Abstract
Background:
Successful reconstruction of periodontal tissues destroyed due to periodontitis has been an evasive goal for the periodontists. Several GTR materials and bone grafts have been tried with varied success rates.
Aims and Objectives:
The aim of the present study was to evaluate and compare the efficacy of non-resorbable (GoreTex®) and bioabsorbable (Resolut Adapt®) membranes in combination with bioactive glass (PerioGlas®) in the treatment of periodontal intrabony defects.
Materials and Methods:
Ten chronic periodontitis patients having bilateral matched intrabony defects were treated with non-resorbable membrane (GoreTex®) and bioactive glass or the bioresorbable membrane (Resolut Adapt®) and bioactive glass in split mouth design. Clinical parameters like plaque index, gingival index, probing pocket depth, clinical attachment level, and gingival recession were recorded at baseline and 9 months post-operatively. Similarly, radiographic (linear CADIA) and intra-surgical (re-entry) measurements were evaluated at baseline and 9 months post-operatively).
Results:
Both the membrane groups showed clinically and statistically significant improvement in clinical parameters i.e., reduction in probing depth (4.6 ± 1.4 mm) vs. 3.7 ± 1.3 mm) and gain in clinical attachment level (4.6 + 1.6 vs. 3.2 ± 1.5 mm) for non-resorbable and bioresorbable membrane groups, respectively. Similar trend was observed when radiographical and intra-surgical (re-entry) measurements were evaluated and compared, pre- and post-operatively at 9 months. However, on comparison between the two groups, the difference was statistically not significant.
Conclusion:
Both the barrier membranes i.e., non-resorbable (Gore-Tex®) and bioabsorbable (Resolut Adapt®) membranes in combination with bioactive glass (PerioGlas®) were equally effective in enhancing the periodontal regeneration.
Keywords: Bioactive glass, guided tissue regeneration, periodontal intrabony defects
Introduction
The ultimate goal of periodontal therapy is the regeneration of periodontal tissues lost due to the periodontal diseases. Several methods have been used to regenerate the periodontal tissues including bone grafts, GTR membranes, and the enamel matrix derivatives. Early animal[1,2] and human studies[3,4] suggested that the predictable restitution of the attachment apparatus can be accomplished by using a treatment, which is based on the principle of guided tissue regeneration (GTR) by using the non-resorbable and bioabsorbable barrier membranes (Nygaard – Ostby et al. 2010).[5]
The expanded polytetrafluoroethylene (ePTFE) membrane is the most widely documented non-resorbable barrier membrane in GTR therapy.[6–9] This porous-non-resorbable membrane is more commonly known as Gore-Tex (W.L. Gore and ASSOC., Flagstaff, Ariz), and features two structural designs;[10] i) open microstructured collar and ii) partially occlusive device, to address the specific needs. This membrane is biocompatible and has been proved to be safe and effective in the treatment of periodontal intrabony defects. Through this membrane possesses ideal characteristics of a barrier, a second surgical procedure is required for its retrieval.
The bioabsorbable membranes are the second generation GTR membranes and were developed to avoid the second surgical procedure to remove the barrier.[11–14] These GTR devices fall into two broad categories, natural products (collagen membrane) and the synthetic (copolymers) materials like Guidor,® (Guidor Co, Bensenville, IL), Vicryl periodontal mesh (Johnson and Johnson Ethicon), Resolut Adapt® and the Atrisorb® GTR barrier. The Resolut Adapt® regenerative material (W.L. Gore and ASSOC, Flagstaff, Ariz) is a composite consisting of degradable polymers of polygycolic and polylactic acid (PRA/PLA Copolymer). It is also supplied with polycaprolate-coated polyglycolic acid (Resolut) sutures. Histologically, it has been demonstrated that this device retains its structure for 4 months and gets resorbed completely within 5-6 months.[10]
Bioactive ceramic glass has been used in medical practice since 1984. Recently, a particulate form of bioactive glass was introduced to the dental profession as an alloplastic bone graft material for the treatment of periodontal infrabony defects. These materials are biocompatible and osteoconductive in nature. However, histologically, it showed evidence of osteoinductive in nature inducing the new cementum formation and attachment, thus preventing apical down growth of junctional epithelium. Clinical studies have shown that the bioactive glass is as effective as DFDBA and other graft materials in the treatment of periodontal infrabony defects.[14,15]
There are few studies reported in the literature wherein the efficacy of non-resorbable or bioabsorbable membranes alone or in combination with bone graft materials (autogenous, DFDBA, tricalcium phosphates, or bioactive glass) were evaluated and compared in periodontal regeneration.[6–9,11–16] However, to the best of our knowledge, there is no study reported in the literature comparing the efficacy of non-resorbable (Gore-Tex®) membrane and the bioabsorbable (Resolut Adapt®) barrier in combination with bioactive glass, in the treatment of periodontal intrabony defects; hence, present study was undertaken.
Materials and Methods
Patient selection
Ten patients (3 males, 7 females) aged 25-55 years (mean age 38 ± 2.5 years) with generalized chronic periodontitis were selected for this clinical study. All the selected patients displayed bilateral--matched intrabony defects with probing depth of ≥ 6 mm and radiographic evidence of angular bone loss.
Inclusion criteria included: i) All patients having non-contributory medical history, ii) no history of antibiotic therapy in the last 6 months, iii) no history of periodontal therapy in the last 3 months, and iv) patient showing optimum compliance in oral hygiene maintenance during the phase-1 (pre-surgical) therapy. Exclusion criteria included: i) Patients with compromised immune system, ii) patients taking drugs known to cause gingival enlargement, iii) pregnant and lactating mothers, and iv) smokers.
The purpose of investigation and the potential benefits and risks of the materials used and procedures to be performed were explained, and each patient signed a written consent form indicating their agreement to participate in the study. The study protocol and consent forms were approved by the institutional ethical committee and review board.
Pre-surgical (phase-1) therapy was performed on all patients, which consisted of motivation and education, oral hygiene instructions, scaling and root planing, and occlusal adjustment when indicated. Re-evaluation of the tissue response and the patients’ plaque control was reinforced 2-4 weeks later.[17] Only those patients showing good compliance in plaque control during the pre-surgical phase were selected for the study: 10 matched pairs of intrabony defects were found suitable for the study.
Study design
Patients demonstrating satisfactory response were considered for the study. In the selected patients, random allocation of experimental site A and experimental site B was done by flip of a coin, and thus a split mouth design with 10 sites in each group was formulated. Exp. site-A received non-resorbable (Gore-Tex®) membrane + bioactive glass (PerioGlas®), whereas Exp. site-B was treated with bioresorbable (Resolut Adapt®) membrane + bioactive glass (PerioGlas®).
Clinical parameters used in this study include: 1) Plaque index (PI); gingival index (GI); probing depth (PD); clinical attachment level; (CAL) and the gingival recession (GR). Radiographic evaluation was done using linear CADIA, whereas the intra-surgical (re-entry) measurements were recorded to evaluate the defect fill and defect resolution. The ancillary parameters like PI and GI were recorded at baseline, 1 month, 3 months, 6 months, and 9 months, whereas the main clinical parameters like PD, CAL, GR, and radiographic and intra-surgical evaluation was performed at baseline and 9 months post-operatively. All the surgical procedures and calibration (measurements) were performed and recorded by single surgeon in order to avoid inter-examiner variability.
Surgical procedure
Adequate anesthesia was achieved by administering 2% xylocaine HCl with adrenaline 1:80,000. After giving intra-sulcular incisions with Bard Parker knife (blade no. 12), the full-thickness mucoperiosteal flaps were reflected using the periosteal elevators. Complete debridement of the defect was done, and a thorough root planing was carried out using the universal (4-R and 4-L) and Gracey (1-14) curettes] [Figure 1]. The surgical area was thoroughly irrigated with saline, surgical templates were made, and pre-suturing[18] was done.
Figure 1.
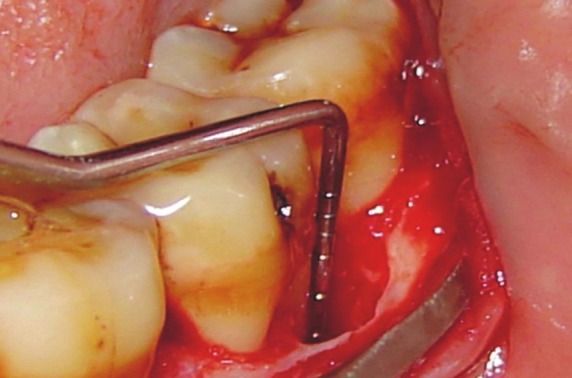
Measurement of defect from CEJ to the defect
Defects in both the sites were filled with bioactive glass (PerioGlas®-NovaBone Products, LLC, Alachua, FI, USA) granules. The required amount of PerioGlas® granules were transferred to dappen dish and moistened with saline, which was then transferred to the defect site with the help of scoop of a Cumine scaler (Hu Friedy, USA), filling the defect to approximate level of crest of the remaining osseous walls.
The membranes were trimmed according to the template size so as to cover the defect and 1-2 mm past the osseous defect margins. Experimental site-A received non-resorbable (Gore-Tex® membrane, W.L. Gore and Associates, Inc. Flagstaff, AZ, USA), whereas the site – B received bioresorbable membrane (Resolut Adapt®-W.L. Gore and Associates, Inc. Flagstaff, AZ, USA) [Figure 2]. In both the places, the membranes fully covered the grafted sites, and the pre-sutured mucoperiosteal flaps were repositioned, sutured, and covered with the periodontal dressing (Coe-pak, GC America).
Figure 2.
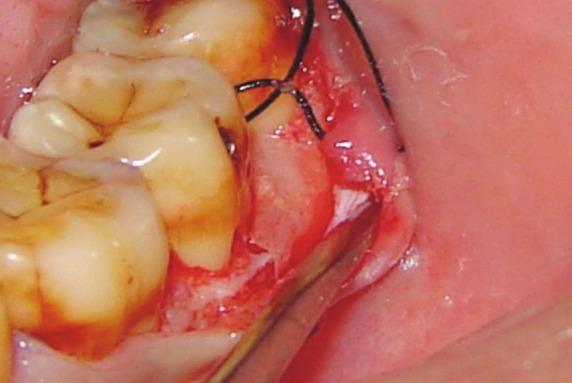
Resolut Adapt® Regenerative membrane to the defect
All patients were prescribed antibiotics (Amoxycillin 500 mg 8 hourly for 5 days) and analgesics (Ibuprofen 400 mg TDS for 3 days). Chlorhexidine mouthwash (0.2%) was advised twice-daily, and all required post-operative instructions were given to the patient.
After 1 week, the periodontal dressing and sutures were removed, and the area was thoroughly irrigated with saline. The re-call appointments were made after 1 month, 3 months, 6 months, and 9 months post-operatively. The non-resorbable membrane was removed after 4-6 weeks [Figure 3].[19]
Figure 3.
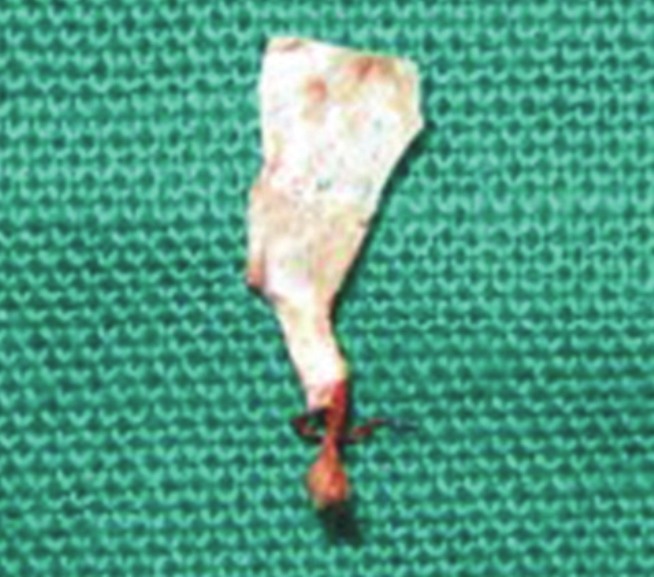
Gore – Tex® Regenerative membrane removed
Surgical re-entry
Nine months post-operatively, the surgical re-entry was performed to record the intra-surgical measurements and to evaluate the bone-fill and defect resolution. After administering the local anesthesia, sulcular incisions were given, mucoperiosteal flaps were reflected, and soft fibrous tissue was removed to facilitate the intra-surgical measurements [Figures 4]. Thorough saline irrigation was done, the flaps were sutured back, and periodontal dressing was placed to be removed after 1 week.
Figure 4.
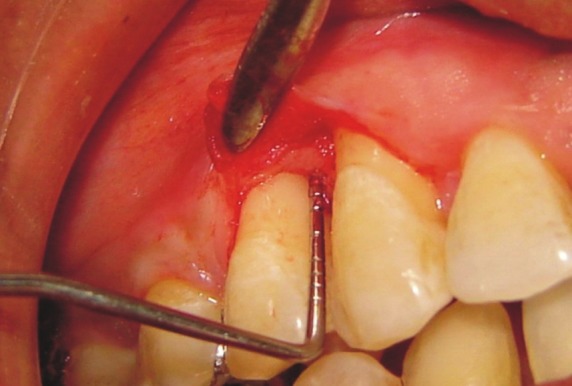
Rentry after 9 months of Expt. site – B
Interpretation of radiographs
All the I.O.P.A radiographs were taken by Long cone projection technique. The I.O.P.A. radiographs were digitalized 4.1 pixel images using Sony DSC – S90 digital camera (Japan) and were then analyzed using the computer-assisted image analysis software.
Statistical analysis
After recording the clinical, radiographic, and intra-surgical parameters, the values were subjected to statistical analysis like paired ‘t’ test, unpaired ‘t’ test, and the Mann-Whitney test. A ‘P’ value of 0.05 or less was considered for the statistical significance.
Results
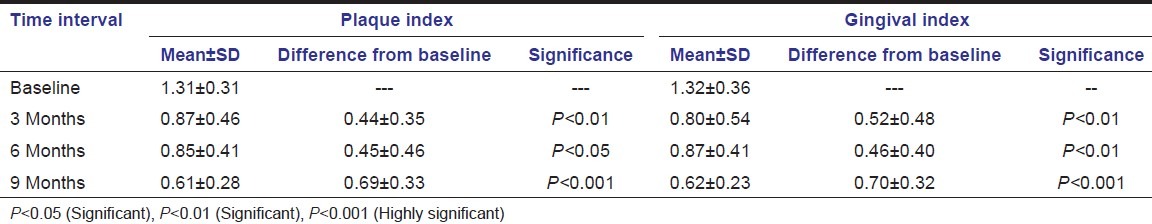
Among the ancillary indices, the mean plaque and gingival scores were reduced significantly (P < 0.001) in both the experimental groups when baseline scores were compared with the 9 months data [Table 1].
Table 1.
Ancillary clinical parameters
The probing depth decreased from baseline (7.7 ± 1.4 mm) to 3.1 ± 0.9 mm with a mean difference of 4.6 ± 1.4 mm for site – A, which was statistically highly significant (P < 0.001). In site – B, the probing depth reduced by 3.9 ± 1.5 mm, which was also statistically highly significant. However, on intergroup comparison, the difference of 0.7 mm was statistically not significant [Table 2].
Table 2.
Clinical parameters
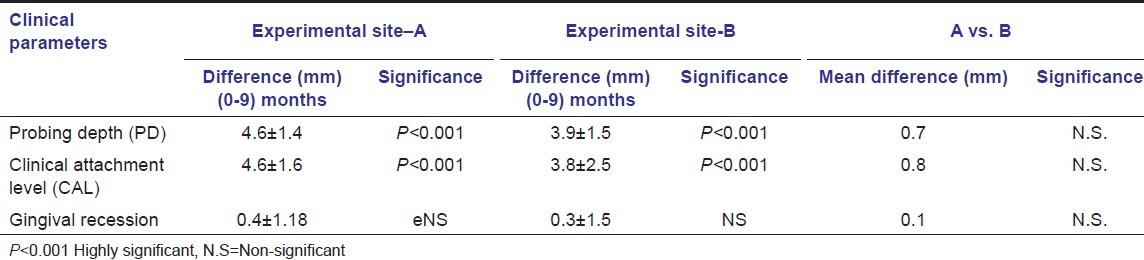
The clinical attachment level (CAL) gain was recorded 4.6 ± 1.6 mm for site – A at 9 month post-operatively, which was statistically highly significant (P < 0.001). Similarly, in site – B, the CAL gain was 3.8 + 2.5 mm for site – B at 9 month post-operatively, which was also statistically highly significant (P < 0.001). However, on intergroup comparison, the difference of 0.8 mm was statistically not significant. [Table 2].
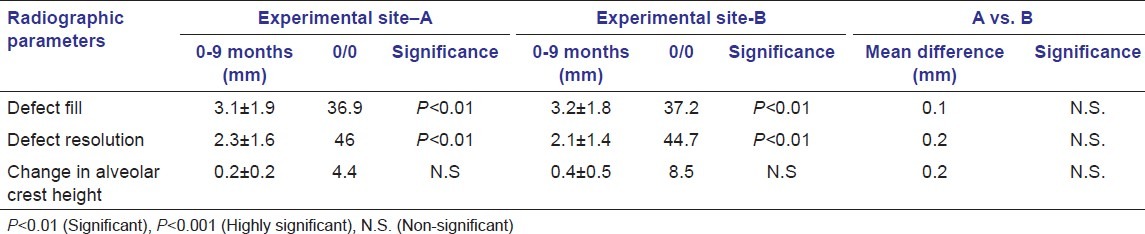
The radiographic bone fill and defect resolution was recorded, and a statistically significant (P < 0.001) improvement was observed in both the groups. However, in intergroup comparison, the difference was statistically non-significant [Table 3].
Table 3.
Radiographic parameters
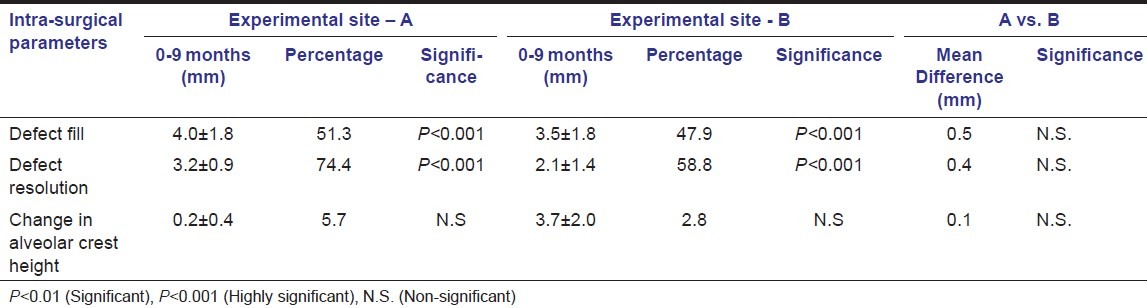
On re-entry procedure, at 9 months post-operatively, the improvement in intra-surgical measurements was highly significant (P < 0.001) in both the groups. However, on inter-group comparison, the difference was statistically non-significant [Table 4].
Table 4.
Intra-surgical (Re-Entry) parameters
Discussion
Regeneration of periodontal osseous defects is the real challenge in periodontal therapy. In earlier studies,[6–9,11] several bone graft materials and barrier membranes alone or in combination have been tried for achieving periodontal regeneration. However, the treatment outcome showed that the combination therapy i.e., GTR membrane + bone graft was more effective and predictable treatment modality than the GTR membrane or bone graft alone. In most of these studies, GTR membrane was combined with allograft (DFDBA), xenograft (Bio-Oss), hydroxyapatite, or the enamel matrix proteins (EMD). However, in recent years, some evidence has been provided that the bioactive glass is also capable of supporting the regenerative healing of the periodontal osseous defects.[12–15] Hence, the present study was undertaken to evaluate and compare the efficacy of two GTR membranes i.e., non-resorbable (Gore-Tex®) vs. bioresorbable (Resolut Adapt®) membranes in combination with the bioactive glass (PerioGlas®) as bone graft material in the treatment of periodontal intrabony defects.
The clinical soft tissue measurements have played a critical role in the evaluation of regenerative procedures. The advantage of this technique is that it provides clinically important information regarding probing depth reduction and relative gain in clinical attachment levels. In the present study, both the groups showed significant (P < 0.001) improvement (P < 0.001) in clinical parameters like probing depth reduction and gain in clinical attachment level when 9 months post-treatment follow-up results were compared with the baseline data, thus signifying the role of GTR material in the periodontal regeneration. The GTR membrane successfully promote the re-growth of the destroyed periodontium;[20] however, there is substantial variation in the clinical predictability, degree of efficacy, and histological outcomes.[21] However, on intergroup comparison, the probing depth reduction in Gore-Tex® membrane group (4.6 ± 1.4 mm) was comparable to that of bioresorbable (Resolut Adapt®) membrane group (3.9 ± 1.5 mm), and the difference between the two groups was statistically non-significant. Similarly, the difference in attachment gain in both the groups (4.6 ± 1.6 mm vs. 3.8 ± 2.5 mm) was not significant at 9 months post-operatively. This mean attachment gain complied exactly with the results in the previous studies.[6–9,11,12]
The radiographic analysis is one of the valid parameters to demonstrate the effectiveness of regenerative procedures. It has played an important role in determining treatment outcome because it offers the only non-invasive method of evaluating the hard tissue response to therapy. In this study, the radiographic analysis was made through the linear CADIA (Computer-Assisted Densitometric Image Analysis), which is an approach developed to combine linear radiographic measurements with CADIA. The results showed comparable defect fill of 36.9% and 37.2% for non-resorbable (Gore-Tex®) and bioabsorbable (Resolut Adapt®) membrane groups, respectively, when 9 month results were compared with the baseline data. Similar trend was observed when change in alveolar crest height and the defect resolutions were compared. These results are consistent with the previous observations made by other authors.[7–9,11–16,22]
The re-entry surgery is among the most common methods used to evaluate the periodontal regeneration. In the present study, there were significant changes in the defect fill between baseline and 9 months intra-surgical data in both the test groups (4.0 ± 1.8 mm vs. 3.5 ± 1.8 mm). However, when both the groups were compared at 9 months, the difference was non-significant. Similar trend was observed when alveolar bone crest height and defect resolution were compared between the two groups. These results are in agreement with the earlier studies reporting significant improvement in regenerative outcome when these GTR materials where compared.[15,16]
The significant improvement in the treatment outcome may also be attributed to the use of bioactive glass as a defect filler and regenerative material in the periodontal regeneration. Studies have shown that treatment of periodontal intrabony defects with bioactive glass leads to significantly greater gain in clinical attachment level and better defect fill. This was also demonstrated in some histological studies wherein the bioactive glass induced significant increase in newly-formed cementum and attachment gain.[16] The bioactive properties guide and promote osteogenesis, allowing rapid and quick formation of new bone.[23]
In conclusion, in this split-mouth clinical study under the given constraints, the combination of bioactive glass (PerioGlas®) with GTR membranes e-PTFE (Gore-Tex®) and PLA/PGA copolymer (Resolut Adapt®) showed enhanced clinical outcome. However, on comparison between the groups, the results obtained from the experimental site A were slightly better than experimental site B, although it was statistically not significant. Further long-term studies are required with larger sample size to determine the efficacy of membranes in combination with bioactive glass that could explain the benefits of this treatment modality in periodontal regeneration.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
References
- 1.Nyman S, Gottlow J, Karring T, Lindhe J. The regeneration potential of the periodontal ligament. An experimental studies in monkeys. J Clin Periodontol. 1982;9:257–65. doi: 10.1111/j.1600-051x.1982.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 2.Aukhil I, Pettersson E, Suggs C. Guided tissue regeneration. An experimental procedure in beagle dogs. J Periodontol. 1986;57:727–34. doi: 10.1902/jop.1986.57.12.727. [DOI] [PubMed] [Google Scholar]
- 3.Nyman S, Lindhe J, Karring T, Rylander H. New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 1982;9:290–6. doi: 10.1111/j.1600-051x.1982.tb02095.x. [DOI] [PubMed] [Google Scholar]
- 4.Gottlow J, Nyman S, Lindhe J, Karring T, Wennström J. New attachment in human periodontium by guided tissue regeneration. J Clin Periodontol. 1986;13:604–16. doi: 10.1111/j.1600-051x.1986.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 5.Nygaard – Ostby P, Bakke V, Nesdal O, Susin C, Wikesjo UM. Periodontal healing following reconstructive surgery effect of guided tissue regeneration using a bioresorbable barrier device when combined with autogenous bone grafting: A randomized controlled trial. 10 year follow-up. J Clin Periodontol. 2010;37:366–73. doi: 10.1111/j.1600-051X.2010.01532.x. [DOI] [PubMed] [Google Scholar]
- 6.Caffesse RG, Mota LF, Duinones CR, Morrison EC. Clinical comparison of resorbable and non-resorbable barriers for guided periodontal tissue regeneration. J Clin Periodontol. 1997;24:747–52. doi: 10.1111/j.1600-051x.1997.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 7.Christgau M, Schmalz G, Rreich E, Wenzel A. Clinical and radiographic split–mouth study on resorbable versus non-resorbable GTR membranes. J Clin Periodontol. 1998;22:306–15. doi: 10.1111/j.1600-051x.1995.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 8.Teparaat T, Solt CW, Clamen LJ, Beck FM. Clinical comparison of bioabsorbable barriers with non-resorbable barriers in guided tissue regeneration in the treatment of human intrabony defects. J Periodontol. 1998;69:632–41. doi: 10.1902/jop.1998.69.6.632. [DOI] [PubMed] [Google Scholar]
- 9.Pretzl B, Kim TS, Holle R, Eickholz P. Long term results of guided tissue regeneration therapy with non-resorbable and bioabsorbable barriers I.V. A case series of infrabony defects after 10 years. J Periodontol. 2008;79:1491–9. doi: 10.1902/jop.2008.070571. [DOI] [PubMed] [Google Scholar]
- 10.Tatakis DN, Promsudthi A, Wikesjo UM. Devices for periodontal regeneration. Periodontol 2000. 1999;19:59–73. doi: 10.1111/j.1600-0757.1999.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 11.Becker W, Becker BE, Mellonig J, Caffesse RG, Warrer K, Caton JG, et al. A prospective multi-center study evaluating periodontal regeneration for Class II furcation invasions and intrabony defects after treatment with a bioabsorbable barrier membrane: 1 year results. J Periodontol. 1996;67:641–9. doi: 10.1902/jop.1996.67.7.641. [DOI] [PubMed] [Google Scholar]
- 12.Mengel R, Softner M, Flores–de–Jacoby L. Bioabsorbable membrane and bioactive glass in the treatment of intrabony defects in patients with generalized aggressive periodontitis – Results of a 12 month clinical and radiological study. J Periodontol. 2003;74:899–908. doi: 10.1902/jop.2003.74.6.899. [DOI] [PubMed] [Google Scholar]
- 13.Gaffane TE. Guided tissue regeneration using a bioabsorbable membrane: A 21 – case series. J Periodontol. 2004;75:1728–33. doi: 10.1902/jop.2004.75.12.1728. [DOI] [PubMed] [Google Scholar]
- 14.Pretzl B, Kim TS, Steinbrenner H, Dorfer C, Himmer K, Eikholz P. Guided tissue regeneration with bioabsorbable barriers. III 10 years results in infrabony defects. J Clin Periodontol. 2009;36:349–56. doi: 10.1111/j.1600-051X.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- 15.Fetner AE, Hartigan MS, Low SB. Periodontal repair using PerioGlas® in non-human primates. Clinical and histological observations. Compendium. 1994;15:932–8. [PubMed] [Google Scholar]
- 16.Karatzas S, Zavras A, Greenspan D, Amar S. Histologic observations of periodontal wound healing after treatment with PerioGlas® in non-human primates. Int J Periodontics Restorative Dent. 1999;19:489–99. [PubMed] [Google Scholar]
- 17.Segelnick SL, Weinberg MA. Reevaluation of initial therapy: When is the appropriate time? J Periodontol. 2006;77:1598–601. doi: 10.1902/jop.2006.050358. [DOI] [PubMed] [Google Scholar]
- 18.Nasr HF, Aichelmann- Reidy ME, Yukna RA. Bone and bone substitute. Periodontol 2000. 1999;19:74–86. doi: 10.1111/j.1600-0757.1999.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 19.Carranza FA, Takei HH, Cochran DL. Carranza's Clinical Periodontology. 10th ed. New Delhi: Elsevier; 2006. Reconstructive periodontal surgery. [Google Scholar]
- 20.Garrett S. Periodontal regeneration around natural teeth. Ann Periodontol. 1996;1:621–66. doi: 10.1902/annals.1996.1.1.621. [DOI] [PubMed] [Google Scholar]
- 21.Bartold PM, McCulloch CA, Narayanan AS, Pitaru S. Tissue engineering: A new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol 2000. 2000;24:253–69. doi: 10.1034/j.1600-0757.2000.2240113.x. [DOI] [PubMed] [Google Scholar]
- 22.Trejo PM, Weltman R, Caffesse RG. Effects of expanded polytetrafluoroethylene and polylactic acid barriers on healthy sites. J Periodontol. 1998;69:14–8. doi: 10.1902/jop.1998.69.1.14. [DOI] [PubMed] [Google Scholar]
- 23.Aichelmann-Reidy ME, Yukna RA. Bone replacement grafts. The bone substitutes. Dent Clin North Am. 1998;42:491–503. [PubMed] [Google Scholar]


