Abstract
The molecular mechanisms that maintain podocytes and consequently, the integrity of the glomerular filtration barrier are incompletely understood. Here, we show that the class III phosphoinositide 3-kinase vacuolar protein sorting 34 (Vps34) plays a central role in modulating endocytic pathways, maintaining podocyte homeostasis. In mice, podocyte-specific conditional knockout of Vps34 led to early proteinuria, glomerular scarring, and death within 3–9 weeks of age. Vps34-deficient podocytes exhibited substantial vacuolization and foot process effacement. Although the formation of autophagosomes and autophagic flux were impaired, comparisons between podocyte-specific Vps34-deficient mice, autophagy-deficient mice, and doubly deficient mice suggested that defective autophagy was not primarily responsible for the severe phenotype caused by the loss of Vps34. In fact, Rab5-positive endosomal compartments, endocytosis, and fluid-phase uptake were severely disrupted in Vps34-deficient podocytes. Vps34 deficiency in nephrocytes, the podocyte-like cells of Drosophila melanogaster, resulted in a block between Rab5- and Rab7-positive endosomal compartments. In summary, these data identify Vps34 as a major regulator of endolysosomal pathways in podocytes and underline the fundamental roles of endocytosis and fluid-phase uptake for the maintenance of the glomerular filtration barrier.
Podocytes are specialized cells of the renal glomerulus that maintain a complex cytoarchitecture to form the glomerular filtration barrier. The concept that podocytes internalize and remove proteins was proposed over 50 years ago.1–3 Multiple electron microscopy studies have shown vesicles in podocytes under normal conditions and further pronounced under pathologic conditions.2–7 However, the physiologic relevance of podocyte uptake mechanisms remained elusive so far. Intriguingly, recent studies indicate a possible role of endocytosis for podocyte homeostasis. A fragment of the (Pro)Renin Receptor (PRR) also known as ATPase H(+)-transporting lysosomal accessory protein 2 (ATP6AP2) is functionally associated with the vacuolar (V-type) adenosine triphosphatase (v-ATPase). Conditional knockout of ATP6AP2 in podocytes leads to impaired lysosomal processing and defective autophagy accompanied by endoplasmic reticulum (ER) stress but also indicates disturbed processing of multivesicular bodies.8 Uptake or endocytosis of extracellular material in membrane-bound vesicles is crucial for a wide range of cellular functions, including antigen presentation, nutrient acquisition, clearance of apoptotic cells, pathogen entry, receptor regulation, hypertension, and synaptic transmission.9,10 Although some studies pinpointed to the requirement of endocytic processes for podocytes,11,12 the signaling crosstalk between endocytosis and signal transduction in this specialized cell population is not yet fully elucidated.
Phosphatidylinositol phosphates (PIPs) and Rab family guanosine triphosphatases coordinate cellular functions like growth, proliferation, migration, differentiation, survival, cell adhesion, or degranulation. They are key regulators of vesicle identity, formation, and trafficking.13,14 Phosphoinositide kinases (PIKs) produce second messenger molecules through phosphorylation of inositolphospholipids at the 3′-OH position of the inositol ring.15 Three classes of PIK are known, with vacuolar protein sorting 34 (Vps34; also called Pik3c3) representing the only known class III PI 3-kinase.16 Vps34 catalyzes phosphate transfer from ATP to lipid (PtdIns) and protein substrates (phosphorylation of PtdIns to PtdIns3P). It is evolutionary conserved and was described first in yeast.16 In mammalian cells, Vps34 is part of a large multiprotein complex consisting of either Beclin1 and UV radiation resistance-associated gene (complex I) or early endosome antigen 1 (EEA1; complex II).17 As part of these distinct multiprotein complexes, Vps34 specifically produces PtdIns(3)P to initiate autophagosome formation and regulate endocytic processes.18 PtdIns3P binding proteins contain a FYVE domain, a specific zinc finger domain (named after the four cysteine-rich proteins: Fab 1 [yeast orthologue of PIKfyve], YOTB [uncharacterized protein YobT], Vac 1 [vesicle transport protein], and EEA1 [Early Endosome Antigen 1]) that allows them to elaborate their function in cellular protein trafficking at specific localizations in cells.19 Concisely, Vps34 controls several vesicular trafficking processes and is required as an early regulator of vesicle docking/fusion at the endosome through the recruitment/activation of components in the PI 3-kinase signaling cascade.20–22 It plays a crucial role for endosome tethering and membrane fusion through EAA1 and other Rab5 effectors, vesicle invagination and cargo selection within multivesicular bodies, and fusion of autophagosomes with lysosomes.23–26
Inhibition of Vps34 has been shown to result in dysfunctional autophagy, vesicular trafficking, and endocytic sorting.18,27–29 Constitutive knockout mice die at embryonic day 7.5–8.30 Conditional knockout of Vps34 in mammals, however, displays organ-specific phenotypes. Conditional knockout in neurons leads to a severe endocytic defect,30 whereas in liver and heart, conditional knockout of Vps34 results in dysfunctional autophagy.31 In humans, Vps34 (encoded by PIK3C3) has been shown to play a role in metabolic disorders, neurodegenerative diseases, and cancer.32 The differences between various species and cell lines in regard to Vps34 deficiency indicate a cell-specific importance of autophagic versus endosmal processes. Here, we show that Vps34 is crucial for the regulation of endocytosis-maintaining podocyte homoeostasis.
Results
Conditional Vps34 Depletion in Mouse Podocytes Induces Massive Proteinuria and Early Lethality
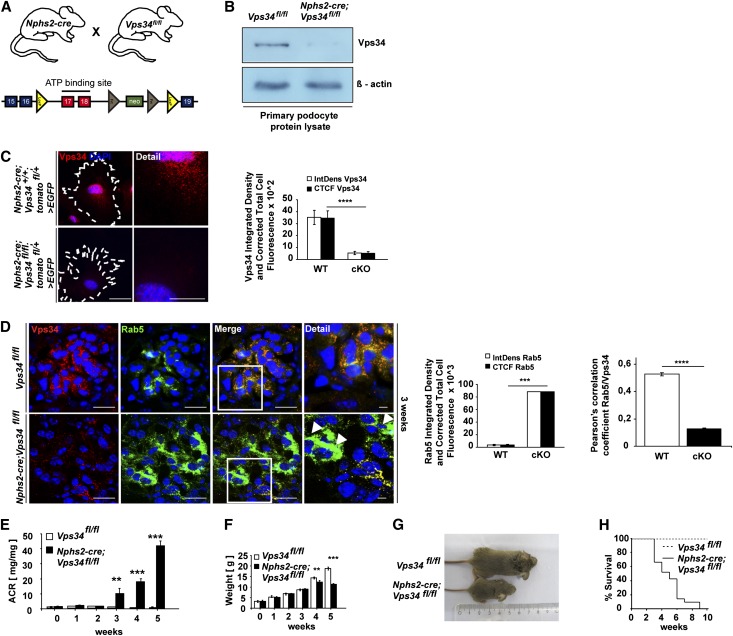
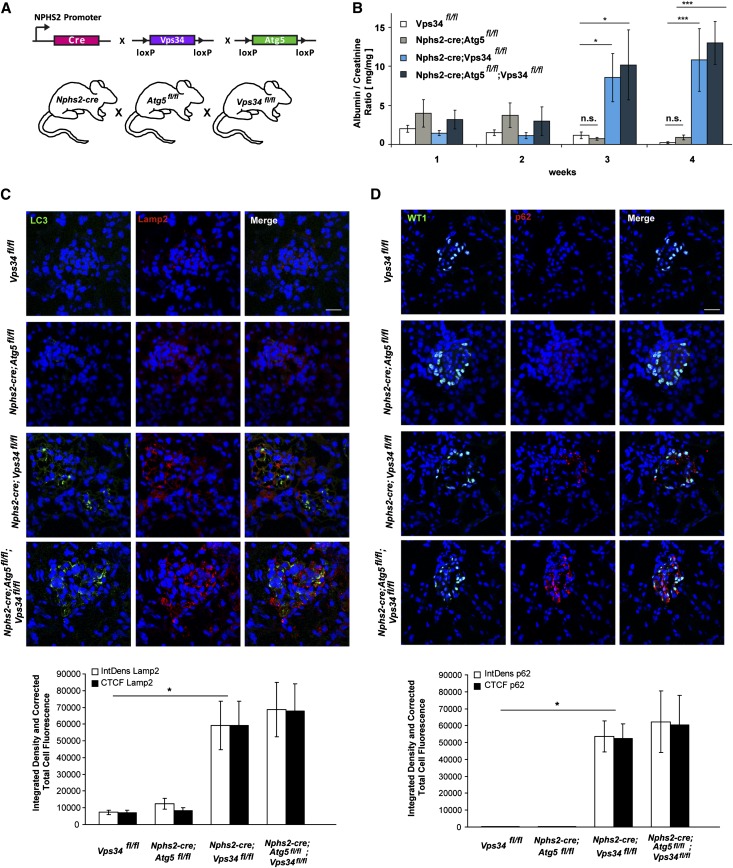
To study the podocyte-specific function of Vps34 in vivo, Vps34fl/fl mice were bred with Nphs2-cre;Vps34fl/+ mice to generate podocyte-specific Nphs2-cre;Vps34fl/fl mice and Vps34fl/fl littermate controls (Figure 1A).30,33 Efficient depletion of Vps34 was confirmed by Western blot analysis of lysates of freshly isolated podocytes (Figure 1B) and immunofluorescence staining of Nphs2-cre;Vps34fl/fl;tomatofl/+>EGFP primary podocytes and wild-type controls (Figure 1C). Colabeling immunofluorescence with the early endosomal marker Rab5 and Vps34 on kidney sections of conditionally Vps34-deficient mice and wild-type controls indicated efficient deletion of Vps34 and a massive accumulation of Rab5 in Vps34-deficient podocytes at 3 weeks of age (Figure 1D, white arrowheads). Tubular Rab5 expression and colocalization with Vps34 are shown in Supplemental Figure 1. Colocalization of Rab5 and Vps34 were quantified using ImageJ software. although Nphs2-cre;Vps34fl/fl mice seemed normal at birth, they developed early-onset proteinuria at 3 weeks of age (Figure 1E) and significant growth retardation at week 4 (Figure 1, F and G). Nphs2-cre;Vps34fl/fl mice all died within 3–9 weeks after birth (Figure 1H).
Figure 1.
Conditional Vps34 depletion in mouse podocytes induces massive proteinuria and early lethality. (A) Mice expressing cre recombinase under control of the podocyte-specific Nphs2 promoter were crossed to Vps34fl/fl mice to generate podocyte-specific Vps34 knockout mice. (B) Western blot analysis of freshly isolated podocyte protein lysates confirmed significant reduction of Vps34 in podocytes of Nphs2-cre;Vps34fl/fl conditional knockout mice. β-actin was used as loading control. (C) Quantification of immunofluorescence microscopy confirms efficient knockout of Vps34 in primary isolated podocytes. Scale bars, 20 µm; 5 µm in detail. (D) Colabeling immunofluorescence staining with Rab5 and Vps34 on kidney sections of conditionally Vps34-deficient mice and wild-type controls. Increased Rab5 expression in Vps34-deficient podocytes at 3 weeks of age (white arrowheads). ****P<0.0001. (E) Nphs2-cre;Vps34fl/fl mice develop early proteinuria. Albumin/creatinine ratios are significantly increased from 3 weeks of age in Vps34fl/fl;Nphs2-cre mice (n=10 per group; ***P<0.001, **P<0.01; two-tailed t test, mean values ± SEM are shown). (F) Weight curve of Vps34fl/fl control and Nphs2-cre;Vps34fl/fl conditional knockout mice; significant differences in weight can be observed by week 4 (n=10 per group; ***P<0.001, **P<0.01, two-tailed t test, mean values ± SEM are shown). (G) Nphs2-cre;Vps34fl/fl mice exhibit significant growth impairment at 5 weeks of age. (H) Kaplan–Meyer survival curve of Vps34fl/fl control and Nphs2-cre;Vps34fl/fl mice (n=35 per group). All Nphs2-cre;Vps34fl/fl mice died within 9–10 weeks after birth; the first death was observed at 3 weeks of age.
Podocyte-Specific Vps34 Deficiency Causes Rapid Podocyte Degeneration and Early-Onset Glomerulosclerosis
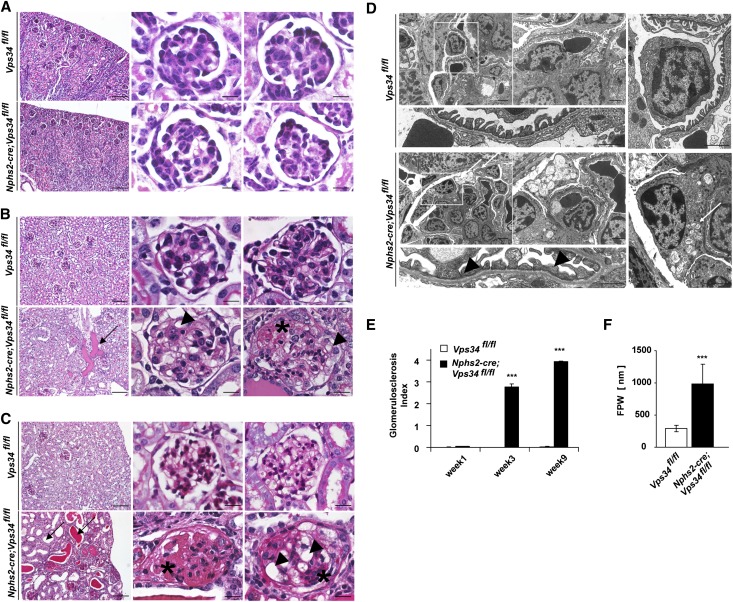
On postnatal day 7, glomeruli of Nphs2-cre;Vps34fl/fl mice were structurally indifferent from glomeruli of littermate controls (Figure 2A and Supplemental Figure 2A). They showed equal distribution of the slit diaphragm proteins nephrin and podocin (Supplemental Figure 3, A and B) and equal numbers of podocytes per visual field (Supplemental Figure 3C). Intriguingly, by 3 weeks of age, Nphs2-cre;Vps34fl/fl mice accumulated tubular protein casts and displayed the first signs of focal glomerulosclerosis (Figure 2, B and D and Supplemental Figure 2B). Semiquantitative histologic analyses showed significantly increased glomerulosclerosis indices by 3 weeks of age (Figure 2E). Electron microscopy confirmed early foot process effacement (Figure 2D and Supplemental Figure 2B) with significantly increased foot process widths (Figure 2F). Vps34-deficient podocytes showed numerous single-membrane vacuoles (Figure 2D and Supplemental Figures 2B and 4); 9 weeks after birth, kidney sections from Nphs2-cre;Vps34fl/fl mice showed tubular dilation, flattened tubular epithelium, tubular protein casts, glomerular vacuolization, and glomerular scarring (Figure 2C and Supplemental Figure 2C). Ultrastructural analysis identified generalized foot process effacement (Supplemental Figure 2C). Podocytes appeared degenerated, with their cell bodies entirely filled with vacuoles (Figure 2D and Supplemental Figure 2, B and C), suggesting a possible defect in cellular vesicular trafficking.
Figure 2.
Podocyte-specific Vps34 deficiency causes rapid podocyte degeneration and early-onset glomerulosclerosis. (A–C) PAS staining of kidney sections of 1-, 3-, and 9-week-old Nphs2-cre;Vps34fl/fl mice and littermate controls. (A) By 1 week after birth, kidney sections do not display significant differences in glomerular architecture. (B) Kidney sections of 3-week-old Nphs2-cre;Vps34fl/fl mice show first signs of tubular protein accumulation (arrow), vacuolization (arrowheads), and segmental sclerosis (asterisk). (C) At 9 weeks of age, kidney sections of Nphs2-cre;Vps34fl/fl mice show undulated renal surface, widespread tubular protein casts with tubular dilatation (arrows), and severe glomerular sclerosis (asterisks). Scale bars, 200 µm in left panels; 10 µm in center and right panels. (D) By 3 weeks of age, electron microscopy of Nphs2-cre;Vps34fl/fl mice showed extensive vacuolization of podocytes (white arrow) and focal foot process fusions (black arrowheads). Scale bars, 5 µm in upper left panels; 2 µm, upper center panel; 1 µm, upper right panel; 500 nm, lower panels. (E) Glomerulosclerosis index of glomerular sections of 1-, 3-, and 9-week-old Nphs2-cre;Vps34fl/fl mice and wild-type controls. (F) Foot process widths of Nphs2-cre;Vps34fl/fl mice and wild-type controls (***P<0.001, two-tailed t test, mean values ± SEM are shown).
Impairment of Autophagy in Nphs2-cre;Vps34fl/fl Mice
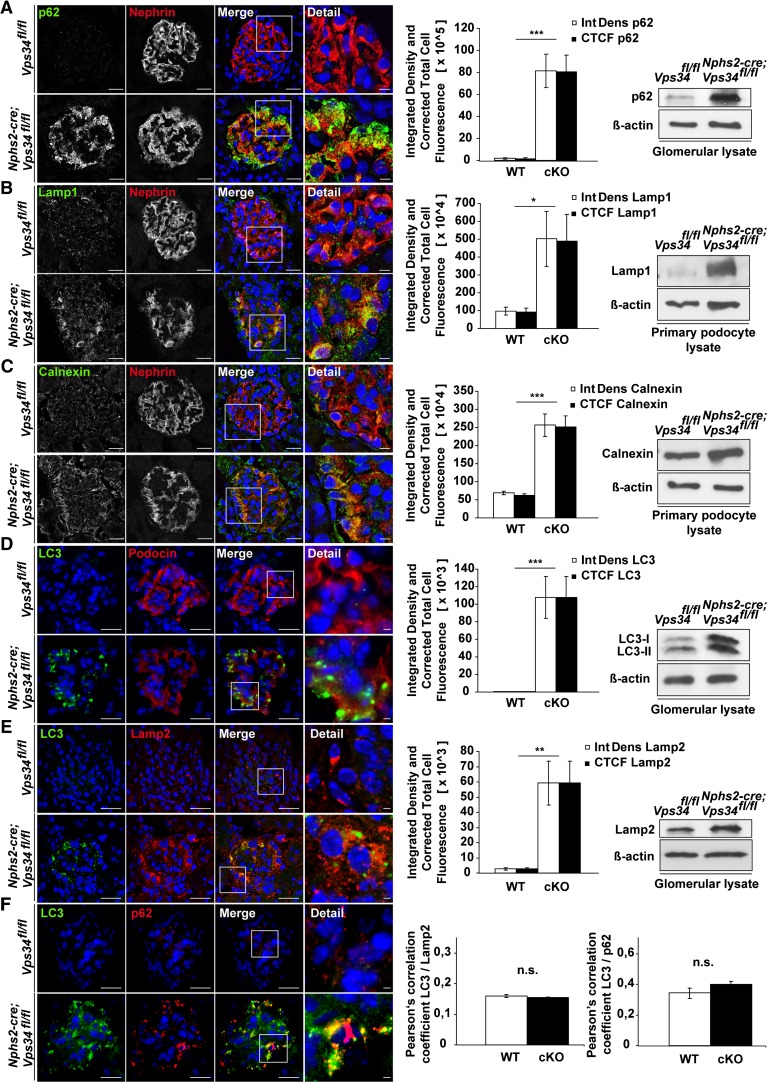
One of the reported functions of Vps34 in intracellular vesicle transport is the induction of autophagy on nutrient deprivation.17 Immunofluorescence staining and Western blot analyses showed increased levels of Lamp1 and -2 in Nphs2-cre;Vps34fl/fl mice, indicating insufficient lysosomal degradation (Figure 3, B and E). During induction of autophagy, microtubule-associated protein 1 light chain 3A (LC3)-I is conjugated to phosphatidylethanolamine to form LC3-II, which is subsequently recruited to form autophagosomes. Immunofluorescence staining and Western blot analysis identified increased LC3-I and -II levels in Nphs2-cre;Vps34fl/fl mice (Figure 3, D–F), indicating that LC3 conjugation can still occur in the absence of Vps34. In agreement with insufficient autophagosome formation, we observed substantial increase of polyubiquitin binding protein p62/SQSTM1 (referred to as p62) in podocytes of Nphs2-cre;Vps34fl/fl mice (Figure 3, A and F). p62 identifies toxic cellular waste that is usually consumed through autophagy.34 Accumulation of p62 induces a cellular stress response,35 confirmed by an accumulation of the ER marker Calnexin (Figure 3C). Confocal imaging analysis showed that LC3 did not colocalize with Lamp2 in Vps34-deficient podocytes (Figure 3E). This finding indicates a defect in autolysosomal formation. To further define the localization of LC3 in the podocytes, we performed immunogold–electron microscopy analyses (Supplemental Figure 4). Anti-LC3 gold particles were present on autophagosomal membranes in tubular cells in Nphs2-cre;Vps34fl/fl mice and littermate controls (Supplemental Figure 4 A, i and B, i). However, Vps34-deficient podocytes exhibited a diffuse cytosolic distribution of LC3 lacking any association with membrane structures, which is indicative of a defect in early autophagosome formation (Supplemental Figure 4B). Consistently, the large-sized cargo-containing vacuoles of Vps34-deficient podocytes were not labeled with anti-LC3 gold particles and thus, do not represent autophagosomes.
Figure 3.
Autophagy is impaired in Nphs2-cre;Vps34fl/fl mice. Immunofluorescence staining of kidney sections of 3-week-old Nphs2-cre;Vps34fl/fl and littermate control mice. (A–E) Quantification of confocal immunofluorescence microscopy and Western blot analyses of glomerular or primary podocyte protein lysates displayed significant accumulation of the autophagy marker p62, the lysosomal markers Lamp1 and -2, the ER stress marker Calnexin, and the autophagy marker LC3 in podocytes of Nphs2-cre;Vps34fl/fl mice. (E) Confocal microscopy showed no significant colocalization of LC3 and the lysosomal marker Lamp2 in Vps34-deficient podocytes. (F) Colocalization of LC3 and p62 in Vps34-deficient podocytes in confocal microscopy. *P<0.05, **P<0.01, ***P<0.001. Scale bars, 20 µm; 5 µm in detail.
Abrogated Autophagic Flux Is Not Causative of the Severe Podocyte Phenotype of Vps34-Deficient Podocytes
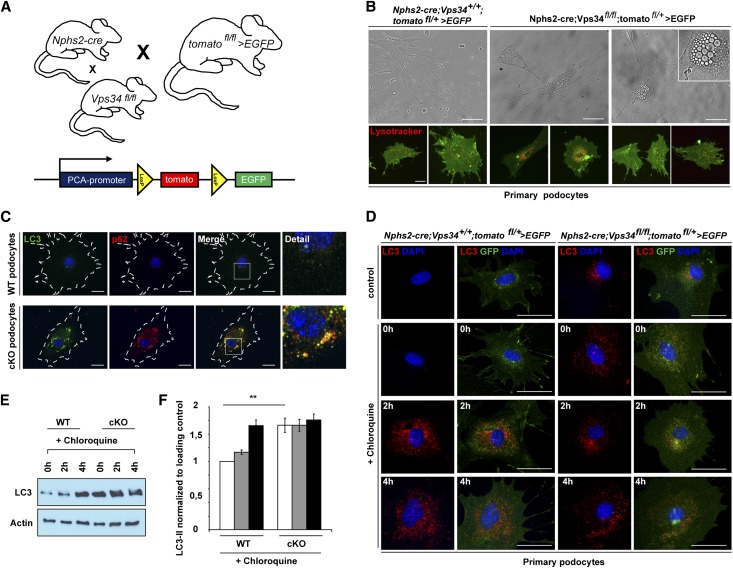
To study the dynamic cellular mechanisms of Vps34, we generated primary podocyte cell cultures (Figure 4, A and B and Supplemental Figure 6). Vps34fl/fl mice were bred to Nphs2-Cre;tomatofl/+>EGFP mice to generate podocyte-specific GFP-positive Vps34-deficient mice (Figure 4A and Supplemental Figure 5A).36 Nphs2-cre;Vps34fl/fl;tomatofl/+>EGFP primary podocytes displayed substantial perinuclear vacuolization (Figure 4B) and a significant reduction in the total cell count after 9 days (Supplemental Figure 6A) compared with GFP-positive control podocytes. To further differentiate and quantify diminished proliferation versus increased cell death in Vps34-deficient podocytes, we isolated primary podocytes of conditionally Vps34-deficient mice and littermate controls and stained cells with Ki-67 as well as cleaved caspase 3 (Supplemental Figure 6, B–E). Isolated primary podocytes from young mice proliferated for one to two cycles and thus, behaved differently from a podocyte in vivo. In vitro, primary wild-type podocytes displayed enhanced Ki-67 staining, whereas Vps34-deficient podocytes did not show signs of proliferation. Active caspase 3 staining, however, suggested increased apoptosis in Vps34-deficient cultured podocytes. To investigate diminished proliferation and increased apoptosis in vivo, we stained sections from 1-, 3-, and 9-week-old mice for Ki-67 and cleaved caspase 3 (Supplemental Figure 6, F–I). Podocytes were costained with wild type 1. In vivo, Ki-67 staining indicated that proliferation activity of neither wild-type nor Vps34-deficient podocytes was present at different time points. Active caspase 3 staining, however, was increased in all glomerular cell types of 9-week-old Nphs2-cre;Vps34fl/fl mice. At early stages of beginning glomerulosclerosis, however, active caspase 3 staining was indifferent from wild type.
Figure 4.
Autophagic flux is abrogated in primary Vps34-deficient podocytes. (A) Nphs2-cre;tomatofl/+>EGFP mice were crossed with Vps34fl/fl and Vps34+/+ mice to obtain Vps34-deficient, GFP-positive podocytes and GFP-positive control podocytes. (B) Primary podocytes at passage 1 were observed by phase/contrast microscopy for 9 days. Vps34-deficient podocytes showed substantial perinuclear vacuolization. Counterstaining with Lysotracker Red was performed to determine if the vacuoles seen in Vps34-deficient podocytes represent accumulation of lysosomal acidic vesicles. Scale bars, 20 µm. (C) Immunofluorescence staining showed massive accumulation of LC3 and p62 in Nphs2-cre;Vps34fl/fl;tomatofl/+>EGFP podocytes. Scale bars, 20 µm. (D–F) Autophagic flux was impaired in Nphs2-cre;Vps34fl/fl;tomatofl/+>EGFP primary podocytes. Serum deprivation induced LC3 accumulation in wild-type GFP-positive primary podocytes that was further enhanced by blockade of autophagosomal degradation with the lysosomal inhibitor chloroquine (10 µM). In contrast, LC3 was already accumulated in Vps34-deficient primary GFP-positive podocytes with no further increase on serum starvation or disruption of autophagosomal–lysosomal fusion by chloroquin. **P<0.01. Scale bars, 50 µm.
To determine if the extensive perinuclear vacuoles seen in Vps34-deficient podocytes represented lysosomal accumulation, vesicles were counterstained with Lysotracker Red (Figure 4B). Equal to our observations in kidney sections of Nphs2-cre;Vps34fl/fl mice and littermate controls (Figure 3, A and D–F), LC3 and p62 accumulated in Nphs2-cre;Vps34fl/fl;tomatofl/+>EGFP podocytes (Figure 4C), which is characteristic for a block in autophagy. To verify impaired autophagic flux, Nphs2-cre;Vps34fl/fl;tomatofl/+>EGFP primary podocytes and wild-type controls were serum-starved in the presence or absence of 10 µM chloroquine (Figure 4, E and F). Chloroquine increases the lysosomal pH, which leads to inhibition of autophagosome–lysosomal fusion and impaired lysosomal protein degradation. Chloroquine treatment of wild-type control cells led to an accumulation of LC3-II over time on starvation representative for autophagic flux (Figure 4, E and F). Nphs2-cre;Vps34fl/fl;tomatofl/+>EGFP primary podocytes, however, already showed high LC3-II levels under normal conditions (Figure 4, E and F) and showed only a little additional increment on treatment with chloroquine (Figure 4, E and F), confirming that autophagic flux is largely abrogated in Vps34-deficient podocytes.
To answer the question of whether autophagy is the main underlying reason for the severe phenotype observed in Nphs2-cre;Vps34fl/fl mice, we generated podocyte-specific Nphs2-cre;Atg5fl/fl mice, Nphs2-cre;Vps34fl/fl mice, and Nphs2-cre;Atg5fl/fl;Vps34fl/fl double conditional knockout mice (Figure 5A) and compared functional data (Figure 5B). Atg5 is essential for the extension and completion of autophagosomes. Our earlier studies showed that specific disruption of autophagy in podocytes by deletion of Atg5 leads to late-onset proteinuria and glomerulosclerosis.37 Nphs2-cre;Atg5fl/fl mice showed no signs of proteinuria, weight loss, or histologic aberration within the first 5 weeks of observation (Figure 5, B–D). Double deficient Nphs2-cre;Atg5fl/fl;Vps34fl/fl mice exhibited an analogous phenotype to Nphs2-cre;Vps34fl/fl single conditional knockout mice (Figure 5B). Impaired autophagy, shown here by increased LC3 deposits and p62 accumulation (Figure 5, C and D), was similar in Nphs2-cre;Vps34fl/fl single conditional knockout mice and Nphs2-cre;Atg5fl/fl;Vps34fl/fl conditional double knockout mice (Figure 5, C and D). The late-onset degeneration of podocytes because of single Atg5 deficiency is, thus, both histologically and phenotypically entirely distinct from the degeneration caused by Vps34 deficiency (Figure 5, B–D).
Figure 5.
Abrogated autophagic flux is not causative for the severe podocyte phenotype of Vps34-deficient podocytes. (A) Vps34fl/fl mice were crossed to Nphs2-cre;Atg5fl/fl mice to obtain Nphs2-cre;Atg5fl/fl;Vps34fl/fl conditional double knockout mice. (B) Albumine/creatinine ratios were significantly increased from 3 weeks of age in Vps34fl/fl;Nphs2-cre and Nphs2-cre;Atg5fl/fl;Vps34fl/fl conditional double knockout mice compared with Nphs2-cre;Atg5fl/fl mice and littermate controls (n=10 per group, *P<0.05, ***P<0.01, two-tailed t test, mean values ± SEM are shown). (C) Confocal microscopy showed accumulation of LC3 and Lamp2 in podocytes of Nphs2-cre;Vps34fl/fl single mutant mice and Nphs2-cre;Atg5fl/fl;Vps34fl/fl conditional double knockout mice. No significant colocalization of LC3 and Lamp2 could be observed. Nphs2-cre; Atg5fl/fl single conditional knockout mice did not show increase of LC3 or Lamp2 in podocytes at 3 weeks of age. Scale bars, 20 µm. (D) p62 accumulates in podocytes of Nphs2-cre;Vps34fl/fl single conditional knockout mice and Nphs2-cre;Atg5fl/fl;Vps34fl/fl conditional double knockout mice. Nphs2-cre;Atg5fl/fl mice did not show increase of p62 in podocytes at 3 weeks of age. Scale bars, 20 µm.
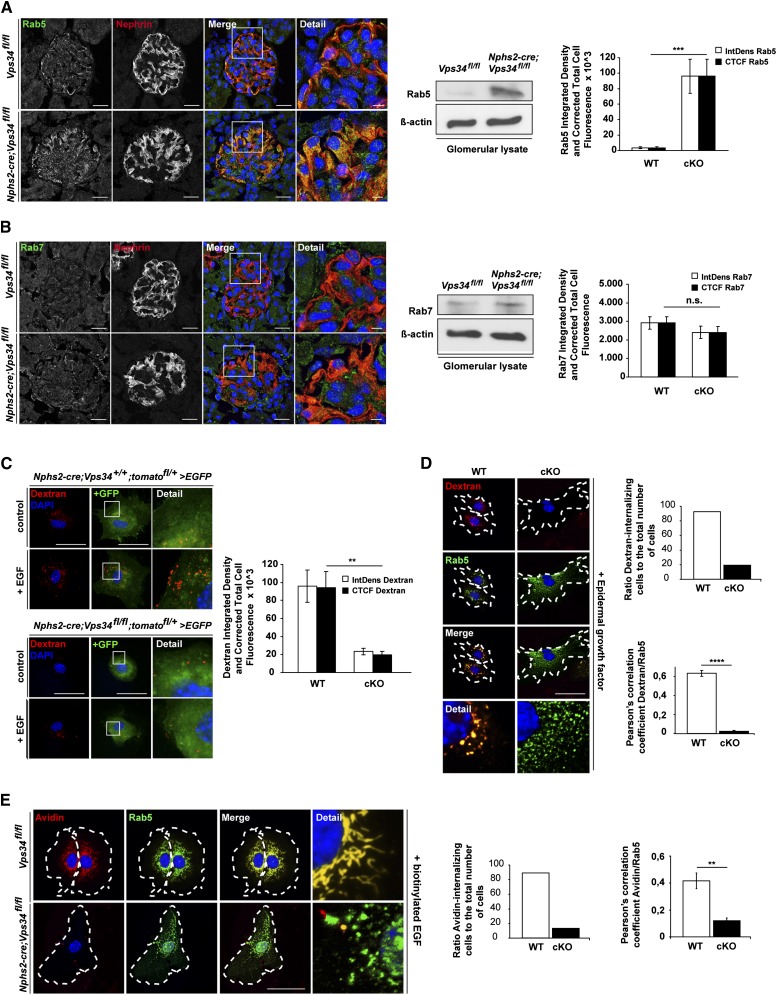
Ablation of Vps34 in Mouse Podocytes Leads to Impaired Endocytosis
Endocytosis of extracellular material in membrane-bound vesicles is crucial for a wide range of cellular functions. Although there is evidence that endocytosis is important for podocytes,8,11,12 the signaling crosstalk between endocytosis and signal transduction in this specialized cell population is still largely unacquainted. Vps34 is implicated in the regulation of intracellular vesicle transport at numerous key positions.17 Because the late-onset degeneration of podocytes caused by Atg5 deficiency is histologically and phenotypically utterly distinct from the degeneration caused by Vps34 deficiency, we hypothesized that, in podocytes, Vps34 deficiency leads to defects in endolysosomal pathways. To study endolysosomal trafficking in Vps34-deficient podocytes, we examined early and late endosomal compartments and PI(3)P-dependent endolysosomal fusion (Figure 6). Rab5 recruits Vps34 to the early endosome to secure the local production of PI(3)P. The presence of PI(3)P is required for the fusion between endocytic vesicles and early endosomes by the recruitment of a subset of proteins (e.g., EEA1).38,39 Similar to the large vacuoles observed in Vps34-deficient mouse podocytes (Figure 2D and Supplemental Figures 2B and 4), Rab5 deficiency causes giant endosomes in cells.40 Vps34-deficient podocytes showed substantial accumulation of Rab5 (Figure 6A). In contrast, Vps34-deficient podocytes displayed no significant differences of the late endosomal marker Rab7 compared with littermate controls (Figure 6B), indicative of a block in endosomal maturation upstream of the late endosomal compartment. To functionally address the question of impaired fluid-phase endocytosis in Vps34-deficient podocytes, we performed an FITC-dextran uptake assay (Figure 6, C and D). Serum-starved Nphs2-cre;Vps34fl/fl;tomatofl/+>EGFP primary podocytes and wild-type controls were stimulated with EGF in the presence of fluorescent-labeled dextran to monitor endosome/macropinosome formation (Figure 6, C and D). In the absence of EGF, dextran was internalized into a few endosomes of variable sizes (Figure 6, C and D). The addition of EGF induced a marked stimulation of dextran uptake at 5 minutes after EGF addition (Figure 6, C and D). Nphs2-cre;Vps34fl/fl;tomatofl/+>EGFP primary podocytes, however, showed impaired uptake of dextran in the presence of EGF, indicating disturbed fluid-phase uptake (Figure 6, C and D). Costaining with the early endosomal marker Rab5 revealed significant overlap with dextran in wild-type podocytes, whereas in Vps34-deficient primary podocytes, accumulated Rab5 did not colocalize with fluorescent-labeled dextran. To functionally address other forms of endocytosis, we evaluated receptor-mediated streptavidin uptake in Vps34-deficient and wild-type primary podocytes (Figure 6E). Serum-starved Nphs2-cre;Vps34fl/fl;tomatofl/+>EGFP primary podocytes and wild-type controls were stimulated with biotinylated EGF in the presence of fluorescent-labeled streptavidin (Figure 6E). In the absence of EGF, streptavidin was internalized into a few endosomes of variable sizes. The addition of biotinylated EGF induced a marked stimulation of streptavidin uptake at 30 minutes after the addition of biotinylated EGF. Nphs2-cre;Vps34fl/fl;tomatofl/+>EGFP primary podocytes, however, showed impaired uptake of streptavidin in the presence of biotinylated EGF, indicating disturbed receptor-mediated endocytosis. Costaining with the early endosomal marker Rab5 indicated a significant overlap with streptavidin in wild-type podocytes, whereas Rab5 did not colocalize with fluorescent-labeled streptavidin in Vps34-deficient primary podocytes.
Figure 6.
Ablation of Vps34 in mouse podocytes leads to impaired endocytosis. (A and B) Confocal immunofluorescence microscopy of kidney sections of 3-week-old Nphs2-cre;Vps34fl/fl and littermate control mice. (A) Rab5, a marker for early endosomes and activator of Vps34, was strongly increased in Nphs2-cre;Vps34fl/fl podocytes at 3 weeks of age. Scale bars, 5 µm. (B) Rab7, a marker for the late endosome, showed no significant differences in podocytes of Nphs2-cre;Vps34fl/fl and Vps34fl/fl mice. Scale bars, 5 µm. (A and B) Western blot analysis of glomerular protein lysates of 3-week-old mice shows that the early endosomal marker Rab5 is accumulated in glomerulus lysates of Nphs2-cre;Vps34fl/fl mice compared with littermate controls. No changes in the late endosomal marker Rab7 can be observed. β-actin was used as loading control. (C and D) Immunofluorescence and quantification of fluid-phase uptake in primary podocytes. Stimulation with 20 nM EGF for 5 minutes induced uptake of dextran in GFP-positive primary control podocytes. In contrast, Vps34-deficient primary GFP-positive podocytes showed impaired uptake of dextran, indicating a blockade in endocytosis. Scale bars, 50 µm. (D) Costaining with Rab5 revealed significant overlap in wild-type podocytes, whereas in Vps34-deficient primary podocytes, Rab5 accumulation did not colocalize with dextran. (E) Immunofluorescence and quantification of streptavidin uptake in primary podocytes. Stimulation of GFP-positive primary wild-type podocytes with 20 nM biotinylated EGF induced uptake of streptavidin coupled to Alexa Fluor 555. In contrast, Vps34-deficient primary GFP-positive podocytes showed impaired uptake of streptavidin, indicating a blockade in receptor-mediated endocytosis. Scale bars, 50 µm. Costaining with Rab5 revealed significant overlap in wild-type podocytes, whereas in Vps34-deficient primary podocytes, Rab5 accumulation did not colocalize with streptavidin. **P<0.01, ***P<0.001, ****P<0.0001.
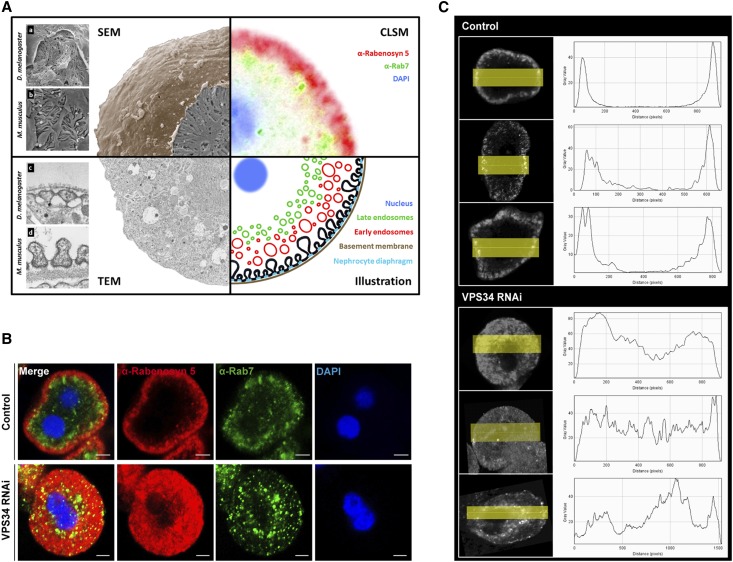
Vps34 Is an Important Regulator of the Early Endosomal Compartment in the Podocyte-Like Drosophila Cell—The Nephrocyte
To test whether these fundamental Vps34-mediated endocytosis mechanisms are a general theme of filtering cells, we analyzed podocyte-like cells in Drosophila melanogaster. Garland cell nephrocytes (GCNs) are highly endocytic active so-called storage kidneys of D. melanogaster (Figure 7A). Immunofluorescence staining with Rabenosyn5, a marker for the early endosome in D. melanogaster, shows the characteristic ring-like pattern of early endosomes in wild-type controls (Figure 7). Underneath this ring, late endosomes can be visualized by immunofluorescence staining with anti-Rab7 antibodies (Figure 7, A and B). On knockdown of Vps34, this pattern was utterly abrogated, and the Rabenosyn5-positive early endosome compartment was significantly extended (Figure 7, B and C), characteristic for a complete early endosomal block in nephrocytes comparable with the observations in Vps34-deficient podocytes (Figure 6).
Figure 7.
Vps34 is an important regulator of the early endosomal compartment in the podocyte-like Drosophila cell—the nephrocyte. (A) Combined schematic of electron microscopy and confocal immunofluorescence microscopy of GCNs. (a–d) Structural comparison of (a) GCN with (b) podocytes and (c) nephrocyte diaphragm with (d) slit diaphragm. The rough surface of the GCN underneath the (a) basement membrane is formed by the (c) nephrocyte diaphragm. In contrast to the mammalian slit diaphragm, which is formed between (b) neighboring podocytes, (a) the Drosophila nephrocyte diaphragm is formed within one GCN. The illustration shows the endocytosis process by formation of early endosomes from invaginations of the nephrocyte diaphragm and their maturation to late endosomes. (B) Confocal immunofluorescence microscopy of Vps34-deficient and wild-type control GCNs. The control shows the thin outer ring of Rabenosyn5-positive early endosomes (red) and the inner ring of Rab7-positive late endosomes (green). On expression of Vps34RNAi, this pattern is disrupted, and the whole cytoplasm is filled with Rabenosyn5-positive endosomes. Control: prospero GAL4/+(2); Vps34RNAi: prospero-GAL4/upstream activating sequence-Vps34-RNAi(2). Scale bars, 5 µm. (C) Distribution profile of the early endosomal marker Rabenosyn5.
Discussion
The podocyte may be the most complicated and intricate cell in the kidney, responsible for maintaining the glomerular filtration barrier and the glomerular basement membrane. The presence of endocytotic vesicles within the podocyte is one of the earliest observations in electron microscopy of the glomerulus.1 Marylin Farquhar and other investigators have hypothesized that these bodies represent transcytotic vesicles of primary urine or protein reabsorption droplets.41–43 Large foot process phagosomes have been identified removing subepithelial immune deposits and giant protamine heparin aggregates.44,45 More recently, it was shown that defects in multivesicular body formation were associated with increased susceptibility to glomerular disease, suggesting the importance of an intact endocytosis machinery for the integrity of the glomerular filter.46 However, the mechanisms and the intracellular pathways of endocytosis in podocytes had remained unclear. Recently, the type 2 phosphoinositide 3-kinase PI3KC2-α has been shown to play a role in glomerular structure, suggesting a function for podocyte maintenance.47 Here, we used a podocyte-specific Vps34-deficient mouse model to identify the fundamental role of endocytosis for podocyte homeostasis. Through the localized phosphorylation of phosphatidylinositol to phosphatidylinositol-3-phosphate (PI[3)]P), Vps34 has a unique function in several vesicular trafficking processes and early vesicle docking/fusion at the endosome.17 It recruits downstream effectors that contain PI(3)P binding motif, such as a FYVE domain (a specific zinc finger domain named after the four cysteine-rich proteins: Fab 1, YOTB, Vac 1 and EEA1) or a PX domain (a phosphoinositide-binding structural domain involved in targeting of proteins to cell membranes), components in the PI 3-kinase signaling cascade to regulate membrane trafficking processes, such as endocystosis and autophagy.20–22,48 Furthermore, Vps34 plays a crucial role in endosome tethering and membrane fusion through EAA1 and other Rab5 effectors, vesicle invagination and cargo selection within multivesicular bodies, and fusion of autophagosomes with lysosomes.23–26
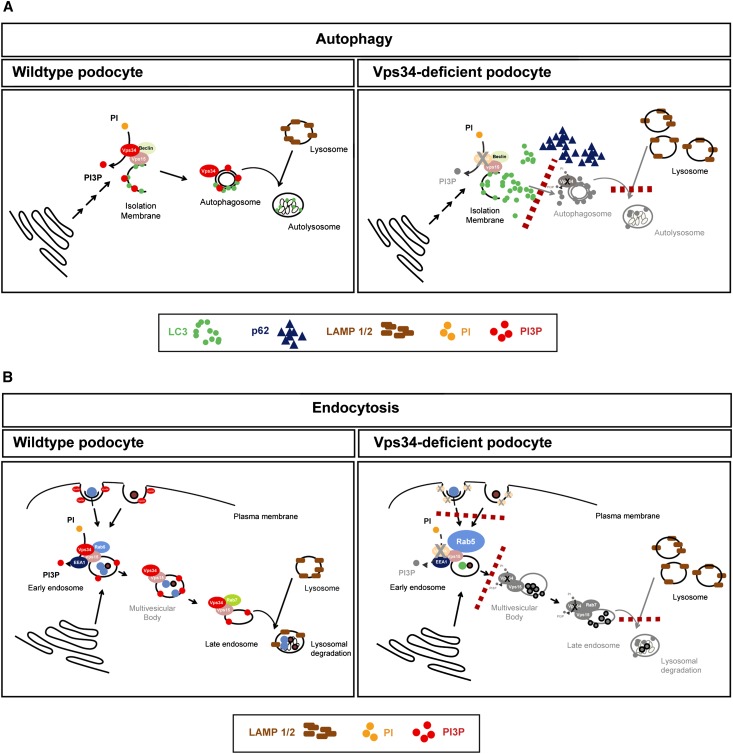
The first study of conditional Vps34 knockout mice described a disruption of endolysosomal pathways in mammalian neurons. Zhou et al.30 observed that, similar to Vps34-deficient podocytes, large-diameter Vps34-deficient sensory neurons showed dramatic defects in endolysosomal pathways leading to rapid degeneration within 5–9 days because of an accumulation of atypical vacuoles and endosomes. However, in mouse liver and heart, depletion of Vps34 shows rather mild phenotypes, similar to the phenotypes seen in autophagy-deficient mice.31,37 Similar to these mice, autophagy is impaired in Vps34-deficient podocytes, and autophagic flux is abrogated. Interference with Vps34 was found to inhibit mammalian target of rapamycin (mTOR) activation. Vps34 can function in a nutrient-sensing pathway upstream of mTOR in several mammalian cell lines, indicating a role for Vps34 in mTOR signaling.49,50,51 Interestingly, Cinà et al.52 have recently shown that deletion of mTOR in podocytes resulted in glomerulosclerosis as a result of decreased autophagic flux. Intriguingly, however, podocyte-specific ablation of Vps34 in mice leads to severe proteinuria and early-onset glomerulosclerosis, showing an entirely distinct phenotype from podocytes that are unable to execute autophagy. We showed that specific disruption of macroautophagy in podocytes by deletion of Atg5 leads to accelerated aging, late-onset proteinuria, and glomerulosclerosis.37 Conditionally, Vps34-deficient mice, however, developed large-sized vacuoles and unprocessed endosomes within the podocyte cytoplasm, resulting in rapid podocyte degeneration and death within 3–9 weeks. The late-onset degeneration of podocytes caused by Atg5 deficiency is, thus, both histologically and phenotypically entirely distinct from the degeneration caused by Vps34 deficiency, indicating that the lack of autophagy is not the main reason underlying the rapid degeneration of Vps34-deficient podocytes (Figure 8). Because of the disparities in Vps34- and autophagy-deficient mice, we hypothesized that Vps34-mediated endocytosis might be of particular importance for podocyte maintenance. In fact, the observed disruption of early endosomal sorting in Vps34-deficient podocytes as well as Vps34-deficient D. melanogaster nephrocytes suggests a block of endocytosis at the level of early endocytosis (Figure 8). A large number of distinct endocytic pathways exist to allow the internalization of nutrients, solutes, and growth factors. The disruption at the early steps of these endocytosis mechanisms is likely to abrogate podocyte-specific functions. To this end, it is not clear which exact subset of endocytic mechanisms is mostly affected in podocyte physiology. Several studies have previously shown that growth factor pathways are significantly regulated by endocytosis of their respective receptors. Hence, essential growth factor signaling axes, such as vascular EGF and EGF pathways, might be affected by the disruption of Vps34-controlled endocytosis in podocytes.53–55 Dysregulation of vascular EGF expression within the glomerulus has been shown in a wide range of primary and acquired renal diseases.54 In our study, we observed an almost complete abrogation of receptor-mediated endocytosis in Vps34-deficient podocytes, emphasizing the importance of receptor-mediated endocytosis for growth factor signaling. Interestingly, we also detected fluid-phase uptake to be seriously compromised in Vps34-deficient podocytes. Larger dextrans are known to be endocytosed through fluid-phase uptake rather than receptor-mediated endocytosis, suggesting that Vps34 deficiency likewise leads to a failure of fluid-phase uptake in podocytes. Because some of the vesicles that can be observed in wild-type podocytes in vivo seem to be nonclathrin-coated, it can be speculated that these vesicles might represent micro- or macropinosomes representing an active podocytic fluid-phase uptake. We speculate that our data point to the importance of receptor-mediated endocytosis and fluid-phase uptake for the maintenance of the filtration barrier. Endocytotic mechanisms are highly conserved features of filtering cells. This result is highlighted by our observation that nephrocytes, podocyte-like cells of D. melanogaster, show an equally severe disruption of the early endosomal compartment on Vps34 knockdown as Vps34-deficient podocytes.56,57 Vps34 deficiency in podocytes as well as nephrocytes disrupts accurate vesicle transport at early stages of endocytosis, causing an increase in unprocessed early endosomes, lysosomes, and impaired endolysosomal fusion, which ultimately results in mislocalization of proteins and ER stress. It is important to note that the alterations in vesicular trafficking pathways in Vps34-deficient podocytes were already detectable at week 1 after birth (accumulation of Rab5, Lamp1/2, and LC3) before a significant phenotype. At this early time point, no functional or histologic changes were yet evident, and secondary effects on cell homeostasis caused by proteinuria can, therefore, be excluded.
Figure 8.
Schematic illustration of Vps34 deficiency in podocytes. (A) Vps34 deficiency leads to incomplete formation of the autophagosomal membrane, resulting in deficient autophagy and autophagosomal fusion. LC3 can still be converted from LC3-I to -II, but no functional autophagosome is formed. It leads to an accumulation of nondegraded LC3 and p62 as well as an accumulation of vacant lysosomes and accumulation of Lamp1/2. The absence of PI(3)P causes a blockade in autophagosomal formation and autophagosomal–lysosomal fusion. (B) Endosomal trafficking is blocked in Vps34-deficient podocytes. Lack of PI(3)P production inhibits fluid-phase uptake, receptor-mediated endocytosis, and maturation of the early endosome to the late endosome, resulting in an accumulation of Rab5. Rab7, a marker for the late endosome, is not affected. Lamp1/2, markers for the lysosome, are upregulated, indicating unused lysosomes because of insufficient endolysosomal fusion.
Here, we provide first evidence that endocytosis is a key mechanism in podocyte homeostasis. Lack of autophagy is not the major reason underlying the rapid degeneration of Vps34-deficient podocytes. Instead, the massive vacuolization and rapid degeneration of Vps34-deficient podocytes that is entirely distinct from autophagy-deficient podocytes point to an early block in endocytic pathways in podocytes caused by impaired vesicle fusion and maturation. Additional investigation is needed to unravel which extracellular and intracellular signals trigger endolysosomal trafficking for podocyte homeostasis.
Concise Methods
Mice
Vps34fl/fl mice (129/B6 mixed background) were a gift from F. Wang, Duke University. Nphs2-cre mice (C56Bl/6 background) and Atg5fl/fl mice (C56Bl/6 background) were previously described.58 STOCK Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice were purchased from JAX (Strain of origin: 129×1/SvJ×129S1/Sv). Vps34fl/fl mice were bred with Nphs2-cre/+ mice to generate podocyte-specific Vps34 knockout mice (referred to as Nphs2-cre;Vps34fl/fl). The podocyte-specific expression of Cre recombinase excises the ATP binding domain of the kinase (encoded by exons 17 and 18) in Vps34fl/fl mice to create a conditional mutant allele resulting in a truncated functionally inactive protein if expressed. Vps34fl/fl littermates served as controls. Nphs2-cre;tomato>EGFPfl/+ mice were crossed with Vps34fl/fl mice to obtain Nphs2-cre;Vps34fl/fl;tomato-EGFPfl/+ mice for the isolation of primary podocytes. Vps34fl/fl mice were bred with Nphs2-cre/+;Atg5fl/fl mice to generate podocyte-specific Vps34fl/fl/Atg5fl/fl conditional double knockout mice. All animal studies were approved by the Committee on Research Animal Care, Regierungspräsidium Freiburg.
Functional Analysis of Podocyte-Specific Vps34-Deficient Mice
Urinary albumin and creatinine were measured at postnatal day 1 and then, one time per week from week 1 to week 9 using mouse albumin-specific (Mikrofluoral Mikroalbumin Test; Progen) and creatinine kits (Creatinine PAP LT-SYS, Labor&Technik; Eberhard Lehmann GmbH) according to the manufacturer’s instructions. Albumin/creatinine ratio was calculated and expressed as milligrams albumin/milligrams creatinine.
Histologic Analysis
Kidneys from 1-, 3-, and 9-week-old Nphs2-cre;Vps34fl/fl and littermate control mice were dissected, fixed in 4% paraformaldehyde, and embedded in paraffin or Lowicryl K4M resin (Electron Microscopy Sciences). Tissues were further processed for periodic acid–Schiff (PAS) staining or electron microscopy, respectively. For PAS staining, 3-µm sections were cut on a Leica microtome and analyzed and photographed with an Axioplan 2 microscope (Zeiss) and an AxioCam camera (Zeiss).
Semiquantitative Histological Analyses
Severity of glomerulosclerosis was evaluated using an index score that includes the percent of glomeruli showing sclerosis and the extension of the glomerulosclerosis within the glomeruli. Glomeruli were graded from 0 to +4: grade 0, normal; grade 1, <25% involvement of the glomerular tuft; grade 2, 25%–50% involvement of the glomerular tuft; grade 3, 50%–75%; grade 4, sclerosis occupying >75% of the glomerular tuft. The glomerulosclerosis score was obtained as follows: (1×number of glomeruli with +1)+(2×number of glomeruli with +2)+(3×number of glomeruli with +3)+(4×number of glomeruli with +4)/total number of glomeruli examined.59
Quantification of Foot Process Effacement
We performed quantification of foot process effacement. Data are means ± SEM. ***P<0.01. Random electron microscopy images were used for morphometric analysis of foot process effacement. Three glomeruli of n=3 mice each were evaluated. Electron images were analyzed using ImageJ software. The quantification of foot process effacement was adapted from van den Berg et al.60 Briefly, from each picture, the mean width of the foot processes (FPWs) was calculated according to the following formula: FPW=π/4×(Σ glomerular basement membrane length/Σ foot process). A foot process was defined as any connected epithelial segment butting on the basement membrane between two neighboring filtration slits.
Immunogold Electron Microscopy
Kidneys of 3-week-old Nphs2-cre;Vps34fl/fl mice and control littermates were perfused transaortically with 4% paraformaldehyde and 0.05% glutaraldehyde in 0.1 phosphate buffer. Kidneys were removed and postfixed in the same fixative (overnight at 4°C). Tissues were washed in PBS, and then, sections (50 μm) were cut on a vibratome and cryoprotected in a solution containing 25% sucrose and 10% glycerol in 50 mM PBS. The sections were freeze-thawed and incubated in blocking solution containing 2% normal goat serum in 50 mM Tris-buffered saline for 1 hour followed by incubation with an anti-LC3 antibody (24 hours at 4°C; Cell Signaling). After the sections were washed, the sections were incubated with 1.4-nm gold-coupled goat anti-mouse secondary antibody (1:100, Nanogold; Nanoprobes, Stony Brook, NY) for immunogold reaction. Immunogold labeling was then enhanced with the HQ Silver Kit (Nanoprobes). After the sections were treated with OsO4, the sections were stained with uranyl acetate, dehydrated, and flat-embedded in epoxy resin (Durcupan ACM, Fluka; Sigma-Aldrich, Gillingham, United Kingdom). Ultrathin sections were cut and analyzed using a Philipps CM 100 electron microscope.
Immunofluorescence Staining of Kidney Sections
Kidneys were snap-frozen in cryogenic Tissue-Tek O.C.T. compound (Electron Microscopy Sciences). The embedded tissue was sectioned at 6 μm with a Leica Kryostat (Leica). The sections were fixed with 4% paraformaldehyde, blocked with PBS containing 5% BSA, and incubated for 1 hour with primary antibodies. After three PBS rinses, fluorophore-conjugated Alexa secondary antibodies (Invitrogen) were applied for 30 minutes. Confocal microscopy and acquisition of images were performed using a Zeiss laser scan confocal microscope. To determine the number of podocytes per glomeruli, kidney sections were stained with the podocyte nuclear marker WT1. WT1-positive cells were counted in 100 glomeruli per mouse (n=3 for Nphs2-cre;Vps34fl/fl and littermate controls).
Quantification of Immunofluorescence and Colocalization
Integrated density and corrected total cell fluorescence was measured using ImageJ software. Ten eight-bits images each were analyzed. Pearson’s correlation coefficient was calculated using ImageJ software (1=perfect colocalization, 0=no colocalization). Three independent experiments were conducted, and the percent inhibition of streptavidin endocytosis was calculated. The cells were classified as cells internalizing avidin or cell not internalizing avidin. The percent inhibition represents the ratio of noninternalizing cells to the total number of cells.
Antibodies
Antibodies were obtained from Abcam (anti-WT1 rabbit pAb, ab15249; anti-Lamp1 rabbit mAb, ab24170; anti-Lamp2 rabbit mAb, ab37024; anti-Rab5 rabbit pAb, ab13253; anti-Rab7 mouse mAb, ab50533), Cell Signaling Biotechnology (anti-LC3B rabbit pAb, 2775; anti-Calnexin rabbit pAb, 2433), Epitomics (anti-Vps34 rabbit mAb, 3838–1), MBL (anti-LC3 mouse mAb, M152–3), Progen (antinephrin guinea pig pAb, GP-N2; anti-p62 guinea pig pAb, GP62-C), Santa Cruz Biotechnology (anti-Rab5 mouse mAb, sc-46692), Sigma-Aldrich (antipodocin rabbit pAb, P0372; anti–β-actin mouse mAb, A5441), and Enzo (anticalnexin rabbit pAb, ADI-SPA-860). Secondary antibodies and nuclear staining reagents were obtained from Invitrogen. For Western blotting, goat anti-rabbit or anti-mouse IgG–horseradish peroxidase (HRP) secondary antibodies were used for the above primary antibodies (anti-rabbit IgG, HRP-linked antibody, 7074; Cell Signaling; anti-mouse IgG, HRP-linked antibody, P0447; Dako). Antibodies for D. melanogaster experiments, rabbit anti-Rab7 and rat anti-Rabenosyn5, were a gift from A. Nakamura, RIKEN Center for Developmental Biology, Japan.
Isolation of Mouse Glomeruli and Primary Podocytes
Glomeruli were isolated from 10-day-old triple transgenic Nphs2-cre;Vps34fl/fl;tomatofl/+>EGFP and littermate control mice and sieved through sieves with decreasing pore sizes (100, 70, and 40 µm). For the isolation of primary podocytes, glomeruli were plated on Collagen IV-coated cell culture dishes. After 4 days, glomerular cells were FACS-sorted for green fluorescent protein (GFP) -positive cells. For Western blot analyses, glomeruli were glass–glass homogenized in lysis buffer (containing 20 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate and 1% Triton X-100)61 and centrifuged at 15,000×g for 15 minutes at 4°C.
Western Blotting
Mouse glomeruli were isolated by either graded sieving or magnetic bead isolation. The proteins were extracted from the isolated glomeruli, lysed in lysis buffer containing EDTA, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, sodium orthovanadate, and protease inhibitors, and quantified by Lowry protein assay (Bio-Rad). Equal amounts of protein samples (25 µg/lane) were separated by SDS-PAGE and blotted onto polyvinylidene fluoride membranes. The membranes were blocked with 5% BSA and incubated overnight at 4°C with primary antibodies as indicated (Concise Methods, Antibodies). The membranes were then incubated with the appropriate secondary antibodies (Concise Methods, Antibodies). After extensive washing in Tris-buffered saline with Tween20, the proteins were visualized using chemiluminescence reagents and exposed to film. For the quantitative analysis, the blots were scanned, and the relative density of each band was calculated and normalized to the density of β-actin using LabImage software.
Primary Podocyte Cell Culture
Primary isolated podocytes from Nphs2-cre;Vps34fl/fl;tomatofl/+>EGFP and littermate control mice were cultured in collagen IV-coated tissue culture flasks (Nunc) in RPMI supplemented with penicillin/streptomycin, FCS (Life Technologies), and insulin-transferrin-sodium selenite supplement (Roche Applied Science) at 37°C in 95% O2 and 5% CO2. For immunofluorescence staining, cells were cultured on collagen IV-coated glass coverslips.
Autophagy Flux Assay
For autophagy flux assays, medium was removed and replaced with serum-free RPMI for 6 hours before the cells were incubated with chloroquine (Sigma Aldrich) dissolved in PBS (25 μM final concentration) for 0, 2, and 4 hours. Western blot quantification was performed using ImageJ software.
Dextran Fluid-Phase Uptake
For dextran uptake assays, medium was removed and replaced with serum-free RPMI for 6 hours before the cells were activated with EGF (20 nM final concentration) and incubated with dextran and Alexa Fluor 555 (Invitrogen) or dextran alone. To visualize dextran uptake, cells were fixed with 4% paraformaldehyde, costained with the early endosomal marker Rab5, and embedded in ProLong Gold Antifade Reagent (Invitrogen) before performing confocal microscopy. Quantification and colocalization analyses were performed using with ImageJ.
Streptavidin Uptake
For avidin uptake assays, medium was removed and replaced with serum-free RPMI for 6 hours before the cells were activated with biotinylated EGF (20 nM final concentration) and incubated with streptavidin coupled with Alexa Fluor 555 (Invitrogen) or streptavidin alone. To visualize avidin uptake, cells were fixed with 4% paraformaldehyde, costained with the early endosomal marker Rab5, and embedded in ProLong Gold Antifade Reagent (Invitrogen) before performing confocal microscopy. Quantification and colocalization analyses were performed using with ImageJ.
D. melanogaster Experiments
D. melanogaster stocks were cultured on standard cornmeal molasses agar food and maintained at 29°C. Virgins of upstream activating sequence-VPS34RNAi (VDRC TID100296) were crossed to prospero-Gal4 (gift from Barry Denholm) for a GCN-specific knockdown of VPS34. GCNs (isolated from wandering third-instar larvae) were dissected in PBS, fixed in 4% paraformaldehyde, washed in PBS and 0.2% Triton X-100, incubated with primary antibodies (in PBS, 0.2% Triton X-100, and 0.05% sodium azide), and mounted in Vectashield (Vector Labs). The mounting medium for GCNs contained 4',6-diamidino-2-phenylindole for nuclear staining. Samples were imaged using NIKON A1 CLEM with inverted microscope Eclipse TI. Rabbit anti-Rab7 and rat anti-Rabenosyn5 were gifts from A. Nakamura, RIKEN Center for Developmental Biology, Japan.
Statistical Analyses
Data are presented as mean ± SEM throughout the text unless otherwise specified. All experiments were performed at least three times. Statistical comparisons were performed using two-tailed t test where applicable. A value of P<0.05 was considered to represent statistically significant differences.
Disclosures
None.
Acknowledgments
We thank F. Wang (Duke University Medical Center Durham) for providing Vps34fl/fl mice, M.J. Möller (University Hospital Aachen) for providing Nphs2-cre mice, and N. Mizushima (University of Tokyo) for providing Atg5fl/fl mice. We thank F. Grahammer (University Hospital Freiburg) for invaluable critical discussions and technical support, C. Meyer (University Hospital Freiburg) for excellent technical assistance, and H. Schachner (Medical University Vienna) for electron microscopy analyses. We thank S. McGoohan (Beth Israel Deaconess Medical Center and Harvard Medical School, Boston) for reading and editing the manuscript.
This research was supported by a Marie Curie Career Integration Grant within the 7th European Community Framework Programme (to W.B.) and Deutsche Forschungsgemeinschaft (DFG) Grants KFO 201 (Project P7; to T.B.H.) and SFB 992 (Project B5; to T.B.H.) as well as the Joint Transnational Grant—Bundesministerium für Bildung und Forschung (BMBF) (to T.B.H.). In addition, the study was supported by Excellence Initiative of the German Federal and State Governments EXC 294 (to T.B.H.) and BMBF GerontoSys2-Project NephAge (to T.B.H.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012070700/-/DCSupplemental.
References
- 1.Farquhar MG, Vernier RL, Good RA: An electron microscope study of the glomerulus in nephrosis, glomerulonephritis, and lupus erythematosus. J Exp Med 106: 649–660, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farquhar MG, Palade GE: Segregation of ferritin in glomerular protein absorption droplets. J Biophys Biochem Cytol 7: 297–304, 1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farquhar MG, Palade GE: Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J Exp Med 114: 699–716, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farquhar MG, Wissig SL, Palade GE: Glomerular permeability. I. Ferritin transfer across the normal glomerular capillary wall. J Exp Med 113: 47–66, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caulfield JP, Farquhar MG: The permeability of glomerular capillaries to graded dextrans. Identification of the basement membrane as the primary filtration barrier. J Cell Biol 63: 883–903, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogt A, Bockhorn H, Kozima K, Sasaki M: Electron microscopic localization of the nephrotoxic antibody in the glomeruli of the rat after intravenous application of purified nephritogenic antibody-ferritin conjugates. J Exp Med 127: 867–878, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venkatachalam MA, Cotran RS, Karnovsky MJ: An ultrastructural study of glomerular permeability in aminonucleoside nephrosis using catalase as a tracer protein. J Exp Med 132: 1168–1180, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riediger F, Quack I, Qadri F, Hartleben B, Park JK, Potthoff SA, Sohn D, Sihn G, Rousselle A, Fokuhl V, Maschke U, Purfürst B, Schneider W, Rump LC, Luft FC, Dechend R, Bader M, Huber TB, Nguyen G, Muller DN: Prorenin receptor is essential for podocyte autophagy and survival. J Am Soc Nephrol 22: 2193–2202, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh M, McMahon HT: The structural era of endocytosis. Science 285: 215–220, 1999 [DOI] [PubMed] [Google Scholar]
- 10.McMahon HT, Boucrot E: Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12: 517–533, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Rastaldi MP, Armelloni S, Berra S, Calvaresi N, Corbelli A, Giardino LA, Li M, Wang GQ, Fornasieri A, Villa A, Heikkila E, Soliymani R, Boucherot A, Cohen CD, Kretzler M, Nitsche A, Ripamonti M, Malgaroli A, Pesaresi M, Forloni GL, Schlöndorff D, Holthofer H, D’Amico G: Glomerular podocytes contain neuron-like functional synaptic vesicles. FASEB J 20: 976–978, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Prabakaran T, Nielsen R, Larsen JV, Sørensen SS, Feldt-Rasmussen U, Saleem MA, Petersen CM, Verroust PJ, Christensen EI: Receptor-mediated endocytosis of α-galactosidase A in human podocytes in Fabry disease. PLoS One 6: e25065, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longatti A, Tooze SA: Vesicular trafficking and autophagosome formation. Cell Death Differ 16: 956–965, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Tooze SA, Yoshimori T: The origin of the autophagosomal membrane. Nat Cell Biol 12: 831–835, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Balla T: Phosphoinositide-derived messengers in endocrine signaling. J Endocrinol 188: 135–153, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Herman PK, Emr SD: Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol 10: 6742–6754, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backer JM: The regulation and function of Class III PI3Ks: Novel roles for Vps34. Biochem J 410: 1–17, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Juhász G, Hill JH, Yan Y, Sass M, Baehrecke EH, Backer JM, Neufeld TP: The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol 181: 655–666, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung PC, Trinkle-Mulcahy L, Cohen P, Lucocq JM: Characterization of a novel phosphatidylinositol 3-phosphate-binding protein containing two FYVE fingers in tandem that is targeted to the Golgi. Biochem J 355: 113–121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemmon SK, Traub LM: Sorting in the endosomal system in yeast and animal cells. Curr Opin Cell Biol 12: 457–466, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Odorizzi G, Babst M, Emr SD: Phosphoinositide signaling and the regulation of membrane trafficking in yeast. Trends Biochem Sci 25: 229–235, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Simonsen A, Stenmark H: PX domains: Attracted by phosphoinositides. Nat Cell Biol 3: E179–E182, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M: Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol 1: 249–252, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Lindmo K, Stenmark H: Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci 119: 605–614, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Eskelinen EL: Maturation of autophagic vacuoles in Mammalian cells. Autophagy 1: 1–10, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Odorizzi G, Babst M, Emr SD: Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95: 847–858, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Kihara A, Noda T, Ishihara N, Ohsumi Y: Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol 152: 519–530, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT: Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182: 685–701, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker S, Chandra P, Manifava M, Axe E, Ktistakis NT: Making autophagosomes: Localized synthesis of phosphatidylinositol 3-phosphate holds the clue. Autophagy 4: 1093–1096, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Zhou X, Wang L, Hasegawa H, Amin P, Han BX, Kaneko S, He Y, Wang F: Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc Natl Acad Sci U S A 107: 9424–9429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, Zong WX: Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A 109: 2003–2008, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stopkova P, Saito T, Papolos DF, Vevera J, Paclt I, Zukov I, Bersson YB, Margolis BA, Strous RD, Lachman HM: Identification of PIK3C3 promoter variant associated with bipolar disorder and schizophrenia. Biol Psychiatry 55: 981–988, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T: p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171: 603–614, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rusten TE, Stenmark H: p62, an autophagy hero or culprit? Nat Cell Biol 12: 207–209, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB: Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120: 1084–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olkkonen VM, Stenmark H: Role of Rab GTPases in membrane traffic. Int Rev Cytol 176: 1–85, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Simonsen A, Lippé R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H: EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394: 494–498, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Stenmark H, Parton RG, Steele-Mortimer O, Lütcke A, Gruenberg J, Zerial M: Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J 13: 1287–1296, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vernier RL, Farquhar MG, Brunson JG, Good RA: Chronic renal disease in children; correlation of clinical findings with morphologic characteristics seen by light and electron microscopy. AMA J Dis Child 96: 306–343, 1958 [PubMed] [Google Scholar]
- 42.Venkatachalam MA, Karnovsky MJ, Cotran RS: Glomerular permeability. Ultrastructural studies in experimental nephrosis using horseradish peroxidase as a tracer. J Exp Med 130: 381–399, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldman JD, Fisher ER: Renal lesions of aminonucleoside nephrosis as revealed by electron microscopy. Lab Invest 8: 371–385, 1959 [PubMed] [Google Scholar]
- 44.Rantala I: Glomerular epithelial cell endocytosis of immune deposits in the nephrotic rat. An ultrastructural immunoperoxidase study. Nephron 29: 239–244, 1981 [DOI] [PubMed] [Google Scholar]
- 45.Sharon Z, Schwartz MM, Pauli BU, Lewis EJ: Kinetics of glomerular visceral epithelial cell phagocytosis. Kidney Int 14: 526–529, 1978 [DOI] [PubMed] [Google Scholar]
- 46.Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS: CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300: 1298–1300, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Harris DP, Vogel P, Wims M, Moberg K, Humphries J, Jhaver KG, DaCosta CM, Shadoan MK, Xu N, Hansen GM, Balakrishnan S, Domin J, Powell DR, Oravecz T: Requirement for class II phosphoinositide 3-kinase C2alpha in maintenance of glomerular structure and function. Mol Cell Biol 31: 63–80, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stenmark H, Aasland R, Driscoll PC: The phosphatidylinositol 3-phosphate-binding FYVE finger. FEBS Lett 513: 77–84, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Byfield MP, Murray JT, Backer JM: hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem 280: 33076–33082, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G: Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A 102: 14238–14243, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ: Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 465: 942–946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cinà DP, Onay T, Paltoo A, Li C, Maezawa Y, De Arteaga J, Jurisicova A, Quaggin SE: Inhibition of MTOR disrupts autophagic flux in podocytes. J Am Soc Nephrol 23: 412–420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE: Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guan F, Villegas G, Teichman J, Mundel P, Tufro A: Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol Renal Physiol 291: F422–F428, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M, Ruiz-Gómez M, Skaer H, Denholm B: The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 322–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denholm B, Skaer H: Bringing together components of the fly renal system. Curr Opin Genet Dev 19: 526–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N: Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441: 885–889, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Raij L, Azar S, Keane W: Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int 26: 137–143, 1984 [DOI] [PubMed] [Google Scholar]
- 60.van den Berg JG, van den Bergh Weerman MA, Assmann KJ, Weening JJ, Florquin S: Podocyte foot process effacement is not correlated with the level of proteinuria in human glomerulopathies. Kidney Int 66: 1901–1906, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]