Abstract
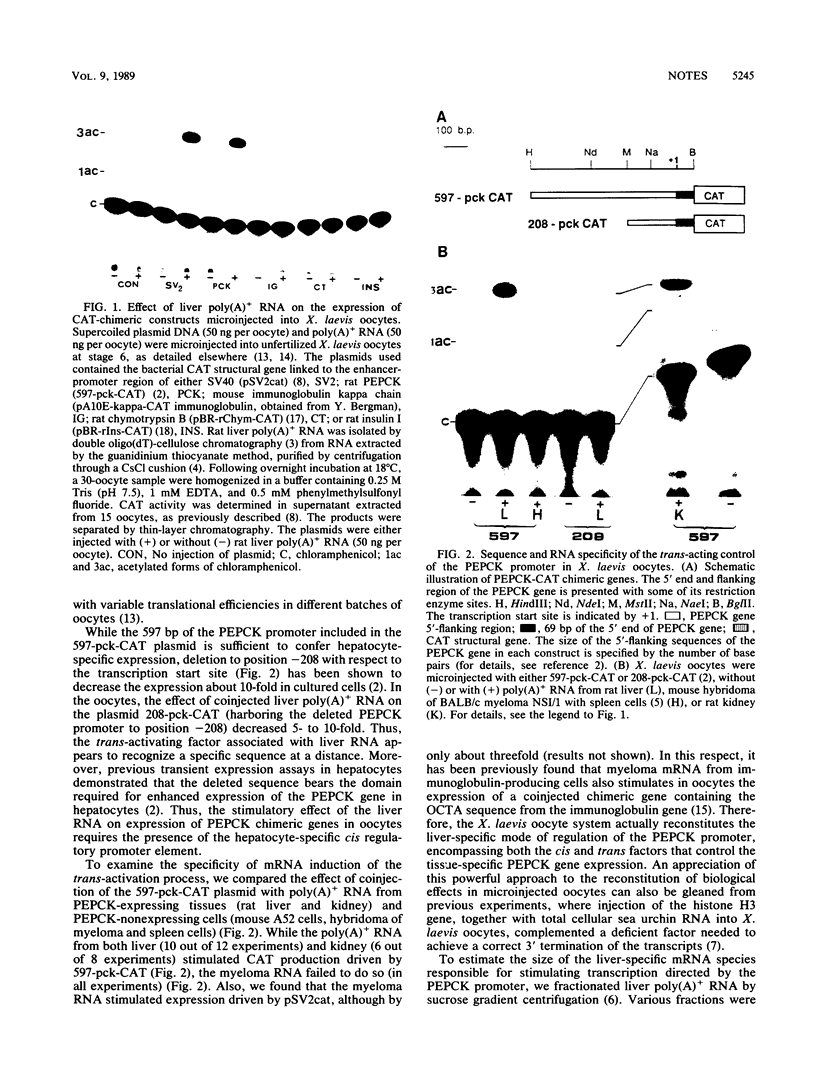
To study the liver-specific trans activation of the rat phosphoenolpyruvate carboxykinase (PEPCK) gene, the PEPCK promoter was linked to a reporter gene and was microinjected into Xenopus laevis oocytes alone or in conjunction with rat liver poly(A)+ RNA. The rat liver mRNA markedly enhanced the expression of the PEPCK-chimeric construct. This effect appeared to be sequence specific, as it was dependent on the presence of the intact promoter. Moreover, the RNA effect was limited to mRNA preparations from PEPCK-expressing tissues only. Finally, microinjection of size-fractionated liver mRNA revealed that the trans-acting factor(s) is encoded by RNA of 1,600 to 2,000 nucleotides, providing a direct bioassay for the gene(s) involved in this tissue-specific trans-activation process.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benvenisty N., Nechushtan H., Cohen H., Reshef L. Separate cis-regulatory elements confer expression of phosphoenolpyruvate carboxykinase (GTP) gene in different cell lines. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1118–1122. doi: 10.1073/pnas.86.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister M., Avivi A., Schlessinger J., Soreq H. Production of EGF-containing polypeptides in Xenopus oocytes microinjected with submaxillary gland mRNA. EMBO J. 1984 Jul;3(7):1499–1505. doi: 10.1002/j.1460-2075.1984.tb02002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Eilat D., Webster D. M., Rees A. R. V region sequences of anti-DNA and anti-RNA autoantibodies from NZB/NZW F1 mice. J Immunol. 1988 Sep 1;141(5):1745–1753. [PubMed] [Google Scholar]
- Farkash Y., Soreq H., Orly J. Biosynthesis of catalytically active rat testosterone 5 alpha-reductase in microinjected Xenopus oocytes: evidence for tissue-specific differences in translatable mRNA. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5824–5828. doi: 10.1073/pnas.85.16.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G., Hofstetter H., Stunnenberg H. G., Birnstiel M. L. Biochemical complementation with RNA in the Xenopus oocyte: a small RNA is required for the generation of 3' histone mRNA termini. Cell. 1983 Oct;34(3):823–828. doi: 10.1016/0092-8674(83)90539-1. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Ikeda T., Coady M., Gundersen C. B., Wright E. M. Expression of size-selected mRNA encoding the intestinal Na/glucose cotransporter in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1987 May;84(9):2634–2637. doi: 10.1073/pnas.84.9.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- McGrane M. M., de Vente J., Yun J., Bloom J., Park E., Wynshaw-Boris A., Wagner T., Rottman F. M., Hanson R. W. Tissue-specific expression and dietary regulation of a chimeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic mice. J Biol Chem. 1988 Aug 15;263(23):11443–11451. [PubMed] [Google Scholar]
- Soreq H., Parvari R., Silman I. Biosynthesis and secretion of catalytically active acetylcholinesterase in Xenopus oocytes microinjected with mRNA from rat brain and from Torpedo electric organ. Proc Natl Acad Sci U S A. 1982 Feb;79(3):830–834. doi: 10.1073/pnas.79.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H. The biosynthesis of biologically active proteins in mRNA-microinjected Xenopus oocytes. CRC Crit Rev Biochem. 1985;18(3):199–238. doi: 10.3109/10409238509085134. [DOI] [PubMed] [Google Scholar]
- Sweeney G. E., Old R. W. Trans-activation of transcription, from promoters containing immunoglobulin gene octamer sequences, by myeloma cell mRNA in Xenopus oocytes. Nucleic Acids Res. 1988 Jun 10;16(11):4903–4913. doi: 10.1093/nar/16.11.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Gurdon J. B. Post-transcriptional processing of simian virus 40 late transcripts in injected frog oocytes. J Mol Biol. 1983 Jan 5;163(1):1–26. doi: 10.1016/0022-2836(83)90027-x. [DOI] [PubMed] [Google Scholar]