Background: Hedgehog signaling is aberrantly activated in malignancies.
Results: The inflammatory cytokine osteopontin causes nonclassical activation of Hedgehog signaling in tumor cells.
Conclusion: The tumor cells become refractory to Smoothened-targeting inhibition and acquire multidrug resistance.
Significance: The outcomes reveal the tumor-extrinsic mechanisms of resistance to Hh inhibitors.
Keywords: Cancer, Cancer Biology, Cancer Therapy, Drug Resistance, Epithelial to Mesenchymal Transition, Osteopontin, Hedgehog, Microenvironment
Abstract
The Hedgehog (Hh) pathway is critical in normal development. However, it has been reported to be up-regulated in numerous cancers and implicated in tumorigenicity and metastasis. Classical activation of Hh signaling initiated by Hh ligands results in activation of Smoothened (SMOH) and culminates in the activation of the GLI transcription factors. Classical Hh signaling is autocrine or paracrine (involving interaction between tumor cells and their stroma/microenvironment). The tumor milieu is rich in inflammatory cytokines that can modulate tumor cell behavior. Here, we show for the first time that the Hh pathway can be nonclassically up-regulated by the inflammatory cytokine, osteopontin (OPN). OPN-initiated Akt-GSK3β signaling mediates the subcellular distribution and activation of GLI1 resulting in the modulation of epithelial mesenchymal plasticity and drug resistance. Interestingly, the SMOH inhibitor cyclopamine was unable to uncouple the effects of OPN on Hh signaling, indicating that OPN nonclassically activates GLI-mediated transcription. Given the fact that OPN is itself transcriptionally activated upon Hh signaling, our current findings highlight the possibility of a feedforward vicious cycle such that the Hh pathway might be turned on nonclassically by stimuli from the tumor milieu. Thus, drugs that target the classical Hh ligand-mediated activation of Hh signaling may be compromised in their ability to interfere with the functioning of the pathway.
Introduction
Hedgehog (Hh)2 signaling in mammalian cells is executed by the GLI family of zinc finger transcription factors comprising GLI1, GLI2, and GLI3 (1). Classical activation of this pathway is initiated by the soluble, lipid-modified morphogens, Sonic Hedgehog (SHH), Indian Hedgehog (IHH), or Desert Hedgehog (DHH) ligands. In the absence of ligands, the receptor Patched (PTCH) binds to and inhibits Smoothened (SMOH), and the pathway is in an off-state. Binding of the ligands releases this inhibitory effect on SMOH which undergoes hyperphosphorylation and locates to the primary cilia, causing activation of GLI followed by its translocation to the nucleus where it activates several target genes including the receptor PTCH, insulin-like growth factor-binding protein, cyclin D2 (2), and OPN (3, 4). The pathway is active in multiple cancer types (5, 6), influences the tumor stromal microenvironment (7), and supports tumor stem cells (8). It is believed that the tumor-reactive stroma responds to the Hh ligands expressed by the tumor cells and produces growth factors that fuel tumor growth. Several inhibitors of this pathway are being tested in clinical trials for their effectiveness in treating multiple malignancies. These are well tolerated for the most part and have demonstrated shrinkage of the tumor. Resistance to the SMOH-targeting inhibitor was characterized by the development of a tumor-intrinsic mutation (for review, see Ref. 4).
The tumor stroma comprises immune-modulatory cells and inflammatory cytokines that engage in a bi-directional relationship with the tumor cells. OPN is a secreted phosphoglycoprotein that is abundant in the tumor microenvironment, forming a notable component of the stroma (9). Increased OPN levels are indicators of advanced breast cancer and multiple other cancer histotypes (10). Nearly 42% of primary breast tumors express moderate to strong levels of OPN. OPN promotes pro-inflammatory conditions of the tumor milieu and acts as an effector of tumor progression and metastasis at several levels (10, 11). OPN signals via multiple receptors including several integrins and CD44, activates NF-κB, PI3-kinase, and Akt signaling (12–14) and enhances malignant attributes.
In the present study, we determined that OPN causes (Hh) ligand-independent nonclassical activation of transcriptional activity of GLI1 in tumor cells, resulting in acquisition of multidrug resistance (MDR) and modulation of epithelial mesenchymal plasticity toward the mesenchymal phenotype. Importantly, this nonclassical signaling induces resistance to SMOH-targeting Hh inhibition. We recently reported that OPN is transcriptionally up-regulated by GLI1. The implications of our study have important bearings on understanding the feedforward activation of Hh signaling and most importantly, on understanding the tumor-extrinsic mechanisms of resistance to Hh inhibitors.
EXPERIMENTAL PROCEDURES
Cell Culture
Cancer cells were cultured in Dulbecco's modified minimum essential medium, F-12 mixture, DMEM/F12, (1:1) (Invitrogen) supplemented with 5% heat-inactivated fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 200 mm sodium pyruvate (Invitrogen) and 20 mm nonessential amino acids (Invitrogen). SUM1315 and SUM159 breast cancer cells (Asterand, Detroit, MI) were cultured in DMEM/F12 supplemented with 5 mg/ml insulin, 5% FBS (Atlanta Biologicals), and either 10 ng/ml EGF or 1 ng/ml hydrocortisone respectively, without antibiotics or antimycotics (15). All cells were maintained in a humidified 5% CO2 environment.
Stable Transfectants
Endogenous GLI1 from MDA-MB-435 cells was stably silenced (KO1) using shRNA (short hairpin RNA) cloned into pSuperior.neo_gfp plasmid or pSuper (OligoEngine, Seattle, WA) (3). SUM159-OPN and MCF7-OPN represent stable transfectants of SUM159 and MCF7 that were engineered to stably express OPN using the OPN-pcDNA3.1 hygromycin− plasmid (16).
Effect of KAAD-Cyclopamine on Cell Proliferation
T47D or SUM1315 cells (5 × 103) were plated in 96-wellplates. The effect of KAAD-cyclopamine (10 μm; Toronto Research Chemicals Inc.) was assessed either in the presence of OPN (100 ng/ml) or without and MTS assay conducted after 24 h.
Western Blotting Analysis
Whole cell lysates were collected in Nonidet P-40 buffer (150 mm NaCl, 50 mm Tris, 1% Nonidet P-40). Isolation of cytosolic and nuclear fractions was done with an NE-PER kit (Pierce) following the manufacturer's protocol. Protein (30 μg) was resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and immunoblotted overnight at 4 °C with antibodies to N-cadherin (4061S; Cell Signaling), keratin 8/18 (4546S; Cell Signaling), vimentin (5741S, Cell Signaling), Twist1 (T5461; Sigma), OPN (905-629; Assay Designs, Ann Arbor, MI), GLI1 (sc-20687; Santa Cruz Biotechnology, Santa Cruz, CA), ABCG2 (AV43649; Sigma), GSK3β (9315; Cell Signaling), phosphoGSK3βS9 (9323; Cell Signaling), phosphoserine-473-Akt (4691; Cell Signaling, Danvers, MA), and total Akt (4060; Cell Signaling). GAPDH (Cell Signaling) was used to confirm equal loading. Anti-rabbit or anti-mouse HRP-conjugated secondary antibody was used for detection, and blots were developed with SuperSignal substrate (Pierce) and exposed using a Fuji LAS3000 imager. The purity of cytosolic and nuclear fractions was confirmed with anti-β-tubulin (2146; Cell Signaling) or anti- histone deacetylase 1 (2062; Cell Signaling) antibodies, respectively.
Luciferase Assay
Cells (40, 000) were transfected with an 8×GLI1 construct in pGL3 promoter plasmid as described previously (17). Empty pGL3 promoter vector was used as control. OPN (100 ng/ml) was added to the well 16 h prior to harvesting the cells (∼33 h of initiation of transfection) for assay. Readings were normalized to total protein content. Each parameter was studied in triplicate and the experiment repeated at least three times. The data are represented as percent luciferase activity, which is derived as a percent of the relative light units in treated groups compared with the untreated groups.
Drug Treatment
Cells (5 × 103) were plated in 96-well microtiter plates. The next day fresh medium was added with indicated concentrations of doxorubicin (APP Pharmaceuticals, LLC, Schaumburg, IL) or cisplatin or paclitaxel (APP Pharmaceuticals) and treated for 24 h. At the end of the treatment the medium was removed, and 100 μl fresh medium was added. MTS assay was done following the manufacturer's protocol (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay; Promega). The cells were placed in an incubator to allow for color development up to 4 h and spectrophotometric readings taken at 490 nm.
Quantitative RT-PCR (qRT-PCR)
RNA was harvested from cells grown in 10-cm plates using Sure Prep Total RNA Purification Kit (Fisher Scientific). cDNA was generated using High Capacity Reverse Transcriptase Kit (Applied Biosystems). Real time PCR was performed using a Bio-Rad iQ5 Real-Time Detection system (Bio-Rad). All reactions were done as three independent replicates and repeated at least once. All assays were done using the TaqMan Gene Expression Assays from Applied Biosystems. The genes queried include GLI1, ABCB1, ABCG2, E-cadherin, N-cadherin, keratin 18, MMP9, SNAI2, Twist1, and vimentin. Transcript levels were normalized to endorse control gene GAPDH using change in the Ct values (ΔCT) to calculate changes in respective expression (2ΔΔCT). To analyze the effect of KAAD-cyclopamine or GANT61 (Tocris Bioscience, Ellisville, MO) treatment on indicators of MDR proteins or epithelial mesenchymal plasticity, untreated samples were set as calibrator (control) and compared with their respective treated samples. Cells were treated with KAAD-cyclopamine (10 μm) or GANT61 (10 μm) for 24 h prior to harvesting RNA.
DIOC2(3) Retention Assay
A modified version of the protocol used by Huo et al. (18) was implemented for this experiment. Cells (1 million) were suspended in 1% BSA in DMEM (assay buffer) without phenol red and then incubated in assay buffer with 2.2 μm DIOC2(3) reagent on ice for 30 min. 25 × 103 cells were plated in black-walled 96-well microtiter plates. The plate was transferred to a 37 °C incubator and incubated for 30 min. Then the cells were washed twice with PBS and resuspended in 250 μl of ice-cold assay buffer to prevent further drug transport. Fluorescence was measured using a fluorescence plate reader at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. Each experiment was conducted in duplicate and repeated three times.
GSK3β Inhibition Assessments
To investigate the role of GSK3β in OPN-induced Hh signaling, tumor cells were treated with a 5 μm concentration of either GSK3β inhibitor, BIO (361551, EMD-Millipore) or the inactive structural analog control, MeBIO (361556, EMD-Millipore). After 2 h of initial treatment OPN (100 ng/ml) was added, and the experiment was terminated after 16 h. For nuclear and cytosolic extracts 5 × 106 cells were utilized whereas 1 × 106 cells were used for RNA extraction.
Statistical Analysis
Statistical differences between groups were assessed after applying the test per the mandates of the experimental design. The tests used include the Mann-Whitney U-test, t test, analysis of variance, using GraphPad Prism 4 software. Statistical significance was determined if the analysis reached 95% confidence. The precise p values are listed in the corresponding figure legends.
RESULTS
OPN Up-regulates GLI1 Expression and Transcriptional Activity
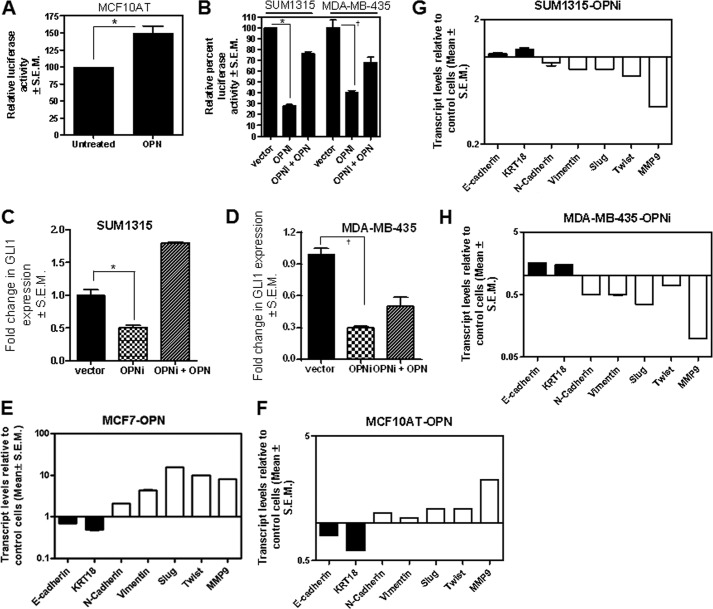
OPN is a cytokine that is commonly present in the reactive tumor stroma. It is produced by the tumor cells as well as the surrounding infiltrating cells. As a promiscuous molecule, OPN can signal through multiple receptors on the cell surface. Exogenous OPN treatment of MCF10AT cells caused an increase in the transcriptional activity of GLI as determined by a significant (p < 0.0001) increase in the activity of a GLI-responsive reporter (Fig. 1A). Conversely, abrogating endogenous OPN from tumor cells (Fig. 1B and supplemental Fig. 1A) significantly decreased (p ≤ 0.01) the transcriptional activity of GLI (Fig. 1C), simultaneous with a notable significant decrease in GLI1 transcript levels (Fig. 1, C and D). Although GLI transcription factors are the ultimate effectors of Hh signaling, the levels of GLI1 are indicative of Hh signaling activity. Exogenous OPN (100 ng/ml) restored both the expression of GLI1 and the transcriptional activity (Fig. 1, B–D). Abrogating OPN also caused a significant decrease (p < 0.0001) in the levels of PTCH, another bona fide GLI transcriptional target gene (supplemental Fig. 1B). Notably, abrogating OPN did not result in any appreciable change in the levels of GLI2 (supplemental Fig. 1B).
FIGURE 1.
OPN regulates GLI1 activity, expression, and induces epithelial-mesenchymal plasticity. A, OPN activates an 8×luciferase GLI reporter in MCF10AT cells (*, p < 0.0001). B, silencing endogenous OPN significantly reduces the activity of 8×GLI1 construct which is recovered upon addition of recombinant OPN in SUM1315 (*, p = 0.01) and MDA-MB-435 (†, p = 0.0002) cells. Cells (3 × 103) were plated in 96-well plates and transfected with 100 ng of the reporter construct. The cells were lysed the following day. Activity is represented as percentage of relative light units normalized to protein. C and D, abrogating OPN notably reduced the expression of GLI1 mRNA in SUM1315 cells (*, p = 0.005) (C) and MDA-MB-435 (†, p = 0.0002) cells (D). Exogenous OPN restored the levels of GLI1. Cells (1 million) were transiently transfected with shRNA targeting OPN. RNA harvested after 16 h of OPN treatment and assessed for GLI1 and GAPDH. E and F, OPN significantly (p < 0.01) modulates the expression of epithelial-mesenchymal markers in MCF7 and MCF10AT cells. Cells were treated with OPN for 16 h followed by harvesting RNA for qRT-PCR. G and H, silencing OPN (OPNi) from SUM1315 (G) or MDA-MB-435 cells (H) caused a moderate but statistically significant increase (p < 0.05) in the expression of epithelial markers, E-cadherin and keratin 18 accompanied by a significant decrease (p ≤ 0.01) in the expression of mesenchymal markers vimentin, Slug, Twist, and MMP9. RNA was assessed by qRT-PCR for E-Cad, N-Cad, vimentin, Slug, TWIST, MMP9, and KRT 18 transcript levels. Error bars, S.E.
OPN Regulates Epithelial-Mesenchymal Transition (EMT) Markers via Modulating Hh Signaling
Previous reports from our laboratory and others (3, 19) have shown that Hh signaling regulates epithelial-mesenchymal transition of tumor cells; specifically, Hh signaling promotes the mesenchymal phenotype. Modulating OPN-initiated signaling impacted the expression of phenotypic monikers of the epithelial-mesenchymal characteristics. Specifically, activating OPN-mediated signaling in the tumorigenic, epithelial breast cancer cells, MCF7 and MCF10AT resulted in decreased expression of E-cadherin and keratin 18 simultaneous with an increase in the expression of N-cadherin, vimentin, Twist, Slug, and MMP9 (Fig. 1, E and F). Conversely, abrogating endogenous OPN from SUM1315 and MDA-MB-435 cells resulted in gain of expression of E-cadherin and keratin 18 concurrent with decreased expression of mesenchymal markers, N-cadherin, vimentin, Twist, and Slug (Figs. 1, G and H). Overall, the data suggest that OPN promotes the expression of mesenchymal markers (supplemental Fig. 1C).
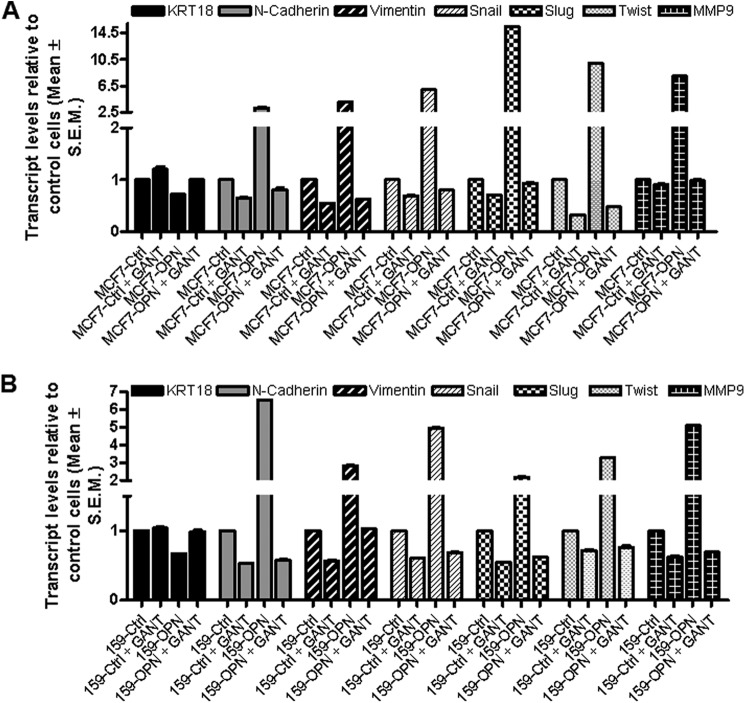
To assess a role for Hh signaling in this activity, we treated cells with GANT61, a specific inhibitor of GLI-mediated transcriptional activity (20). GANT61 is a specific inhibitor of GLI1- and GLI2-mediated transcription and acts by altering the conformation of the GLI molecules such that their binding to DNA is reduced (21). GANT61 not only caused an increase in the expression of epithelial marker keratin 18, but it also antagonized the inhibitory effects of OPN on keratin 18 expression (Figs. 2, A and B, and supplemental Fig. 2A). Interestingly, GANT61 alone was able to reduce the expression of the mesenchymal markers, N-cadherin, vimentin, Snail, Twist, Slug, and MMP9 and a simultaneous increase in the epithelial marker keratin 18, directly implicating a role for GLI in regulating epithelial-mesenchymal plasticity. GANT61 also strongly antagonized the stimulatory effects of OPN on all these mesenchymal markers (Fig. 2, A and B), suggesting that the effects of OPN on these markers are mediated by GLI-mediated transcriptional activation. Silencing endogenous OPN in the MDA-MB-435 cells mirrored the effects of inhibiting GLI (supplemental Fig. 2). Collectively, the data strongly suggest that OPN regulates epithelial-mesenchymal transition by up-regulating GLI-mediated transcription.
FIGURE 2.
OPN-induced epithelial-mesenchymal plasticity is mediated by GLI transcriptional activity. A, MCF7 control and OPN-expressing stable cells were treated with either vehicle control or GANT61 (10 μm). B, SUM159 control cells or SUM159-OPN expressing stable cells were treated with GANT61 (10 μm). The expression of the indicated molecules was assessed by qRT-PCR. In MCF7 cells, there was a significant up-regulation of KRT18 (p < 0.05) and a significant down-regulation of all of the mesenchymal markers (p < 0.01) following GANT61 treatment. Conversely, OPN-expressing cells showed a notable reduction in the levels of KRT18 concomitant with an increase in the mesenchymal markers (p < 0.05). In the SUM159 cells GANT61 did not cause an appreciable change in KRT18, but caused a significant (p < 0.01) decrease in the mesenchymal markers. OPN-expressing cells showed a notable decrease in KRT18 and an increase (p < 0.05) in all of the mesenchymal markers assessed.
OPN Enhances Drug Resistance through GLI1-dependent Regulation of ABCB1 and ABCG2 MDR Genes
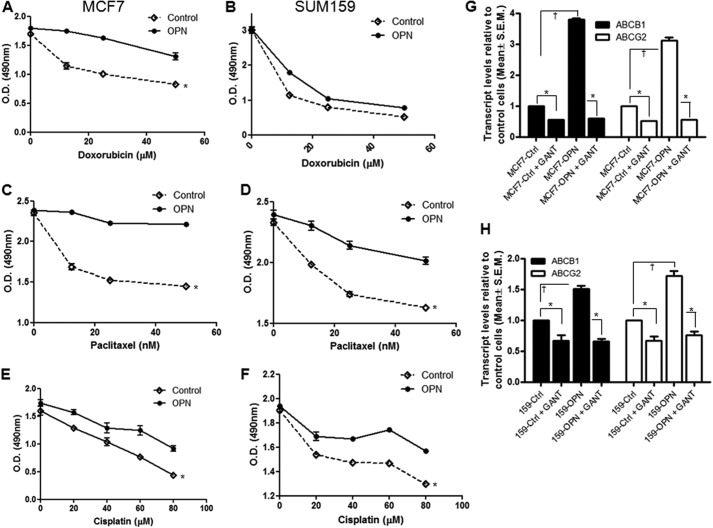
Epithelial-mesenchymal transition is accompanied by the acquisition of a drug-resistant phenotype (22–25). Accordingly, OPN-initiated signaling notably enhanced the ability of MCF7 (Fig. 3, A, C, and E) and SUM159 (Fig. 3, B, D, and F) cells to resist the cytotoxic effects of doxorubicin, paclitaxel, and cisplatin. OPN also caused an appreciable increase in the multidrug resistance proteins, ABCB1 (MDR1) and ABCG2 (BCRP) (Fig. 3 and supplemental Fig. 3A) that was dependent on GLI-mediated transcription because GANT61 interfered with the ability of OPN to up-regulate these genes (Fig. 3, G and H, and supplemental Fig. 3B). Further, silencing endogenous OPN from MDA-MB-435 cells had an effect similar to inhibiting GLI-mediated transcription with GANT61 or silencing endogenous GLI1 (GLI1-KO), indicating overlapping regulatory pathways (supplemental Fig. 3C).
FIGURE 3.
OPN influences drug resistance through the regulation MDR genes, ABCB1 and ABCG2. A–F, exogenous OPN induced resistance to doxorubicin (A and B), paclitaxel (C and D), and cisplatin (E and F) in MCF7 (*, p = 0.01; p = 0.0002; p = 0.02) and SUM159 (*, p > 0.05; p = 0.01; p = 0.01); cells, respectively. The response to the indicated drug concentrations was assessed by MTS assay. G, blocking the transcriptional activity of GLI using GANT61 significantly decreased expression of ABCB1 and ABCG2 in MCF7 (*, p < 0.0001 for both ABC proteins) and SUM159 (*, p = 0.01 and p = 0.002, respectively) cells (H). OPN caused a notable increase in ABCB1 and ABCG2 in MCF7 (†, p < 0.0001 for both ABC proteins) and SUM159 (†, p = 0.0002 and p = 0.0009, respectively) cells. GANT61 negated the effect of OPN on the expression of both MDR genes (*, p < 0.0001), ABCB1 and ABCG2. MCF7 cells or SUM159 cells were treated with either GANT61 (10 μm) or OPN (100 ng/ml) or OPN and GANT61. RNA was assessed for the expression of ABCB1 and ABCG2 by qRT-PCR. Error bars, S.E.
OPN Inactivates GSK3β and Promotes Nuclear Accumulation of GLI1
OPN-mediated signaling resulting in phosphorylation of Akt is well characterized to be the leading cause of the pro-malignant properties of OPN (26). Further, as seen in Fig. 4A, OPN also inactivates GSK3β by phosphorylating it at the serine 9 position (27). It is understood that activation of Hh signaling results in release of the GLI transcription factors from a multiprotein complex comprising Fused, Costal2, PKA, casein kinase I and GSK3β, followed by nuclear translocation of the GLI transcription factors (28). We treated SUM1315 cells with the GSK3β inhibitor BIO and found that in contrast to treatment with the MeBIO inactive analog, BIO caused GLI1 in the cytoplasmic compartment of cells to sharply disappear concomitant with an increased accumulation in the nucleus (Fig. 4B). Being such a potent GSK3β inhibitor, BIO overrides any effect of exogenous OPN.
FIGURE 4.
Inactivation of GSK3β by OPN causes accumulation of GLI1 in the nucleus and enhances GLI-mediated transcriptional activity. A, OPN induced phosphorylation of Akt at Ser-473 and phosphorylation of GSK3β at Ser-9. SUM1315 and T47D cells were cultured as described earlier, cells lysed for total protein and assayed for AKT, phospho-Akt, GSK3β, and phospho-GSK3β. GAPDH was used as control for equal loading. B, inactivating GSK3β facilitated accumulation of GLI1 in the nucleus in SUM1315 cells. Cells were treated with OPN and BIO or MeBIO and processed to enrich nuclear and cytosolic fractions. These were immunoblotted for GLI1. Histone deacetylase 1 (HDAC) and tubulin serve to ascertain the enrichment of the nuclear and cytosolic fractions, respectively. C and D, OPN caused a significant increase (*, p < 0.0001) in the transcript levels of GLI1 in SUM1315 and T47D cells. Inhibiting GSK3β using BIO increased the levels of GLI1 (*, p < 0.0001). This was further increased upon addition of recombinant OPN. E, SUM1315 or T47D cells were transfected with 8×GLI1 plasmid followed by treatment with OPN and BIO or MeBIO. Inhibiting GSK3β with BIO significantly (*, p < 0.0001) increased GLI-mediated transcriptional activity.
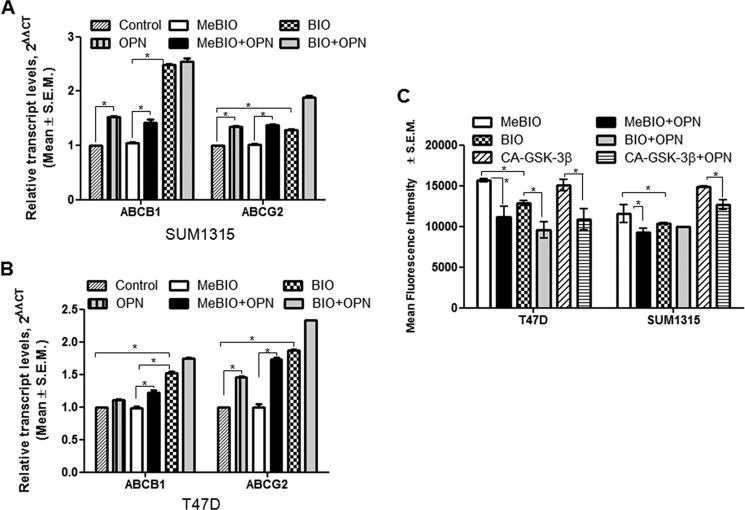
Inactivation of GSK3β Enhances GLI1 Transcriptional Activity and Expression of ABC Proteins and Reduces Drug Retention
We saw a concomitant increase in GLI1 levels when GSK3β activity is inhibited. Relative to MeBIO treatment, BIO caused a modest, but significant increase in the levels of GLI1 in the SUM1315 cells, but a robust increase in the T47D cells. OPN further augmented this increase in the GLI1 levels (Fig. 4, C and D). This was also reflected in the enhanced transcriptional activity of GLI when GSK3β is inhibited. However, OPN was unable to override the effects of inhibiting GSK3β with BIO and cause any further increase in GLI1 levels, indicating complete inhibition of GSK3β activity in the cells (Fig. 4E).
Given the enhanced drug resistance upon inhibition of GSK3β activity, we queried levels of the ABC proteins and determined that OPN enhanced the expression of ABCB1 and ABCG2 proteins when the cells were treated with the MeBIO analog. Although OPN was able to augment the impact of BIO on the expression of ABCG2, there was no further increase in ABCB1 expression in the SUM1315 cells. The T47D cells exhibited similar outcomes (Fig. 5, A and B, and supplemental Fig. 3D). There was no change observed with experimental control inhibitor MeBIO comparison with the untreated cells.
FIGURE 5.
Inhibiting GSK3β increases the expression of MDR genes ABCB1 and ABCG2 and the drug efflux activity of cells. A and B, SUM1315 (A) and T47D (B) cells (1 × 106) were treated with 5 μm GSK3β inhibitor BIO or its control MeBIO for 2 h followed by OPN (100 ng/ml) as described above, and RNA was collected from the cells and assessed for ABCB1 and ABCG2 transcript levels. Relative to control, OPN treatment resulted in an increase in the transcript levels of ABCB1 and ABCG2 (*, p < 0.0001). BIO caused a significant increase in the levels of both ABC proteins (p < 0.0001) relative to the MeBIO inactive analog. C, OPN decreased the efflux ability of cells (*, p < 0.0001). Blocking the activity of GSK3β facilitated efflux activity of cells; OPN further decreased the efflux activity of BIO-treated cells. Transient transfection of constitutively active GSK3β (CA-GSK3β) had effects opposite that of blocking GSK3β activity. Efflux of DIOC2(3) was assessed as a surrogate of drug efflux. The readings were directly proportional to the amount of DIOC2(3) retained by the cells.
Given our finding that OPN caused enhanced drug resistance by up-regulating ABC proteins that pump out drugs, we measured the impact of inhibiting GSK3β on the functional ability of cells to exclude cytotoxic drugs using DIOC2 as a surrogate indicator. The mean fluorescence intensity is indicative of the amount of DIOC2 that is retained in the cells. When cells were treated with OPN the drug retention capacity of these cells decreased, indicative of an enhanced drug effluxing activity. Inhibiting the activity of GSK3β with BIO resulted in decreased drug retention (relative to MeBIO); this was further compounded by OPN. In contrast, cells that were transiently transfected with constituently active GSK3β (CA-GSK3β) displayed greater drug retention ability (less drug efflux) that was diminished by OPN (Fig. 5C).
OPN-induced Signaling Makes Tumor Cells Refractory to SMOH-targeting Hh Inhibition
Classical activation of Hh signaling involves inhibition of the 7-pass transmembrane protein, SMOH. Most of the Hh inhibitors characterized and all the ones in clinical trials target SMOH. Pharmacological inhibition of Hh signaling results in decreased proliferation in vitro and in tumor shrinkage and normalization of tumor vasculature in vivo (29). Thus, we assessed the impact of OPN on the response of tumor cells to KAAD-cyclopamine, a well characterized SMOH-targeting Hh inhibitor. We first assessed the impact on epithelial-mesenchymal plasticity. As seen in Fig. 6, KAAD-cyclopamine caused a modest but significant increase in the expression of keratin 18 and a notable decrease in most of the mesenchymal markers (supplemental Fig. 4A). Surprisingly, OPN antagonized the effects of KAAD-cyclopamine on the cells completely reversing the restored epithelial phenotype elicited by KAAD-cyclopamine. As such, in both MCF7 and SUM159 cells, OPN was able to override the effects of Hh inhibition. Whereas the levels of the ABC proteins were significantly decreased in response to cyclopamine, OPN induced a robust increase in the expression of both ABC proteins even in presence of cyclopamine (Fig. 7, A and B, and supplemental Fig. 4B). One of the well known effects of Hh inhibition is a reduction in proliferation. Accordingly, we noted an overall decreased tumor cell proliferation in response to cyclopamine that was completely annulled by OPN (Fig. 7C). This was also reflected in the strikingly different response of the cells to loss of endogenous OPN (OPNi) in the MDA-MB-435 cells. Abrogation of OPN or treatment with KAAD-cyclopamine caused a significant decrease in the levels of ABCB1 and ABCG2; silencing OPN enhanced the effects of KAAD-cyclopamine on reducing the expression of these MDR proteins (supplemental Fig. 4C). Abrogating OPN also facilitated the ability of KAAD-cyclopamine to cause a collective reduction of mesenchymal markers concomitant with a gain in the expression of the epithelial marker, keratin 8/18 (supplemental Fig. 5). Finally, abrogating OPN (OPNi) or GLI1 (KO1 and kd2) from the tumor cells improved the susceptibility of the cells to all three cytotoxic chemotherapeutics. Taken together, the data strongly suggest a role for OPN-mediated activation of GLI transcriptional activity in up-regulating MDR gene expression leading to multidrug resistance (supplemental Fig. 6). Cumulatively, our results suggest that OPN-induced signaling makes tumor cells resistant to Hh inhibitory strategies.
FIGURE 6.
OPN effaces the inhibitory effect of SMOH targeting KAAD-cyclopamine inhibitor. MCF7 (A) and SUM159 (B) cells overexpressing OPN were treated with KAAD-cyclopamine (10 μm) in serum-free medium overnight and RNA collected from cells. RNA was assessed by real-time RT-PCR for KRT18, N-cadherin, vimentin, SNAI1, Slug, Twist, and MMP9 transcript levels. Whereas KAAD-cyclopamine treatment increases expression of KRT18 (p < 0.0001) simultaneous with a moderate but statistically significant decrease in the mesenchymal markers, OPN notably (p < 0.01) overrides the effect of KAAD-cyclopamine and reverses the trend of expression of the epithelial and mesenchymal markers.
FIGURE 7.
OPN reverses the inhibitory effect of SMOH-targeting cyclopamine on expression of ABC transporter proteins and on inhibition of proliferation. A and B, MCF7 (A) and SUM159 (B) cells overexpressing OPN were treated with KAAD-cyclopamine (10 μm) in serum-free medium overnight and RNA assessed by qRT-PCR for ABCB1 and ABCG2 transcript levels. *, p < 0.05 comparing the KAAD-cyclopamine treated cells with the control (untreated) cells. †, p < 0.01 when contrasting the OPN-expressing, KAAD-cyclopamine-treated cells with control cells treated with KAAD-cyclopamine. C, KAAD-cyclopamine caused a notable (*, p < 0.001) decrease in the proliferation rate; OPN countered this effect (†, p < 0.01). Error bars, S.E.
DISCUSSION
During development differential polarized epithelial cells, upon environmental cues, undergo profound morphogenetic changes collectively referred to as the EMT. The mesenchymal features gained by the resultant cells include motility, invasiveness, and a heightened resistance to apoptosis. Epithelial and mesenchymal cells can be distinguished by a number of classical markers; for example, epithelial markers include adherens and tight junction proteins such as E-cadherin and ZO-1, whereas mesenchymal markers include the extracellular matrix component fibronectin and the intermediate filament protein vimentin. Tumor cells also execute the EMT transdifferentiation program during progression to an invasive or metastatic state. EMT is generally induced in epithelial cells by heterotypical signals, specifically those released by the mesenchymal cells that constitute the stroma of normal and neoplastic tissues. Heterotypical signaling pathways that originate in the activated tumor-associated stroma comprising the tumor microenvironment trigger the EMT program. EMT-inducing signaling pathways typically include the TGF-β, Wnt, Notch, and Hh pathways (30, 31).
The Hh signaling pathway is a critical mediator of segmental patterning during embryonic development, and it regulates the proliferation, migration, and differentiation of target cells in a spatial, temporal, and concentration-dependent manner. This pathway is aberrantly activated in many cancer types due to either activating mutations in the tumor or due to up-regulation of the ligands. Tumor cells also activate Hh signaling in their surrounding stromal cells thereby modifying their milieu in a paracrine manner (3, 17). Likewise, the inflammatory tumor milieu harbors growth factors such as vascular endothelial growth factor (VEGF)-A and -C, basic fibroblast growth factor (bFGF), tumor necrosis factor (TNF), hepatocyte growth factor (HGF), epidermal growth factor (EGF) family members, platelet-derived growth factor (PDGF), and chemokines such as CXCL12 and interleukin (IL)-8 and inflammatory cytokines including COX-2, IL-6, and OPN (32, 33).
By virtue of its ability to signal via multiple integrins and CD44 variants, OPN signals promiscuously to activate various growth- and survival-promoting pathways. In doing so, it causes phosphorylation and inactivation of GSK3β (27). GSK3, a multifunctional serine/threonine kinase, is an important component of diverse signaling pathways involved in the regulation of cell fate, protein synthesis, glycogen metabolism, cell mobility, proliferation, and survival (35, 36). Dysregulation of GSK3β has also been implicated in tumorigenesis and cancer progression (37, 38). We see that GSK3β serves as a critical nexus between OPN and activation of Hh signaling. In vertebrates GSK3β together with Fused, Costal2, PKA, and casein kinase I is understood to sequester the GLI transcription factors of the Hh pathway in a complex. This complex dissociates upon the binding of Hh ligands to the receptor PTCH and release GLI for nuclear translocation, the mechanistics of which are being unraveled in vertebrates and mammals. We show that OPN inactivates GSK3β; as a result the GLI transcription factors accumulate in the nucleus. As a consequence, OPN initiates nonclassical activation of canonical (GLI-mediated) Hh signaling resulting in the acquisition of mesenchymal characteristics. EMT is characterized by drug-resistant properties (22). Our work shows that OPN up-regulates the expression of the ABC transporter MDR proteins in a GLI-dependent manner. The ABC (ATP-binding cassette) proteins act as ATP-dependent efflux pump that reduce intracellular drug accumulation rendering the drug ineffective (39). Consequently, OPN causes resistance to cytotoxic drugs including doxorubicin, paclitaxel, and cisplatin. Although cisplatin is not a direct substrate of ABCB1 and ABCG2, members of the ABC protein family have been known to induce changes in cell regulatory pathways, such as inhibiting cisplatin-induced caspase-3 activation (40), independent of their drug efflux ability. It is likely that these changes in such cell regulatory pathways could impact resistance to cisplatin in the perspective of increased levels of the ABC proteins under conditions of nonclassical activation of Hh signaling. The GANT61 small molecule compound was able to reverse all of the effects that OPN exhibited, indicating clearly that the effects of OPN were mediated by the transcriptional activity of the GLI transcription factor(s). Abrogating endogenous GLI1 phenocopied the effects of GANT61.
While Hh inhibitors targeting GLI (such as GANT61) are being evaluated further, several of the SMOH-targeting compounds are being investigated in clinical trials. Therefore, it was essential to determine the effect of OPN on the response of tumor cells to a SMOH-targeting compound, KAAD-cyclopamine. We made the striking observation that OPN could abolish the effects of cyclopamine on the tumor cells, making them resistant to cyclopamine. This also indicated that the signaling induced by OPN did not involve the classical SMOH-dependent signaling, but rather shunted to GLI1 via GSK3β. Due to the involvement of GSK3β, it is likely that OPN can also impact other signaling pathways involving GSK3β. Indeed, Robertson and Chellaiah have reported that OPN activates the Wnt/β-catenin pathway (27). This pathway also has been reported to activate the expression of various P-glycoproteins in colon cancer and in neuroblastoma (34). In our system although we cannot rule this out, the effects of OPN are distinctly mediated by GLI factors because antagonizing the transcriptional activity of GLI or abolishing the endogenous pool of GLI1 negated the effects of OPN.
As a secreted molecule, OPN has the capability to profoundly influence the tumor milieu and consequently impact tumor cell behavior. In fact, our previous work showed that OPN itself is transcriptionally up-regulated as a result of classical Hh signaling (3). Although efforts are being made to target tumor-intrinsic targets, it is necessary also to factor in the role of the complex molecular networks and cross-talk between different components of the tumor microenvironment that can result in the emergence of resistance to therapy. Our work shows that OPN causes the feedforward activation of a program which results in the translocation of GLI transcription factors into the nucleus and transcription of target genes that induce multidrug resistance. Abrogation OPN-initiated signaling reinstates drug sensitivity. Most importantly, we also demonstrate an alternate signaling mechanism that uncouples Hh signaling via SMOH that manifests as resistance to Hh (SMOH-targeting) inhibitors. As such, our findings have an important bearing on appreciating the need to mitigate the effects of tumor microenvironment to combat drug resistance.
This work was supported, in whole or in part, by National Institutes of Health Grants R01CA138850 (to L. A. S.) and R01CA140472 (to R. S. S.) from NCI. This work was also supported by a Mayer Mitchell award (to L. A. S.) and a University of Alabama at Birmingham Center for Metabolic Bone Disease pilot grant (to L. A. S.).

This article contains supplemental Figs. 1–6.
- Hh
- hedgehog
- ABCB1
- ATP-binding cassette subfamily B member 1
- ABCG2
- ATP-binding cassette subfamily G member 2
- Akt
- protein kinase B (PKB)
- BIO
- (2′Z,3′E)-6-bromoindirubin-3′-oxime
- CD44
- cluster of differentiation 44/hyaluronate receptor
- DIOC2(3)
- 3,3′-diethyloxacarbocyanine iodide
- EMT
- epithelial-mesenchymal transition
- GANT61
- 2,2′-[[dihydro-2-(4-pyridinyl)-1,3(2H,4H)-pyrimidinediyl]bis(methylene)]bis[N,N-dimethylbenzenamine]
- GSK3β
- glycogen synthase kinase-3β
- KAAD-cyclopamine
- 3-keto-N-(aminoethyl-aminocaproyl-dihydrocinnamoyl)cyclopamine
- MDR
- multidrug resistance
- MeBIO
- 1-methyl-BIO
- MMP9
- matrix metallopeptidase 9
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OPN
- osteopontin
- PTCH
- Patched
- qRT-PCR
- quantitative real-time RT-PCR
- SMOH
- Smoothened.
REFERENCES
- 1. Ruiz i Altaba A., Mas C., Stecca B. (2007) The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 17, 438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yoon J. W., Kita Y., Frank D. J., Majewski R. R., Konicek B. A., Nobrega M. A., Jacob H., Walterhouse D., Iannaccone P. (2002) Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J. Biol. Chem. 277, 5548–5555 [DOI] [PubMed] [Google Scholar]
- 3. Das S., Harris L. G., Metge B. J., Liu S., Riker A. I., Samant R. S., Shevde L. A. (2009) The Hedgehog pathway transcription factor GLI1 promotes malignant behavior of cancer cells by up-regulating osteopontin. J. Biol. Chem. 284, 22888–22897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harris L. G., Samant R. S., Shevde L. A. (2011) Hedgehog signaling: networking to nurture a promalignant tumor microenvironment. Mol. Cancer Res. 9, 1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evangelista M., Tian H., de Sauvage F. J. (2006) The Hedgehog signaling pathway in cancer. Clin. Cancer Res. 12, 5924–5928 [DOI] [PubMed] [Google Scholar]
- 6. Jiang J., Hui C. C. (2008) Hedgehog signaling in development and cancer. Dev. Cell 15, 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mukherjee S., Frolova N., Sadlonova A., Novak Z., Steg A., Page G. P., Welch D. R., Lobo-Ruppert S. M., Ruppert J. M., Johnson M. R., Frost A. R. (2006) Hedgehog signaling and response to cyclopamine differ in epithelial and stromal cells in benign breast and breast cancer. Cancer Biol. Ther. 5, 674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang J., Leong N. L., Mung J. C., Hidaka C., Lu H. H. (2008) Interaction between zonal populations of articular chondrocytes suppresses chondrocyte mineralization and this process is mediated by PTHrP. Osteoarthritis Cartilage 16, 70–82 [DOI] [PubMed] [Google Scholar]
- 9. Craig A. M., Nemir M., Mukherjee B. B., Chambers A. F., Denhardt D. T. (1988) Identification of the major phosphoprotein secreted by many rodent cell lines as 2ar/osteopontin: enhanced expression in H-ras-transformed 3T3 cells. Biochem. Biophys. Res. Commun. 157, 166–173 [DOI] [PubMed] [Google Scholar]
- 10. Shevde L. A., Samant R. S., Paik J. C., Metge B. J., Chambers A. F., Casey G., Frost A. R., Welch D. R. (2006) Osteopontin knockdown suppresses tumorigenicity of human metastatic breast carcinoma, MDA-MB-435. Clin. Exp. Metastasis 23, 123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shevde L. A., Das S., Clark D. W., Samant R. S. (2010) Osteopontin: an effector and an effect of tumor metastasis. Curr. Mol. Med. 10, 71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Das R., Mahabeleshwar G. H., Kundu G. C. (2003) Osteopontin stimulates cell motility and nuclear factor κB-mediated secretion of urokinase type plasminogen activator through phosphatidylinositol 3-kinase/Akt signaling pathways in breast cancer cells. J. Biol. Chem. 278, 28593–28606 [DOI] [PubMed] [Google Scholar]
- 13. Philip S., Bulbule A., Kundu G. C. (2004) Matrix metalloproteinase-2: mechanism and regulation of NF-κB-mediated activation and its role in cell motility and ECM-invasion. Glycoconj. J. 21, 429–441 [DOI] [PubMed] [Google Scholar]
- 14. Rangaswami H., Bulbule A., Kundu G. C. (2006) Nuclear factor-inducing kinase: a key regulator in osteopontin-induced MAPK/IκB kinase-dependent NF-κB-mediated promatrix metalloproteinase-9 activation. Glycoconj. J. 23, 221–232 [DOI] [PubMed] [Google Scholar]
- 15. DiMeo T. A., Anderson K., Phadke P., Fan C., Feng C., Perou C. M., Naber S., Kuperwasser C. (2009) A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 69, 5364–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cook A. C., Tuck A. B., McCarthy S., Turner J. G., Irby R. B., Bloom G. C., Yeatman T. J., Chambers A. F. (2005) Osteopontin induces multiple changes in gene expression that reflect the six “hallmarks of cancer” in a model of breast cancer progression. Mol. Carcinog. 43, 225–236 [DOI] [PubMed] [Google Scholar]
- 17. Harris L. G., Pannell L. K., Singh S., Samant R. S., Shevde L. A. (2012) Increased vascularity and spontaneous metastasis of breast cancer by Hedgehog signaling mediated up-regulation of cyr61. Oncogene 31, 3370–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huo H., Magro P. G., Pietsch E. C., Patel B. B., Scotto K. W. (2010) Histone methyltransferase MLL1 regulates MDR1 transcription and chemoresistance. Cancer Res. 70, 8726–8735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li X., Deng W., Lobo-Ruppert S. M., Ruppert J. M. (2007) Gli1 acts through Snail and E-cadherin to promote nuclear signaling by β-catenin. Oncogene 26, 4489–4498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mazumdar T., Devecchio J., Agyeman A., Shi T., Houghton J. A. (2011) Blocking Hedgehog survival signaling at the level of the GLI genes induces DNA damage and extensive cell death in human colon carcinoma cells. Cancer Res. 71, 5904–5914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lauth M., Rohnalter V., Bergström A., Kooshesh M., Svenningsson P., Toftgård R. (2010) Antipsychotic drugs regulate Hedgehog signaling by modulation of 7-dehydrocholesterol reductase levels. Mol. Pharmacol. 78, 486–496 [DOI] [PubMed] [Google Scholar]
- 22. Kalluri R., Weinberg R. A. (2009) The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mani S. A., Guo W., Liao M. J., Eaton E. N., Ayyanan A., Zhou A. Y., Brooks M., Reinhard F., Zhang C. C., Shipitsin M., Campbell L. L., Polyak K., Brisken C., Yang J., Weinberg R. A. (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weinberg R. A. (2008) Twisted epithelial-mesenchymal transition blocks senescence. Nat. Cell Biol. 10, 1021–1023 [DOI] [PubMed] [Google Scholar]
- 25. Yang J., Weinberg R. A. (2008) Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818–829 [DOI] [PubMed] [Google Scholar]
- 26. Chakraborty G., Jain S., Behera R., Ahmed M., Sharma P., Kumar V., Kundu G. C. (2006) The multifaceted roles of osteopontin in cell signaling, tumor progression and angiogenesis. Curr. Mol. Med. 6, 819–830 [DOI] [PubMed] [Google Scholar]
- 27. Robertson B. W., Chellaiah M. A. (2010) Osteopontin induces β-catenin signaling through activation of Akt in prostate cancer cells. Exp. Cell Res. 316, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scales S. J., de Sauvage F. J. (2009) Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol. Sci. 30, 303–312 [DOI] [PubMed] [Google Scholar]
- 29. Olive K. P., Jacobetz M. A., Davidson C. J., Gopinathan A., McIntyre D., Honess D., Madhu B., Goldgraben M. A., Caldwell M. E., Allard D., Frese K. K., Denicola G., Feig C., Combs C., Winter S. P., Ireland-Zecchini H., Reichelt S., Howat W. J., Chang A., Dhara M., Wang L., Rückert F., Grützmann R., Pilarsky C., Izeradjene K., Hingorani S. R., Huang P., Davies S. E., Plunkett W., Egorin M., Hruban R. H., Whitebread N., McGovern K., Adams J., Iacobuzio-Donahue C., Griffiths J., Tuveson D. A. (2009) Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zavadil J., Haley J., Kalluri R., Muthuswamy S. K., Thompson E. (2008) Epithelial-mesenchymal transition. Cancer Res. 68, 9574–9577 [DOI] [PubMed] [Google Scholar]
- 31. Zhou B. P., Hung M. C. (2005) Wnt, Hedgehog and Snail: sister pathways that control by GSK-3β and β-Trcp in the regulation of metastasis. Cell Cycle 4, 772–776 [DOI] [PubMed] [Google Scholar]
- 32. Benelli R., Lorusso G., Albini A., Noonan D. M. (2006) Cytokines and chemokines as regulators of angiogenesis in health and disease. Curr. Pharm. Des. 12, 3101–3115 [DOI] [PubMed] [Google Scholar]
- 33. Robinson S. C., Coussens L. M. (2005) Soluble mediators of inflammation during tumor development. Adv. Cancer Res. 93, 159–187 [DOI] [PubMed] [Google Scholar]
- 34. Chikazawa N., Tanaka H., Tasaka T., Nakamura M., Tanaka M., Onishi H., Katano M. (2010) Inhibition of Wnt signaling pathway decreases chemotherapy-resistant side-population colon cancer cells. Anticancer Res. 30, 2041–2048 [PubMed] [Google Scholar]
- 35. Doble B. W., Woodgett J. R. (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grimes C. A., Jope R. S. (2001) The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Prog. Neurobiol. 65, 391–426 [DOI] [PubMed] [Google Scholar]
- 37. Manoukian A. S., Woodgett J. R. (2002) Role of glycogen synthase kinase-3 in cancer: regulation by Wnts and other signaling pathways. Adv. Cancer Res. 84, 203–229 [DOI] [PubMed] [Google Scholar]
- 38. Ougolkov A. V., Billadeau D. D. (2006) Targeting GSK-3: a promising approach for cancer therapy? Future Oncol. 2, 91–100 [DOI] [PubMed] [Google Scholar]
- 39. Sims-Mourtada J., Izzo J. G., Ajani J., Chao K. S. (2007) Sonic Hedgehog promotes multiple drug resistance by regulation of drug transport. Oncogene 26, 5674–5679 [DOI] [PubMed] [Google Scholar]
- 40. Gibalová L., Sereš M., Rusnák A., Ditte P., Labudová M., Uhrík B., Pastorek J., Sedlák J., Breier A., Sulová Z. (2012) P-glycoprotein depresses cisplatin sensitivity in L1210 cells by inhibiting cisplatin-induced caspase-3 activation. Toxicol. in Vitro 26, 435–444 [DOI] [PubMed] [Google Scholar]