Abstract
Context:
Walking bleach technique uses 30% hydrogen peroxide and sodium perborate, and this paste mixture causes loosening of the coronal temporary restorative materials and thus decreasing its clinical effectiveness and causing irritation to the patients oral tissues. In the present study, sealing ability of hygroscopic coronal temporary restorative materials were compared with the other commonly used temporary restorative materials.
Aim:
To evaluate the effects of walking bleach material on the marginal sealing ability and coronal microleakage of the hydrophilic temporary restorative materials with that of the other commonly used temporary restorative materials in endodontic practice.
Materials and Methods:
Seventy-five extracted human maxillary central incisor teeth were prepared chemo-mechanically and obturated with gutta-percha in lateral condensation technique. Surface of each tooth was double coated with cyanoacrylate glue. All the teeth were randomly divided in to five groups. Out of 15 teeth in each group, 10 teeth served as experimental specimens, in which bleaching agent was placed in the pulp chamber and 5 teeth served as control, in which no bleaching agent was placed. The access cavities were restored with temporary restorative materials being tested per each group respectively. The specimens were then immersed in 1% India ink dye and subjected to thermo cycling for 7 days. All the teeth were longitudinally sectioned and observed with stereomicroscope and were graded according to the depth of linear dye penetration.
Statistical Analysis Used:
Mann-Whitney U test and Kruskal-Wallis test.
Results:
Hydrophilic temporary restorative materials Cavit G and Coltosol F have shown minimal coronal dye leakage with better sealing ability when exposed to walking bleach paste mixture in the dye penetration tests compared to other commonly used temporary restorative materials.
Conclusion:
Marginal sealing ability of Cavit G and Coltosol F were not influenced by the effects of bleaching agent compared to other temporary restorative materials used in the study.
Keywords: Bleaching agent, dye leakage, dye penetration, microleakage, sealing ability, walking bleach
Introduction
Walking bleach technique uses 30% hydrogen peroxide as the primary oxidizing agent. An increase in pressure inside the root canal space may occur due to liberation of oxygen gas from the bleaching paste mixture resulting in loosening of coronal temporary restorative materials. The paste components may leak into the patients’ oral cavity causing acidic taste, irritation, localized sloughing of the adjacent gingival tissues and decreases the effectiveness of the bleaching agent. So the clinical choice of a coronal temporary restorative material becomes utmost important for the success of bleaching treatment.[1]
Materials and Methods
Seventy-five freshly extracted sound human maxillary central incisors were collected.
All the teeth were cleaned of calculus, surface deposits and were stored in 10% neutral formalin and used within 1 month of extraction. Endodontic access cavity preparations were made on all teeth using a high speed rotary hand piece (NSK Dental Corp, Nakanishi, Japan) and an Endo access bur no: 2 (Dentsply, Malleifer, Tulsa Dental, Tulsa, USA). Necrotic pulp tissue was removed with a barbed broach (Dentsply, Malleifer, Tulsa Dental, Tulsa, USA). A No: 10-K file (Dentsply, Malleifer, Tulsa Dental, Tulsa, USA) was placed in the canal to establish the patency of the foramen and working length for biomechanical preparation was determined by substracting 1 mm from the length achieved with the tip of the trail file just visible at the apical foramen of the tooth and the radicular space preparation was performed upto No: 40 size K-file following step back technique in all the teeth. During the process 19% ethylene diamine tetra acetic acid (EDTA) (MD-ChelCream, META Dental corp, Biomed, Cheongju, Korea), copious amounts of 3% sodium hypochlorite (Vishal Dentocare Pvt Ltd, Ahmedabad, Gujarat, India) and normal salines were alternatively used to irrigate the canal between the changes in the file size.
Canals were then dried with absorbent paper points and were coated with a thin mix of AH [Amine Hydroxy] plus sealer (Dentsply, Maillefer, USA). In all the teeth, no: 40 size gutta-percha points (Dentsply, Maillefer, USA) were used as master apical cone and were obturated in lateral condensation technique using accessory gutta-percha points. Surfaces of all the teeth were double coated with cyanoacrylate glue except 1 mm around the circumference of the access cavity.
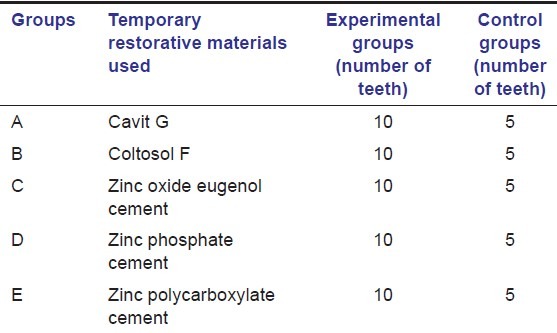
All the teeth were equally divided into five groups following stratified randomization and double-blinded trail. Each group consists of 15 teeth, of which 10 teeth were served as experimental specimens and 5 teeth as control specimens as summarized in Table 1.
Table 1.
Experimental and control groups
In all the experimental group specimens, 30% hydrogen peroxide liquid (Dentsply De trey, Knstanz, Germany) and sodium perborate monohydrate powder (Degussa, Hanau, Germany) were freshly mixed in volume ratio of 1:3 into a paste form and were placed in the pulp chamber.
In all the control group specimens, no bleaching agent was placed in the pulp chamber instead dry cotton pellets were placed.
Access cavity openings of all the teeth in each group were sealed with three millimeter thickness of one of the five temporary tooth restorative materials being tested respectively, irrespective of either experimental or control group specimens as summarized in Table 1.
The five coronal temporary restorative materials used in the present study were cavit G (3M ESPE Dental-Medizin GmbH Co, Seafeld, Germany), coltosol F (Coltene AG, Altstatten, Switzerland), zinc oxide eugenol cement (DPI Dental, The bombay burma trading corporation limited, India), zinc phosphate cement (Harvard Dental International GmbH, Germany) and zinc polycarboxylate cement (Poly-F, Dentsply, De Trey GmbH, Germany). All the temporary restorative materials were manipulated and allowed to set according to manufacturer's instructions. After the final setting of the restorative materials, all the teeth were immersed in 1% India ink dye (AB Chemi, Glasco, UK) and in the dye solution the teeth were subjected to thermocycling at 4° ± 2°C and 56° ± 2°C for 500, 2 min cycles with a dwell time of 60 s for 7 days.[2]
The teeth were then removed from the dye solution and were thoroughly rinsed under running tap water for 5 min, dried and the cyanoacrylate glue was completely removed with a scalpel. All the teeth were longitudinally sectioned in a mesio-distal direction with a diamond disc of 0.3 mm in thickness (Salvin dental, Oklahoma, USA) at a speed of 20,000 rpm under copious water irrigation. The depth of linear dye penetration was observed with stereomicroscope (Stemy SV8 Zeiss, Germany) of magnification ×10 equipped with a micromeasure grid and the measurements of dye penetration were carried out by the same operator.
They were ranked by graded scores of 0-3 [Figure 1]:[3]
Figure 1.
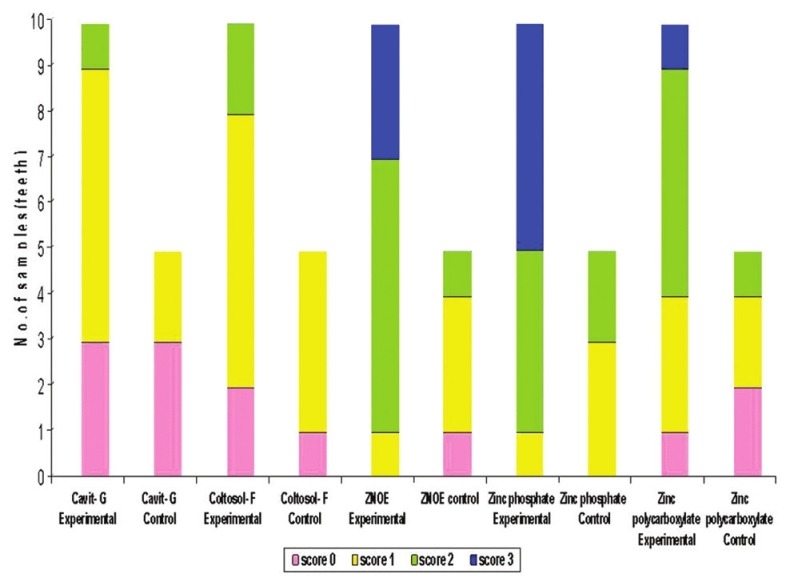
Bar diagram representing the number of samples with varying scores of dye leakage of the experimental and control groups
No dye penetration.
Dye penetration not reaching the pulp chamber.
Dye penetration reaching the pulp chamber.
Loss of temporary restorative material from the occlusal access.
Statistical methods used
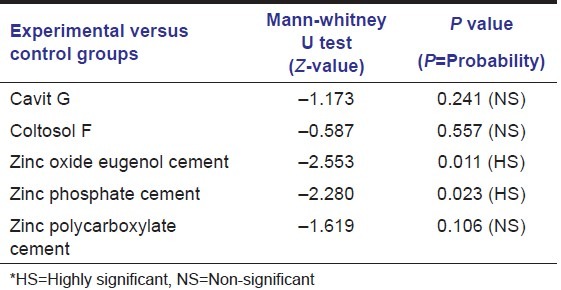
Statistical analysis was done using Kruskal–Wallis test and Mann Whitney U test. Comparative analysis of the experimental and control group specimens within each group was done using Mann-Whitney U test and is presented in Table 2.
Table 2.
Mann-Whitney U test for comparison of the experimental and control group specimens and their P value
Kruskal-Wallis test was done to see whether there was any significant difference between the experimental groups and the statistical analysis showed that there was a significant difference between the experimental groups of five different temporary restorative materials used.
Results
Kruskal-Wallis test between the experimental groups shows that Cavit G exhibited the minimal leakage values in the dye penetration tests with a mean of 13.30 followed by Coltosol F with 16.20, zinc polycarboxylate cement with 25.70, zinc oxide eugenol cement with 34.80 and maximum leakage value of dye penetration was exhibited by zinc phosphate cement with a mean of 37.50.
The mean value among the experimental groups showed a highly significant difference as the P value was 0.001.
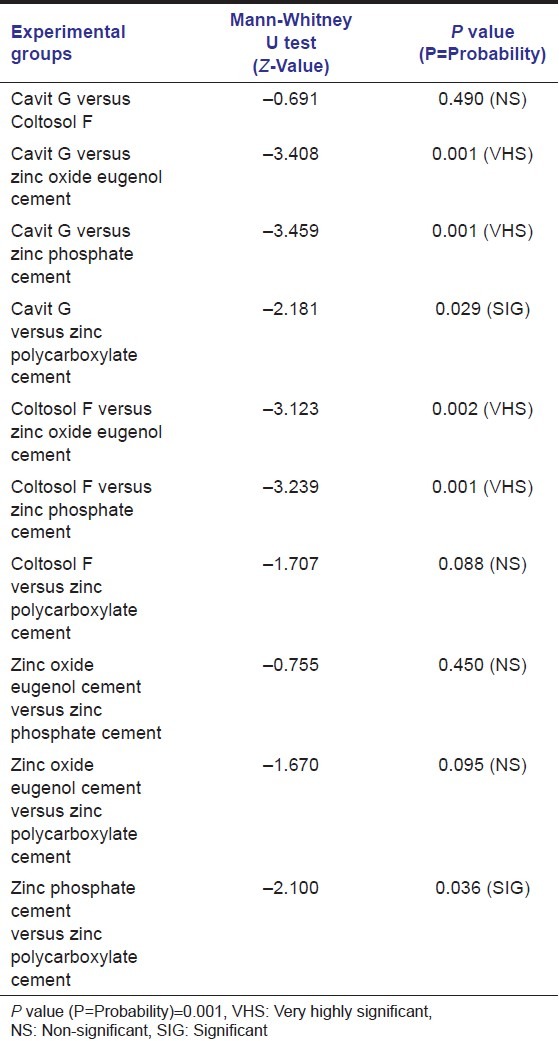
So to find out where the significant difference was, Mann-Whitney U test was done comparing the dye leakage values of only the experimental group specimens and is presented in Table 3.
Table 3.
Intergroup comparison of the experimental specimens using Mann-Whitney U test
Kruskal-Wallis test between the control groups shows that Cavit G exhibited the minimal leakage values in the dye penetration tests with a mean of 8.20, followed by Coltosol F with 12.10, zinc polycarboxylate cement with 12.40, zinc oxide eugenol cement with 14.20, zinc phosphate cement with 18.10.
The mean value among the control groups was not significant as the P value was 0.202. So it was not required to go for the Mann-Whitney U test between the control groups.
Discussion
Non-vital bleaching technique suggested by Spasser[4] in the year 1961, uses a mixture of sodium perborate and water in paste form placed in the pulp chamber of tooth between dental appointments. Perborate in this state breaks down into sodium metaborate and hydrogen peroxide. The later finally degrades into water, free radicals and oxygen thus allowing the release of the primary oxidizing agent.
Nutting and Poe[5] in 1963 suggested mixing of sodium perborate with 30% hydrogen peroxide to enhance the bleaching effects. This method was referred to as the walking bleach technique and it was believed that the combination of these two oxidizing agents would be synergistic and more effective in bleaching the tooth.
Microleakage between the restorative materials and tooth surface can be evaluated by several methods and they include dye penetration tests, fluid filtration methods, radioisotopes, electrochemical methods, fluroscent microscopy, resin infiltration methods, and chemical tracers. Of these, dye penetration method was the most widely used as it is simple and reproducible.[6] So in the present study, linear dye penetration method was used and the dye utilized was 1% India's ink. Usually methylene blue or rhodamine-B solutions are used for dye penetration tests. However, these two dye solutions were decolorized by the bleaching paste mixture when used in our pilot study and 1% India's ink dye was scarcely discolored and thus we chose it to use in our study.
To simulate fluctuations in temperature changes of the oral cavity, thermocycling was included in the present study as it has been reported by Bobotis[7] and McInerney[8] that temperature fluctuations can adversely affect the marginal sealing ability of temporary restorative materials.
As Webber[9] suggested a minimum thickness of 3 mm for any temporary restorative material to achieve a superior marginal sealing ability. So in the present study we standardized the thickness of all coronal temporary restorative materials used to 3 mm.
Both Cavit G and Coltosol F are hydrophilic restorative materials with a higher linear coefficient of expansion. The coefficient of linear expansion of Cavit G and Coltosol F was almost double that of zinc oxide eugenol cement, which explains its effectiveness as a coronal temporary restorative material in walking bleach techniques and even after subjecting to thermocycling, they maintained a leak-proof seal both at room temperature and also at temperatures ranging from 4°C to 55°C.[9]
Marosky[10] found a correlation between ease of handling and sealing ability of the temporary restorative materials. It was noted that the premixed cements allowed less marginal leakage and showed better sealing ability, as fewer chances for manipulative errors with premixed cements than those cements that depend upon the investigators skills in mixing powder-liquid components of the cements.
Cavit G is a premixed radiopaque, grey color temporary restorative material composed of zinc oxide, calcium sulphate, zinc sulphate, glycol acetate, polyvinylacetate resins, polyvinyl chloride acetate, triethanolamine and synthetic resin filler material with no eugenol content.[1]
Coltosol F is a recently introduced premixed radiopaque, white color temporary restorative material composed of zinc oxide, zinc sulphate-1-hydrate, calcium sulphate-hemihydrate, diatomaceous earth, natrium fluoride, synthetic resin material with no eugenol content and with peppermint aroma.[1]
However, there was no statistically significant difference in the leakage values following dye penetration tests between Cavit G [Figure 2] and Coltosol F neither in their experimental nor in control group specimens of group A and B.
Figure 2.
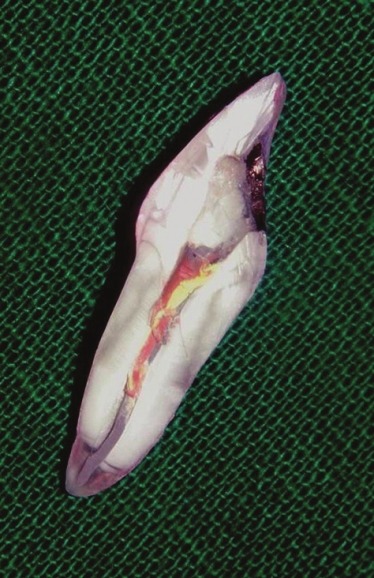
Minimal dye leakage was seen in experimental tooth specimens of group A
Bonding of zinc phosphate cement to tooth structure occurs by mechanical interlocking and not by chemical interactions. Zinc phosphate cement is available in powder: Liquid system. Powder is composed of zinc oxide (90%), magnesium oxide (8.2%), silicone dioxide (1.4%), bismuth trioxide (0.1%) and traces of barium oxide, barium sulphate and calcium oxide. Liquid is composed of phosphoric acid (free acid) (38.2%), phosphoric acid combined with aluminium and zinc (16.2%), aluminium (2.5%), water (36%), zinc (7.1%). Recommended powder: Liquid ratio is 1.4 g powder to 0.5 ml of liquid.[11]
Naoum and Chandler[11] reported that the sealing ability of zinc phosphate cement is inferior to Cavit G, zinc oxide eugenol cements and Gilles et al,[12] reported that, when Cavit G and zinc oxide eugenol cements were subjected to thermocycling, Cavit G showed minimal dimensional changes, whereas zinc oxide eugenol cement showed large dimensional fluctuations following thermocycling. However, Krakow et al,[13] in an in-vivo study evaluated the sealing ability of Cavit, Caviton, Gutta-percha, zinc phosphate cement and zinc oxide eugenol cements and reported that Cavit, Caviton and zinc phosphate cements showed no or minimal dye leakage and Gutta-percha showed the maximum dye leakage in the dye penetration tests.
In five of the experimental specimens of group D, where zinc phosphate cement was used as temporary restorative material, there was a loss of restorative material from the occlusal access. Whereas in control group specimens, there was no loss of restorative material from the occlusal access. However, there was a significant difference in the leakage values between experimental and control group specimens as leakage values were minimal in the control group compared to experimental group specimens, which indicates the definite effect of the bleaching paste mixture on its contact with the zinc phosphate cement [Figure 3].
Figure 3.
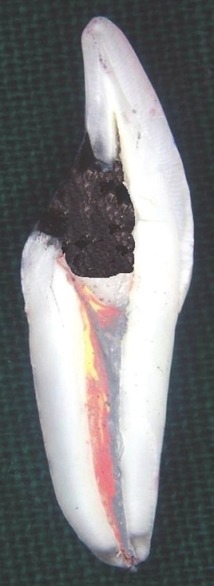
Maximum dye leakage was seen in experimental tooth specimens of group D
Zinc oxide eugenol cement is available as powder: Liquid system. Powder is composed of zinc oxide (100%) and liquid is composed of eugenol (100%).[11]
In three of the experimental specimens of group C, where zinc oxide eugenol cement was used as temporary restorative material, there was a loss of restorative material from the occlusal access. Whereas in control group specimens, there was no loss of restorative material from the occlusal access, which indicates the definite effect of the bleaching paste mixture on its contact with the zinc oxide eugenol cement.
So in the present ex-vivo study, it can be inferred that zinc oxide eugenol and zinc phosphate cements were more strongly influenced by the effects of bleaching agent and to a certain extent, zinc polycarboxylate cement also got affected from the bleaching agent.
As leakage values were minimal and there was no loss of temporary restorative material from the occlusal access of both experimental and control groups specimens of group E. Zinc polycarboxylate cement is available as powder: Liquid system. Powder is composed of zinc oxide (90%), stannic oxide (8.2%), silicone dioxide (1.4%) and traces of bismuth and aluminium. Liquid is mainly composed of aqueous solution of polyacrylic acid and minute quantities of itaconic or tartaric acid. The cement bonds chemically to tooth structure by chelation.[11]
Noguera[14] in an in-vitro study compared the sealing ability of Cavit G, IRM [Intermediate restorative material and TERM [Temporary endodontic restorative material], with TERM showed the least dye leakage, compared to Cavit G and IRM at the tooth-restoration interface in the dye penetration tests.
Waite[15] evaluated the sealing ability of TERM as interim restorative material for walking bleach technique when using either sodium perborate/water or sodium perborate/superoxol. TERM provided an unsatisfactory seal when used with walking bleach technique. However, when used over dry cotton pellets, TERM provided an excellent seal.
Deveaux[16] evaluated the sealing ability of Cavit, IRM and TERM in bacterial micro-leakage in-vitro study involving thermo-cycling. IRM and Cavit showed maximum bacterial penetration, whereas TERM remained leak-proof.
However, in the present study, the hydrophilic temporary restorative materials Cavit G and Coltosol F were not affected by the effects of bleaching paste mixture and also withstood temperature changes much better compared to the other temporary restorative materials used.
So both Cavit G and Coltosol F are not only be used as temporary restorative materials in contemporary endodontics, but also offers tremendous scope for their use as coronal temporary restorative materials in walking bleach techniques.
Ex-vivo studies definitely cannot duplicate the environment that exists in-vivo. However, these ex-vivo evaluations provide information that aids the clinician in the selection of the best material for specific clinical situations.
Conclusion
Both zinc phosphate and zinc oxide eugenol cements were more strongly influenced by the effects of bleaching agent and thermo-cycling on their sealing ability, as they showed maximum leakage values in the dye penetration tests of experimental group specimens compared to the control group specimens.
Minimal leakage values in the dye penetration tests were observed with hydrophilic pre-mixed temporary restorative materials of both experimental and control group specimens. As Cavit G and Coltosol F were not influenced by the effects of bleaching agent and thermo-cycling, they offer tremendous scope for their use as coronal temporary restorative materials for the successful outcome of the walking bleach treatment.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
References
- 1.Hosoya N, Cox CF, Arai T, Nakamura J. The walking bleach procedure: An in vitro study to measure microleakage of five temporary sealing agents. J Endod. 2000;26:716–8. doi: 10.1097/00004770-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Popoff DA, Goncalves FS, Ferreira RC, Magalhaes CS, Moreira AN, Mjor IA. Repair of amalgam restorations with conventional and bonded amalgam. An in-vitro study. J Dent Sci. 2010;25:154–8. [Google Scholar]
- 3.De Moor RJ, De Bruyne MA. The long-term sealing ability of AH 26 and AH plus used with three gutta-percha obturation techniques. Quintessence Int. 2004;35:326–31. [PubMed] [Google Scholar]
- 4.Spasser HF. A simple bleaching technique using sodium perborate. NY State Dent J. 1961;27:332–4. [Google Scholar]
- 5.Nutting EB, Poe GS. A new combination for bleaching teeth. J South Calif Dent Assoc. 1963;31:289–91. [Google Scholar]
- 6.Mohan KM, Lakshminarayanan L. Methods of detecting microleakage. J Conserv Dent. 2004;7:79–87. [Google Scholar]
- 7.Bobotis HG, Anderson RW, Pashley DH, Pantera EA., Jr A microleakage study of temporary restorative materials used in endodontics. J Endod. 1989;15:569–72. doi: 10.1016/S0099-2399(89)80151-7. [DOI] [PubMed] [Google Scholar]
- 8.McInerney ST, Zillich R. Evaluation of internal sealing ability of three materials. J Endod. 1992;18:376–8. doi: 10.1016/s0099-2399(06)81222-7. [DOI] [PubMed] [Google Scholar]
- 9.Webber RT, del Rio CE, Brady JM, Segall RO. Sealing quality of a temporary filling material. Oral Surg Oral Med Oral Pathol. 1978;46:123–30. doi: 10.1016/0030-4220(78)90446-2. [DOI] [PubMed] [Google Scholar]
- 10.Marosky JE, Patterson SS, Swartz M. Marginal leakage of temporary sealing materials used between endodontic appointments and assessed by calcium 45 – An in vitro study. J Endod. 1977;3:110–3. doi: 10.1016/S0099-2399(77)80205-7. [DOI] [PubMed] [Google Scholar]
- 11.Naoum HJ, Chandler NP. Temporization for endodontics. Int Endod J. 2002;35:964–78. doi: 10.1046/j.1365-2591.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 12.Gilles JA, Huget EF, Stone RC. Dimensional stability of temporary restoratives. Oral Surg Oral Med Oral Pathol. 1975;40:796–99. doi: 10.1016/0030-4220(75)90450-8. [DOI] [PubMed] [Google Scholar]
- 13.Krakow AA, de Stoppelaar JD, Gron P. In vivo study of temporary filling materials used in endodontics in anterior teeth. Oral Surg Oral Med Oral Pathol. 1977;43:615–20. doi: 10.1016/0030-4220(77)90117-7. [DOI] [PubMed] [Google Scholar]
- 14.Noguera AP, McDonald NJ. Comparative in vitro coronal microleakage study of new endodontic restorative materials. J Endod. 1990;16:523–7. doi: 10.1016/s0099-2399(07)80214-7. [DOI] [PubMed] [Google Scholar]
- 15.Waite RM, Carnes DL Jr, Walker WA., 3rd Microleakage of TERM used with sodium perborate/water and sodium perborate/superoxol in the “walking bleach” technique. J Endod. 1998;24:648–50. doi: 10.1016/S0099-2399(98)80147-7. [DOI] [PubMed] [Google Scholar]
- 16.Deveaux E, Hildelbert P. Bacterial microleakage of Cavit, IRM, and TERM. Oral Surg Oral Med Oral Pathol. 1992;74:634–43. doi: 10.1016/0030-4220(92)90358-w. [DOI] [PubMed] [Google Scholar]


