Abstract
Objective:
The purpose of this study was to evaluate the effects of synthetically processed hydroxyapatite particles in remineralization of the early enamel lesions in comparison with 2% sodium fluoride.
Materials and Methods:
Thirty sound human premolars were divided into nanohydroxyapatite group (n = 15) and the sodium fluoride group (n = 15). The specimens were subjected to demineralization before being coated with 10% aqueous slurry of 20 nm nanohydroxyapatite or 2% sodium fluoride. The remineralizing efficacy of the materials was evaluated using surface microhardness (SMH) measurements, scanning microscopic analysis and analysis of the Ca/P ratio of the surface enamel. Data analysis was carried out using paired t-test and independent t-test.
Results:
The results showed that the nanohydroxyapatite group produced a surface morphology close to the biologic enamel, the increase in mineral content (Ca/P ratio) was more significant in the nanohydroxyapatite group (P < 0.05) and the SMH recovery was closer to the baseline level in the nanohydroxyapatite group (P < 0.05). Both the groups did not show any significant difference in thickness (P > 0.05).
Conclusion:
The use of biomimetic nanohydroxyapatite as a remineralizing agent holds promise as a new synthetic enamel biocompatible material to repair early carious lesions.
Keywords: Biomimetic, enamel, microhardness, mineral content, nanohydroxyapatite, remineralization, sodium fluoride
Introduction
Prevention of dental caries has always been difficult to tackle. Dental professionals have actively participated in caries prevention through plaque removal and dental hygiene techniques; reinforcing the need for reduction in cariogenic refined carbohydrates ingestion; topical application of fluoridated dentrifices, rinses and gels; systemic water fluoridation; placement of pit and fissure sealants.[1]
Caries is a dynamic process with interspersed periods of demineralization and remineralization. The phenomenon of reversal of incipient or early enamel caries forms an important part of prevention leading to apparent repair of the lesion.[2] Several investigators have worked towards developing the ideal remineralizing agent, which diffuses into the subsurface or delivers calcium and phosphate into the subsurface.[3]
Fluorides, over the past 25 years, have been instrumental in causing the decline of dental caries experience in most industrialized countries. Topical fluorides promote the formation of flourapatite in the presence of calcium and phosphate ions produced during enamel demineralization. However, for every 2 fluoride ions, 10 calcium ions, and 6 phosphate ions are required to form one-unit cell of fluorapatite [Ca10 (PO4)6 F2 ].[4] Hence, the availability of calcium and phosphate ions can be the limiting factor for net remineralization to occur. Also given the mechanism of remineralization by topical fluorides they attempt only to reduce apatite dissolution rather than aiming to promote mineralization of apatite crystal or replacement of the lost minerals.[5]
The structure of enamel is too complex to be remodeled and the basic enamel building blocks are generally 20-40 nm hydroxyapatite (HA [Ca10 (PO4)6 (OH)2) nanoparticles. Therefore, the remineralization of enamel minerals by using synthetic apatite or HA, that resembles enamel HA may be beneficial.[5,6] The aim of this study thus was to explore the effects of synthetically processed nanosized biomimetic HA particles in causing remineralization of the early enamel lesions in comparison with 2% sodium fluoride.
Materials and Methods
This is a triple blind study carried out after obtaining approval from the institutional ethics committee.
Specimen preparation
Thirty human premolars extracted for orthodontic reasons were selected for the study. Sound non-carious teeth without any restorations were included and teeth with deformities, defects, fractures were excluded from the study. The teeth thus selected were thoroughly cleaned of debris and stored in saline (0.9% sodium chloride solution) until required. The crown was separated from the root and sectioned into two halves using a high speed diamond disc. The abraded surfaces were polished using pumice polishing paste. The specimens were randomly divided into a control group and a treatment group, each consisting of 15 specimens.
Lesion formation
Each specimen were placed in 15 ml of demineralizing solution of the composition 2.2 mM CaCl2, 0.05 M lactic acid, and 0.5 ppm F - adjusted to pH 4.5 with 50% NaOH for 48 h. At the end of 48 h chalky white incipient caries like lesions developed on the surface of the specimens.
Remineralization regimen
Nanohydroxyapatite group
Nanohydroxyapatite powder was procured from M/s. Dynamic Orthopedics (P) Ltd., Cochin, India. The nanohydroxyapatite powder had a crystal dimension of 50-100 nm in length and 20-40 nm in width. It was ensured that these nanohydroxyapatite crystals are similar to apatite crystals in human enamel by X-ray diffraction analysis to guarantee their biomimetic property.[7] The demineralized specimens were coated with 10% aqueous slurry of nanohydroxyapatite and immersed in artificial saliva[8] (Fusayama Meyer's artificial saliva of the composition KCl 0.4 g/l, NaCl 0.4 g/l, CaCl2·2H2 O 0.906 g/l, NaH2 PO4·2H2 O 0.690 g/l, Na2 S·9H2 O 0.005 g/l, Urea 1 g/l; pH 6.5) for 10 days.
Sodium fluoride group
2% neutral sodium fluoride solution was prepared by dissolving 20 g of sodium fluoride powder in 1 l of distilled water. Fifteen demineralized specimens were immersed in the freshly prepared 2% sodium fluoride solution followed by immersion in artificial saliva for 10 days.
Scanning electron microscopic analysis
Environmental scanning electron microscope (SEM) (Carl Zeiss, Oxford Instrument, INCA- Oxford Software package) was used for analyzing the surface morphology and enamel thickness of the specimens at the baseline level, after demineralization and remineralization treatments. The procedures were carried out under extended pressure (100 Pa air pressure) with LaB6 filament. Enamel thickness was measured at three points from the dentinoenamel junction to the enamel surface by drawing tangents and an average was taken out of the three readings.
Energy dispersive X-ray analysis
The calcium and phosphate content in the surface enamel was measured with the aid of SEM fitted with energy dispersive X-ray analysis. The readings were then converted to Ca/P ratio (wt%). Ca/P ratio was assessed before and after demineralization and after remineralization to evaluate the change in the mineral density.
Surface microhardness analysis
The surface microhardness (SMH) of the specimens were measured as Vikers hardness number (VHN) during three stages of this study: Before demineralization, after demineralization, and after remineralization using an automatic digital microhardness tester (Micro Vickers Hardness Tester, Omni Tech). Three indentations were made on each sample during each stage of the study using a single load of 200gf (gram force) with a holding period of 15 s and the SMH data obtained were the average values of three measurements. A minimum distance of 150 μm was ensured between adjacent indentations in order to avoid measurement errors.
Statistical analysis
The data were computerized and analyzed using SPSS (Software Package used for Statistical Analysis) version 11.5. The results obtained on enamel thickness, mineral content, and microhardness determination between each step in the same group was analyzed using paired t-test followed by analysis using independent sample's t-test to compare the results between both the groups.
Results
Surface morphologic analysis
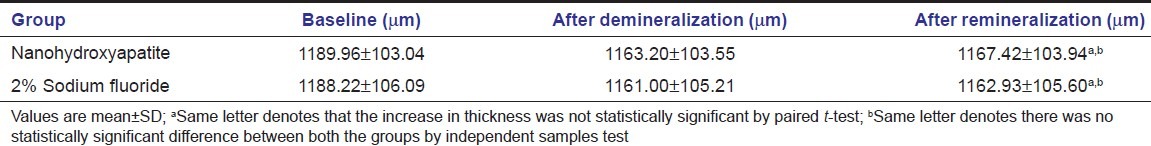
Observable surface changes were seen in the SEM analysis of the enamel. Figure 1a depicts the normal smooth and intact enamel surface before demineralization with the typical key hole patterns of enamel prisms. The surface structure changed to irregular with voids and numerous micropores after demineralization [Figure 1b]. The specimens treated with 2% sodium fluoride showed deposition of calcium fluoride globules on the demineralized enamel surfaces [Figure 2]. The surfaces of the teeth treated with nanohydroxyapatite displayed nucleation of apatitic crystals in the pores created by demineralization [Figure 3]. The porous interprismatic and prismatic enamel structures were completely hidden by a thick, uniform apatitic layer with typical fastened needle-like HA crystallites [Figure 4] in contrast to the specimens treated by 2% sodium fluoride which exhibited evidence of uncovered porous surfaces.
Figure 1.
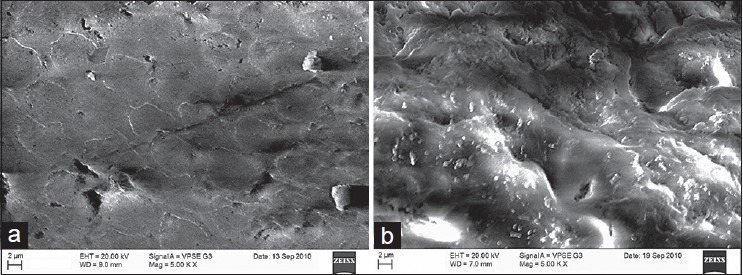
(a) Enamel surface before demineralization at ×5000; (b) enamel surface after demineralization at ×5000
Figure 2.
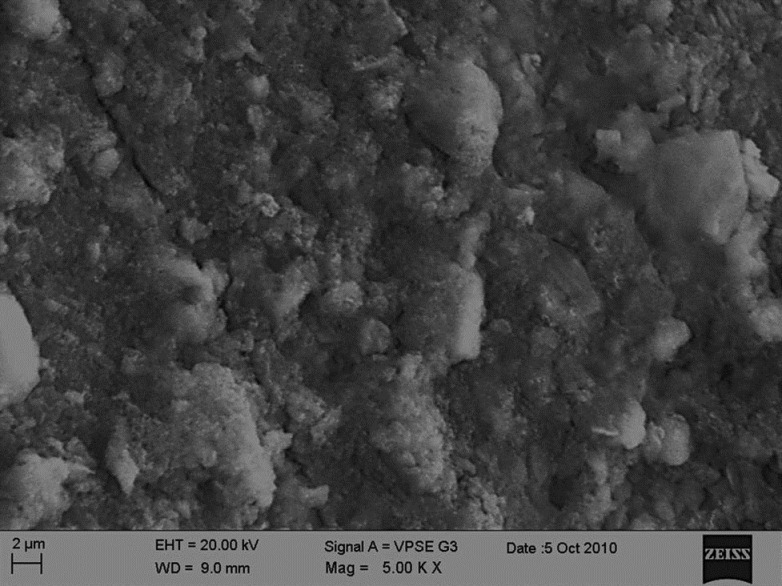
Enamel surface treated with sodium fluoride at ×5000 showing calcium fluoride deposition
Figure 3.
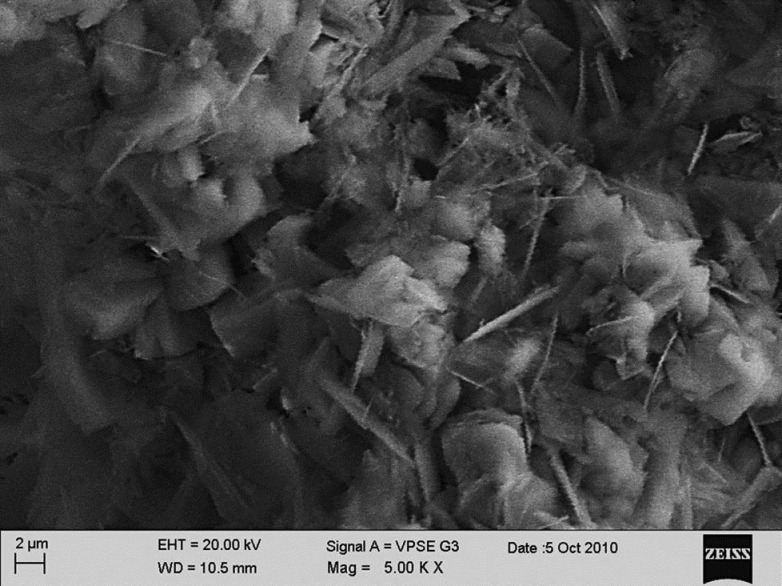
Apatite layer being formed on the demineralized surface
Figure 4.

Enamel surface treated with nanohydroxyapatite at ×1470 showing nucleating hydroxyapatite crystallites occupying the pores
Surface mineral content analysis
The Ca/P ratio fell to a considerable level in both the groups after demineralization. Though both the groups showed a significant increase in Ca/P ratio (P < 0.05), it was found that the recovery of mineral content was more in HA group than in 2% sodium fluoride group and the difference was statistically significant (P < 0.05) [Table 1].
Table 1.
Surface zone mineral content of the enamel at various stages of the study (Ca/P ratio)

Enamel thickness analysis
There was a considerable reduction in the thickness of the surface enamel after demineralization [Table 2]. Treatment with both 2% sodium fluoride and nanohydroxyapatite resulted in a slight increase in thickness of the surface enamel. Nanohydroxyapatite group had a better increase in enamel thickness, but the difference was not statistically significant (P > 0.05).
Table 2.
Enamel thickness analysis at various stages of the study
Surface microhardness analysis
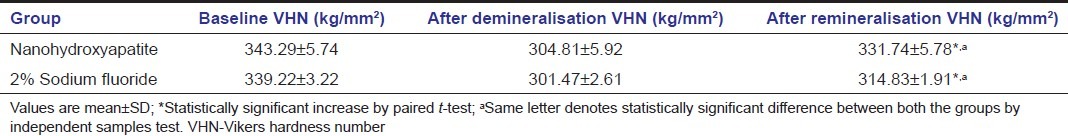
The results of enamel SMH analysis of the specimens are shown in Table 3. The specimens in both the treatment groups had experienced a decrease in microhardness after demineralization and had rehardened after remineralization. The increase in SMH was statistically significant in both the groups (P < 0.05). SMH recovery was more pronounced in HA group than in sodium fluoride group and was statistically significant (P < 0.05).
Table 3.
Surface microhardness analysis of the enamel at various stages of the study
Discussion
Fluorides have been the most favored remineralizing agent. Topically applied sodium fluoride solution has been known to cause remineralization, mainly by reducing apatite dissolution by forming less soluble fluorapatite and increasing SMH, but are unable to reconstruct the lost mineral structure.[9,10] Nanostructured HA crystals exhibit high levels of biomimetic properties due to their composition, structure, morphology, bulk and surface physical–chemical properties. 20 nm HA used in this study are bioinspired molecules that has a surface area of 100 m2/g, which makes them possess strong affinity to the demineralized surfaces.[6] The SEM analysis of the surface after remineralization induced by nanohydroxyapatite reflects this observation where HA nanocrystals were found to adhere to the pores created by demineralization. These adherent nanocrystals were found to aggregate and grow into microclusters and form a uniform apatite layer on the demineralized surface. The surface also revealed the newly formed apatite layer to be completely covering the prismatic and interprismatic enamel structures. The same observation was noticed in an earlier study where 100 nm carbonated HA particles were found to cover the demineralized enamel surfaces more effectively when compared with fluoridated toothpaste.[11]
The fall in the surface Ca/P ratio following demineralization did not practically increase after remineralizing with 2% sodium fluoride application. This finding suggests that only structural modification of apatite occurs and is restricted to a partial hydroxyl group replacement by fluoride ions without influencing the Ca and phosphate content. The specimens treated with nanohydroxyapatite exhibited surface Ca/P ratio close to that of the biological enamel and the synthetic nanohydroxyapatite used indicating an apatite coating deposition on the demineralized enamel surface. An earlier study[11] done on remineralization by carbonated nanohydroxyapatite crystals expressed that the enamel slabs brushed with the material showed an increase in Ca/P ratio, though not closer to the biological enamel which might be attributed to the absence of salivary effect on the remineralization. The study also depicts the Ca/P ratio of the specimens treated with fluoride did not resemble the Ca/P ratio of biologic enamel.
The significant increase in SMH in the nanohydroxyapatite group establishes that this new material rehardens the softened enamel by gradual deposition of the mineral that precipitates and nucleates in the dark zone of demineralization thereby offering complete biomimetic regeneration of the lost enamel crystallites. A 48 h remineralization study[12] using nanohydroxyapatite revealed a similar increase in SMH after treatment with the material. The earlier study also pictures that the increase in SMH did not reach close enough to the biologic enamel surface hardness which might be because of the decreased contact time with the test material and also the blockade of the surface layer.[13]
Conclusion
10% biomimetic nanohydroxyapatite of the particle size 20 nm has the potential to remineralize initial enamel caries under in vitro conditions when compared with 2% sodium fluoride. This documented biomimetic apatite coating on the demineralized enamel suffices the need for a synthetic enamel biocompatible material able to repair early enamel lesions. Nanohydroxyapatite would therefore be beneficial in promoting remineralization with regular daily usage.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
References
- 1.Hicks J, Flaitz C. Role of remineralizing fluid in in vitro enamel caries formation and progression. Quintessence Int. 2007;38:313–9. [PubMed] [Google Scholar]
- 2.Pearce EI, Moore AJ. Remineralization of softened bovine enamel following treatment of overlying plaque with a mineral-enriching solution. J Dent Res. 1985;64:416–21. doi: 10.1177/00220345850640030401. [DOI] [PubMed] [Google Scholar]
- 3.Walsh LJ. Contemporary technologies for remineralization therapies: A review. Int Dent SA. 2009;11:6–16. [Google Scholar]
- 4.Reynolds EC. Calcium phosphate-based remineralization systems: Scientific evidence? Aust Dent J. 2008;53:268–73. doi: 10.1111/j.1834-7819.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 5.Roveri N, Foresti E, Lelli M, Lesci IG. Recent advances in preventing teeth health hazard: The daily use of hydroxyapatite instead of fluoride. Recent Pat Biomed Eng. 2009;2:197–215. [Google Scholar]
- 6.Li L, Pan H, Tao J, Xu X, Mao C, Gu X, et al. Repair of enamel by using hydroxyapatite nanoparticles as the building blocks. J Mater Chem. 2008;18:4079–84. [Google Scholar]
- 7.Wang M. Developing bioactive composite materials for tissue replacement. Biomaterials. 2003;24:2133–51. doi: 10.1016/s0142-9612(03)00037-1. [DOI] [PubMed] [Google Scholar]
- 8.Meyer JM, Nally JK. Influence of artificial salivas on the corrosion of dental alloys. J Dent Res. 1975;54:678. [Google Scholar]
- 9.Newby CS, Creeth JE, Rees GD, Schemehorn BR. Surface microhardness changes, enamel fluoride uptake, and fluoride availability from commercial toothpastes. J Clin Dent. 2006;17:94–9. [PubMed] [Google Scholar]
- 10.Nobre-dos-Santos M, Rodrigues LK, Del-Bel-Cury AA, Cury JA. In situ effect of a dentifrice with low fluoride concentration and low pH on enamel remineralization and fluoride uptake. J Oral Sci. 2007;49:147–54. doi: 10.2334/josnusd.49.147. [DOI] [PubMed] [Google Scholar]
- 11.Roveri N, Battisella E, Bianchi CL, Foltran I, Foresti E, Iafisco M, et al. Surface enamel remineralization: Biomimetic apatite nanocrystals and fluoride ions different effects. J Nanomater. 2009;2009:1–9. [Google Scholar]
- 12.Jeong SH, Jang SO, Kim KN, Kwon HK, Park YD, Kim BI. Remineralisation potential of new toothpaste containing nano-hydroxyapatite. Key Eng Mater. 2006:537–540. [Google Scholar]
- 13.Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater. 2009;4:1–6. doi: 10.1088/1748-6041/4/3/034104. [DOI] [PubMed] [Google Scholar]


