors in a general population: results of the Swiss SAPALDIA study. Nephrol Dial Transplant 21: 935–944, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Verhave JC, Hillege HL, Burgerhof JG, Gansevoort RT, de Zeeuw D, de Jong PE, PREVEND Study Group : The association between atherosclerotic risk factors and renal function in the general population. Kidney Int 67: 1967–1973, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Verhave JC, Gansevoort RT, Hillege HL, Bakker SJ, De Zeeuw D, de Jong PE, PREVEND Study Group : An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int Suppl 92: S18–S21, 2004 [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 23.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S: Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int 65: 1870–1876, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Wadsworth ME, Butterworth SL, Hardy RJ, Kuh DJ, Richards M, Langenberg C, Hilder WS, Connor M: The life course prospective design: An example of benefits and problems associated with study longevity. Soc Sci Med 57: 2193–2205, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Stafford M, Black S, Shah I, Hardy R, Pierce M, Richards M, Wong A: Kuh D on behalf of the NSHD scientific & data collection teams: Using a birth cohort to study ageing: Representativeness and response rates in the National Survey of Health and Development [epub ahead of print January 22, 2013]. Eur J Ageing doi: 10.1007/s10433-013-0258-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirosh A, Shai I, Afek A, Dubnov-Raz G, Ayalon N, Gordon B, Derazne E, Tzur D, Shamis A, Vinker S, Rudich A: Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med 364: 1315–1325, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuh D, Pierce M, Adams J, Deanfield J, Ekelund U, Friberg P, Ghosh AK, Harwood N, Hughes A, Macfarlane PW, Mishra G, Pellerin D, Wong A, Stephen AM, Richards M, Hardy R, NSHD scientific and data collection team : Cohort profile: updating the cohort profile for the MRC National Survey of Health and Development: A new clinic-based data collection for ageing research. Int J Epidemiol 40: e1–e9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wills AK, Lawlor DA, Muniz-Terrera G, Matthews F, Cooper R, Ghosh AK, Kuh D, Hardy R, FALCon Study Team : Population heterogeneity in trajectories of midlife blood pressure. Epidemiology 23: 203–211, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wills AK, Hardy RJ, Black S, Kuh DJ: Trajectories of overweight and body mass index in adulthood and blood pressure at age 53: The 1946 British birth cohort study. J Hypertens 28: 679–686, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Rockhill B, Newman B, Weinberg C: Use and misuse of population attributable fractions. Am J Public Health 88: 15–19, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flegal KM, Carroll MD, Kit BK, Ogden CL: Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307: 491–497, 2012 [DOI] [PubMed] [Google Scholar]
- 35.StataCorp : Stata statistical software: release 12, College Station, Texas, StataCorp LP, 2011 [Google Scholar]