Abstract
In older adults, measurements of physical performance assess physical function and associate with mortality and disability. Muscle wasting and diminished physical performance often accompany CKD, resembling physiologic aging, but whether physical performance associates with clinical outcome in CKD is unknown. We evaluated 385 ambulatory, stroke-free participants with stage 2–4 CKD enrolled in clinic-based cohorts at the University of Washington and University of Maryland and Veterans Affairs Maryland Healthcare systems. We compared handgrip strength, usual gait speed, timed up and go (TUAG), and 6-minute walking distance with normative values and constructed Cox proportional hazards models and receiver operating characteristic curves to test associations with all-cause mortality. Mean age was 61 years and the mean estimated GFR was 41 ml/min per 1.73 m2. Measures of lower extremity performance were at least 30% lower than predicted, but handgrip strength was relatively preserved. Fifty deaths occurred during the median 3-year follow-up period. After adjustment, each 0.1-m/s decrement in gait speed associated with a 26% higher risk for death, and each 1-second longer TUAG associated with an 8% higher risk for death. On the basis of the receiver operating characteristic analysis, gait speed and TUAG more strongly predicted 3-year mortality than kidney function or commonly measured serum biomarkers. Adding gait speed to a model that included estimated GFR significantly improved the prediction of 3-year mortality. In summary, impaired physical performance of the lower extremities is common in CKD and strongly associates with all-cause mortality.
CKD is a growing global health problem that affects >25 million US adults.1 CKD leads to the retention of metabolic waste products and hormonal disturbances that adversely affect multiple target organ systems, including skeletal muscle. A major consequence of loss of skeletal muscle (sarcopenia) is skeletal muscle dysfunction, which is associated with impaired mobility and reduced physical performance. Among general older adult populations, decreased physical performance is independently associated with subsequent disability, fracture, falls, hospitalization, and mortality.2–4 In particular, usual gait speed has been used as an adjunct for risk stratification by quantifying the burden of recognized and unrecognized multisystem comorbidity, resulting in a strong prognostic marker for subsequent mortality.2,5–7 Other tests of lower extremity function, such as the short physical performance battery, chair raises, and corridor walks are also used to effectively capture clinical and subclinical disease burden and predict future risks of death and disability.8
Physical performance measures may be particularly helpful for assessing health risks in the setting of CKD. First, CKD represents a catabolic state of oxidative damage, inflammation, and malnutrition that culminates in skeletal muscle wasting and diminished function.9–11 Second, kidney disease is also linked with a disproportionately high burden of subclinical and clinical cardiovascular disease that can directly impair physical performance.12,13 Previous studies of physical performance among persons with CKD have generally focused on dialysis-dependent ESRD patients or community-based cohorts that exclude advanced CKD. Associations of physical performance with survival among individuals who have moderate to severe CKD not treated with maintenance dialysis are less well understood.
We hypothesized that persons with CKD not treated with maintenance dialysis would have decreased physical performance and that physical performance would be associated with risk of all-cause mortality independent of known comorbidity and kidney function. We prospectively measured a comprehensive battery of physical performance tests to measure lower and upper extremity function in two cohorts of patients with stage 2–4 CKD. We compared results of each physical performance test to predicted normative values and estimated associations of physical performance with all-cause mortality.
Results
Characteristics of the Cohort
There were 385 participants who had sufficient physical performance data from the three study sites (Supplemental Table 1). The mean age of the cohort was 61±13 years; 84% of participants were men. Among the difference study sites, mean age was 70±8 years at the University of Maryland (UMD) and Baltimore Veterans Affairs Medical Center (VAMC), 62±11 years at the Seattle VAMC, and 53.4±12 years at Harborview Medical Center (HMC). The mean estimated GFR (eGFR) for the combined cohort was 41±19 ml/min per 1.73 m2. Among the different study sites, the mean eGFR was 36±10.6 ml/min per 1.73 m2 at the UMD and Baltimore VAMC cohort, 39±18 ml/min per 1.73 m2 at the Seattle VAMC, and 46±22 ml/min per 1.73 m2 at HMC. Compared with participants who were included in the analyses, those participants who never had a timed up and go (TUAG) assessment (most common performance measure) were, on average, older (69±11 years versus 59±13 years), had greater mobility disability (54% versus 21%), and had lower eGFR (35±18 ml/min per 1.73 m2 versus 42±19 ml/min per 1.73 m2). When the study sample was divided into those with faster TUAG (<12 seconds) compared with slower TUAG (≥12 seconds), those with slower TUAG were older (66±14 years versus 58±12 years) and a higher proportion were women (Table 1). Other notable differences among participants who had slower TUAG times included a greater prevalence of cardiovascular diseases and disability, and a lower mean eGFR (38±18 ml/min per 1.73 m2 versus 44±20 ml/min per 1.73 m2). Those with slower TUAG also tended to have slower gait speed, 6-minute walk distance (6MWD), and weaker handgrip strength (HGS).
Table 1.
Characteristics of participants in the overall cohort with at least one completed physical performance task and those with completed TUAG assessments
| Factor | Missing (n) | Overall (n=385) | Fast TUAG (n=240) | Slow TUAG (n=122) |
|---|---|---|---|---|
| Demographic data | 0 | |||
| Age (yr) | 61±13 | 57.7±12 | 66.4±12 | |
| Female | 63 (16) | 33 (14) | 26 (21) | |
| HMC | 158 (41) | 87 (36) | 56 (46) | |
| Seattle VAMC | 169 (44) | 117 (49) | 45 (37) | |
| Baltimore VAMC | 58 (15) | 36 (15) | 21 (17) | |
| Race | 0 | |||
| White | 239 (62) | 149 (62) | 73 (60) | |
| Other | 146 (38) | 91 (38) | 49 (40) | |
| Education | 30 | |||
| Some high school or less | 25 (7) | 12 (5) | 11 (10) | |
| Completed high school | 238 (67) | 144 (65) | 78 (70) | |
| Completed college or more | 92 (26) | 65 (29) | 23 (21) | |
| Current smoking | 10 | 62 (17) | 35 (15) | 24 (20) |
| Current alcohol use | 64 | 111 (35) | 79 (40) | 28 (28) |
| Physical examination data | ||||
| Systolic BP (mmHg) | 0 | 132.9±20.7 | 131.6±19.8 | 134.2±21.4 |
| BMI (kg/m2) | 0 | 31±6.9 | 30.2±6.3 | 32.5±7.7 |
| Waist circumference (in.) | 0 | 42.2±6.7 | 40.9±6 | 44.4±7.3 |
| Laboratory values | ||||
| eGFRcysc (ml/min per 1.73 m2)a | 58 | 47.6±23.3 | 51.7±24.8 | 41.1±18.3 |
| eGFR CKD-EPI (ml/min per 1.73 m2) | 0 | 41.3±19.3 | 43.6±19.9 | 37.8±17.5 |
| Creatinine (mg/dl) | 0 | 2.2±1.3 | 2.1±1.2 | 2.2±1.3 |
| Albuminuria (mg/g Cr) | 6 | 87.6 [11–630] | 94.7 [9.7–632] | 62.7 [11.7–575] |
| Hemoglobin (g/dl) | 16 | 12.8±1.9 | 13.1±1.9 | 12.5±1.9 |
| Bicarbonate (mmol/L) | 0 | 24.6±3.5 | 24.8±3.3 | 24.2±3.9 |
| CRP (mg/dl) | 29 | 5.2±7.7 | 4.5±7.1 | 5.8±7.6 |
| Cholesterol (mg/dl) | 0 | 177±56.6 | 181.1±57.7 | 170.9±55.3 |
| LDL (mg/dl) | 17 | 104.6±41.8 | 107.7±43.3 | 99.5±38 |
| HDL (mg/dl) | 0 | 40.3±17.3 | 41.3±19.2 | 38±13.6 |
| Triglycerides (mg/dl) | 0 | 163.4±120 | 169±131.8 | 159.5±102.5 |
| Albumin (mg/dl) | 0 | 3.8±0.6 | 3.9±0.6 | 3.8±0.6 |
| Phosphate (mg/dl) | 1 | 3.8±0.8 | 3.8±0.8 | 3.9±0.9 |
| Physical performance | ||||
| 4-m walk (m/s) | 63 | 0.9±0.2 | 1±0.2 | 0.7±0.2 |
| TUAG (sec) | 23 | 11.2±4.5 | 8.8±2 | 15.9±4.5 |
| 6-min walk (m) | 76 | 400±100.3 | 436.8±81.9 | 308.5±78.9 |
| Grip strength (kg) | 4 | 36.15±10.6 | 38.7±10.2 | 32.4±9.7 |
| Exercise (times per week)a | 70 | |||
| Never | 83 (26) | 41 (21) | 31 (33) | |
| <1 | 34 (11) | 24 (12) | 7 (8) | |
| 1 | 46 (15) | 28 (14) | 16 (17) | |
| 2–3 | 71 (23) | 46 (23) | 23 (25) | |
| >3 | 81 (26) | 61 (31) | 16 (17) | |
| Prevalent disease | 0 | |||
| Diabetes | 213 (55) | 118 (49) | 75 (61) | |
| Any CAD | 99 (26) | 48 (20) | 41 (34) | |
| Cancer | 54 (16) | 32 (15) | 17 (17) | |
| Disability | ||||
| Use of assistive device | 14 | 69 (19) | 18 (8) | 35 (29) |
| ≥1 ADL task | 54 | 27 (8) | 13 (6) | 10 (10) |
| ≥1 IADL taska | 65 | 112 (35) | 52 (26) | 49 (50) |
| ≥1 mobility taska | 64 | 77 (24) | 26 (13) | 37 (38) |
Values for categorical variables given as n (%), whereas values for continuous variables given as mean ± SD or median [25th– 75th percentile]. ADL, activity of daily living; IADL, instrumental activity of daily living.
Measured only in the SKS cohort.
Description of Physical Performance Measurements
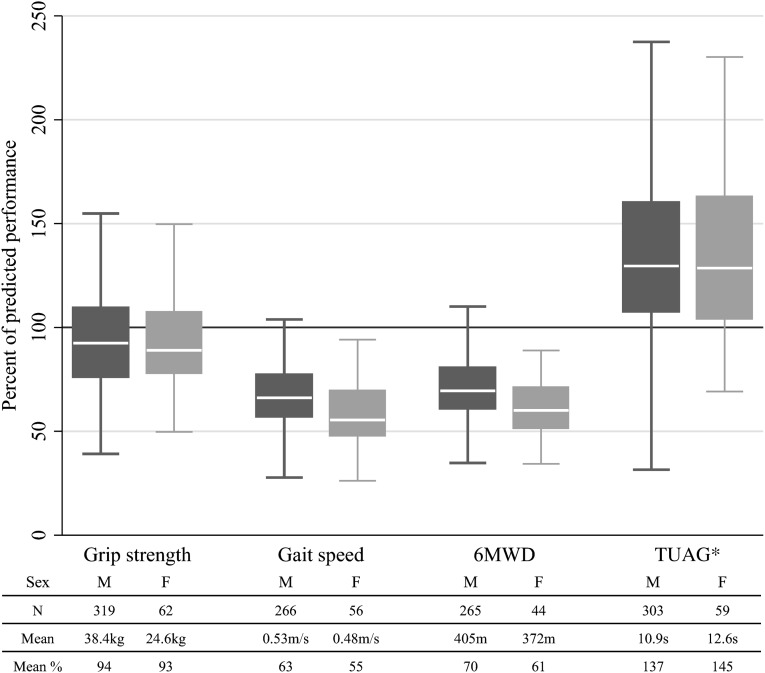
Lower extremity physical performance measures were moderately correlated to each other (correlation coefficient, −0.67 to 0.57) but had a weaker correlation to eGFR (correlation coefficient, 0.07–0.18; P<0.001 for all correlations). There was weak correlation between HGS and the lower extremity physical performance measures (correlation coefficient, 0.15–0.33; P<0.001). All of the lower extremity physical performance measures were diminished in CKD patients compared with normative control values (Figure 1; P<0.001 for all measures). Specifically, gait speed, TUAG, and 6MWD were 30%–39% lower than predicted, with greater decrements observed among women. In contrast, HGS, the only measurement of upper extremity function, was not impaired in CKD patients, compared with normative controls.
Figure 1.
Percentage of predicted performance for each measure by sex. Numbers under bars represent number of participants in each group and mean performance. Note that gait speeds are normalized to height. *For TUAG, a higher percentage predicted indicates worse and slower performance.
Association of Physical Performance Measures with Survival
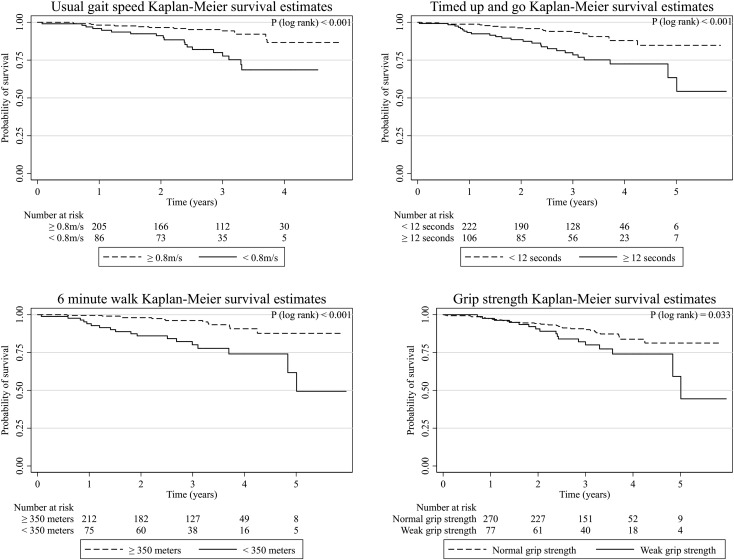
Median follow-up time was 3 years (interquartile range, 2–3.7 years). There were 50 deaths (13%) during follow-up (overall mortality rate of 47 per 1000 person-years). Lower performance across each of the performance measures was associated with greater mortality in unadjusted analyses (Figure 2 and Table 2). After adjustment for age, sex, race, smoking, body mass index (BMI), eGFR, diabetes, and prevalent coronary artery disease (CAD), gait speed and TUAG, but not HGS or 6MWD, remained associated with mortality when analyzed per incremental change in performance. However, 6MWD <350 m was significantly associated with higher risk of mortality compared with ≥350 m. After full adjustment, each 0.1-m/s slower gait speed was associated with an estimated 26% greater risk of death (95% confidence interval [95% CI], 9%, 47%) and each 1-second longer TUAG result was associated with an estimated 8% greater risk of death (95% CI, 1%, 14%). The association of lower extremity physical performance with mortality also persisted after adjustment for renal function measured by eGFRcysc instead of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in the subgroup of participants with cystatin C measurements (Supplemental Table 2).
Figure 2.
Kaplan–Meier survival estimates for each physical performance measure.
Table 2.
Upper and lower extremity physical performance measures and risk of death
| Measure | Performance | Deaths/At Risk (n) | Mortality Rate (per 1000 person-years) | Hazard Ratio (95% CI) | |
|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||
| Gait speed | >0.8 m/s | 15/222 | 25 (15–41) | Reference | Reference |
| ≤0.8 m/s | 19/100 | 79 (50–123) | 3.24 (1.47–7.18) | 2.45 (1.09–5.54) | |
| Per 0.1-m/s slower | 1.31 (1.13–1.52) | 1.26 (1.09–1.47) | |||
| TUAG | Fast (<12 s) | 19/240 | 27 (17–43) | Reference | Reference |
| Slow (≥12 s) | 26/122 | 79 (54–116) | 2.08 (1.06–4.08) | 1.81 (0.92–3.56) | |
| Per 1-s slower | 1.08 (1.03–1.14) | 1.08 (1.01–1.14) | |||
| 6-min walk | ≥350 m | 13/223 | 19 (11–33) | Reference | Reference |
| <350 m | 18/86 | 77 (49–122) | 3.61 (1.71–7.63) | 2.82 (1.17–6.92) | |
| Per 50-m decrease | 1.22 (1.07–1.40) | 1.15 (0.98–1.36) | |||
| Grip | Stronger grip | 32/295 | 38 (27–54) | Reference | Reference |
| Weak gripa | 17/86 | 71 (44–114) | 1.50 (0.82–2.72) | 1.30 (0.71–2.37) | |
| Per 5-kg decrease | 1.17 (1.02–1.33) | 1.07 (0.92–1.24) | |||
Model 1 included age, sex, race, study site. Model 2 added smoking, BMI, diabetes, prevalent CAD, and eGFR (CKD-EPI equation per 10 ml/min per 1.73 m2).
Grip strength cut-offs defined by sex- and BMI-specific cut-offs from the Cardiovascular Health Study (34).
Further adjustment for hemoglobin, C-reactive protein (CRP), and education only minimally affected the estimated associations of gait speed or TUAG with mortality. Associations of gait speed and TUAG with all-cause mortality were not attenuated after exclusion of 77 participants with prevalent mobility disability (among 321 participants with completed mobility disability assessment). Censoring deaths in the first 180 days of follow-up also minimally affected the association between gait speed and TUAG and mortality.
Predictive Ability of Physical Performance Measures
Among those with complete measurements of handgrip strength, gait speed, and TUAG (n=311), receiver operating characteristic curves for 3-year mortality among the four physical performance measures indicated the greatest area under the curve (AUC) for usual 6MWD (0.80; 95% CI, 0.70, 0.90), followed by gait speed (0.78; 95% CI, 0.70, 0.86) and TUAG (0.74; 95% CI, 0.64, 0.84). By comparison, HGS (0.66; 95% CI, 56, 0.75) had the lowest AUC for predicting death. Each of the lower extremity physical performance tests had an AUC that was superior to each of the individual biomarkers of CKD, which included eGFR (0.63; 95% CI, 0.52, 0.74), serum bicarbonate (0.53; 95% CI, 0.39, 0.67), hemoglobin (0.57; 95% CI, 0.44, 0.69), CRP (0.48; 95% CI, 0.36, 0.60), albumin (0.52; 95% CI, 0.40, 0.65), and phosphate (0.65; 95% CI, 0.54, 0.77). When gait speed was added to a base model including age, sex, and eGFR, there was a significant improvement in prediction of 3-year mortality from an AUC of 0.67 (95% CI, 0.54, 0.81) to an AUC of 0.83 (95% CI, 0.74, 0.96) (P<0.001) compared with an AUC of 0.80 (95% CI, 0.74, 0.96) when TUAG was added to the base model (P<0.01 compared with base model). Further addition of TUAG and 6-minute walk to gait speed did not appreciably improve discrimination of 3-year mortality.
Discussion
In a cohort of persons with CKD not treated with maintenance dialysis, we found substantially diminished performance in lower extremity function, but relatively preserved upper extremity muscle strength compared with normative values. Specifically, performance on usual gait speed, TUAG, and 6MWD was at least 30% lower than predicted. After adjustment for demographics and comorbidity, usual gait speed and TUAG were associated with all-cause mortality. These associations were also observed when the subgroup with baseline self-reported mobility disability was excluded. Moreover, lower extremity performance measures more strongly predicted mortality than estimates of kidney function or other common biomarkers. Taken together, these findings suggest that simple objective physical performance measures may be useful for risk stratification of CKD patients not treated with renal replacement therapy and may represent important therapeutic targets in this population.
To our knowledge, this is the first comprehensive study to quantify the decrements in physical performance compared with published normative values and to describe associations of each of these measures with mortality in a referred population with CKD. Large studies of community-dwelling older adults have shown declines in both upper and lower extremity physical performance among those with mild to moderate CKD.14 Our study demonstrated that persons with CKD suffer diminished lower extremity physical performance that corresponds with increased risk of all-cause mortality. The magnitude of excess risk associated with slower gait speed in these CKD patients was similar to that reported recently in a large meta-analysis of community-dwelling older adults,2 and highlights the importance of objective measures of usual gait speed in assessing risk of death in adults with nondialysis CKD.
In contrast to prior studies in older adult population, HGS was not associated with mortality, and the severity of impairment in this test of upper extremity performance was much more modest. HGS has traditionally been viewed as a strong predictor of mortality in older adults independent of physical activity and muscle mass15 and was more recently described to be associated with increased risk of mortality and initiation of dialysis in a small study of Taiwanese stage 1–5 CKD patients.16 We found that adjustment for eGFR markedly attenuated the crude associations of HGS with mortality. The lack of association was also noted in the analysis of HGS as a dichotomous variable using generally accepted sex- and BMI-specific cut-offs from the Cardiovascular Health Study to ensure generalizability. Our results suggest that lower extremity function may better capture the disease burden of CKD; however, more data are needed to clarify this distinction.
The higher prevalence of clinical and subclinical multisystem comorbidities related to the metabolic abnormalities and vascular dysfunction associated with CKD may in part help explain the strong association observed between physical performance and mortality. In older adults, the ability of gait speed and TUAG to capture comorbid burden highlights the importance of the multiple interactions among several different systems (e.g., nervous, cardiopulmonary, and musculoskeletal systems) involved in coordinating gait and balance. For example, slower gait speed and gait variability have been associated with subclinical cerebrovascular disease even in apparently high-functioning older adults,17–19 a condition that is markedly more common in older adults with impaired renal function. Metabolic abnormalities associated with mild to moderate CKD and uremia may also affect the vascular, neurologic, and musculoskeletal systems culminating in both subclinical and overt cardiovascular disease and physical impairment.20,21 Indeed, adverse effects of uremia on muscle metabolism22 may act to augment the insulin resistance and inflammation/oxidative stress associated with CKD.23,24 In the general population of older adults, insulin resistance in particular has been associated with decreased physical performance6,25 and with risk for cerebrovascular disease26 and cardiovascular disease in nonpatients with diabetes,27 but the role of insulin resistance to physical performance and vascular disease in CKD patients is less clear. Nonetheless, given the well described association between CKD, cognitive impairment, and subclinical cerebrovascular disease,28–30 it is possible that simple, objective measures of lower extremity physical performance reflect the cumulative multisystem comorbid burden associated with CKD and improve assessment of mortality risk.
Our study had several limitations. First, caution must be taken against ascribing a causal relationship between lower extremity physical performance and mortality risk from this observational study. Associations of lower extremity function with mortality may have been confounded by unmeasured characteristics or by the severity of comorbid conditions that could not be precisely captured by standard assessment. The presence of confounding weakens the case for impaired lower extremity performance as a direct cause for mortality but does not detract from the argument that lower extremity performance captures the complex disease manifestations of CKD. Second, the relatively low number of deaths increases the imprecision regarding the true magnitude of associations in the nondialysis CKD population at large, motivating a need for additional studies to replicate our findings in similar populations. Third, it is also possible that our follow-up time was not sufficiently long enough to capture significant differences in survival between those with strong and weak HGS. Finally, the incomplete assessment of self-reported exercise, which was not collected in the University of Maryland cohort, limits any assessment of the effect that planned exercise activity may have on the association between physical performance and mortality. Furthermore, from our results it cannot be determined whether lower physical activity is a consequence of or a cause of lower physical performance in persons with CKD.
In conclusion, our study demonstrates that lower extremity physical performance is substantially impaired in persons with CKD not treated with dialysis and is associated with all-cause mortality after adjustment. Associations with mortality were similar in magnitude to kidney function and were stronger than traditionally measured biomarkers of CKD. Measurements of lower extremity function are relatively easy to perform and may capture a complex set of skeletal muscle and neurologic impairments that develop in CKD patients and substantially affect their survival. These results argue for further investigation into the principle biologic mechanisms underlying decreased physical performance in CKD patients and evaluating whether interventions improving physical performance in CKD translate to improvements in overall comorbid burden and clinical outcomes.
Concise Methods
This analysis combined data from two distinct prospective cohort studies at two different institutions. Both studies were designed to investigate the role of physical performance measures in nondialysis CKD patients, and used similar procedures to measure each of the physical performance measures. Each study had its own protocol/manual of operations for data collection procedures.
Seattle Kidney Study
The Seattle Kidney Study (SKS) is a clinic-based, prospective cohort study of nondialysis CKD patients based in Seattle, Washington. Beginning in 2004, participants were recruited from outpatient nephrology clinics at HMC and the Veterans Affairs Puget Sound Medical Center. Inclusion criteria are age >18 years and CKD defined by eGFR <90 ml/min per 1.73 m2 or an albumin to creatinine ratio of ≥30 mg/g, based on blood and urine specimens provided at the first study visit. Exclusion criteria are an expected initiation of renal replacement therapy or the expectation to leave the area within 3 months, previous kidney transplantation, dementia, institutionalization, participation in a clinical trial, or inability to undergo the informed consent process. All participants in this study gave written informed consent.
UMD Study of CKD
The UMD study is an observational study of physical performance in older community-dwelling adults with stage 3–4 CKD. Participants were recruited from nephrology clinics at the UMD Medical Center and the Baltimore VAMC. Inclusion criteria were age 60–85 years and eGFR 15–60 ml/min per 1.73 m2 based on outpatient serum creatinine concentrations. Exclusion criteria included renal transplantation, dementia, institutionalization, prior stroke or carotid artery revascularization, non-English speaking, known HIV, severe anemia (hemoglobin <9 g/dl), and uncontrolled diabetes (glycated hemoglobin >11%).
The studies were approved by institutional review boards at the University of Washington, University of Maryland, Baltimore, and Veterans Affairs Health Care System. All participants provided written informed consent.
Control Population for TUAG Measurements
Controls for the TUAG test were older volunteer participants in studies of physical function at the University of Maryland Claude Pepper Older Americans Independence Center and the Baltimore Veterans Affairs Geriatric Research Education and Clinical Center. These participants were recruited from general medical clinics in the Baltimore VAMC and from community-dwelling adults in the greater Baltimore region responding to recruitment materials. They were free of CKD, CAD, stroke, chronic obstructive pulmonary disease, and HIV. Those who had hypertension and noninsulin-dependent diabetes were included.
For the purpose of this study, we combined data from SKS and UMD study participants who had an eGFR <90 ml/min per 1.73 m2 per the CKD-EPI equation, were not receiving chronic renal replacement therapy at the time of physical performance assessment, were stroke-free, were not using a wheelchair, and had completed at least one physical performance measurement. Data from both the SKS and UMD studies were used for survival analyses.
Physical Performance Measurements
Physical performance testing was conducted from September 2006 until June 2010 in the SKS cohort and March 2006 through June 2011 in the UMD cohort. Study coordinators at each study site performed the following four established tests of upper and lower extremity functioning: usual gait speed assessment, TUAG, HGS, and 6MWD. Usual gait speed was measured by asking participants to walk at their usual pace over a marked 4-m course, with the faster of two trials entered for analysis. TUAG was measured by recording the time to get up from a fully seated position, walk around a cone placed 4 m away, and then return to a seated position. The faster of two trials entered the analysis. HGS was assessed in the participant’s dominant hand using a Takei dynamometer (Takei Kiki Kogyo, Japan); the mean from three consecutive efforts entered the analysis. For 6MWD, coordinators asked participants to walk as fast as they could along a marked indoor corridor and recorded the total distance traveled after 6 minutes. If the participant could not complete the full 6-minute walk, the total distance completed was used. A single 6MWD was performed per participant study visit.
Assessment of Mortality
In the SKS cohort, vital status was assessed semi-annually via phone calls to study participants or their emergency contact. If contact was unsuccessful, then vital status was assessed using the Social Security Death Index. In the Maryland cohort, death was assessed via chart review.
Assessment of Other Study Data
Comorbid conditions were defined based on baseline participant responses to the study questionnaires and chart review (see the Supplemental Methods for prevalent disease definitions). Physical activity was measured only among participants in the SKS cohort using self-reported frequency of exercise, regarded as a planned, structured, and repetitive activity. This was categorized as never exercise, <1 time per week, 1 time per week, 2–3 times per week, and >3 times per week. Medication use was assessed using the inventory method at the HMC study site or using the electronic pharmacy database at the Maryland and VA study sites; missing medication data were verified by chart review. Coordinators measured BP and collected serum, plasma, and urine samples; these samples were performed on the same day as the physical performance evaluation in the SKS cohort and within 90 days of performance evaluation in the UMD cohort. Three seated BP measurements were recorded 5 minutes apart using an automated sphygmomanometer and the average of the last two readings was used for analysis. Blood samples were centrifuged for 20 minutes at 3300 rpm, transferred to cryovials, and stored at −80°C. General chemistries were measured from frozen serum using a Beckman-Coulter DXC autoanalyzer. In the SKS cohort only, serum cystatin C and CRP concentrations were measured using the Siemens Nephelometer, which utilizes a particle-enhanced immunonephelometric assay (N Latex Cystatin C).31 Routine calibration was performed using standards obtained from the manufacturer along with daily quality controls. We measured urinary albumin concentration by immunoturbidimetry and urinary creatinine concentration by the modified Jaffe method.
For primary analysis, we used the CKD-EPI equation to estimate GFR32 because serum creatinine concentrations were available for both cohorts. In sensitivity analysis, we used cystatin C to estimate GFR among the subset of SKS participants. Both equations provide similar precision and accuracy compared with gold-standard radioisotope dilution methods20,21; however, cystatin C-based eGFR may be preferable for studies of muscle function due to the interdependence of serum creatinine and muscle mass. We used the following equation to estimate GFR by cystatin C31:
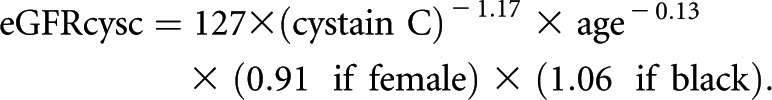 |
Assessment of Disability
Disability was assessed in the 327 participants in the SKS cohort by querying participants about difficulties with 15 tasks of daily life, including activities of daily living, instrumental activities of daily living, and mobility tasks.33 Mobility tasks included the ability to walk from room to room, walk up one flight of stairs, and walk one-half mile. Disability was defined as needing some help or being unable to perform a particular task. In keeping with previous studies, we categorized activities of daily living, instrumental activities of daily living, and mobility disabilities as the presence of ≥1 disability versus none.34
Statistical Analyses
We compared each participant’s physical performance score to predicted values for HGS, gait speed, TUAG, and 6MWD using published data and normative equations to determine the percentage of predicted value. HGS was compared with the predicted value based on age and sex in the general population.35 Usual gait speed was compared with published predicted values based on sex, age, and height.36 For TUAG, a normative equation was derived from 78 non-CKD controls at the Baltimore VAMC (Supplemental Table 1). The percentage of predicted performance was calculated as follows: [(observed performance)/predicted performance]×100. For TUAG, values >100% of predicted indicated worse performance because longer time to complete the task reflected slower movement. For 6MWD, normative equations were obtained from published literature for 6MWD.37 We used a paired t test to test differences in actual physical performance from predicted values by testing the difference between the natural log of the percentage of predicted for each individual from the natural log of 100% for each performance task.
For survival analysis, participants began accruing risk time from the time they completed physical performance measurement and were followed until death or censoring due to the end of available follow-up time. In these analyses, physical performance was measured as continuous and dichotomous variables using established cut points from previous studies (Supplemental Table 2).2,34,38,39 Given that a substantial portion of participants from the SKS cohort are aged <65 years and have a high prevalence of heart failure (24%), diabetes (55%), and coronary heart disease (26%), we selected a 6MWD cut-point based on previous studies of patients with cardiopulmonary disorders. Previously published studies of participants with cardiopulmonary disorders have demonstrated consistent associations of 6MWD <350 m with mortality. Moreover, 350 m corresponded to the lowest 30th percentile of performance on the 6-minute walk in our cohort.
We calculated unadjusted mortality rates for each measure as the number of deaths per 1000 person-years and used the Kaplan–Meier method to estimate unadjusted cumulative survival during follow-up, with the log-rank test to assess for statistical differences in survival. We used the Cox proportional hazards regression models to estimate adjusted hazard ratios for all-cause mortality associated with each physical performance measure. The proportional hazards assumption was confirmed using by Schoenfeld global test. Two sets of adjustment covariates were selected before analysis. Model 1 included age, sex, race, and study site. Model 2 added comorbidities and cardiovascular risk factors including eGFR, smoking, BMI, diabetes, and prevalent CAD. In a first sensitivity analysis, we additionally adjusted for education, hemoglobin, and CRP. In a second sensitivity analysis, we excluded individuals who had baseline mobility disability from the analysis. In the third sensitivity analysis, we censored those participants who died in the first 180 days at the time of their death in order to exclude those at imminent risk of death. We performed multiple imputation for education and CRP using chained equations given 8% missing data for these covariates.40
We plotted receiver operating characteristic curves to estimate AUCs for 3-year mortality for each physical performance measure, eGFR, and traditionally measured CKD biomarkers restricting the analysis to participants with completed grip strength, gait speed, and TUAG assessments to ensure a consistent study sample for comparisons between different physical performance measurements. Differences in AUCs for these models were used to assess the added discriminatory value of the physical performance measure for 3-year mortality. The hold-out cross-validation method was used to obtain an unbiased estimate of predictive ability using a random sample composed of 66% of the cohort.41 Final unbiased AUC results for multivariate logistic regression models were obtained using repeated random subsampling validation in which 10 randomly selected validation datasets each composed of 66% of the cohort were used to evaluate the predictive ability of the model. The AUCs and SEMs derived from these randomly generated datasets were averaged to arrive at a final AUC and 95% CI. The likelihood ratio test was used to test for statistically significant differences between nested multivariate models.
Disclosures
None.
Acknowledgments
We thank study coordinators Noah Citron, Georgia Galvin, and Jamie Giffuni for their contributions to the study.
This work was supported by grants from the National Institutes of Health (R01-HL070938 to J.H. and B.K. and K23-DK063079 to S.S.), the Kidney Research Institute, the National Institute of Diabetes and Digestive and Kidney Diseases (T32-DK007467-28 and F32-DK093235 to B.R.), the VA Rehabilitation R&D Merit Review (to S.S. and L.K.), the National Institute on Aging Claude D. Pepper Older Americans Independence Center (P30-AG028747), the Department of Veterans Affairs, and the Baltimore VAMC Geriatric Research Education and Clinical Center, as well as an unrestricted grant from the Northwest Kidney Center Foundation. A.J.L. was supported by a VA Rehabilitation Research and Development Career Development Award (#6982). This study is the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, Washington, and the VA Maryland Healthcare System, Baltimore, Maryland.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Physical Performance and All-Cause Mortality in CKD,” on pages 689–690.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012070702/-/DCSupplemental.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 299: 2038–2047, 2007 [DOI] [PubMed]
- 2.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J: Gait speed and survival in older adults. JAMA 305: 50–58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L: Midlife hand grip strength as a predictor of old age disability. JAMA 281: 558–560, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Sasaki H, Kasagi F, Yamada M, Fujita S: Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med 120: 337–342, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Atkinson HH, Rosano C, Simonsick EM, Williamson JD, Davis C, Ambrosius WT, Rapp SR, Cesari M, Newman AB, Harris TB, Rubin SM, Yaffe K, Satterfield S, Kritchevsky SB, Health ABC study : Cognitive function, gait speed decline, and comorbidities: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci 62: 844–850, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Kuo CK, Lin LY, Yu YH, Wu KH, Kuo HK: Inverse association between insulin resistance and gait speed in nondiabetic older men: Results from the U.S. National Health and Nutrition Examination Survey (NHANES) 1999-2002. BMC Geriatr 9: 49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB: A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49: M85–M94, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB: Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 295: 2018–2026, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Yeun JY, Levine RA, Mantadilok V, Kaysen GA: C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 35: 469–476, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C: Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int 55: 648–658, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Elbaz A, Ripert M, Tavernier B, Février B, Zureik M, Gariépy J, Alpérovitch A, Tzourio C: Common carotid artery intima-media thickness, carotid plaques, and walking speed. Stroke 36: 2198–2202, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Inzitari M, Naydeck BL, Newman AB: Coronary artery calcium and physical function in older adults: The Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 63: 1112–1118, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odden MC, Chertow GM, Fried LF, Newman AB, Connelly S, Angleman S, Harris TB, Simonsick EM, Shlipak MG, for the HABC Study : Cystatin C and measures of physical function in elderly adults: The Health, Aging, and Body Composition (HABC) Study. Am J Epidemiol 164: 1180–1189, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Metter EJ, Talbot LA, Schrager M, Conwit R: Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 57: B359–B365, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Chang YT, Wu HL, Guo HR, Cheng YY, Tseng CC, Wang MC, Lin CY, Sung JM: Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrol Dial Transplant 26: 3588–3595, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB: Special article: Gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci 63: 1380–1388, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosano C, Brach J, Longstreth WT, Jr, Newman AB: Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology 26: 52–60, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Rosano C, Brach J, Studenski S, Longstreth WT, Jr, Newman AB: Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology 29: 193–200, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwong YT, Stevens LA, Selvin E, Zhang YL, Greene T, Van Lente F, Levey AS, Coresh J: Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis 56: 39–49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conjard A, Ferrier B, Martin M, Caillette A, Carrier H, Baverel G: Effects of chronic renal failure on enzymes of energy metabolism in individual human muscle fibers. J Am Soc Nephrol 6: 68–74, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Landau M, Kurella-Tamura M, Shlipak MG, Kanaya A, Strotmeyer E, Koster A, Satterfield S, Simsonick EM, Goodpaster B, Newman AB, Fried LF, Health, Aging and Body Composition Study : Correlates of insulin resistance in older individuals with and without kidney disease. Nephrol Dial Transplant 26: 2814–2819, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siew ED, Ikizler TA: Insulin resistance and protein energy metabolism in patients with advanced chronic kidney disease. Semin Dial 23: 378–382, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Barzilay JI, Cotsonis GA, Walston J, Schwartz AV, Satterfield S, Miljkovic I, Harris TB, Health ABC Study : Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged >or=70 years. Diabetes Care 32: 736–738, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thacker EL, Psaty BM, McKnight B, Heckbert SR, Longstreth WT, Jr, Mukamal KJ, Meigs JB, de Boer IH, Boyko EJ, Carnethon MR, Kizer JR, Tracy RP, Smith NL, Siscovick DS: Fasting and post-glucose load measures of insulin resistance and risk of ischemic stroke in older adults. Stroke 42: 3347–3351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith NL, Barzilay JI, Shaffer D, Savage PJ, Heckbert SR, Kuller LH, Kronmal RA, Resnick HE, Psaty BM: Fasting and 2-hour postchallenge serum glucose measures and risk of incident cardiovascular events in the elderly: The Cardiovascular Health Study. Arch Intern Med 162: 209–216, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Kurella M, Chertow GM, Fried LF, Cummings SR, Harris T, Simonsick E, Satterfield S, Ayonayon H, Yaffe K: Chronic kidney disease and cognitive impairment in the elderly: The health, aging, and body composition study. J Am Soc Nephrol 16: 2127–2133, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Yaffe K, Ackerson L, Kurella Tamura M, Le Blanc P, Kusek JW, Sehgal AR, Cohen D, Anderson C, Appel L, Desalvo K, Ojo A, Seliger S, Robinson N, Makos G, Go AS, Chronic Renal Insufficiency Cohort Investigators : Chronic kidney disease and cognitive function in older adults: Findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc 58: 338–345, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto Y, Ohara T, Nagakane Y, Tanaka E, Morii F, Koizumi T, Akiguchi I: Chronic kidney disease, 24-h blood pressure and small vessel diseases are independently associated with cognitive impairment in lacunar infarct patients. Hypertens Res 34: 1276–1282, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Erlandsen EJ, Randers E, Kristensen JH: Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest 59: 1–8, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitti JE, Kovar MG: The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat 1 (21): 1–115, 1987 [PubMed] [Google Scholar]
- 34.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group : Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Mathiowetz V, Weber K, Volland G, Kashman N: Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am 9: 222–226, 1984 [DOI] [PubMed] [Google Scholar]
- 36.Bohannon RW: Comfortable and maximum walking speed of adults aged 20-79 years: Reference values and determinants. Age Ageing 26: 15–19, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Troosters T, Gosselink R, Decramer M: Six minute walking distance in healthy elderly subjects. Eur Respir J 14: 270–274, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE: The six-minute walk test: A useful metric for the cardiopulmonary patient. Intern Med J 39: 495–501, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Bischoff HA, Stähelin HB, Monsch AU, Iversen MD, Weyh A, von Dechend M, Akos R, Conzelmann M, Dick W, Theiler R: Identifying a cut-off point for normal mobility: A comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing 32: 315–320, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Royston P: Multiple imputation of missing values. Stata J 4: 227–241, 2004 [Google Scholar]
- 41.Arlot S, Celisse C: A survey of cross-validation procedures for model selection. Stat Surv 4: 40–79, 2010 [Google Scholar]