Background: Metalloprotease FtsH is involved in quality control of membrane proteins.
Results: Simultaneous (or sequential) expression of misfolded DrrAB and FtsH results in significant recovery of DrrAB-mediated doxorubicin efflux function.
Conclusion: FtsH facilitates refolding of previously misassembled DrrAB.
Significance: This is the first study showing that FtsH contains both protease and refolding functions and plays a dual role in biogenesis of membrane proteins.
Keywords: ABC Transporter, Chaperone Chaperonin, Drug Transport, Protein Assembly, Protein Folding, AAA+ Family, DrrAB, FtsH
Abstract
This study provides the first direct evidence for the dual role of the metalloprotease FtsH in membrane protein biogenesis. Using the physiological substrate DrrAB, it is shown that FtsH is not only responsible for proteolysis of unassembled DrrB protein but also plays a much broader role in biogenesis of the DrrAB complex. Previous studies showed that the stable expression of DrrB in the membrane depends on simultaneous expression of DrrA. Here we show that DrrB is proteolyzed by FtsH when it is expressed alone. Moreover, DrrA and DrrB proteins expressed together in a temperature-sensitive ftsH mutant strain of Escherichia coli were found to be nonfunctional due to their incorrect assembly. Simultaneous expression of wild-type FtsH in trans resulted in normal doxorubicin efflux. Strikingly, doxorubicin efflux could be restored in mutant cells irrespective of whether FtsH was expressed simultaneously with DrrAB or expressed after these proteins had already accumulated in an inactive conformation, thus providing crucial evidence for the ability of FtsH to refold the misassembled proteins. Complementation experiments also showed that the catalytic AAA domain of FtsH contains a chaperone-like activity, however, unlike wild-type FtsH, it was unable to restore function. Our results therefore show for the first time that FtsH contains the protease as well as refolding functions, and both the AAA and the proteolytic domains of FtsH are required for each of these activities.
Introduction
Membrane proteins play essential roles in cell physiology. They carry out import of nutrients, export of toxins, antibiotics and drugs, and play important roles in energy and signal transduction. Improper assembly of membrane proteins is known to result in various diseases. However, because of the complexity of the assembly process and the diverse array of factors involved, understanding mechanisms of membrane protein assembly poses a serious challenge. The bacterial doxorubicin resistance proteins A and B (DrrAB)2 system is an attractive model for studying assembly of ABC (ATP-binding cassette) transporters. In this system, the catalytic function (DrrA) and the transport function (DrrB) are present on separate subunits (1), which together form a tetrameric complex in the membrane (2) and carry out ATP-dependent efflux of the anti-cancer drugs doxorubicin (Dox) and daunorubicin (3). Previous studies from this lab suggested that for proper function of the DrrAB complex the DrrA and DrrB proteins may be required to co-assemble (4). It was also shown that interaction between DrrA and DrrB is essential for stable maintenance of DrrB in the membrane so that the expression of DrrB is undetectable in the absence of a simultaneous expression of DrrA (2). Co-expression of DrrA in cis or trans restores the wild-type levels of DrrB expression, therefore suggesting that DrrA protects DrrB from proteolysis by a cellular protease. The nature of the protease and whether it plays a specific role in quality control and biogenesis of the DrrAB complex has so far remained uncharacterized. In this study, we examine the role of FtsH (filament temperature-sensitive protein H) in this process.
FtsH is a zinc-dependent metalloprotease, which belongs to the AAA (ATPases associated with diverse cellular activities) family of proteins. Along with other proteases, such as ClpAP, ClpXP, HsIUV, and Lon, these proteins form the large AAA+ superfamily of proteins, members of which share a similar AAA-ATPase domain (5). FtsH is evolutionarily conserved with more than 40% sequence identity observed between bacterial, yeast, and human homologs (6). Escherichia coli FtsH is the best studied of all known members, and it has been shown to be the only growth-essential protease in E. coli. Yeast cells lacking the three FtsH orthologs (two m-AAA and one i-AAA) were also found to be nonviable, demonstrating the essential function of this enzyme in eukaryotic cells (6, 7). FtsH is unique in being embedded in the cell membrane in E. coli, and in the mitochondrial inner membrane in eukaryotes (8), where it forms homohexameric ring-like structures. The major role of FtsH is believed to be in the quality control of specific membrane proteins, such as degradation of the unassembled SecY and subunit “a” of F0 sector of the ATP synthase, in addition to modulating levels of some soluble regulatory proteins (8–10).
FtsH contains two transmembrane helices at the N terminus, followed by a cytoplasmic domain containing the catalytic AAA domain in the middle and the proteolytic domain at the C terminus. The AAA domain (residues 144–398) consists of the conserved Walker A, Walker B, and SRH (second region of homology) motifs, which are essential for ATP binding and hydrolysis (8). The proteolytic domain contains the conserved Zn2+ binding motif 417HEXXH421, the third Zn2+-ligand residue Glu479, and the coiled-coil leucine-zipper sequence (8). FtsH carries out proteolysis of polypeptides in an ATP- and Zn2+-dependent manner, whereas other AAA+ proteases, such as Lon and ClpA/P, are serine peptidases (11). To initiate proteolysis of a membrane protein, the putative polypeptide binding site in the catalytic AAA domain of FtsH is believed to capture the cytoplasmic tail (either at the N or the C terminus) of the membrane substrate, followed by dislocation and processive unfolding of the protein to an open structure (8). Therefore, both the catalytic AAA domain and the proteolytic domain are required for proteolysis.
Although the major function of the AAA+ proteases is in proteolysis, they also exhibit chaperone-like activities, which allow them to monitor the folding status of a protein, promote disassembly or unfolding (12), and specifically degrade non-native proteins (13). For a long time, it has been speculated that the AAA+ proteases may also have the ability to refold their substrate proteins (14), which remains an open question (15). FtsH was originally identified by Ito and co-workers (16) in a screen to isolate factors, which may assist in membrane protein assembly. They used SecY-PhoA fusion to screen for stop-transfer defect mutations, and found that such a mutation lies in the ftsH gene. Depletion of FtsH also resulted in significant export defects of β-lactamase and OmpA in E. coli in addition to causing a strong stop-transfer defect phenotype. Together, these studies indicated that FtsH may be involved in protein assembly into and through the membrane and may play a role in determining orientation of membrane proteins (16). In another study, FtsH orthologs Yta10 and Yta12 in yeast mitochondria were shown to be required for the formation of a 48-kDa assembly intermediate of the F0 subunit 9 (17). Finally, in vitro studies showed that the purified AAA domain of Yme1, a yeast mitochondrial homolog of FtsH, suppresses aggregation of a model polypeptide (18). Despite these observations, however, no direct evidence for the role of FtsH or its homologs in functional assembly of membrane proteins has been obtained so far.
In this study, we provide the first direct evidence that FtsH is a dual function enzyme containing both the protease and assembly functions. We show that not only is FtsH responsible for removal of the unassembled DrrB but that it is actually able to refold previously misassembled DrrAB proteins and restore Dox efflux function of the complex. Our results also show that although the AAA domain of FtsH provides recognition and specificity for binding of the substrate, both ATP hydrolysis and the proteolytic functions of FtsH are used concurrently for refolding of DrrAB and restoration of function. Our studies, therefore, not only shed light on the mechanism of assembly of the DrrAB complex but also further elucidate the function of the FtsH protease.
EXPERIMENTAL PROCEDURES
Strains and Plasmids
The E. coli strains and plasmids are described in Table 1.
TABLE 1.
Bacterial strains, plasmids, and antisera
| Name | Description | Ref. or source |
|---|---|---|
| Bacterial strains | ||
| TG1 | K-12Δ (lac-pro) supE thi hsdD5/F′ tra36 proA+B+ lacIq lacZΔM15 | 28 |
| HMS174(DE3) | F− recA1 hsdR (rK12- mK12+) (DE3) (Rif R) | 28 |
| AR796 | F−, zhd-33::Tn10, araD139, (argF-lac)U169, rpsL150, rpsE, relA1, f1bB5301, deoC1, ptsF25, rbsR, zgj3198::Tn10kan. | 29 |
| AR797 | F−, zhd-33::Tn10, araD139, (argF-lac)U169, rpsL150, rpsE, relA1, f1bB5301, deoC1, ptsF25, rbsR, zgj3198::Tn10kan. ftsH1 | 29 |
| SG1110 | Δlon-510, zba-1091::ΔTn10 (λ) | 30 |
| SG1126 | Δarg, clpA319::Δkan | 30 |
| Plasmids | ||
| pSU2718 | Cloning vector, pACYC184 derivative, Cmr | 1 |
| pKY326 | groES/groEL in pHSG575, Cmr | 19 |
| pDX101 | drrAB in pSU2718, Cmr | 1 |
| pDX103 | drrB in pSU2718, Cmr | 1 |
| pUCftsH | ftsH in pUC18, Ampr, restriction sites: EcoRI, HindIII | This study |
| pUCftsH(K198N) | pUC18 containing ftsH with mutation of Lys198 to Asn, Ampr | This study |
| pUCftsH(HEH) | pUC18 containing ftsH with mutations of His417-Glu418-His421 to Ala417-Gln418-Ala421, Ampr | This study |
| pUCftsH(AAA) | pUC18 containing ftsH with deletion of proteolytic domain, Ampr | This study |
| pUCgroESL | groES/groEL in pUC18, Ampr, restriction sites: NdeI, HindIII | This study |
| pETftsH | ftsH in pET28a with C-terminal histag, Kanr | This study |
| pETFftsH(K198N) | ftsH(K198N) in pET28a with C-terminal His tag, Kanr | This study |
| pETftsH(HEH) | ftsH(HEH) in pET28a with C-terminal His tag, Kanr | This study |
| pETftsH(AAA) | ftsH(AAA) in pET28a with C-terminal His tag, Kanr | This study |
| pBADftsH | ftsH in pBAD/HisA, Ampr | This study |
Media and Growth Conditions
E. coli cells were normally grown in LB medium at 30 or 37 °C, unless indicated otherwise. Chloramphenicol, kanamycin, or ampicillin was added to a final concentration of 20, 30, or 100 μg/ml, respectively, where indicated. E. coli cells used for the Dox efflux assay were grown in TEA medium (3).
Site-directed Mutagenesis of ftsH
Site-directed mutagenesis of the ftsH gene was carried out by a QuikChange Multisite-directed mutagenesis kit (Stratagene, La Jolla, CA). Using pUC18/ftsH plasmid as the template, Lys198, located in the conserved Walker A motif of the AAA domain, was changed to aspargine. The resulting plasmid was named pUCftsH(K198N). Another mutant, named ftsH(HEH), was obtained by substituting His417-Glu418-His421 in the conserved HEAGH motif present in the proteolytic domain to Ala417-Gln418-Ala421.
Subcloning of the AAA Domain of ftsH into pUC18 Vector
To completely remove the proteolytic domain, a fragment of ftsH corresponding to the first 1194 base pairs was PCR-amplified and ligated into the pUC18 vector using the EcoRI and HindIII restriction enzymes resulting in pUC18(AAA). This construct is referred to as ftsH(AAA) in this article.
Subcloning of ftsH and the groESL Genes
Wild-type ftsH, ftsH(K198N), ftsH(HEH), and ftsH(AAA) genes were subcloned into pET28a vector using SnaBI and HindIII restriction enzymes. The resulting plasmids were named pETftsH, pETftsH(K198N), pETftsH(HEH), and pETftsH(AAA), respectively. The groES/L genes were subcloned from pKY326 (19) into pUC18 vector using EcoRI and SmaI restriction enzymes resulting in pUCgroESL.
Cloning of ftsH into the pBAD Vector
Using pBAD/HisA (Invitrogen) as a template, a 3.9-kb fragment between NcoI and HindIII sites was amplified. The NcoI site was substituted with the XhoI site in the primers used for amplification. This resulted in deletion of the multiple cloning sites and the polyhistidine region. The ftsH gene was amplified from pUCftsH using primers containing XhoI and HindIII sites and ligated to the above fragment.
Growth and Protein Expression
E. coli TG1, AR796, AR797, E. coli SG1110, and E. coli SG1126 cells containing the indicated plasmids were grown at 30 or 37 °C to mid-log phase (A600 nm = 0.6). The proteins were induced by addition of 0.1 or 0.25 mm IPTG, and the incubation was continued at 30, 37, or 42 °C for 3 h. The cells were spun down, resuspended in 5 ml of lysis buffer (2 mm Tris-Cl, pH 7.5, 20% glycerol, 2 mm EDTA, 1 mm DTT) and lysed by a single passage through a French pressure cell at 20,000 p.s.i. After centrifugation at 10,000 × g for 15 min, the pellet represented the inclusion body fraction. The supernatant was centrifuged at 100,000 × g for 1 h to separate the supernatant (cytosolic fraction) and the pellet (membrane fraction). The membrane, cytosol, and the inclusion body fractions were analyzed by 12% SDS-PAGE, followed by Western blot analysis using anti-DrrA or anti-DrrB antibodies (2).
Purification of the FtsH Protein
FtsH protein was purified according to published protocols (9) with modifications. E. coli HMS174(DE3) cells containing the pETftsH plasmid or its variants were inoculated in 1 liter of LB medium supplemented with 30 μg/ml of kanamycin at 37 °C. The cells were grown to mid-log phase and induced with 0.25 mm IPTG at 20 °C overnight. The membrane fraction was prepared as described earlier (2). 5 mg of the membrane fraction was solubilized with 5 ml of solubilization buffer (50 mm Tris-Cl, pH7.5, 500 mm KCl, 0.5% (w/v) Nonidet P-40, 15% (w/v) glycerol, 2.9 mm 2-mercaptoethanol). The solubilized protein was purified using a nickel-nitrilotriacetic acid-agarose column and eluted with a gradient of 50 to 500 mm imidazole. Fractions containing FtsH were collected and dialyzed against 2 liters of the dialysis buffer (10 mm Tris-Cl, pH 7.5, 15% (w/v) glycerol, 50 mm KCl, 0.5% (w/v) Nonidet P-40, 5 mm MgCl2, 1 mm dithiothreitol) for 12 h. The protein was stored at −80 °C until used.
ATPase Activity Assay
The ATPase activity of FtsH was detected by the malachite green-ammonium molybdate colorimetric assay (9).
In Vivo FtsH Proteolytic Assay
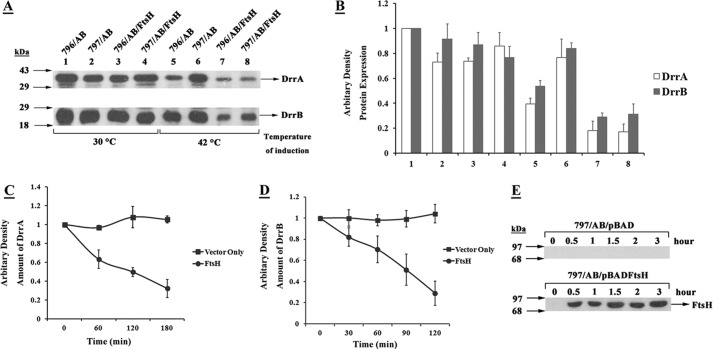
Membrane-bound DrrAB proteins were used as a substrate to determine the proteolytic activity of FtsH. pBAD vector or the pBADftsH plasmid was transformed into AR797 cells containing pDX101 (pSU2718drrAB). The cells were grown in LB medium to mid-log phase and the DrrAB proteins were induced with 0.25 mm IPTG at 42 °C for 1 h. To terminate the synthesis of DrrAB, 1000 μg/ml of chloramphenicol was added to the cell culture and incubated at 42 °C for 30 min. The cells were washed extensively to remove chloramphenicol and IPTG. After resuspending the cells in fresh medium, synthesis of FtsH from the pBADftsH plasmid was induced by addition of 0.2% arabinose at 42 °C. An aliquot of cell culture was taken out at 0, 30, 60, 90, 120, and 180 min. Membrane fractions were prepared as described above, and 20 μg of total membrane protein was loaded onto 12% SDS-PAGE, followed by Western blot analysis using anti-DrrA, anti-DrrB, or anti-FtsH antibodies.
In Vitro FtsH Proteolytic Assay
α-Casein was used as a substrate in vitro to demonstrate the proteolytic activity of purified FtsH and the FtsH(HEH) protein (20). 40 μg of α-casein (Sigma) was mixed with 40 μg of purified FtsH or FtsH (HEH) in 150 μl of protease buffer (50 mm Tris-Cl, pH 8.0, 20 mm KCl, 5 mm MgCl2, 12.5 μm Zn(OAc)2, 0.5% (w/v) Nonidet P-40, 10% (w/v) glycerol, 1 mm dithiothreitol) at 42 °C. The reaction was initiated by adding 8 mm ATP, and an aliquot (20 μl) of the sample was taken out at the indicated time points. The reaction was terminated by adding 7 μl of 4 × SDS sample buffer. The samples were analyzed by 12% SDS-PAGE, followed by Coomassie Brilliant Blue staining.
Whole Cell Dox Efflux Assay
The whole cell Dox efflux assay was carried out according to the protocol published previously (3). The fluorescence spectra were recorded on an Alphascan-2 spectrofluorometer (Photon Technology International, London, Ontario, Canada). The slope of the Dox efflux curve of the positive control (the first sample) in each panel was designated as 1.0. The efficiency of Dox efflux of each sample within one panel was calculated by dividing the slope of the efflux curve by the slope of sample 1. The average data obtained from three independent experiments were plotted in the histograms.
RESULTS
FtsH Is Responsible for the Proteolysis of Unassembled DrrB
Previous studies from this laboratory showed that DrrB is undetectable in wild-type E. coli membranes in the absence of simultaneous expression of DrrA (2) (Fig. 1A, lane 2), however, stable expression of DrrB is seen when both DrrA and DrrB are expressed together (lane 1). These results suggest that DrrB is completely degraded when not complexed with DrrA. Review of recent literature suggested that proteins of the AAA+ family, especially FtsH, may be involved in the quality control of membrane proteins (13). To determine whether this is true for DrrB, three proteases, including Lon, ClpA/ClpP, and FtsH, were investigated. If any of these proteases are responsible for degradation of unassembled DrrB, stable expression of DrrB will be observed in cells deficient in that protease as compared with the wild-type cells. The FtsH-deficient E. coli AR797 strain (Table 1) used in this study contains a temperature-sensitive mutation in the ftsH gene, therefore it was grown at 30 °C but the temperature was switched to 42 °C to inactivate FtsH. The Lon− (SG1110) and ClpA− (SG1126) cells were grown normally at 30 or 37 °C. The isogenic E. coli AR796 parent strain was used as a control. Of the 3 protease-deficient strains tested, only the FtsH-deficient E. coli cells showed stable expression of DrrB in the absence of DrrA (Fig. 1, B, lanes 1–3, C, lane 2), whereas the Lon− and ClpA− cells showed no effect on the stability of DrrB (Fig. 1C, lanes 3 and 4). Because DrrB was not seen in AR796 (wild-type) cells at either 30 or 42 °C (Fig. 1B, lanes 1 and 2), but was stably expressed in the 797 (FtsHts) cells at 42 °C (lane 3), these results show that FtsH degrades unassembled DrrB. In contrast to the expression of DrrB alone, when both DrrA and DrrB were expressed together in wild-type 796 cells at 30 or 42 °C, stable expression of DrrB was seen (Fig. 1B, lanes 4 and 5), confirming that DrrA protects DrrB against FtsH proteolysis. Note that the amount of DrrA and DrrB in the wild-type cells was less at 42 °C as compared with at 30 °C (Fig. 1B, lanes 4 and 5), suggesting that the DrrAB complex acquires a more open conformation at higher temperature resulting in partial proteolysis by endogenous FtsH. Protection of DrrB from FtsH proteolysis by DrrA was also seen in the ClpA− and Lon− backgrounds (Fig. 1C, lanes 5 and 6). Together, the data in Fig. 1 show that FtsH plays an important role in quality control of the DrrB protein when DrrA is absent. To rule out the possibility that DrrA or DrrB may aggregate when their expression is induced at 42 °C, the membrane, cytosol, and inclusion body fractions were prepared from both wild-type and FtsHts cells and analyzed by Western blotting using anti-DrrA and anti-DrrB antibodies. The data in Fig. 2 show that although some DrrB protein is present in the inclusion body fraction in both wild-type (lower panel, lane 3) and FtsHts cells (lane 9) at 30 °C, the induction of either strain at 42 °C did not result in any increase in the amount of inclusion body formation (lower panel, lanes 6 and 12). Moreover, no aggregated DrrAB proteins were seen in the stacking region of the gel in any of the fractions of wild-type or FtsHts cells, indicating absence of any significant aggregation under the conditions used in these experiments. Note that the anti-DrrB antibody is an anti-peptide antibody, therefore it shows some cross-reactivity with epitopes in some other E. coli proteins, as explained in a previous publication (1).
FIGURE 1.
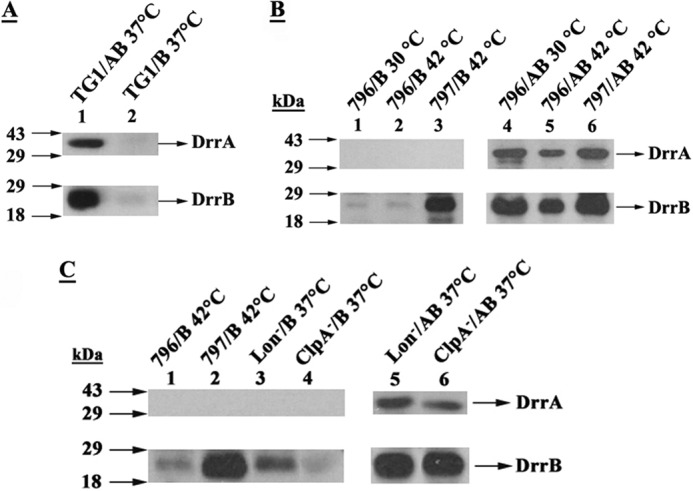
Role of DrrA and FtsH in stable maintenance of DrrB. A, DrrA is required for stable expression of DrrB. Wild-type E. coli (TG1) cells expressing DrrAB (pDX101) or DrrB alone (pDX103) were grown at 37 °C to mid-log phase (A600 nm = 0.6) and induced with 0.25 mm IPTG for 3 h. 30 μg of membrane proteins were loaded onto 12% SDS-PAGE gels, followed by Western blotting against anti-DrrA (upper panel) and anti-DrrB (lower panel) antibodies. B, FtsH is responsible for proteolysis of unassembled DrrB. Wild-type E. coli (796) or the E. coli 797 (ftsHts) cells expressing DrrAB (pDX101) or DrrB alone (pDX103) were grown at 30 °C to mid-log phase and induced with 0.25 mm IPTG at either 30 or 42 °C for 3 h. Western blot analysis of the membrane fraction was carried out as in A. C, ClpA or the Lon protease is not involved in quality control of DrrB. Wild-type E. coli (796), E. coli 797 (ftsHts), E. coli SG1110 (Lon−), and E. coli SG1126 (ClpA−) cells expressing DrrAB (pDX101) or DrrB alone (pDX103) were grown at 30 or 37 °C to mid-log phase and induced with 0.25 mm IPTG at either 42 or 37 °C for 3 h, as indicated. Analysis was carried out as described in B above.
FIGURE 2.
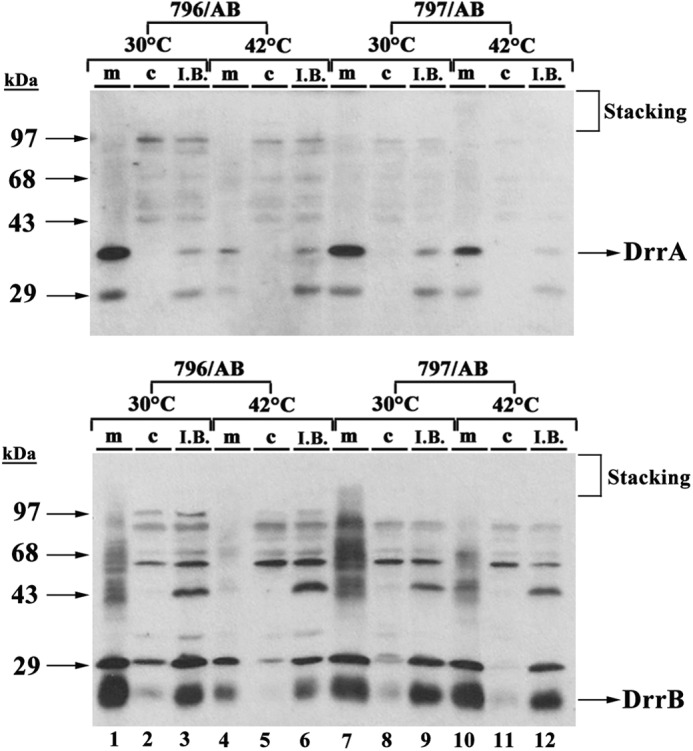
Western blot analysis of the cytosol, membrane, and inclusion body fractions of E. coli 796 or 797 cells expressing DrrAB at 30 or 42 °C. Wild-type E. coli 796 or E. coli 797 (ftsHts) cells expressing DrrAB (pDX101) were grown at 30 °C to mid-log phase and induced with 0.25 mm IPTG at either 30 or 42 °C for 3 h. Cell fractions were prepared, as described under “Experimental Procedures.” 20 μg of each fraction was loaded onto 12% SDS-PAGE gels, followed by Western blotting against anti-DrrA (upper panel) and anti-DrrB (lower panel) antibodies (m, membrane; c, cytosol; IB, inclusion body). Note that the anti-DrrB antibody is an antipeptide antibody, therefore it shows some cross-reactivity with epitopes in other E. coli proteins, as explained in a previous publication (1).
Expression of DrrB Alone or DrrAB Together in FtsH-deficient Cells Results in Growth Inhibition
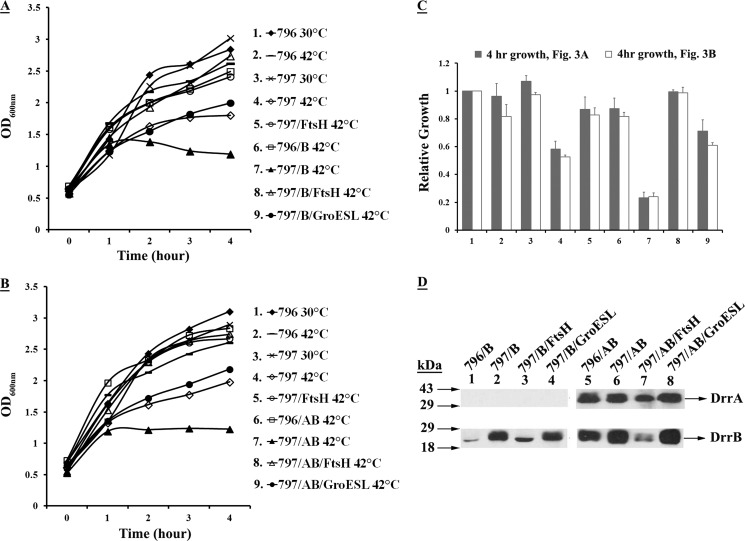
The growth analysis was carried out for wild-type and FtsHts cells expressing DrrB or DrrAB at 30 or 42 °C (Fig. 3, A and B). The relative growth of various strains at the 4-h time point was plotted in a histogram (Fig. 3C). The data in Fig. 3A show that the growth of mutant FtsHts cells is compromised at 42 °C (Fig. 3, A/797, open diamonds, C, column 4) as compared with the growth of wild-type cells under similar conditions (Fig. 3, A/796, lines, C, column 2). This result is not surprising due to the essential nature of E. coli FtsH. Interestingly, the expression of DrrB alone in FtsHts cells at 42 °C further inhibited the growth of these cells (Fig. 3, A, compare 797, open diamonds with 797/B, filled triangles; C, column 7). However, growth inhibition was not seen when DrrB was expressed in wild-type cells at 42 °C (Fig. 3, compare 796, lines with 796/B, open rectangles, C, column 6). Because DrrB accumulates in FtsHts cells but is proteolyzed in wild-type cells (Fig. 1B), it allows us to conclude that the growth defect seen in mutant cells is caused by accumulation of unassembled DrrB protein. Surprisingly, however, growth inhibition was also seen in FtsHts cells expressing DrrA and DrrB together at 42 °C (Fig. 3, B, 797A/B, filled triangles, C, column 7). This effect was unexpected because DrrA and DrrB can be expressed together in the wild-type cells at 42 °C without any negative effect on their growth (Fig. 3, B, 796A/B, open rectangles, C, column 6). These data indicate that the DrrAB proteins expressed in FtsHts cells at 42 °C may be misfolded, and the accumulation of misfolded membrane proteins results in growth inhibition. It was also observed that if the FtsHts cells initially induced at 42 °C were shifted down to 30 °C, cell growth resumed, albeit slowly. After a 43-h shift-down of temperature, the final growth was about half as compared with the cells induced and maintained at 30 °C (data not shown), indicating that growth inhibition of these cells is quite severe. In summary, the data in Fig. 3 suggest that FtsH is not only responsible for removing unassembled DrrB (in the absence of DrrA) but it may also be critical for proper assembly of the DrrAB complex in the membrane.
FIGURE 3.
Growth inhibition resulting from expression of DrrB alone or DrrAB together in E. coli 797 cells can be relieved by overexpression of FtsH or GroESL. A, effect of overexpression of FtsH or GroESL on growth of 797 cells expressing DrrB. 797 cells containing pDX103 (DrrB) and pUCftsH or pUCgroESL were grown at 30 °C and induced at 42 °C, as described in the legend to Fig. 1B. The growth was monitored at A600 nm for 4 h after induction. B, effect of overexpression of FtsH or GroESL on growth of 797 cells expressing DrrAB. 797 cells containing pDX101 (DrrAB) and pUCftsH or pUCgroESL were anayzed, as described in A above. C, quantitation of the growth of 796 or 797 cells expressing DrrB or DrrAB at 4 h after induction. The growth of sample #1 at 4 h in A or B was designated as 1.0. The relative growth of each culture at 4 h was calculated. The histogram represents an average of 3 experiments. Columns 1–9 correspond to cultures 1–9 in A (filled) and B (open). D, Western blot analysis. The membrane fractions were prepared from samples 6–9 collected at the 4-h time point in A and B. Western blot analysis was carried out as described in the legend to Fig. 1A.
The growth defect of the FtsHts cells expressing DrrB or DrrAB at 42 °C could be rescued by overexpression of FtsH in trans (Fig. 3, A and B, open triangles, C, column 8), indicating that the absence of functional FtsH was solely responsible for this defect. The growth defect in each case was also suppressed by overexpression of the chaperone GroESL (Fig. 3, A and B, filled circles, C, column 9) but not to the same extent as seen with FtsH. Western blot analysis of the membrane fractions (prepared from the 4-h cultures of samples 6–9 in Fig. 3, A and B) showed that whereas FtsH overexpression resulted in significant proteolysis of DrrAB (Fig. 3D, lanes 3 and 7), overexpression of GroESL did not (lanes 4 and 8). This might imply that FtsH restores growth by simply removing misfolded DrrAB proteins, whereas GroESL is able to alter their conformation, thus alleviating growth inhibition.
FtsH Preferentially Proteolyzes Misfolded DrrAB
To determine whether FtsH discriminates between properly assembled and misfolded DrrAB, the effect of overexpression of FtsH proteolysis was compared in wild-type or FtsHts cells at 30 or 42 °C. Interestingly, overexpression of FtsH in either wild type (Fig. 4, A and B, compare lanes 1 and 3) or FtsHts cells (lanes 2 and 4) produced no significant proteolysis of DrrA and DrrB expressed at 30 °C, showing that FtsH does not proteolyze properly assembled DrrAB. However, when the DrrAB proteins were expressed in wild-type cells at 42 °C, simultaneous overexpression of FtsH resulted in significant proteolysis (Fig. 4, A and B, compare lanes 3 and 7). These data suggest that the DrrAB proteins must acquire a partially unfolded conformation at a higher temperature (as also seen in Fig. 1B), thus making them more susceptible to proteolysis by overexpressed FtsH. As expected, overexpression of FtsH in FtsHts cells also showed significant proteolysis of DrrAB expressed at 42 °C (Fig. 4, A and B, lanes 4 and 8). This is consistent with the data in Fig. 3, which suggested that the DrrAB proteins expressed at 42 °C in FtsHts cells are misfolded. (Please note that the conformation of the DrrAB proteins in wild-type cells at 42 °C is completely different from the DrrAB expressed in mutant FtsHts cells at 42 °C even though they are both sensitive to overexpressed FtsH. In a later experiment in Fig. 8, it is shown that the DrrAB proteins expressed in wild-type cells at 42 °C retain normal function, whereas the DrrAB expressed in FtsHts cells are inactive due to their misfolding.)
FIGURE 4.
FtsH preferentially degrades misfolded DrrAB. A, E. coli 796 or 797 cells containing pDX101(DrrAB) and pUCftsH were grown and analyzed by Western blotting, as described in the legend to Fig. 1A. B, quantitative analysis of the amounts of DrrA and DrrB. The intensity of the bands on the nitrocellulose membrane in Fig. 4A was determined by densitometric scanning. The intensity of DrrA and DrrB in lane 1 was designated as 1. The data represent an average of 3 experiments. Columns 1–8 correspond to lanes 1–8 in Fig. 4A. C–E, in vivo FtsH proteolytic assay. The assay was carried out as described under “Experimental Procedures.” The membrane fractions were prepared from cells collected at the indicated time points after addition of arabinose. 20 μg of total membrane protein was loaded onto 12% SDS-PAGE, followed by Western blot with anti-DrrA, anti-DrrB, or anti-FtsH antibodies. The intensity of the bands on the nitrocellulose membrane was determined by densitometric scanning. The intensity of DrrA and DrrB on the membrane at 0 min was designated as 1.0. C, quantitation of DrrA in the membrane at the indicated time points after addition of arabinose. The data shown represent an average of 3 experiments. D, quantitation of DrrB in the membrane. E, Western blot analysis showing the amount of FtsH present in the membrane at various times after addition of arabinose. Top panel, cells containing pBAD vector only. Bottom panel, cells containing the plasmid pBADftsH.
FIGURE 8.
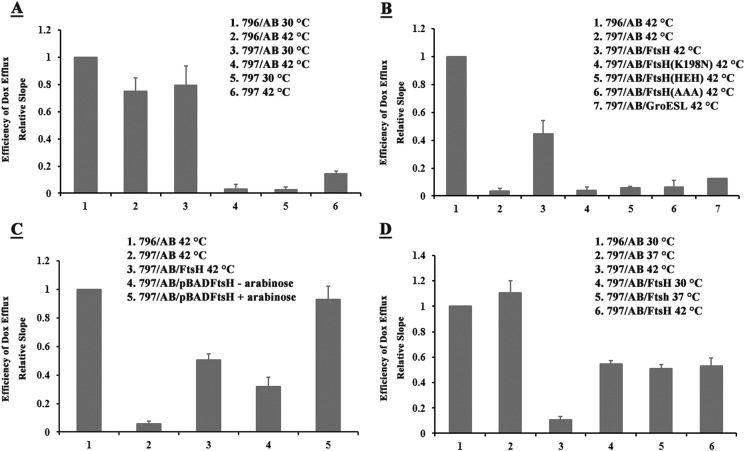
DrrAB-mediated Dox efflux. A, Dox efflux in E. coli 796 or 797 cells. The cells containing pDX101 (DrrAB) or the empty vector were grown at 30 °C to mid-log phase and induced with 0.1 mm IPTG at either 30 or 42 °C for 1 h. The washed cells were loaded with Dox, and efflux was initiated by addition of glucose and detected fluorometrically. The slope of the efflux curve of sample 1 in each panel was designated as 1.0. The efficiency of Dox efflux was then calculated by dividing the slope of each efflux curve by the slope of sample 1. The average data obtained from three independent experiments are shown in the histograms. B, complementation of the DrrAB-mediated Dox efflux in 797 cells by simultaneous overexpression of wild-type FtsH or its variants. E. coli 797 cells containing pDX101(DrrAB) and pUCftsH (wild-type FtsH), pUCftsH(K198N), pUCftsH(HEH), pUCftsH(AAA), or pUCgroESL were analyzed as described under A. C, effect of sequential expression of DrrAB and FtsH on complementation of Dox efflux. E. coli 797 cells containing pDX101(DrrAB) and pBADftsH were grown at 30 °C to mid-log phase and induced with 0.1 mm IPTG for 1 h at 42 °C. The cells were washed extensively to remove IPTG and divided into two halves. One-half was kept at 42 °C for 1 h without any induction (−ara, 42 °C) and the other half was induced by 1% arabinose at 42 °C (+ara, 42 °C) for 1 h. As controls, 796/AB, 797/AB, and 797/AB/pUCftsH were grown at 30 °C to mid-log phase and induced with 0.1 mm IPTG at 42 °C for 2 h. The cells were loaded with Dox, and efflux was measured. The data were analyzed as described under A. D, effect of simultaneous overexpression of FtsH on Dox efflux by DrrAB proteins expressed at different temperatures. 797 cells containing pDX101(DrrAB) and pUCftsH were grown at 30 °C to A0.6, induced with 0.1 mm IPTG at 30, 37, or 42 °C for 1 h, and analyzed as in A.
The rate of proteolysis of misfolded DrrAB by FtsH was analyzed in a separate time course experiment. The misfolded DrrAB proteins were first allowed to accumulate in the membranes of FtsHts cells by induction with IPTG for 1 h at 42 °C. Chloramphenicol was added to stop further synthesis, as described under “Experimental Procedures.” The synthesis of FtsH from pBADftsH was induced by addition of arabinose, and the proteolysis of DrrA and DrrB by FtsH was determined by Western blot analysis. The data in Fig. 4, C and D (filled circles), show that synthesis of FtsH (Fig. 4E, lower panel) resulted in increasing proteolysis of misfolded DrrA and DrrB from the membrane. At 120 min after addition of arabinose, about 75–80% of DrrA and DrrB were removed from the membrane. These observations are in agreement with the dislocation model proposed previously for the activity of FtsH (8). No significant proteolysis of DrrAB was seen in the absence of FtsH synthesis (Fig. 4, C and D, filled rectangles).
The AAA Domain of FtsH contains a Chaperone-like Activity, But It Is Not Sufficient by Itself to Restore the Dox Efflux Function
The growth experiment in Fig. 3B suggested that FtsH may be critical for the assembly of the DrrAB complex. Further support for this idea was obtained by comparing the rate of assembly of the DrrAB complex in wild-type and FtsHts cells. The data in Fig. 5 show that the assembly of DrrA and DrrB in the membrane of FtsHts cells is significantly compromised already at 30 °C as compared within the wild-type cells. A significant difference in the amounts of DrrAB in the membrane of wild-type and mutant cells was seen at all time points tested (Fig. 5, compare lanes 1–3 with 7–9). However, this difference is most evident at the early time points, which suggests that the rate of assembly of DrrAB is affected by FtsH. This is most likely due to the partial defect of FtsH function in FtsHts cells already at 30 °C, leading to low efficiency of DrrAB complex formation.
FIGURE 5.
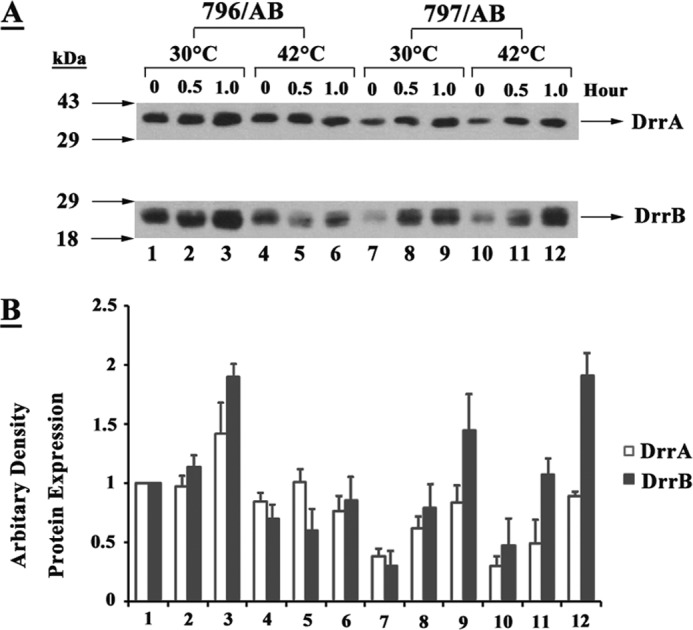
FtsH promotes assembly of the DrrAB complex. A, E. coli 796 or 797 cells expressing DrrAB were grown at 30 °C to mid-log phase and the culture was divided into two halves. One-half was induced with 0.25 mm IPTG at 30 °C (lanes 1–3 for 796 and lanes 7–9 for 797) and the other half was induced at 42 °C (lanes 4–6 for 796 and lanes 10–12 for 797). Aliquots of each sample were collected at 30-min intervals. Hours of induction are shown at the top of the gel. 0 h represents A600 nm = 0.6 at which time IPTG was added. Western blot analysis was carried out as described in the legend to Fig. 1A. B, quantitative analysis of DrrA and DrrB. The intensity of bands on the nitrocellulose membrane was determined by densitometric scanning. The intensity of DrrA and DrrB in lane 1 was designated as 1. The data represent an average of 3 experiments.
To determine whether the ability to promote assembly of DrrAB resides in the AAA domain of FtsH, variants containing mutations in the Walker A motif of the AAA domain (K198N mutation) or the conserved amino acids in the proteolytic domain (the HEH mutation and the AAA subclone are described under “Experimental Procedures”) were created. The K198N allele contained a defective AAA domain, whereas the HEH mutant and the AAA subclone contained an intact AAA domain. As expected, the K198N mutation resulted in a significantly reduced ATPase activity, however, the HEH mutant and the AAA subclone were unaffected (Table 2). The in vitro proteolytic activity assay showed that although wild-type FtsH completely proteolyzed α-casein in 1 h, no significant reduction in the α-casein level was seen with the HEH mutant even after 2 h of incubation (Fig. 6). These analyses confirmed that the AAA and the proteolytic domain mutants behave as expected. Therefore, they were used in two different complementation experiments (described below) to determine whether the AAA domain by itself is sufficient for promoting assembly of the DrrAB complex.
TABLE 2.
The ATPase activity of purified FtsH and its variants
The proteins were purified, and their ATPase activity was determined as described under “Experimental Procedures.”
| FtsH variant | ATPase activity |
|---|---|
| nmol Pi/min/mg | |
| Wild-type FtsH | 139.5 |
| FtsH(K198N) | 18.1 |
| FtsH(HEH) | 147.8 |
| FtsH(AAA) | 155.6 |
FIGURE 6.
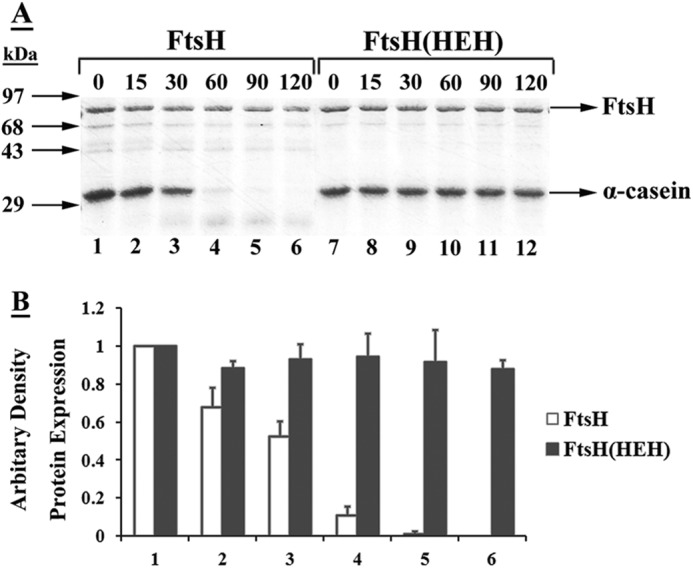
In vitro digestion of α-casein by purified FtsH or FtsH(HEH). A, α-casein was mixed with purified wild-type FtsH or FtsH(HEH) protein in the protease buffer as described under “Experimental Procedures.” The samples were analyzed by 12% SDS-PAGE, followed by Coomassie Brilliant Blue staining. B, quantitative analysis of the amounts of α-casein. The intensity of the bands on the nitrocellulose membrane was determined by densitometric scanning. The intensity of α-casein in lane 1 was designated as 1. The intensities represent an average of 3 experiments.
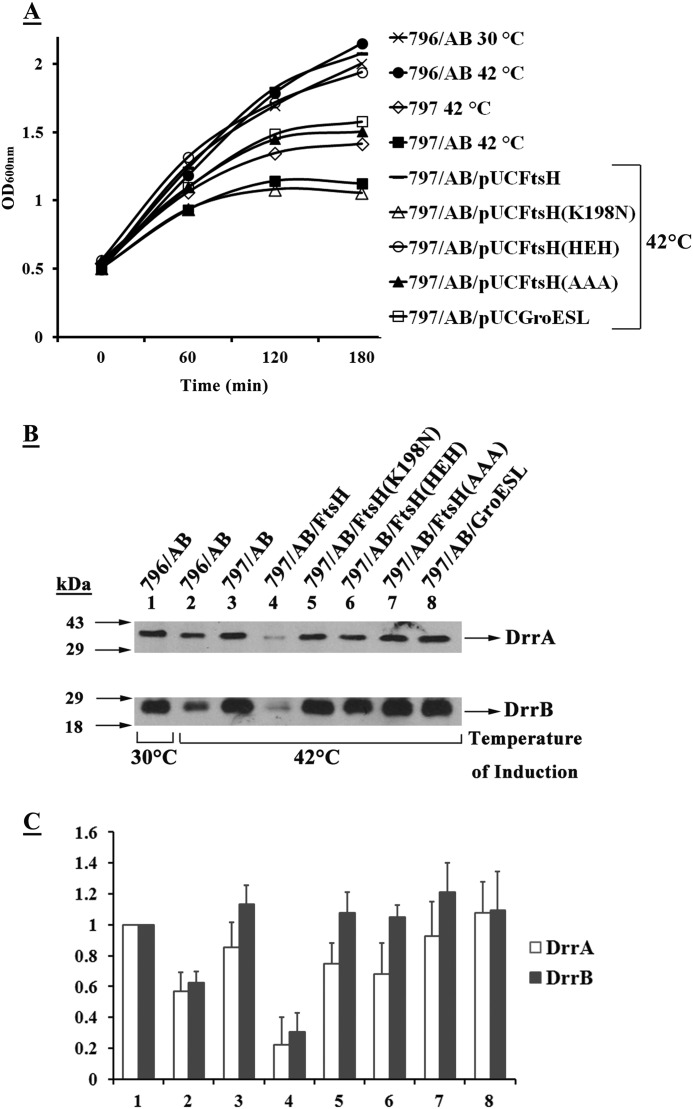
Expression of the DrrAB proteins in FtsHts cells was previously shown to result in severe growth inhibition (Fig. 3B, filled triangles). This inhibition was reversed by simultaneous expression of FtsH (Fig. 3B, open triangles). In the next experiment, we asked whether co-expression of the HEH allele or the AAA subclone can rescue FtsHts cells from growth inhibition resulting from DrrAB expression. The data in Fig. 7 show that the simultaneous expression of either the HEH mutant (Fig. 7A, open circles) or the AAA subclone (Fig. 7A, filled triangles) with DrrAB can complement the growth defect of FtsHts cells, indicating that the AAA domain of FtsH indeed contains a chaperone-like activity. Interestingly, the HEH mutant showed much better complementation of the growth defect as compared with the AAA subclone, perhaps due to a more native conformation of the full-length HEH protein as compared with the AAA subclone. The K198N mutation, on the other hand, showed no growth complementation effect (Fig. 7A, open triangles), showing that the ATPase activity associated with the AAA domain is important for chaperone function of FtsH. Western blot analysis showed that the DrrAB complex is membrane-associated in FtsH(HEH), FtsH(AAA), and FtsH (K198N)-containing strains (Fig. 7, B and C, lanes 5–7), and the amounts of DrrA and DrrB in these cells were comparable with the levels in their absence (lane 3). Therefore the restoration of growth by HEH and AAA clones must result from a change in conformation of the DrrAB proteins brought about by the functional AAA domain present in these two clones. Whether the HEH and AAA variants of FtsH can also restore function of the DrrAB complex is addressed in the next experiment.
FIGURE 7.
The AAA domain of FtsH is sufficient to complement the growth defect resulting from the expression of DrrAB in 797 cells. E. coli 797 cells containing the drrAB genes (pDX101) and the wild-type or a mutated version (K198N, HEH, or AAA subclone) of the ftsH gene on a compatible plasmid were grown at 30 °C and induced at 42 °C, as in Fig. 1B. A, growth analysis at 600 nm. B, Western blot analysis. Membranes prepared from the cells collected at the 3-h time point were analyzed, as described in the legend to Fig. 1A. C, quantitative analysis of DrrA and DrrB. The intensity of the bands on the nitrocellulose membrane in B was determined by densitometric scanning. The intensity of DrrA and DrrB in lane 1 was designated as 1.0. The data represent an average of 3 experiments. Columns 1–8 correspond to samples 1–8 in B.
We previously showed that wild-type DrrA and DrrB proteins together carry out ATP-dependent efflux of the anticancer drug doxorubicin (3). Here, we investigated whether co-expression of the AAA subclone or HEH allele can restore Dox efflux function of the misassembled DrrAB proteins expressed in FtsHts cells at 42 °C (Fig. 8). The data in Fig. 8A indicate that the rate of DrrAB-mediated Dox efflux at 30 °C in wild-type 796 and FtsHts 797 cells are comparable (columns 1 and 3). Induction of wild-type cells at 42 °C showed only a slight reduction in Dox efflux (column 2), which is likely due to the destabilization effect produced by high temperature on DrrAB, as seen earlier in Figs. 1 and 4. By contrast, the FtsHts cells induced at 42 °C showed very little or no DrrAB-mediated Dox efflux (Fig. 8A, column 4), which was comparable with the efflux seen with control cells containing empty vector (Fig. 8A, columns 5 and 6). These data confirm that the DrrAB proteins are misfolded in the absence of functional FtsH. Simultaneous overexpression of FtsH variants, K198N, HEH, or the AAA subclone, in FtsHts cells did not restore function of misassembled DrrAB (Fig. 8B, columns 4–6), although restoration of growth by HEH and the AAA subclone was earlier seen in Fig. 7A. Similarly, overexpression of GroESL also did not complement the DrrAB-mediated Dox efflux in FtsHts cells (Fig. 8B, column 7). One possible explanation for these data could be that although the AAA domain of FtsH (or GroESL) is able to alter the conformation of the DrrAB proteins and relieve growth inhibition, it is not sufficient by itself to restore proper conformation required for full function of the complex.
Wild-type FtsH Can Refold Previously Misassembled DrrAB and Restore Function
Surprisingly, co-expression of wild-type FtsH restored the Dox efflux function of the misassembled proteins expressed in FtsHts cells at 42 °C resulting in a significant recovery (about 45%) of Dox efflux by the DrrAB complex (Fig. 8B, compare columns 2 and 3). Because this could only have resulted if FtsH expressed in trans facilitated assembly of the complex, these results imply that the AAA and proteolytic domains of FtsH must work hand-in-hand to bring about functional assembly of the DrrAB complex. In this experiment, however, FtsH and the DrrAB proteins were expressed simultaneously by IPTG induction, therefore it was not possible to determine whether FtsH assists only the newly synthesized DrrAB to achieve proper conformation, or if it can also bring about refolding of the DrrAB proteins that have already been misfolded.
Therefore, in the next experiment, DrrAB and wild-type FtsH were expressed in FtsHts cells in a sequential manner. The ftsH gene subcloned under control of the araBAD promoter was induced by arabinose, whereas the drrAB genes remained under control of the lac promoter induced by IPTG. The FtsHts cells containing both plasmids (797/AB/pBADftsH) were grown at 30 °C to mid-log phase. The DrrAB proteins were first induced with IPTG at 42 °C for 1 h (this condition inactivates FtsH and renders DrrAB inactive as seen in Fig. 8, A and B). The cells were then washed several times to remove extracellular IPTG and stop further synthesis of DrrAB. The expression of FtsH was induced by arabinose for 1 h at 42 °C, and the cells were subjected to the Dox efflux assay. To maintain the chromosomally encoded FtsH in an inactive conformation, the temperature was maintained at 42 °C throughout the duration of the experiment. As previously seen in Fig. 8B, simultaneous expression of DrrAB and FtsH resulted in restoration of Dox efflux (Fig. 8C, 797/AB/pUCftsH, column 3). More interestingly, however, even greater restoration of the DrrAB-mediated Dox efflux was seen when FtsH was induced after DrrAB proteins had been pre-synthesized in these cells (Fig. 8C, 797/AB/pBADftsH+ara, column 5). In the absence of arabinose induction of FtsH, much lower restoration of Dox efflux was seen (Fig. 8C, 797/AB/pBADftsH −ara, column 4). These data, therefore, show that the sequential expression of DrrAB and FtsH can still restore the function of the previously misfolded DrrAB proteins to the same (or even higher) extent as seen with simultaneous expression.
Finally, the effect of overexpression of FtsH on Dox efflux function of DrrAB expressed at different temperatures was investigated. Irrespective of whether the DrrAB proteins were induced at 30, 37, or 42 °C in FtsHts cells, simultaneous expression of FtsH resulted in a very similar final Dox efflux efficiency (Fig. 8D). At 42 °C, co-expression of FtsH enhanced Dox efflux of misfolded DrrAB 5-fold (compare columns 3 and 6), yielding about 45% Dox efflux efficiency. Interestingly, overexpression of FtsH at 30 or 37 °C reduced the efficiency by about half (compare columns 1 and 2 with 4 and 5), once again yielding final Dox efflux efficiency of about 45%. These results imply that FtsH produces an optimal level of functional complexes in the membrane perhaps by exerting both proteolytic and refolding effects concurrently.
DISCUSSION
Non-native proteins, especially unassembled membrane proteins, interfere with cellular processes and are known to become toxic to the cells. Therefore, quality control systems, consisting of chaperones and proteases, play essential roles by monitoring their folding and either refolding or degrading misfolded proteins (21–23). Hsp60 (GroEL/ES) and Hsp70 (DnaK) proteins provide classical examples of ATP-dependent chaperones that prevent aggregation of newly translated proteins and promote their refolding (22). A special class of chaperones (e.g. ClpB in bacteria and its homologs Hsp78 and Hsp104 in eukaryotes) is known to resolubilize protein aggregates and, in cooperation with the Hsp70 chaperones (specifically DnaKJE), can result in regaining function of the affected protein (15). On the other end of the spectrum are proteins classically defined as proteases, for example, Lon, ClpA/P, ClpX/P, and FtsH, whose major function is considered to be removal of irreversibly damaged proteins from the cell (6, 14, 24). Despite their differences, however, both classical chaperones and proteases share common features. For example, both have the ability to recognize and bind non-native polypeptides and both bring about unfolding of their substrates, which are subsequently refolded (by a chaperone) or degraded (by a protease)(12, 22, 25). Because of the ATP-dependent unfolding function of AAA+ proteases, it has been speculated that they may also have the ability to refold substrate proteins and may participate in protein biogenesis. However, very little direct evidence is available for the role of FtsH or other AAA+ proteases in biogenesis, especially of membrane protein complexes. In this study, we provide clear evidence that E. coli FtsH is able to both degrade and refold misassembled DrrAB proteins, resulting in regaining the Dox efflux function of the membrane complex.
We show that in the absence of the DrrA protein, DrrB acquires an FtsH-sensitive conformation and is completely proteolyzed. However, in the absence of functional FtsH, the DrrB protein accumulates even in the absence of DrrA confirming that FtsH monitors the folding status of DrrB and removes it if it is improperly assembled. The molecular details of proteolysis of DrrB by FtsH are currently unknown, however, based on the prevalent model for its action (8, 26) we assume that FtsH could initiate proteolysis of DrrB either at the N- or the C-terminal end (both of which are found in the cytoplasm (27)). Cross-linking studies previously showed that the N terminus of DrrB is the major site of interaction with DrrA (3, 27); therefore we propose that proteolysis of DrrB initiates at its N-terminal tail, and binding of DrrA to this region of DrrB protects it from proteolysis by FtsH.
Interestingly, we found that the function of FtsH is not limited to proteolysis of unassembled DrrB, but it also plays an essential role in folding and assembly of the DrrAB complex. This conclusion is supported by several lines of evidence presented in this paper. First, the expression of DrrA and DrrB together in FtsHts cells at 42 °C (which results in inactivation of FtsH) was found to be growth inhibitory (Fig. 3B) suggesting that the complex is improperly assembled in the absence of a functional FtsH. Second, the rate of assembly of the DrrAB complex in the FtsHts cells at 30 °C was found to be significantly reduced as compared with the wild-type cells (Fig. 5). Third, functional analysis showed complete absence of the DrrAB-mediated Dox efflux in FtsHts cells under conditions of FtsH inactivation, suggesting that most or all of the DrrAB proteins expressed in these cells at 42 °C are misassembled. By contrast, the DrrAB proteins expressed in wild-type cells at 42 °C retained on average 85–90% of the Dox efflux activity (Fig. 8A). Finally, co-expression of FtsH in trans in FtsHts cells restored the ability of DrrAB to carry out Dox efflux, confirming that FtsH facilitates assembly of the DrrAB complex (Fig. 8B). Nevertheless, this result was surprising because FtsH contains a functional proteolytic domain. Overexpression in FtsHts cells results in proteolysis of the misfolded DrrAB proteins (as seen in Fig. 4), however, the data in Fig. 8B show that FtsH also facilitated some folding resulting in about 45% recovery of the Dox efflux activity. Either the AAA domain by itself or the GroESL chaperone was unable to complement the Dox efflux function of DrrAB in FtsHts cells, even though each was able to alleviate the growth defect. Therefore, together these data suggest that both the AAA and protease domains of FtsH are essential for promoting functional assembly of DrrAB.
The most crucial evidence for the refolding function of FtsH, however, came from the sequential expression studies. Irrespective of whether FtsH was expressed simultaneously with DrrAB or expressed after the nonfunctional DrrA and DrrB proteins had already accumulated in FtsHts cells, it was able to restore the function of the complex (Fig. 8C), thus showing conclusively that FtsH not only facilitates assembly of the DrrAB complex but is also actively involved in refolding previously misassembled DrrAB proteins. Interestingly, we also found that the sequential expression of DrrAB and FtsH resulted in a significantly higher recovery of the Dox efflux function of DrrAB as compared with simultaneous expression (Fig. 8C). This finding suggests that FtsH treats its substrate differently during its synthesis as compared with after it has already been synthesized.
In summary, our studies confirm that the AAA domain of FtsH can recognize and bind substrates and change their conformation, which is in agreement with the previous studies (18). However, we also show that the two activities (ATPase and proteolytic) of FtsH must be present simultaneously and occur in a coordinated manner to facilitate assembly and refolding of DrrAB. Much more extensive analysis will be required in the future to understand the nature of the molecular processes involved in refolding of DrrA and DrrB and to determine whether other factors also play a role in the assembly of the DrrAB complex. Further studies will also provide clues about how degradation and assembly of multisubunit complexes are regulated, and whether other AAA+ proteases may also contain chaperone activity. This study raises intriguing questions about the distinction between classical chaperones like GroESL (that can prevent aggregation of many proteins) and the classical proteases like FtsH that not only carry out proteolysis but also actively participate in refolding of their specific substrates, as shown in this study. Bukau and co-workers (22) previously coined four terms to describe the various activities of chaperones and proteases: Holders (small heat shock proteins, Hsps), Folders (GroESL and DnaK), Unfolders (ClpA, ClpX, and ClpB), and Proteases (Lon, ClpP, and FtsH). In light of the findings reported in this article, we propose a new term “specific refolder” to describe the function of FtsH and possibly other AAA+ proteases that may be shown in the future to contain such an activity.
Acknowledgments
We thank Koreaki Ito for the E. coli 796 and 797 strains as well as the ClpA− and Lon− strains. We thank P. C. Tai for critical reading of the manuscript and for constructive comments and suggestions that made this a much stronger manuscript. Thanks are also due to Ling Wei for technical help during the early stages of this work.
This work was supported, in whole or in part, by National Institutes of Health Service Award RO1 GM51981-09 (to P. K.).
- DrrAB
- doxorubicin resistance proteins A and B
- Dox
- doxorubicin
- AAA
- ATPase associated with diverse cellular activities
- ABC
- ATP-binding cassette
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside.
REFERENCES
- 1. Kaur P. (1997) Expression and characterization of DrrA and DrrB proteins of Streptomyces peucetius in Escherichia coli. DrrA is an ATP binding protein. J. Bacteriol. 179, 569–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaur P., Russell J. (1998) Biochemical coupling between the DrrA and DrrB proteins of the doxorubicin efflux pump of Streptomyces peucetius. J. Biol. Chem. 273, 17933–17939 [DOI] [PubMed] [Google Scholar]
- 3. Zhang H., Pradhan P., Kaur P. (2010) The extreme C terminus of the ABC protein DrrA contains unique motifs involved in function and assembly of the DrrAB complex. J. Biol. Chem. 285, 38324–38336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pradhan P., Li W., Kaur P. (2009) Translational coupling controls expression and function of the DrrAB drug efflux pump. J. Mol. Biol. 385, 831–842 [DOI] [PubMed] [Google Scholar]
- 5. Snider J., Thibault G., Houry W. A. (2008) The AAA+ superfamily of functionally diverse proteins. Genome Biol. 9, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnold I., Langer T. (2002) Membrane protein degradation by AAA proteases in mitochondria. Biochim. Biophys. Acta 1592, 89–96 [DOI] [PubMed] [Google Scholar]
- 7. Langer T. (2000) AAA proteases. Cellular machines for degrading membrane proteins. Trends Biochem. Sci. 25, 247–251 [DOI] [PubMed] [Google Scholar]
- 8. Ito K., Akiyama Y. (2005) Cellular functions, mechanism of action, and regulation of FtsH protease. Annu. Rev. Microbiol. 59, 211–231 [DOI] [PubMed] [Google Scholar]
- 9. Akiyama Y., Kihara A., Tokuda H., Ito K. (1996) FtsH (HflB) is an ATP-dependent protease selectively acting on SecY and some other membrane proteins. J. Biol. Chem. 271, 31196–31201 [DOI] [PubMed] [Google Scholar]
- 10. Akiyama Y. (2009) Quality control of cytoplasmic membrane proteins in Escherichia coli. J. Biochem. 146, 449–454 [DOI] [PubMed] [Google Scholar]
- 11. Truscott K. N., Lowth B. R., Strack P. R., Dougan D. A. (2010) Diverse functions of mitochondrial AAA+ proteins. Protein activation, disaggregation, and degradation. Biochem. Cell Biol. 88, 97–108 [DOI] [PubMed] [Google Scholar]
- 12. Sauer R. T., Bolon D. N., Burton B. M., Burton R. E., Flynn J. M., Grant R. A., Hersch G. L., Joshi S. A., Kenniston J. A., Levchenko I., Neher S. B., Oakes E. S., Siddiqui S. M., Wah D. A., Baker T. A. (2004) Sculpting the proteome with AAA+ proteases and disassembly machines. Cell 119, 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weibezahn J., Bukau B., Mogk A. (2004) Unscrambling an egg. Protein disaggregation by AAA+ proteins. Microb. Cell Fact. 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suzuki C. K., Rep M., van Dijl J. M., Suda K., Grivell L. A., Schatz G. (1997) ATP-dependent proteases that also chaperone protein biogenesis. Trends Biochem. Sci, 22, 118–123 [DOI] [PubMed] [Google Scholar]
- 15. Sauer R. T., Baker T. A. (2011) AAA+ proteases. ATP-fueled machines of protein destruction. Annu. Rev. Biochem. 80, 587–612 [DOI] [PubMed] [Google Scholar]
- 16. Akiyama Y., Ogura T., Ito K. (1994) Involvement of FtsH in protein assembly into and through the membrane. I. Mutations that reduce retention efficiency of a cytoplasmic reporter. J. Biol. Chem. 269, 5218–5224 [PubMed] [Google Scholar]
- 17. Arlt H., Tauer R., Feldmann H., Neupert W., Langer T. (1996) The YTA10–12 complex, an AAA protease with chaperone-like activity in the inner membrane of mitochondria. Cell 85, 875–885 [DOI] [PubMed] [Google Scholar]
- 18. Leonhard K., Stiegler A., Neupert W., Langer T. (1999) Chaperone-like activity of the AAA domain of the yeast Yme1 AAA protease. Nature 398, 348–351 [DOI] [PubMed] [Google Scholar]
- 19. Shirai Y., Akiyama Y., Ito K. (1996) Suppression of ftsH mutant phenotypes by overproduction of molecular chaperones. J. Bacteriol. 178, 1141–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asahara Y., Atsuta K., Motohashi K., Taguchi H., Yohda M., Yoshida M. (2000) FtsH recognizes proteins with unfolded structure and hydrolyzes the carboxyl side of hydrophobic residues. J. Biochem. 127, 931–937 [DOI] [PubMed] [Google Scholar]
- 21. Mogk A., Haslberger T., Tessarz P., Bukau B. (2008) Common and specific mechanisms of AAA+ proteins involved in protein quality control. Biochem. Soc. Trans. 36, 120–125 [DOI] [PubMed] [Google Scholar]
- 22. Dougan D. A., Mogk A., Bukau B. (2002) Protein folding and degradation in bacteria. To degrade or not to degrade? That is the question. Cell. Mol. Life Sci. 59, 1607–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horwich A. L., Fenton W. A., Chapman E., Farr G. W. (2007) Two families of chaperonin. Physiology and mechanism. Annu. Rev. Cell Dev. Biol. 23, 115–145 [DOI] [PubMed] [Google Scholar]
- 24. Gottesman S. (2003) Proteolysis in bacterial regulatory circuits. Annu. Rev. Cell Dev. Biol. 19, 565–587 [DOI] [PubMed] [Google Scholar]
- 25. Pak M., Hoskins J. R., Singh S. K., Maurizi M. R., Wickner S. (1999) Concurrent chaperone and protease activities of ClpAP and the requirement for the N-terminal ClpA ATP binding site for chaperone activity. J. Biol. Chem. 274, 19316–19322 [DOI] [PubMed] [Google Scholar]
- 26. Chiba S., Akiyama Y., Ito K. (2002) Membrane protein degradation, by FtsH can be initiated from either end. J. Bacteriol. 184, 4775–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gandlur S. M., Wei L., Levine J., Russell J., Kaur P. (2004) Membrane topology of the DrrB protein of the doxorubicin transporter of Streptomyces peucetius. J. Biol. Chem. 279, 27799–27806 [DOI] [PubMed] [Google Scholar]
- 28. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 29. Kihara A., Akiyama Y., Ito K. (1995) FtsH is required for proteolytic elimination of uncomplexed forms of SecY, an essential protein translocase subunit. Proc. Natl. Acad. Sci. U.S.A. 92, 4532–4536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maurizi M. R., Trisler P., Gottesman S. (1985) Insertional mutagenesis of the lon gene in Escherichia coli. lon is dispensable. J. Bacteriol. 164, 1124–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]