Abstract
Exosomes are the newest family member of ‘bioactive vesicles’ that function to promote intercellular communication. Exosomes are derived from the fusion of multivesicular bodies with the plasma membrane and extracellular release of the intraluminal vesicles. Recent studies have focused on the biogenesis and composition of exosomes as well as regulation of exosome release. Exosomes have been shown to be released by cells of hematopoietic and non‐hematopoietic origin, yet their function remains enigmatic. Much of the prior work has focused on exosomes as a source of tumor antigens and in presentation of tumor antigens to T cells. However, new studies have shown that exosomes might also promote cell‐to‐cell spread of infectious agents. Moreover, exosomes isolated from cells infected with various intracellular pathogens, including Mycobacterium tuberculosis and Toxoplasma gondii, have been shown to contain microbial components and can promote antigen presentation and macrophage activation, suggesting that exosomes may function in immune surveillance. In this review, we summarize our understanding of exosome biogenesis but focus primarily on new insights into exosome function. We also discuss their possible use as disease biomarkers and vaccine candidates.
Keywords: antigens, biomarkers, exosomes, immunity, multivesicular bodies, mycobacteria, vaccine
The number of studies concerning bioactive vesicles has significantly increased in recent years with much of this increase stemming from work on exosomes. This class of bioactive vesicles is formed through the fusion of multivesicular bodies (MVBs) with the plasma membrane and release of intraluminal vesicles (ILVs) as exosomes. Originally observed as a mechanism to remove transferrin receptor during reticulocyte maturation (1), more recent studies have focused on the role of exosomes in antigen presentation (reviewed in 2). The presence of antigens on exosomes released from tumor cells and the ability of these exosomes to stimulate anti‐tumor responses in vitro and in vivo suggest that their use may hold therapeutic promise for cancer patients. An area of very recent interest has been on exosomes role in the spread of pathogens as well as their potential function in promoting or regulating an immune response upon infection. Studies have shown that exosomes may function in the cell‐to‐cell spread of HIV 3, 4, although this may simply reflect analogous biogenesis/trafficking of exosome and envelope proteins (5). Exosomes have also been successfully used as vaccines against various pathogens. Finally, studies have suggested that exosomes released from cells infected with intracellular pathogens such as Mycobacterium tuberculosis (M. tb) contain microbial components and that these exosomes have immune modulatory activity. In this review, we give a general summary of MVBs and exosomes but focus primarily on their diverse functions as well as their potential usefulness as vaccines and disease biomarkers.
MVB: Biogenesis and Functions
Eukaryotic cells secrete proteins from the biosynthetic pathway by constitutive exocytosis of secretory vesicles or by regulated release of secretory/storage granules upon appropriate stimulation. However, recently, the endocytic network has also been demonstrated to contain an alternative secretory pathway (6). Pan and Johnstone were the first to describe this pathway in reticulocytes 7, 8. Subsequent to its discovery in reticulocytes, it has been shown to be present in many cell types including B lymphocytes (9), dendritic cells (DCs) (10), platelets (11), epithelial cells (12) and neurons (13). The studies in reticulocytes demonstrated that this alternative secretory pathway involves the fusion of MVBs with the plasma membrane and extracellular release of the material present within the ILVs. The MVB is an intermediate but a well‐defined compartment that is formed from endosomes by invagination of the limiting endosomal membrane.
Although it has been almost 30 years since electron microscopy (EM) studies suggested the presence of MVBs in cells, there are still significant gaps in our understanding of MVB biogenesis (14). The process has to be well co‐ordinated as it dictates the composition and the fate of ILVs. Only recently have certain mechanisms for protein sorting to MVBs been elucidated and include (i) ubiquitination of the target and (ii) preferential aggregation. Using a Saccharomyces cerevisiae experimental system, it has been shown that monoubiquitination of endosomal proteins serves as a signal for sorting to MVBs (15). Some studies have also suggested that oligoubiquitination may also be a sorting signal for trafficking to MVBs, which may increase MVB sorting efficiency (16). A key player in MVB biogenesis is the hetero‐oligomeric protein complex, endosomal sorting complex required for transport (ESCRT). ESCRT‐I, ‐II and ‐III recognize monoubiquitinated cargoes and promote their inclusion in MVBs (14). Once completed, the ESCRT complex dissociates from the MVB membrane aided by the adenosine triphosphatase vacuolar protein sorting 4 (Vps4) and is recycled for subsequent cargo (17). As summarized in Figure 1, hepatocyte growth factor regulated tyrosine kinase substrate (HRS) binds ubiquitinated cargo while simultaneously recruiting the ESCRT family of proteins and mobilizes the cargo for inclusion into MVBs.
Figure 1.

MVB biogenesis and exosome release. Monoubiquitination or aggregation provides the signal for trafficking of proteins and lipids to MVBs. The machinery for sorting ubiquitinated proteins involves the multi‐domain Vps27/HRS protein that acts as a bridge between monoubiquitinated transmembrane proteins and clathrin on endosomes (94). ESCRT is also a key player in MVB biogenesis. ESCRT‐I, ‐II and ‐III recognize monoubiquitinated cargoes and promote their inclusion in MVBs (95). Once completed, the ESCRT complex dissociates from the MVB membrane. Interestingly, the proteins within the ILVs are enriched for ubiquitin, indicating that not all of the ubiquitin is removed from proteins upon targeting to the MVB. Fusion of the MVB and release of the ILVs as exosomes are regulated and are known to require PLD, calcium and Rab11. PLD, phospholipase D.
However, some proteins such as the transferrin receptor are present in ILVs but are not ubiquitinated. These proteins, which lack the sorting signal for ubiquitination, are partitioned into the ILVs based on their intrinsic physical properties and preference to segregate into raft‐like microdomains (18). Molecular aggregation of transferrin receptor in reticulocytes reroutes the receptor from the recycling compartment to the MVB (19). Studies by Geminard et al. indicate that the transferrin receptor can interact with the ESCRT machinery despite the lack of ubiquitination (20). Additional studies have demonstrated that protein aggregation induced by antibodies led to the defective sorting of antigens to MVBs (19). Thus, protein clustering appears to be a major determinant in protein trafficking to the MVB. Recent studies by Theos et al. demonstrated a trafficking of melanosomal protein Pmel17 to MVBs in an ubiquitin‐ and ESCRT‐independent manner (21). In summary, not all cargoes are recruited to MVBs by the same mechanism, and there are still significant gaps in our understanding of how different cargoes are targeted to ILVs and how the ILV formation occurs.
In contrast to our limited knowledge of MVB biogenesis, their function has been well defined and plays a central role in endocytic trafficking. In most cell types, the MVBs fuse with the lysosomal compartment and thus shuttle MVB cargo for degradation. However, MVBs can also fuse with the plasma membrane and release their ILVs as ‘exosomes’(22).
Exosomes are 30‐ to 100‐nm lipid bilayer vesicles with a density of 1.13 g/mL (for B cell derived) to 1.19 g/mL (for intestinal cell derived). Biophysically, exosomes are equivalent to cytoplasm enclosed in a lipid bilayer with the external domains of transmembrane proteins exposed to the extracellular environment. EM studies have demonstrated the fusion of the limiting membrane of MVB with the plasma membrane as well as release of ILVs in different cell types of hematopoietic origin, such as Epstein–Barr virus (EBV)‐transformed B cells (9), mastocytes (23), DCs 10, 24, platelets (11), macrophages (25) and cells of non‐hematopoietic origin like neurons and epithelial cells (13).
Exosome Composition
The lipid and protein content of exosomes has been extensively analyzed by various techniques including Western blotting, fluorescence‐activated cell sorting, immuno‐EM and mass spectrometry. Exosome composition varies depending on the cell type of origin. Nevertheless, exosomes contain a number of common protein components (26). As shown in Figure 2, the cytosolic proteins present on exosomes include Rabs, which promote exosome docking and the membrane fusion events (27). The annexins, including annexin I, II, V and VI, may regulate membrane cytoskeleton dynamics and membrane fusion events (28). Several adhesion molecules such as intercellular adhesion molecule‐1, CD146, CD9, milk‐fat‐globule EGF‐factor VIII (MFG‐E8), CD18, CD11a, CD11b, CD11c, CD166 and LFA‐3/CD58 have also been identified in exosomal preparations 26, 27. In addition, several proteins involved in apoptosis are present on exosomes including thioredoxin peroxidase II, Alix, 14‐3‐3 and galectin 3. Exosomes also contain heat‐shock proteins Hsp70 and Hsp90, which can facilitate peptide loading onto major histocompatibility complex (MHC)I and MHCII (29). One of the characteristic features of exosomes is the tetraspanins, which include CD9, CD63, CD81 and CD82. Exosomes also carry some cell‐specific proteins like MHCII and CD86 present only on exosomes isolated from antigen‐presenting cells (APCs) (30) and MFG‐E8/lactadherin present on exosomes from immature DCs (31). Exosomes are also enriched in proteins that participate in vesicle formation and trafficking like the lyso‐bisphosphatidic acid (LBPA)‐binding protein Alix (28). Other proteins detected on exosomes are the metabolic enzymes such as peroxidases, pyruvate and lipid kinases and enolase‐1 (32). Consistent with their endosomal origin, exosomes typically do not contain endoplasmic reticulum, mitochondria or nuclear proteins.
Figure 2.
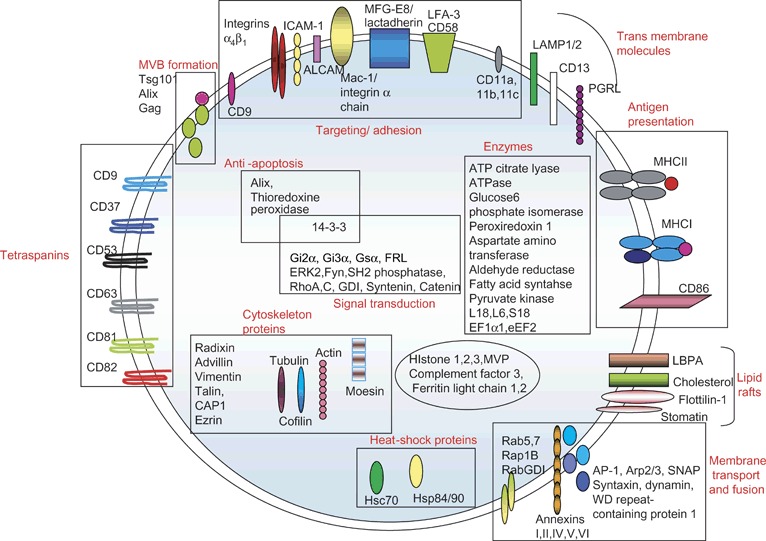
Protein composition of exosomes indicating their name, their location (i.e. membrane bound or soluble) and in some cases their function. GDI, GTP dissociation inhibitor; ICAM1, intercellular adhesion molecule‐1; CAP‐1, adenylyl cyclase‐associated protein; LAMP, lysosomal associated membrane protein‐1; PGRL, PG regulatory‐like protein.
Similar to proteins, lipids present on exosomes are characteristic of the cell origin, with most of the lipid analytical work being performed on exosomes derived from reticulocytes (33), mast cells (34), B lymphocyte cell lines (35) and human DCs (34). The typical lipid composition of mast cell‐derived exosomes includes lysophosphatidylcholine, sphingomyelin, phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, cholesterol and diglyceride (34). Although most of these lipids are also present on exosomes isolated from other cell types, and the ratios of these lipids vary. For instance, the cholesterol/phospholipid ratio is higher in B‐cell‐derived exosomes compared with exosomes from mast cells and reticulocytes (36). Phospholipids like LBPA accumulate in MVBs and appear to play a key role in ILV formation (37). Recent studies by de Gassart et al. have demonstrated the presence of lipid rafts on the exosomes (18). These low‐density, Triton‐insoluble fractions are enriched in cholesterol and glycosphingolipids and contain different acylated proteins such as glycosyl‐phosphatidylinositol‐anchored proteins and tyrosine kinases of the Src family (38).
Exosome Function
The release of exosomes provides another mechanism of intercellular communication. Exosome function will depend on the cell type from which they were derived and the composition of exosomes in terms of lipids, carbohydrates and proteins. Because exosomes contain a spectrum of surface molecules, they provide a mechanism to engage different cell receptors simultaneously and for exchange of material between cells.
Alternative secretion of proteins by exosomes
Exosome release was initially characterized as a mechanism to eliminate obsolete proteins during reticulocyte maturation and differentiation. Johnstone et al. showed that sheep red blood cells lose their transferrin receptor during in vivo and in vitro maturation by exosome release (39). The transferrin receptor is tightly associated with Hsp70 on exosomes, and the heat‐shock protein may play a role in exosome release from reticulocytes. How soluble proteins that lack a signal sequence are released from cells has been the focus of considerable research, and some mechanisms have recently been defined. One method is through association with exosomes, as observed for the translationally controlled tumor protein (TCTP), a cytoplasmic protein that facilitates an inflammatory response by stimulating histamine release. Tumor suppressor activated pathway 6 (TSAP6), a p53‐inducible 5–6 transmembrane protein, has been reported to associate with exosomes as well as with cytoplasmic TCTP, thus facilitating the release of TCTP through exosomes (40). A more recent study by Yu et al. demonstrated that exosomes function in the release of some p53‐regulated extracellular proteins and that this effect stems from the upregulation of TSAP6 expression by p53 (41). Another function attributed to exosomes includes the constitutive extracellular release of tumor necrosis factor (TNF) receptor 1 (42). This established a new mechanism for release of soluble cytokine receptors, which could compete for ligand binding. The cytokine interleukin (IL)‐1β and possibly the chemokine regulated activation normal T cell expressed and secreted (RANTES) may also be secreted by exosomes (43). Other studies have defined a role for exosomes in ectodomain shedding and consequently a vehicle for the cellular export of soluble molecules like L1 (CD171) and CD44 in ovarian carcinoma cells (44). A study by Azevedo et al. showed that in sepsis, nitric oxide and bacterial elements are responsible for type‐specific platelet‐derived exosome generation (45). These exosomes play an active role in vascular signaling as redox active particles, which induced endothelial cell caspase‐3 activation and apoptosis of vascular cells (46).
Antigen presentation
Pioneering studies by Raposo et al. showed that exosomes secreted by EBV‐transformed B cells can stimulate human CD4+ T‐cell clones in an antigen‐specific manner (9). Their studies were the first to document the release of intact MHCII on the exosomes secreted by both human and murine B‐cell lines. Subsequent studies have shown that exosomes produced by mouse DCs pulsed with tumor peptides induced the rejection of established tumors in a T‐cell‐dependent fashion (10). Exosomes can also transfer antigens from tumor cells to DCs (47) and therefore functions in antigen cross‐presentation. Like DC‐derived exosomes, exosomes from tumor cells carry MHC molecules along with tumor antigens like melan‐A/MART1 (melanoma tumor), which can be recognized by T cells (27). Together, the data implicate that exosomes can function in presenting tumor antigens to sensitized T cells and can promote tumor rejection in vivo 10, 43. A number of excellent reviews are available on exosomes role in tumor immunity 48, 49, 50. Interestingly, recent studies by Muntasell et al. showed that in a 24‐h period, ∼12% of the surface‐bound peptide–MHCII complex is endocytosed, trafficked to MVBs and released on exosomes, suggesting that exosome discharge may be a common mechanism to seed secondary lymphoid organs with membrane‐bound antigen (51).
In addition to their role in antigen presentation, exosomes have also been implicated in immune suppression. This was nicely demonstrated by Peche et al. who showed that injecting donor‐haplotype exosomes from bone marrow DCs before transplantation leads to a significantly prolonged heart allograft survival in congenic and fully MHC‐mismatched Lewis rats (52). Moreover, in vivo studies showed a significant decrease in CD4+ T cells in the exosome‐treated recipient, suggesting an immunotolerance effect (52). Exosomes isolated from immature DCs treated with cytokines, such as IL‐4 and IL‐10, when injected into mice reduced the severity of established collagen‐induced arthritis 53, 54. DCs that were virally transduced to produce Fas ligand (FasL) also produced exosomes with anti‐inflammatory activity (55). Therefore, the use of exosomes may be a better therapeutic approach compared with DCs for the treatment of autoimmune diseases such as rheumatoid arthritis. ‘Tolerosomes’ corresponding to exosome‐like structures are produced by intestinal epithelial cells and could induce tolerance to oral antigens 56, 57, 58. Moreover, exosomes from T cells, melanoma cells and ovarian tumor cells have been shown to carry FasL, which could induce T‐cell apoptosis 59, 60. Tumor‐derived exosomes may also impair DC development and induce myeloid‐suppressive cells (61).
Shuttle for RNA
Elegant studies by Valadi et al. showed that exosomes are enriched in messenger RNA and micro RNA. The exosomes derived from a human (HMC‐1) and mouse (MC/9) mast cell lines were found to transport RNA to neighboring mast cells, which was then translated indicating that the transferred RNA was biologically active. The RNA transferred through exosomes (exosomal shuttle RNA) can confer new functions to the cells (62).
Shuttle for infectious agents
The cellular process associated with MVB biogenesis and release has been commandeered during evolution by various pathogens, including viruses, to provide an escape mechanism from the host immune response. Indeed, an evolutionary link between retroviruses and exosome biogenesis has been proposed (63). Studies with retroviruses have revealed the ability of viruses to hijack the intracellular machinery of MVBs for their budding at the cell surface (64). HIV utilizes MVBs as the major site for accumulation in human macrophages 25, 63, and the viruses released have markers commonly found on exosomes. However, although the HIV particles and exosome contain similar components, they may have different origins (5). Interestingly, recent studies in HIV‐1‐infected macrophages have suggested that HIV‐1 is present within internally sequestered CD63‐positive plasma membrane domains but not in endosomes (65).
New studies have revealed an unexpected role for exosomes in the spread of prions. Prion diseases are fatal neurodegenerative disorders that affect both humans and animals. Raposo and colleagues have demonstrated that prion protein (PrP) in both its normal (PrPc) and its scrappie (PRPsc) conformation are trafficked to MVBs and released on exosomes (66).
Pathogen immune surveillance – a novel function of exosomes
Recent work has yielded substantial insight into the immune responses required for controlling an infection and how pathogens circumvent these mechanisms. It is now apparent that innate effector mechanisms are initiated through specific detection of microbial patterns, which facilitate an immune response. These microbial signatures are referred to as pathogen‐associated molecular patterns (PAMPs) and are specifically recognized by the host’s pattern recognition receptors (67). Therefore, PAMPs expressed on the surface or released by the pathogen play an essential role in stimulating immunity. By their nature, intracellular pathogens show a more limited exposure to the immune system compared with extracellular pathogens, and this includes limited exposure of PAMPs. We and others hypothesized that release of exosomes from infected cells may be one mechanism by which this sequestration of PAMPs can be overcome.
The first evidence to support this hypothesis came from a series of insightful experiments by Russell and colleagues where they identified a number of the mycobacteria cell wall components that were trafficked inside the infected cell (68). EM studies by Beatty et al. identified mycobacterial PAMPs including lipoarabinomannan (LAM) and phosphatidyl‐myo‐inositol mannosides (PIM) in the endocytic compartment of Mycobacterium bovis BCG‐infected macrophages (68). Density gradient electrophoresis analysis of the infected cells also suggested that released mycobacterial lipids coalesce in the late endosomal/lysosomal compartments, including MVBs (68). Studies directed toward the trafficking of Mycobacterium avium glycopeptidolipids (GPL) also indicated a similar release and trafficking of the GPL inside the macrophages (69). Moreover, these studies indicated that the mycobacterial PAMPs were not confined to the infected cells but were also trafficked to the neighboring bystander cells 68, 69, 70, 71. These results raised an important question about the mechanism of mycobacterial component secretion.
Beatty et al. originally determined that vesicles the size of exosomes contain mycobacterial components (68). Additional studies indicated that the mycobacterial components, including GPL, LAM, PIM, trehalose dimycolate and phenolic glycolipids, were released from mycobacterial‐infected macrophages through exosomes (Figure 3A) 69, 71 and that these exosomes were captured by the bystander uninfected cells. Interestingly, some of these lipids have been shown to induce a proinflammatory response when introduced to uninfected macrophages. For instance, PIM2 coated on microspheres could induce TNF‐α and mycobacteria‐containing phagosome‐1 in interferon‐γ‐primed bone marrow macrophages or thioglycollate‐elicited peritoneal macrophages (71). Moreover, as indicated above, exosomes can function to modulate immune responses, including immune stimulation and immune suppression. Our recent studies demonstrated that exosomes derived from Mycobacterium‐infected cells were capable of inducing a proinflammatory response as indicated by the TNF‐α and RANTES secretion and inducible nitric oxide synthase (iNOS) induction in naïve cells (Figure 3B) 69, 72. This response was completely dependent on MyD88, an adaptor molecule required for most Toll‐like receptor signaling (69). The stimulatory activity of exosomes were also replicated in vivo where mice injected with exosomes derived from M. bovis bacille Calmette–Guérin (BCG) or M. tb‐infected cells induced a TNF‐α and IL‐12p40 response as well as neutrophil and alveolar macrophage recruitment into the bronchoalveolar lavage fluid (BALF) (69). Exosomes have been shown to be released in the biological fluids like urine (73), amniotic fluid (74), BALF (75) and plasma (76). Exosomes isolated from the BALF of the mice infected with M. bovis BCG also contained PAMPs and could induce a proinflammatory response in treated macrophages (72). These results, for the first time, reveal exosomes ability to induce a proinflammatory response both in vitro and in vivo.
Figure 3.
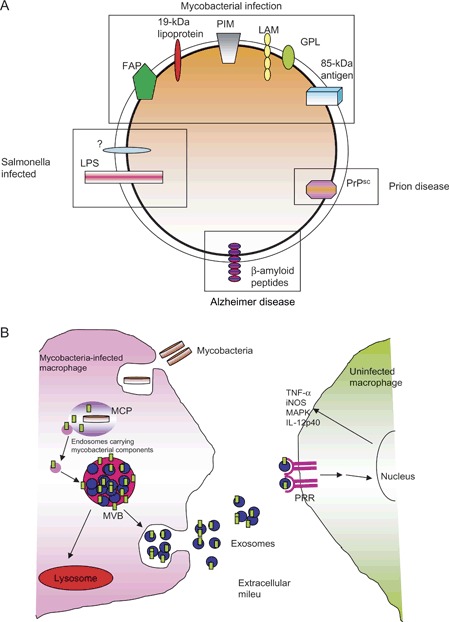
Composition and trafficking of exosomes from infected macrophages with a focus on mycobacterial‐infected cells. A) A list of microbial or infectious components that have been shown to be on exosomes. These include ‘infectious’ proteins such as prions as well as HIV. Also shown are microbial molecules, which have been released from the pathogen and trafficked to MVBs and present on exosomes. B) General diagram of how mycobacterial PAMPs are released from the mycobacteria within a phagosome and transported to the MVB for release on exosomes. The exosomes containing the PAMPs bind to pattern recognition receptors (PRRs) on surrounding macrophages leading to macrophage activation. LPS, lipopolysaccharide; MCP, mycobacteria‐containing phagosome; FAP, fibronectin attachment protein; iNOS, inducible nitric oxide synthase; MAPK, mitogen activated protein kinase.
These experiments supported a role for exosomes in the intercellular transport of mycobacterial components; however, which component(s) induced the proinflammatory response in naïve macrophages was not defined. Insight into this question was provided by our recent studies using the M. tb H37Rv lspA knockout strain, which lacks the 19‐kDa lipoprotein (77). Previous studies have indicated that the 19‐kDa lipoprotein of M. tb interacts with TLR2 and can induce IL‐12p40 production in macrophages (78). Exosomes isolated from macrophages infected with the LspA‐deficient M. tb failed to induce TNF‐α secretion or iNOS production in uninfected macrophages, whereas cells treated with exosomes from wild‐type M. tb or the LspA‐complemented M. tb strain induced both TNF‐α secretion and iNOS production (Figure 4). Together, these data indicate that the 19‐KDa lipoprotein is present on exosomes from M. tb‐infected macrophages and is responsible, at least in part, for the exosomes proinflammatory activity.
Figure 4.
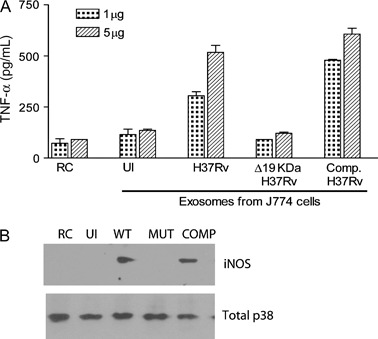
Exosomes isolated from macrophages infected with an LspA‐deficient H37Rv fail to stimulate macrophage activation. Exosomes were isolated from infected J774 cells as described (81) and used to treat bone marrow‐derived murine macrophages. After 24 h, supernatants were isolated and analyzed for TNF‐α by enzyme‐linked immunosorbent assay, and cells were harvested and analyzed for iNOS expression by Western blot as described (81). Total MAPK p38 was used as a loading control. COMP, the LspA mutant complemented with the wild‐type (WT) LspA gene; MUT, H37Rv mutant that lacks LspA and therefore fails to make the 19‐KDa lipoprotein; RC, untreated cells; UI, exosomes from uninfected cells.
Interestingly, this phenomenon of immune surveillance in the host by exosomes was not unique to the Mycobacterium genus as exosomes isolated from Salmonella typhimurium‐ or Toxoplasma gondii‐infected macrophages also stimulated a proinflammatory response in uninfected cells (72). The exosomes isolated from S. typhimurium‐infected cells contained lipopolysaccharide, a major bacterial PAMP. Although T. gondii maintains an intracellular niche in macrophages quite distinct from mycobacteria or Salmonella, exosomes released from infected cells also appear to contain T. gondii PAMPs (72). Nevertheless, the true generality of this mechanism for extracellular release of PAMPs from cells infected with intracellular pathogens remains to be determined, as is the effect these exosomes have on the immune response.
Exosomes as vaccine candidates
The use of exosomes has garnered considerable interest as vaccine candidates for tumor immunotherapy (79). Much of this interest stems from the difficulty associated with DC‐based immunotherapy and how an exosome‐based approach can overcome some of these difficulties. Tumor cell‐derived exosomes containing tumor antigens plus MHC class I molecules can transfer tumor antigens to DCs to induce a CD8+ T‐cell dependent anti‐tumor immune response (80). Exosomes released from DCs pulsed with tumor antigens were also shown to elicit strong anti‐tumor responses. Data obtained in mice have shown that exosomes obtained from DCs pulsed with tumor peptides could prime specific cytotoxic T lymphocytes (CTLs) in vivo and limit or suppress growth of established murine tumors in a T‐cell‐dependent manner 10, 81. Interestingly, tumor‐derived exosomes may have broader activity than previously believed as one study showed that exosomes isolated from different tumors inhibited not only syngeneic but also allogenic tumor growth, indicating that tumor‐derived exosomes may harbor some common tumor antigens (47). Together, these studies indicate that exosomes can be isolated from tumor cells or from DCs pulsed with tumor antigens to deliver a target immunogen capable of inducing an effective immune response and that they may represent a new cell‐free vaccine. Some phase I clinical trials have been completed, and although problems with their use remain, the data suggest that exosome‐based therapy is a viable approach (82).
The successful use of exosomes in cancer immunotherapy has lead to the hypothesis that they could function as vaccine candidates in the context of infectious diseases. Aline et al. demonstrated that exosomes derived from DCs pulsed with T. gondii tachyzoite sonicates could induce a protective immune response against T. gondii infection. These exosomes primed an antigen‐specific cellular and humoral immune response, which provided a good protection against both acute and chronic toxoplasmosis (83). Moreover, CBA/J mice vaccinated with exosomes isolated from T. gondii antigen‐pulsed DCs exhibited significantly fewer brain cyst (84). Another application of exosomes in immunotherapy has been implicated in the treatment of pneumococcal infection in mice (85). Colino and Snapper showed that murine bone marrow‐derived DCs (BMDCs) pulsed in vitro with intact diphtheria toxin (DT)‐released exosomes, which upon injection into mice induce immunoglobulin G (IgG)2b and IgG2a responses specific for DT (86). Exosomes have also been evaluated in the context of Streptococcus infections. Invasive strains of Streptococcus pneumoniae are leading causes of meningitis and major causes of otitis media and bacteremia in children and pneumonia in the elderly (87). Vaccine‐mediated protection against S. pneumoniae infection is based on humoral immunity specific for S. pneumoniae capsular polysaccharides (Cps) (88). Similar to the DT exosomes, BMDCs treated with Cps14 released exosomes enriched in Cps14. These purified exosomes could induce a S. pneumoniae‐protective Cps14‐specific immunoglobulin M and IgG3 response in naive recipients (85). Exosomes isolated from M. bovis BCG‐infected macrophages could stimulate splenic T cells isolated from BCG‐infected mice (J. S. S., unpublished data), but whether these exosomes can function as vaccine candidates awaits further study. Exosomes as a vaccine has also been explored for atypical severe acute respiratory syndrome (SARS) caused by the positive‐stranded RNA virus, SARS‐associated cornavirus (SARS‐CoV). Studies by Kuate et al. showed that exosomes containing spike S protein of SARS‐CoV induced neutralizing antibody titres (89). This immune response was further accentuated by priming with the SARS‐S exosomal vaccine and then boosting with the currently used adenoviral vector vaccine (89).
In addition to the potential use of exosomes as vaccines against infectious diseases, exosomes have also proved useful in treatment of autoimmune diseases in animal models. This is illustrated in studies by Kim et al. who showed that administration of exosomes derived from DCs‐expressing recombinant IL‐4 was able to modulate the activity of APC and T cells in vivo, partly through a FasL/Fas‐dependent mechanism, resulting in effective treatment against collagen‐induced arthritis through suppression of the delayed‐type hypersensitivity inflammatory response (90).
Exosomes as biomarkers
The proteins associated with renal diseases could be detected on exosomes isolated from urine, indicating a possible use for urine exosomes as biomarkers (91). For instance, Pisitkun et al. demonstrated the excretion of exosomes containing aquaporin‐2 protein in autosomal dominant and autosomal recessive nephrogenic diabetes insipidus patients (73). Similar proteomic studies performed on urinary exosomes generated a long list of molecular signatures, illustrating valuable potential for diagnostic, prognostic and pathophysiological discovery (92). Similar to reno pathologies, exosomes are also an attractive biomarker candidate for cancer diagnosis with most of the focus centered on bladder cancer. The differentially expressed proteins include psoriasin, kertain‐14, galectin‐7, epidermal fatty acid binding protein (E‐FABP), migration inhibitor factor‐related protein (MRP8) and 14 and stratifin, which may be useful markers for the diagnosis of bladder cancer (91). Exosomes may also be valuable as biomarkers for infectious diseases, mainly in the context of defining treatment success. Unfortunately, to date, this has not been tested. Exosomes may be particularly useful in the context of tuberculosis (TB) as the time required to test a new TB drug treatment protocol is extensive, leading to high drug development cost as well as delays in the introduction of new medication. A major limitation in developing an efficient drug treatment for TB is the lack of available methodology to identify an early infection as well as determine drug treatment efficacy. Currently, a major goal is to identify disease biomarkers in biological fluids that can be measured relatively inexpensively for early diagnosis of disease and treatment success. We hypothesize that exosomes, whose composition may change during the course of an M. tb infection and treatment, may be such a biomarker.
Exosome display technology
Exosome display technology is a novel technique of manipulating the molecular composition of the exosomes and tailoring exosomes with new desirable properties. Recently, exosome display was applied for the induction of epitope‐specific antibody response against tumor biomarkers (93). This technology opens up new possibilities in designing novel therapies and generating new diagnostic tools. Exosome display has been used to prepare recombinant vesicles carrying cytokines as well as tumor antigens that may or may not have been previously found on exosomes (79). The targeted co‐delivery of antigens with the activators of DCs, B‐, T‐ or natural killer cells may also accentuate the exosomes efficacy as a vaccine.
Concluding Remarks and Future Direction
It has become increasingly clear, as new exosome studies are published, that these small bioactive vesicles are important in a number of biological functions. From their original discovery in the removal of unwanted proteins from maturing reticulocytes to their role in immune surveillance, the inventory of functions continues to grow. As cancer phase I clinical trails have shown, our knowledge of exosomes can be applied therapeutically and the use of exosomes in treatment and diagnostics is also likely to grow. Nevertheless, there are still many unanswered questions including (i) how is fusion of a MVB with the plasma membrane and release of exosomes regulated, (ii) under what circumstances do exosomes function in vivo, and what are the consequences of their expression and (iii) how can we modify exosome composition to maximize their efficacy as vaccines or therapeutic agents. As our understanding of exosome formation and function continues to expand, answers to these and many other questions should be forthcoming.
Acknowledgments
The LspA mutant was kindly provided by Dr Joel Ernst (New York University School of Medicine, New York, NY, USA). This work was supported through grants AI056979 and AI052439 from the National Institute of Allergy and Infectious Diseases (J. S. S.).
References
- 1. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 1987;262:9412–9420. [PubMed] [Google Scholar]
- 2. Couzin J. Cell biology: the ins and outs of exosomes. Science 2005;308:1862–1863. [DOI] [PubMed] [Google Scholar]
- 3. Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proc Natl Acad Sci U S A 2003;100:10592–10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. Higher‐order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol 2007;5:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen BJ, Lamb RA. Mechanisms for enveloped virus budding: can some viruses do without an ESCRT? Virology 2007;372:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Niel G, Porto‐Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem 2006;140:13–21. [DOI] [PubMed] [Google Scholar]
- 7. Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 1985;101:942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan BT, Blostein R, Johnstone RM. Loss of the transferrin receptor during the maturation of sheep reticulocytes in vitro. An immunological approach. Biochem J 1983;210:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen‐presenting vesicles. J Exp Med 1996;183:1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi‐Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell‐free vaccine: dendritic cell‐derived exosomes. Nat Med 1998;4:594–600. [DOI] [PubMed] [Google Scholar]
- 11. Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha‐granules. Blood 1999;94:3791–3799. [PubMed] [Google Scholar]
- 12. Wilson JM, Whitney JA, Neutra MR. Biogenesis of the apical endosome‐lysosome complex during differentiation of absorptive epithelial cells in rat ileum. J Cell Sci 1991;100:133–143. [DOI] [PubMed] [Google Scholar]
- 13. Marzesco AM, Janich P, Wilsch‐Brauninger M, Dubreuil V, Langenfeld K, Corbeil D, Huttner WB. Release of extracellular membrane particles carrying the stem cell marker prominin‐1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci 2005;118:2849–2858. [DOI] [PubMed] [Google Scholar]
- 14. Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol 2007;23:519–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katzmann DJ, Sarkar S, Chu T, Audhya A, Emr SD. Multivesicular body sorting: ubiquitin ligase Rsp5 is required for the modification and sorting of carboxypeptidase S. Mol Biol Cell 2004;15:468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Niel G, Wubbolts R, Ten Broeke T, Buschow SI, Ossendorp FA, Melief CJ, Raposo G, Van Balkom BW, Stoorvogel W. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity 2006;25:885–894. [DOI] [PubMed] [Google Scholar]
- 17. Yeo SC, Xu L, Ren J, Boulton VJ, Wagle MD, Liu C, Ren G, Wong P, Zahn R, Sasajala P, Yang H, Piper RC, Munn AL. Vps20p and Vta1p interact with Vps4p and function in multivesicular body sorting and endosomal transport in Saccharomyces cerevisiae. J Cell Sci 2003;116:3957–3970. [DOI] [PubMed] [Google Scholar]
- 18. De Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid raft‐associated protein sorting in exosomes. Blood 2003;102:4336–4344. [DOI] [PubMed] [Google Scholar]
- 19. Vidal M, Mangeat P, Hoekstra D. Aggregation reroutes molecules from a recycling to a vesicle‐mediated secretion pathway during reticulocyte maturation. J Cell Sci 1997;110:1867–1877. [DOI] [PubMed] [Google Scholar]
- 20. Geminard C, De Gassart A, Blanc L, Vidal M. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic 2004;5:181–193. [DOI] [PubMed] [Google Scholar]
- 21. Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS. A lumenal domain‐dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell 2006;10:343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 2000;113:3365–3374. [DOI] [PubMed] [Google Scholar]
- 23. Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell 1997;8:2631–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi‐Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell‐derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 1999;147:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nguyen DG, Booth A, Gould SJ, Hildreth JE. Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J Biol Chem 2003;278:52347–52354. [DOI] [PubMed] [Google Scholar]
- 26. Thery C, Boussac M, Veron P, Ricciardi‐Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell‐derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 2001;166:7309–7318. [DOI] [PubMed] [Google Scholar]
- 27. Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, Patel P, Selby PJ, Banks RE. Proteomic analysis of melanoma‐derived exosomes by two‐dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics 2004;4:4019–4031. [DOI] [PubMed] [Google Scholar]
- 28. Futter CE, White IJ. Annexins and endocytosis. Traffic 2007;8:951–958. [DOI] [PubMed] [Google Scholar]
- 29. Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface‐positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 2005;65:5238–5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Segura E, Amigorena S, Thery C. Mature dendritic cells secrete exosomes with strong ability to induce antigen‐specific effector immune responses. Blood Cells Mol Dis 2005;35:89–93. [DOI] [PubMed] [Google Scholar]
- 31. Veron P, Segura E, Sugano G, Amigorena S, Thery C. Accumulation of MFG‐E8/lactadherin on exosomes from immature dendritic cells. Blood Cells Mol Dis 2005;35:81–88. [DOI] [PubMed] [Google Scholar]
- 32. Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am J Pathol 2004;164:1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vidal M, Sainte‐Marie J, Philippot JR, Bienvenue A. Asymmetric distribution of phospholipids in the membrane of vesicles released during in vitro maturation of guinea pig reticulocytes: evidence precluding a role for “aminophospholipid translocase”. J Cell Physiol 1989;140:455–462. [DOI] [PubMed] [Google Scholar]
- 34. Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, Kobayashi T, Salles JP, Perret B, Bonnerot C, Record M. Mast cell‐ and dendritic cell‐derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J 2004;380:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W. Proteomic and biochemical analyses of human B cell‐derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem 2003;278:10963–10972. [DOI] [PubMed] [Google Scholar]
- 36. Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie 2007;89:205–212. [DOI] [PubMed] [Google Scholar]
- 37. Chu Z, Witte DP, Qi X. Saposin C‐LBPA interaction in late‐endosomes/lysosomes. Exp Cell Res 2005;303:300–307. [DOI] [PubMed] [Google Scholar]
- 38. Echarri A, Muriel O, Del Pozo MA. Intracellular trafficking of raft/caveolae domains: insights from integrin signaling. Semin Cell Dev Biol 2007;18:627–637. [DOI] [PubMed] [Google Scholar]
- 39. Johnstone RM, Adam M, Pan BT. The fate of the transferrin receptor during maturation of sheep reticulocytes in vitro. Can J Biochem Cell Biol 1984;62:1246–1254. [DOI] [PubMed] [Google Scholar]
- 40. Amzallag N, Passer BJ, Allanic D, Segura E, Thery C, Goud B, Amson R, Telerman A. TSAP6 facilitates the secretion of translationally controlled tumor protein/histamine‐releasing factor via a nonclassical pathway. J Biol Chem 2004;279:46104–46112. [DOI] [PubMed] [Google Scholar]
- 41. Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res 2006;66:4795–4801. [DOI] [PubMed] [Google Scholar]
- 42. Zhang HG, Liu C, Su K, Yu S, Zhang L, Zhang S, Wang J, Cao X, Grizzle W, Kimberly RP. A membrane form of TNF‐alpha presented by exosomes delays T cell activation‐induced cell death. J Immunol 2006;176:7385–7393. [DOI] [PubMed] [Google Scholar]
- 43. Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, Cao X. Efficient induction of antitumor T cell immunity by exosomes derived from heat‐shocked lymphoma cells. Eur J Immunol 2006;36:1598–1607. [DOI] [PubMed] [Google Scholar]
- 44. Stoeck A, Keller S, Riedle S, Sanderson MP, Runz S, Le Naour F, Gutwein P, Ludwig A, Rubinstein E, Altevogt P. A role for exosomes in the constitutive and stimulus‐induced ectodomain cleavage of L1 and CD44. Biochem J 2006;393:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Azevedo LC, Janiszewski M, Pontieri V, Pedro MA, Bassi E, Tucci PJ, Laurindo FR. Platelet‐derived exosomes from septic shock patients induce myocardial dysfunction. Crit Care 2007;11:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gambim MH, Carmo AO, Marti L, Verissimo‐Filho S, Lopes LR, Janiszewski M. Platelet‐derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care 2007;11:R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor‐derived exosomes are a source of shared tumor rejection antigens for CTL cross‐priming. Nat Med 2001;7:297–303. [DOI] [PubMed] [Google Scholar]
- 48. Andre F, Schartz NE, Chaput N, Flament C, Raposo G, Amigorena S, Angevin E, Zitvogel L. Tumor‐derived exosomes: a new source of tumor rejection antigens. Vaccine 2002;20 (Suppl. 4):A28–A31. [DOI] [PubMed] [Google Scholar]
- 49. Chaput N, Flament C, Viaud S, Taieb J, Roux S, Spatz A, Andre F, Lepecq JB, Boussac M, Garin J, Amigorena S, Thery C, Zitvogel L. Dendritic cell derived‐exosomes: biology and clinical implementations. J Leukoc Biol 2006;80:471–478. [DOI] [PubMed] [Google Scholar]
- 50. Andre F, Escudier B, Angevin E, Tursz T, Zitvogel L. Exosomes for cancer immunotherapy. Ann Oncol 2004;15 (Suppl. 4):iv141–iv144. [DOI] [PubMed] [Google Scholar]
- 51. Muntasell A, Berger AC, Roche PA. T cell‐induced secretion of MHC class II‐peptide complexes on B cell exosomes. EMBO J 2007;26:4263–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peche H, Heslan M, Usal C, Amigorena S, Cuturi MC. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell‐derived exosomes modulates allograft rejection. Transplantation 2003;76:1503–1510. [DOI] [PubMed] [Google Scholar]
- 53. Kim SH, Lechman ER, Bianco N, Menon R, Keravala A, Nash J, Mi Z, Watkins SC, Gambotto A, Robbins PD. Exosomes derived from IL‐10‐treated dendritic cells can suppress inflammation and collagen‐induced arthritis. J Immunol 2005;174:6440–6448. [DOI] [PubMed] [Google Scholar]
- 54. Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL‐4. J Immunol 2007;179:2242–2249. [DOI] [PubMed] [Google Scholar]
- 55. Kim SH, Bianco N, Menon R, Lechman ER, Shufesky WJ, Morelli AE, Robbins PD. Exosomes derived from genetically modified DC expressing FasL are anti‐inflammatory and immunosuppressive. Mol Ther 2006;13:289–300. [DOI] [PubMed] [Google Scholar]
- 56. Karlsson M, Lundin S, Dahlgren U, Kahu H, Pettersson I, Telemo E. “Tolerosomes” are produced by intestinal epithelial cells. Eur J Immunol 2001;31:2892–2900. [DOI] [PubMed] [Google Scholar]
- 57. Van Niel G, Mallegol J, Bevilacqua C, Candalh C, Brugiere S, Tomaskovic‐Crook E, Heath JK, Cerf‐Bensussan N, Heyman M. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut 2003;52:1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mallegol J, Van Niel G, Lebreton C, Lepelletier Y, Candalh C, Dugave C, Heath JK, Raposo G, Cerf‐Bensussan N, Heyman M. T84‐intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology 2007;132:1866–1876. [DOI] [PubMed] [Google Scholar]
- 59. Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, Arancia G, Gentile M, Parmiani G et al Induction of lymphocyte apoptosis by tumor cell secretion of FasL‐bearing microvesicles. J Exp Med 2002;195:1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Martinez‐Lorenzo MJ, Anel A, Gamen S, Monle NI, Lasierra P, Larrad L, Pineiro A, Alava MA, Naval J. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. J Immunol 1999;163:1274–1281. [PubMed] [Google Scholar]
- 61. Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour‐released exosomes and their implications in cancer immunity. Cell Death Differ 2008;15:80–88. [DOI] [PubMed] [Google Scholar]
- 62. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654–659. [DOI] [PubMed] [Google Scholar]
- 63. Pelchen‐Matthews A, Raposo G, Marsh M. Endosomes, exosomes and Trojan viruses. Trends Microbiol 2004;12:310–316. [DOI] [PubMed] [Google Scholar]
- 64. Jouve M, Sol‐Foulon N, Watson S, Schwartz O, Benaroch P. HIV‐1 buds and accumulates in “nonacidic” endosomes of macrophages. Cell Host Microbe 2007;2:85–95. [DOI] [PubMed] [Google Scholar]
- 65. Deneka M, Pelchen‐Matthews A, Byland R, Ruiz‐Mateos E, Marsh M. In macrophages, HIV‐1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J Cell Biol 2007;177:329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Porto‐Carreiro I, Fevrier B, Paquet S, Vilette D, Raposo G. Prions and exosomes: from PrPc trafficking to PrPsc propagation. Blood Cells Mol Dis 2005;35:143–148. [DOI] [PubMed] [Google Scholar]
- 67. Medzhitov R. Toll‐like receptors and innate immunity. Nat Rev Immunol 2001;1:135–145. [DOI] [PubMed] [Google Scholar]
- 68. Beatty WL, Rhoades ER, Ullrich HJ, Chatterjee D, Heuser JE, Russell DG. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic 2000;1:235–247. [DOI] [PubMed] [Google Scholar]
- 69. Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem 2007;282:25779–25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Beatty WL, Ullrich HJ, Russell DG. Mycobacterial surface moieties are released from infected macrophages by a constitutive exocytic event. Eur J Cell Biol 2001;80:31–40. [DOI] [PubMed] [Google Scholar]
- 71. Rhoades E, Hsu F, Torrelles JB, Turk J, Chatterjee D, Russell DG. Identification and macrophage‐activating activity of glycolipids released from intracellular Mycobacterium bovis BCG. Mol Microbiol 2003;48:875–888. [DOI] [PubMed] [Google Scholar]
- 72. Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007;110:3234–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 2004;101:13368–13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Keller S, Rupp C, Stoeck A, Runz S, Fogel M, Lugert S, Hager HD, Abdel‐Bakky MS, Gutwein P, Altevogt P. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int 2007;72:1095–1102. [DOI] [PubMed] [Google Scholar]
- 75. Admyre C, Grunewald J, Thyberg J, Gripenback S, Tornling G, Eklund A, Scheynius A, Gabrielsson S. Exosomes with major histocompatibility complex class II and co‐stimulatory molecules are present in human BAL fluid. Eur Respir J 2003;22:578–583. [DOI] [PubMed] [Google Scholar]
- 76. Caby MP, Lankar D, Vincendeau‐Scherrer C, Raposo G, Bonnerot C. Exosomal‐like vesicles are present in human blood plasma. Int Immunol 2005;17:879–887. [DOI] [PubMed] [Google Scholar]
- 77. Banaiee N, Kincaid EZ, Buchwald U, Jacobs WR Jr, Ernst JD. Potent inhibition of macrophage responses to IFN‐gamma by live virulent Mycobacterium tuberculosis is independent of mature mycobacterial lipoproteins but dependent on TLR2. J Immunol 2006;176:3019–3027. [DOI] [PubMed] [Google Scholar]
- 78. Stewart GR, Wilkinson KA, Newton SM, Sullivan SM, Neyrolles O, Wain JR, Patel J, Pool KL, Young DB, Wilkinson RJ. Effect of deletion or overexpression of the 19‐kilodalton lipoprotein Rv3763 on the innate response to Mycobacterium tuberculosis. Infect Immun 2005;73:6831–6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Delcayre A, Le Pecq JB. Exosomes as novel therapeutic nanodevices. Curr Opin Mol Ther 2006;8:31–38. [PubMed] [Google Scholar]
- 80. Hao S, Bai O, Yuan J, Qureshi M, Xiang J. Dendritic cell‐derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell‐derived exosomes. Cell Mol Immunol 2006;3:205–211. [PubMed] [Google Scholar]
- 81. Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T‐lymphocyte responses and antitumour immunity. Immunology 2007;120:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hao S, Moyana T, Xiang J. Review: cancer immunotherapy by exosome‐based vaccines. Cancer Biother Radiopharm 2007;22:692–703. [DOI] [PubMed] [Google Scholar]
- 83. Aline F, Bout D, Amigorena S, Roingeard P, Dimier‐Poisson I. Toxoplasma gondii antigen‐pulsed‐dendritic cell‐derived exosomes induce a protective immune response against T. gondii infection. Infect Immun 2004;72:4127–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Beauvillain C, Ruiz S, Guiton R, Bout D, Dimier‐Poisson I. A vaccine based on exosomes secreted by a dendritic cell line confers protection against T. gondii infection in syngeneic and allogeneic mice. Microbes Infect 2007;9:1614–1622. [DOI] [PubMed] [Google Scholar]
- 85. Colino J, Snapper CM. Dendritic cell‐derived exosomes express a Streptococcus pneumoniae capsular polysaccharide type 14 cross‐reactive antigen that induces protective immunoglobulin responses against pneumococcal infection in mice. Infect Immun 2007;75:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Colino J, Snapper CM. Exosomes from bone marrow dendritic cells pulsed with diphtheria toxoid preferentially induce type 1 antigen‐specific IgG responses in naive recipients in the absence of free antigen. J Immunol 2006;177:3757–3762. [DOI] [PubMed] [Google Scholar]
- 87. Lopez R. Pneumococcus: the sugar‐coated bacteria. Int Microbiol 2006;9:179–190. [PubMed] [Google Scholar]
- 88. Makwana N, Riordan FA. Bacterial meningitis: the impact of vaccination. CNS Drugs 2007;21:355–366. [DOI] [PubMed] [Google Scholar]
- 89. Kuate S, Cinatl J, Doerr HW, Uberla K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology 2007;362:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. MHC class II+ exosomes in plasma suppress inflammation in an antigen‐specific and Fas ligand/Fas‐dependent manner. J Immunol 2007;179:2235–2241. [DOI] [PubMed] [Google Scholar]
- 91. Pisitkun T, Johnstone R, Knepper MA. Discovery of urinary biomarkers. Mol Cell Proteomics 2006;5:1760–1771. [DOI] [PubMed] [Google Scholar]
- 92. Hoorn EJ, Pisitkun T, Zietse R, Gross P, Frokiaer J, Wang NS, Gonzales PA, Star RA, Knepper MA. Prospects for urinary proteomics: exosomes as a source of urinary biomarkers. Nephrology (Carlton) 2005;10:283–290. [DOI] [PubMed] [Google Scholar]
- 93. Delcayre A, Estelles A, Sperinde J, Roulon T, Paz P, Aguilar B, Villanueva J, Khine S, Le Pecq JB. Exosome Display technology: applications to the development of new diagnostics and therapeutics. Blood Cells Mol Dis 2005;35:158–168. [DOI] [PubMed] [Google Scholar]
- 94. Katzmann DJ, Stefan CJ, Babst M, Emr SD. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J Cell Biol 2003;162:413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Teo H, Perisic O, Gonzalez B, Williams RL. ESCRT‐II, an endosome‐associated complex required for protein sorting: crystal structure and interactions with ESCRT‐III and membranes. Dev Cell 2004;7:559–569. [DOI] [PubMed] [Google Scholar]


