Background: Multiple Wnts are expressed and play an important role in tooth development.
Results: Inactivation of Gpr177 in dental epithelium leads to inhibition of Wnt signaling activity and arrest of early tooth development.
Conclusion: Intra-epithelial action of Wnt signaling is essential for early tooth development.
Significance: Dental epithelium is the primary target of epithelial-derived Wnts during tooth development.
Keywords: Epithelium, Gene Knockout, Secretion, Tooth Development, Wnt Signaling, Gpr177, Wnt, Enamel Knot, Intra-epithelial Signaling, Tooth Morphogenesis
Abstract
Multiple Wnt ligands are expressed in the developing tooth and play important and redundant functions during odontogenesis. However, the source of Wnt ligands and their targeting cells and action mechanism in tooth organogenesis remain largely elusive. Here we show that epithelial inactivation of Gpr177, the mouse Wntless (Wls) whose product regulates Wnt sorting and secretion, leads to arrest of tooth development at the early cap stage and abrogates tooth-forming capability of the dental epithelium. Gpr177 in the epithelium is necessary for the activation of canonical Wnt signaling in the dental epithelium and formation of a functional enamel knot. Epithelial deletion of Gpr177 results in defective gene expression and cellular behavior in the dental epithelium but does not alter odontogenic program in the mesenchyme. Furthermore, deletion of Axin2, a negative intracellular regulator of canonical Wnt signaling, rescues the tooth defects in mice carrying Gpr177 mutation in the dental epithelium. Together with the fact that active Wnt canonical signaling is present predominantly in the dental epithelium during tooth development, our results demonstrate that Gpr177-mediated Wnt ligands in the dental epithelium act primarily in an intra-epithelial context to regulate enamel knot formation and subsequent tooth development.
Introduction
The epithelial-mesenchymal interaction is essential for development of many organs, including the tooth (1). In mouse, tooth development is initiated by localized oral epithelial thickening, forming the dental placode at embryonic day 11.5 (E11.5). At E12.5 and E13.5, the placode invaginates into the underlying cranial neural crest-derived mesenchyme to form a tooth bud. Active cell proliferation further drives folding movement of the dental epithelium, leading to the formation of cap-like structure at E14.5. Meanwhile, the primary enamel knot that acts as a signaling center to regulate tooth morphogenesis and patterning forms at the tip of the dental epithelium (2). This transient structure lacks cell proliferation, disappears through apoptosis, and is subsequently replaced by the secondary enamel knots at the bell stage when odontogenic differentiation begins.
It has been well established that the odontogenic processes are regulated by a number of signaling molecules, including Shh, FGFs, BMPs,2 and Wnts, which act to mediate epithelial-mesenchymal interactions (1, 3). The secreted glycoproteins of Wnt family contain at least 19 members in mammals (4, 5). In signal-producing cells, synthesized Wnt proteins undergo posttranslational modification and maturation and intracellular trafficking and are finally secreted to the extracellular microenvironment. Through binding to the Fzd and/or Lpr5/6 receptors, Wnt signals elicit diverse intracellular responses in signal-receiving cells. For the canonical Wnt pathway, β-catenin is the master signaling transducer. In the absence of Wnt, β-catenin, maintained at low levels, is targeted for proteasome degradation through a destruction complex mediated by Axin1 and Axin2 (6, 7). In the presence of Wnt, the Axin-dependent complex is sequestered, allowing β-catenin to enter the nucleus and interact with Lef/Tcf transcription factors to activate downstream target genes (8). One of these targets is Axin2, which attenuates β-catenin signaling in a negative feedback mechanism (9–13).
During tooth morphogenesis, the canonical Wnt ligands and signaling regulators contribute to the core of the multi-signaling hub to mediate the epithelial-mesenchymal interaction (14–20). Multiple Wnt ligands, including Wnt-3, -4, -5a, -6, -7b, -10a, and -10b, are expressed in the developing tooth germ (21, 22). Disruption of Wnt signaling either by β-catenin deletion (16) or ectopic expression of Dkk-1 in the oral epithelium resulted in the arrest of tooth development at the early bud stage (16, 23). Conversely, constitutive stimulation of β-catenin signaling in the oral epithelium led to ectopic tooth formation (15, 16). Wnt/β-catenin signaling was shown to be critical for morphological transition from the bud to the cap stage through activation of Fgf4 (14). A recent report further demonstrated a feedback regulatory circuit of Bmp4 and Wnt that is essential for signaling cross-talk between the dental epithelium and underlying mesenchyme (19). Wnt, acting in parallel to and together with Bmp4, is a master orchestrator for signaling interplay across the two different tissue layers. Wnt is likely one of the earliest signaling regulators, acting upstream of other odontogenic pathways to orchestrate their interactions (16–18). The expression patterns of Wnt ligands, predominantly in the epithelial components, also suggest that the epithelium may be the source of Wnts during tooth morphogenesis (21, 22). However, due to overlapped expression of many Wnts in the developing tooth, specific gene inactivation may not be practical and likely encounters issues related to functional redundancy. In addition, the tissue component that is responsible for secretion of Wnts required for activation of Wnt/β-catenin signaling in the dental epithelium remains elusive.
Disruption of Wnt secretion in signal-producing cells promises new insights into the mechanism underlying tooth development. We have recently identified Gpr177 as the mouse orthologue of Drosophila Wls/Evi/Srt essential for proper sorting and secretion of Wnt (24–28). In flies, Wls/Evi/Srt regulates the secretion of all Wnts, except WntD, due to its exclusion from lipid modifications (29, 30). In mice, genetic studies have suggested that the Gpr177-mediated regulation of canonical and noncanonical Wnts is required for different cell types and tissues (26, 27, 31). Abrogation of Wnt secretion caused by Gpr177 deficiency may provide an excellent strategy to determine the source of Wnt and its targeting cells during organogenesis. To further decipher Wnt signaling regulation in tooth development, we created a mouse model with epithelial-specific disruption of Gpr177 because canonical Wnt ligands are expressed predominantly in the dental epithelium. We report here that the epithelial ablation of Gpr177 impairs tooth morphogenesis at the early cap stage due to a lack of intraepithelial Wnt-mediated β-catenin signaling. The fact that inactivation of Axin2 alleviates the tooth phenotypes in Gpr177 mutants further supports a requirement of the canonical Wnt signaling in the dental epithelium for normal tooth development. This study reveals a requirement of intraepithelial supply of Wnts during tooth morphogenesis.
EXPERIMENTAL PROCEDURES
Animals
All animals used by this study were hosted in a standard specific pathogen-free mouse facility, and use of the animals was approved by the Committee of Laboratory Animals, Hangzhou Normal University. Gpr177 conditional mice were generated by flanking exon 3 of the Gpr177 gene with the LoxP sequences as described before (27). Mouse lines for TgKRT14-Cre (32), Osr2+/− (33), Axin2LacZ (11), TopGal (34), and R26R-LacZ (35) used in this study were purchased from The Jackson Laboratory in Bar Harbor, Maine. Genotyping of these mouse lines was performed accordingly. All mice were bred on a mixed 129/C57b6 background. The morning when the virginal plug was detected was designed as embryonic day 0, and embryos were harvested from timed pregnant females.
Histology, in Situ Hybridization, Immunohistochemistry, and X-Gal Staining
Samples were fixed in 4% paraformaldehyde, PBS at 4 °C overnight, dehydrated through gradient ethanol series, paraffin-embedded, and sectioned at 10 μm. Sections were subjected to hematoxylin/eosin staining for histological analysis or to nonradioactive in situ hybridization as described previously (36). Standard immunohistochemical staining was performed using antibody against Gpr177 as described previously (26) and antibody against active β-catenin (anti-abc, from Millipore). For X-gal staining, samples were fixed 4% paraformaldehyde and cryosectioned at 12 μm. Sections were subjected to standard X-gal staining and counterstained with 0.1% eosin.
In Vitro Organ Culture, Tissue Recombination, and Kidney Capsule Culture
Organ culture and tissue recombination were performed as described previously (36, 37). Briefly, for tooth tissue recombination, molar tooth germs (or incisor tooth germ unless specified) were dissected from E13.5 wild type and mutant embryos and incubated with 0.1% collagenase for 20 min at 37 °C, and the dental epithelia were separated from the dental mesenchyme. Wild type dental epithelium was recombined with the mutant dental mesenchyme and vice versa. As positive controls, wild type dental epithelium was recombined with wild type dental mesenchyme. Recombinants were culture on Nucleopore Track-Etch Membrane (0.2 μm) in a Trowell type organ culture dish for 24 h before being grafted underneath kidney capsule of host mice, as described previously (36, 38). Samples were retrieved after 3 weeks in kidney capsule culture and processed for further analysis. For the TopFlash-like tissue recombination assay, dental epithelia from E14.5 wild type molar or Gpr177K1cre molar were isolated and recombined with oral mesenchyme isolated from the mandibular arch of E13.5 TopGal reporter mice, respectively. Tissue recombinants were placed in Trowell-type organ culture for 24 h, fixed in 4% paraformaldehyde, cryosectioned, and then subjected to X-gal staining.
Cell Proliferation and TUNEL Assays
Cell proliferation rate was measured by BrdU labeling, and apoptosis was determined by TUNEL assay, as described previously (39). Briefly, timed pregnant mice were injected intraperitoneally with BrdU solution (3 mg/100 g body weight) from BrdU labeling and detection kit (Roche Applied Science) 30 min before being sacrificed. Embryonic heads (n = 3 for both wild type and mutant) were fixed in 4% paraformaldehyde and then processed for paraffin sections at 7 μm for immunohistochemical staining according to the manufacturer's instruction. BrdU-positive cells counted (≥20 consecutive fields at 40× magnification from 3 samples for both wild type and mutants) and were calculated as percentage of labeled cells among total nuclear stained cells within a defined arbitrary area. Statistical significance was calculated using Student's t test. Apoptosis was assayed by TUNEL staining using the In Situ Cell Death Assay kit according to manufacturer's instruction (Roche Applied Science).
Co-Immunoprecipitation and Western Blotting Assays
Mouse Gpr177 cDNA was amplified by RT-PCR and subcloned into pFLAG-CMV-1 (5 terminal FLAG epitope vector, Sigma). Mouse Wnt cDNAs cloned by RT-PCR were subcloned into pCMV-Myc (C-terminal Myc epitope vector, Clontech). The sequences of all constructs were confirmed by DNA sequencing. HEK293-T cells were maintained in DMEM containing 10% fetal bovine serum (Invitrogen) and transfected with the indicated constructs by Escort™ IV Transfection Reagent (Sigma). Cell pellets were harvested separately at 36–48 h after transfection. Immunoprecipitation assays were performed as described previously (40). Briefly, cells were washed with ice-cold PBS, lysed with cell lysis buffer for Western assay and immunoprecipitation (Beyotime), and centrifuged to collect supernatant. About 5% of the supernatant was reserved for analyzing protein expression, and the remainder was incubated with Anti-FLAG® M2 magnetic beads at 4 °C for 2 h. The beads were collected using a magnetic Stand (Promega), washed 3 times, and incubated with FLAG peptide (0.4 mg/ml, Sigma) to release immunoprecipitates. Immunoprecipitates or total proteins isolated from HEK293 cells were resolved by SDS-PAGE and subjected to immunoblotting analyses with anti-FLAG (Cell Signaling), Myc (Cell Signaling), Wnt3 (Abcam), Wnt4 (R&D), Wnt6 (R&D), and Wnt10b (Abcam) antibodies followed by incubation with each corresponding horseradish peroxidase-linked secondary antibody (Santa Cruz). For functional secretion assays of Wnts, HEK 293 cells were co-transfected with Wnt-Myc and either pCMV-FLAG or FLAG-Gpr177 plasmid and grown in DMEM supplemented with 10% FBS for 24 h. The culture medium was then replaced with serum-free DMEM medium for another 24 h before the conditioned medium was collected and concentrated using an Amicon Ultra-15 centrifugal filter (Millipore). The Wnt secreted into medium was detected by Western blotting with anti-Myc antibody (cell signaling) using the NOVEX ECL CHEMI Kit (Invitrogen).
RESULTS
Dental Epithelial Gpr177 Is Required for Tooth Morphogenesis
We first examined the expression pattern of Gpr177 to determine the cell type potentially responsible for the production of Wnt ligands during early tooth development. At E11.5, Gpr177 transcripts were strongly detected in the dental epithelium but were found at relatively lower levels in the dental mesenchyme of both incisor and molar (Fig. 1; data not shown). This Gpr177 expression pattern was maintained similarly at the bud and cap stages (Fig. 1), suggesting its potential role in the regulation of Wnt production in both epithelial and mesenchymal components during early tooth development.
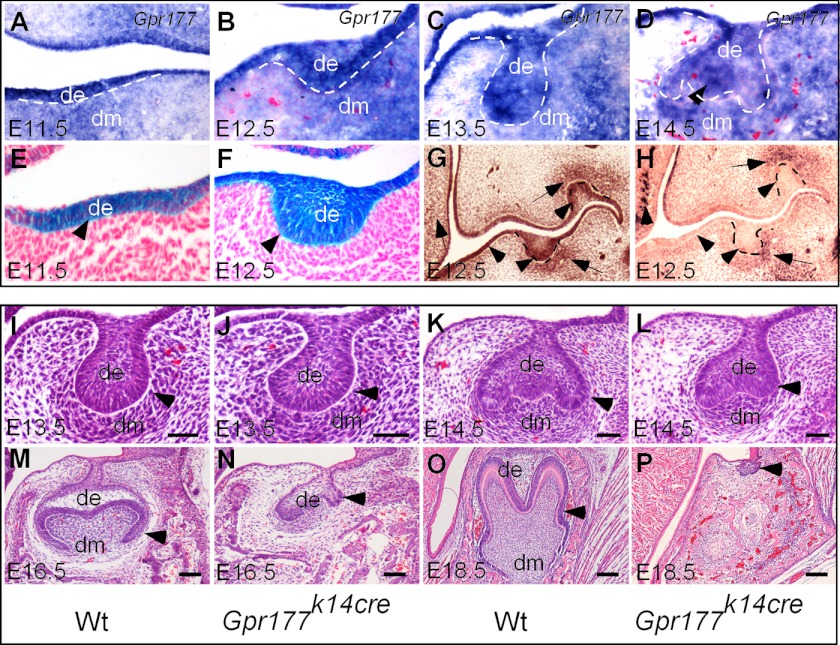
FIGURE 1.
Gpr177 is required for mouse tooth morphogenesis. A–D, in situ hybridization shows Gpr177 expression in the molar epithelium and mesenchyme throughout early tooth development. E and F, X-gal staining shows Cre activity in the dental epithelium of K14Cre;Rosa26R molar germs at the lamina (E) and early bud stage (F). G and H, immunohistochemical staining using antibodies against Gpr177 shows Gpr177 expression in the wild type molar (G) but absent Gpr177 staining in the dental and adjacent oral epithelium of Gpr177K14cre molar germ (H) at E12.5. I–P, histological analyses show that Gpr177K14cre molar developed normally to the late tooth bud stage (J) but was arrested at the early cap stage at E14.5 (L) and E16.5 (N) and became regressed at E18.5 (P; pointed out by the arrowhead). de, dental epithelium; dm, dental mesenchyme. Scale bars, 50-μm in panels I–L; 100-μm in panels M and N; 200-μm in panels O and P.
Because Wnt ligands are expressed predominantly in the dental epithelium and Gpr177 is also strongly expressed there, we examined the requirement of Gpr177 in the dental epithelium for tooth development by generating mice carrying the K14-Cre transgenic allele and homozygous Gpr177fx allele (Gpr177K14cre). The K14-Cre line (32) exhibited Cre recombination activity in the oral ectoderm at E11.5, and the activity became much stronger at E12.5 (Fig. 1). To ensure a successful deletion of Gpr177, we examined Gpr177 protein expression in Gpr177K14cre mutants by immunohistochemical staining. As shown in Fig. 1, Gpr177 protein was indeed absent in the dental epithelium at the E12.5 bud stage. We then analyzed tooth phenotype in Gpr177K14cre mice from E11.5 to newborn (P0) by histology, since the mutants died a few hours after birth for unknown reason. We found that both molar and incisor germs appeared normal at the initiation stage in Gpr177K14cre mutants, as assessed by normal tooth bud structure at E13.5 (Fig. 1; data not shown). However, at the E14.5 cap stage, the mutant tooth germs failed to progress further, being arrested at the early cap stage, and becoming abortively regressed subsequently (Fig. 1). These observations indicate a requirement for Gpr177 in the dental epithelium for normal tooth development. Because a similar phenotype was found in the incisor and molar, we chose molar as the model in our further studies.
Epithelial Deletion of Gpr177 Leads to Failed Secretion of Wnt Ligands and Absent Wnt Canonical Signaling Activity in the Dental Epithelium
To confirm that the tooth phenotype observed in Gpr177K14cre mice is indeed attributed to the lack of Wnt ligand secretion from the dental epithelium, we first examined canonical Wnt signaling activity in the developing tooth by compounding the TopGal transgenic allele onto the Gpr177K14cre background. In wild type mice, TopGal activity is normally detected only at the tip of the molar epithelial bud where the enamel knot will form at E13.5 and becomes restricted to the enamel knot at E14.5 (Fig. 2A; Ref. 16). However, in the mutant, residual TopGal activity was detected in the dental epithelium at the E13.5 bud stage and was completely absent in the dental epithelium at the E14.5 cap stage (Fig. 2A), indicating that deletion of Gpr177 abolishes the canonical Wnt signaling in the dental epithelium.
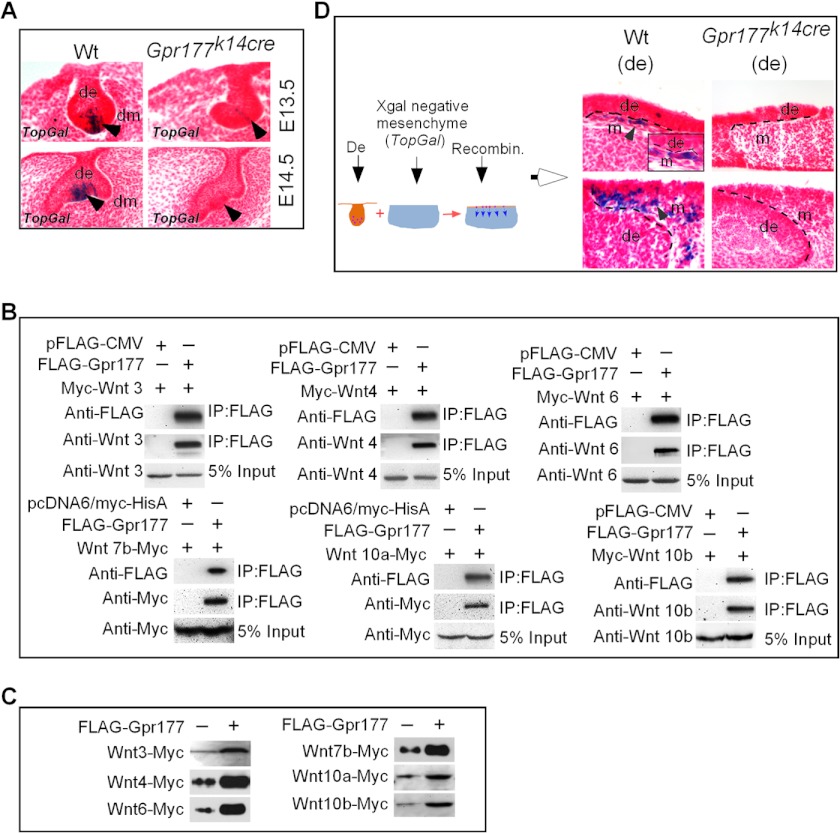
FIGURE 2.
Gpr177 is required for Wnt secretion and activation of canonical Wnt signaling in the dental epithelium. A, X-gal staining shows abolished TopGal reporter activity in the developing molars of Gpr177K14cre mice at the bud and cap stages as compared with the wild type counterparts. B, co-immunoprecipitation (IP) demonstrates physical interaction of Gpr177 with all the Wnt ligands known to be expressed in the dental epithelium of developing tooth. C, Western blotting shows enhanced secretion of representative Wnt ligands in culture media co-transfected with Gpr177 expression vector. Each Myc-tagged Wnt expression vector was co-transfected with pFLAG-CMV as controls. D, tissue recombination using dental epithelium from E14.5 wild type or Gpr177K14cre molars and oral mesenchyme of the E13.5 TopGal embryo shows activation of TopGal activity in the adjacent oral mesenchymal cells by the wild type dental epithelium but not by the mutant dental epithelium (de).
Several Wnt ligands are expressed in the epithelium of developing tooth. Although it has been shown that Gpr177-mediated regulation of canonical and noncanonical Wnts is required in different cell types and tissues (26, 27, 31), we wanted to ensure that Gpr177 indeed interacts with and regulates all of the Wnt ligands expressed in the dental epithelium. We conducted co-immunoprecipitation experiments to determine physical association of Gpr177 with each of the epithelia-expressed Wnts, including Wnt-3, -4, -6, -7b, -10a, and -10b. As shown in Fig. 2B, Gpr177 formed complex with each individual Wnt tested, indicating a physical interaction between Gpr177 and these tested Wnt proteins. We then further tested the function of Gpr177 in the secretion of Wnt proteins. We collected and concentrated media from cell cultures co-transfected with tagged Wnt construct and Gpr177 expression vector or control vector and performed a Western blotting assay for secreted Wnt proteins. Our results showed that the overexpression of Gpr177 significantly enhances the level of Wnts in the media (Fig. 2C).
To further demonstrate that the absence of Gpr177 abolishes the secretion of Wnt ligands from the dental epithelium, we performed a TopFlash-like assay in tissue recombination experiments. We isolated and recombined the dental epithelium from the first molars of E14.5 Gpr177K14cre embryos with oral mesenchyme from E13.5 TopGal mice where no reporter activity is ever detected. E14.5 wild type molar epithelia were used as control. In this assay system TopGal activity in the mesenchyme would be turned on, as assessed by X-gal staining, if Wnt ligands are secreted from the dental epithelium. Our results clearly showed that the dental epithelium from wild type, but not Gpr177K14cre mice, was able to activate TopGal reporter expression in the mesenchymal cells (Fig. 2D), providing additional evidence for the lack of Wnt secretion from the dental epithelium of Gpr177K14cre mice.
Aberrant Expression of Enamel Knot Signaling Molecules in the Absence of Gpr177
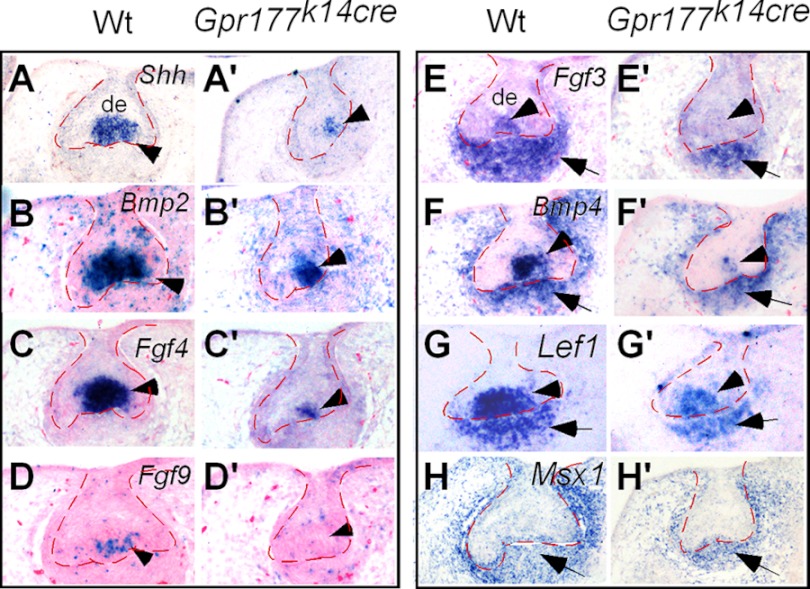
To further elucidate the mechanism underlying early tooth development mediated by Gpr177, we examined the expression of several regulatory genes known to be important for tooth development at E14.5 when the phenotype became discernible. We found that the expression of the enamel knot-specific genes, including Shh, Bmp2, Fgf4, and Fgf9, was significantly down-regulated or absent in the mutants (Fig. 3). In addition, the enamel knot expression component of Fgf3, Bmp4, and Lef1 (2, 41, 42) was also dramatically down-regulated or diminished in the mutants (Fig. 3), indicating a disruption of the primary enamel knot formation. In contrast, the expression of the dental mesenchymal marker Msx1 as well as the mesenchymal expression component of Fgf3, Lef1, and Bmp4 was retained (Fig. 3), suggesting that the absence of Gpr177 in the dental epithelium primarily affects the dental epithelial development. Dysregulation of these signaling molecules in the epithelium leads to the formation of a defective enamel knot that is insufficient to support further tooth morphogenesis.
FIGURE 3.
Lack of Gpr177 leads to altered gene expression in the enamel knot. In the absence of Gpr177 in the dental epithelium, the expression levels of Shh (A, A′), Bmp2 (B, B′) Fgf4 (C, C′), Bmp4 (F, F′), and Lef1 (G, G′) in the enamel knot are reduced significantly, and the expression of Fgf9 (D, D′) and Fgf3 (E, E′) is abolished completely. However, the expression of Fgf3 (E, E′), Bmp4 (F, F′), Lef1 (G, G′), and Msx1 (H, H′) in the dental mesenchyme, although slightly reduced, is retained.
Gpr177 Deficiency Causes Defective Cell Proliferation in the Dental Epithelium
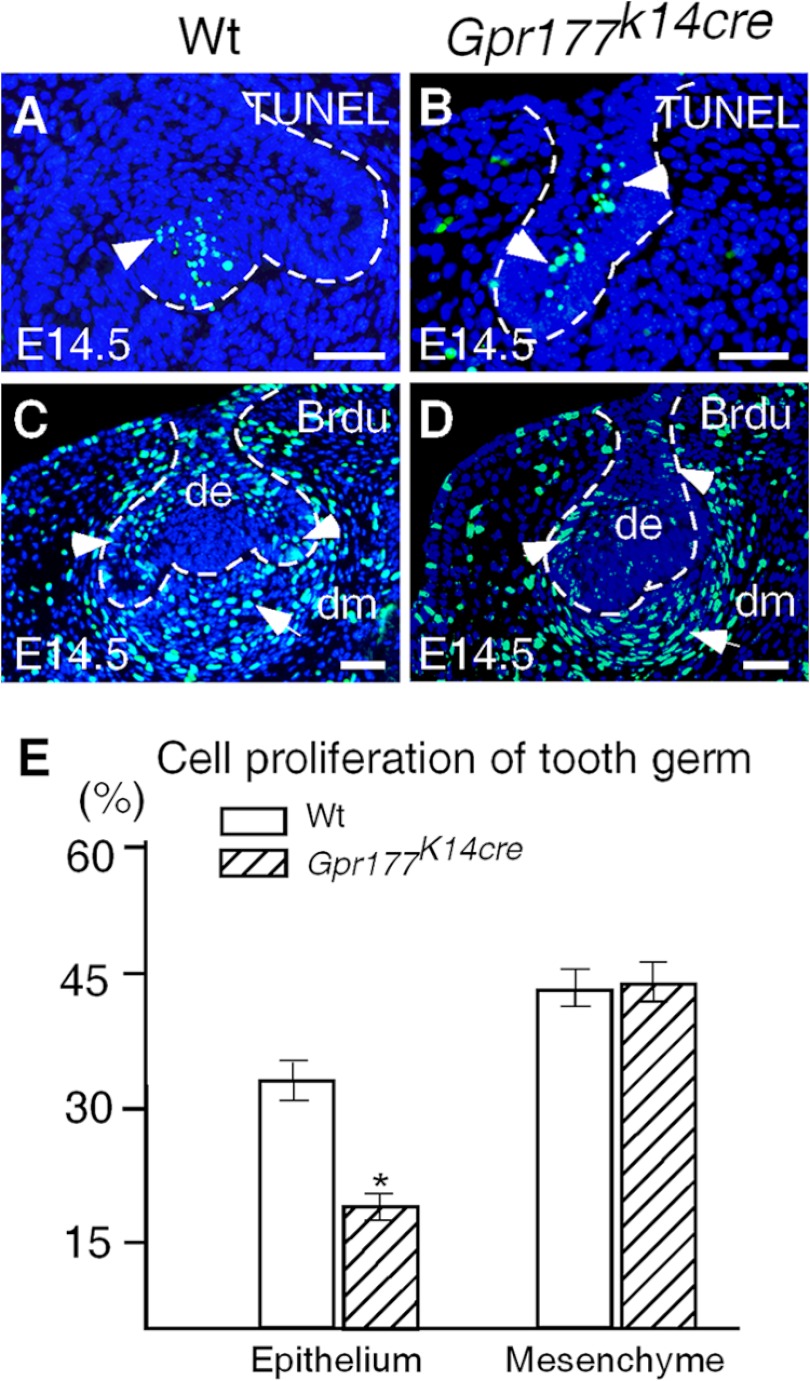
To reveal cellular processes underlying the defective tooth development in Gpr177K14cre mice, we performed BrdU labeling and apoptotic assays. Cell proliferation is a determining factor to drive dental epithelial folding to advance tooth morphogenesis from the bud to the cap stage, and FGFs from the enamel knot are responsible for stimulating cell proliferation (1, 43). Our results showed that at the E14.5 cap stage, the wild type littermates exhibited an extensive amount of BrdU-positive cells in the dental epithelium, including the cervical loop as well as the surrounding dental mesenchyme (Fig. 4). In contrast, the number of BrdU-positive cells was reduced significantly (p < 0.02) in the dental epithelium but not in the mesenchyme of Gpr177K14cre mice at the same stage (Fig. 4). To determine whether Gpr177 plays a role in cell survival, we examined programmed cell death in the developing tooth at E14.5 by TUNEL assay. In the wild type controls, apoptotic cells were found restrictedly in the enamel knot (Fig. 4). However, in E14.5 Gpr177K14cre tooth germs, scattered apoptotic cells were found primarily in the stellate reticulum, further indicating a defective enamel knot in the mutant (Fig. 4). These findings are consistent with the gene expression patterns and further support our notion that the tooth defect observed in Gpr177K14cre mice is attributed to the malformed primary enamel knot.
FIGURE 4.
Aberrant apoptosis and cell proliferation in tooth germs lacking epithelial Gpr177. A and B, TUNEL assays show normal cell apoptosis in the enamel knot of an E14.5 wild type molar (A) but apoptotic cells in the stellate reticulum of an E14.5 Gpr177K14cre molar (B). C and D, BrdU labeling shows reduced cell proliferation rate in the dental epithelium of an E14.5 Gpr177K14cre molar (D) as compared with the wild type control (C). E, shown is a comparison of BrdU-labeled cells in the designated areas of dental epithelium and dental mesenchyme of E14.5 molars in controls and mutants. *, p < 0.02. Scale bars, 50 μm.
Gpr177 Deficiency in Dental Epithelium Does Not Impair Odontogenic Capability in the Mesenchyme
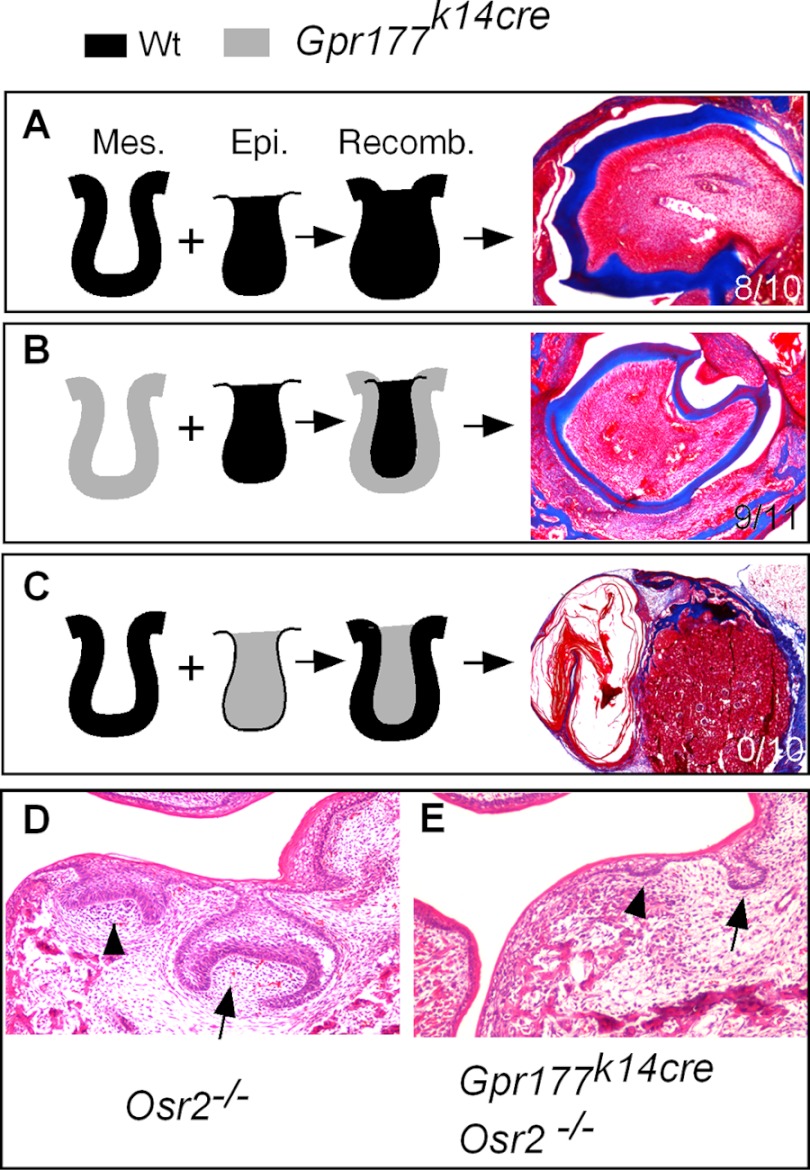
Because Wnt ligands are predominantly expressed in the dental epithelium and a Wnt-Bmp feedback circuit between dental epithelium and mesenchyme was thought to control early tooth development (19), we wondered if a lack of Wnt secretion from the dental epithelium and disruption of this circuit would also alter the odontogenic program in the dental mesenchyme. We performed reciprocal tissue recombination experiments. We isolated E13.5 molar germs from wild type and Gpr177K14cre embryos. The dental epithelium and mesenchyme were separated and recombined reciprocally between the wild type and mutant. As the control, wild type epithelium was recombined with wild type mesenchyme. Recombinants were grafted underneath the kidney capsule of adult mice for 3 weeks after 24 h in the Trowell-type organ culture. The results showed that the recombinants of the wild type epithelium and the Gpr177K14cre mesenchyme produced well-differentiated teeth (Fig. 5) with a success rate (9/11) similar to the controls (8/10). However, the recombinants of the wild type mesenchyme and the Gpr177K14cre epithelium failed to give rise to any tooth structure (0/10), forming keratinized cysts instead (Fig. 5). These observations indicate that the tooth-forming capability was lost in the dental epithelium deficient for Gpr177. However, despite the of lack epithelial Wnt secretion, the dental mesenchyme of Gpr177K14cre mice retains its odontogenic program.
FIGURE 5.
Epithelial deficiency in Gpr177 does not impair odontogenic capability of the dental mesenchyme. A, shown is a tooth formed in the tissue recombinants of E13.5 wild type molar epithelium and mesenchyme. B, shown is a tooth formed in the tissue recombinants of E13.5 molar epithelium and mutant molar mesenchyme. C, shown is a tooth that failed to form in the tissue recombinants of E13.5 wild type molar mesenchyme and mutant molar epithelium. D, shown is an ectopic tooth (arrowhead) formed at the lingual side of the first molar (arrow) in an E16.5 Osr2 mutant. E, shown is a residual ectopic tooth bud (arrowhead) formed at the lingual side of the repressed first molar (arrow) in an E16.5 Gpr177K14cre;Osr2−/− embryo.
Because in Gpr177K14cre mice inactivation of epithelial Gpr177 occurs at E11.5 when tooth development has begun, we asked if Gpr177 is required for epithelial response to odontogenic induction by the dental mesenchyme. To address this question, we took advantage of the Osr2 mutants in which an ectopic tooth lingual to the first molar is induced at E13.5 (36; Fig. 5D). We compounded the Osr2 null alleles onto Gpr177K14cre background. Although ectopic tooth buds were indeed observed in Osr2 and Gpr177 compound mutants (Gpr177K14cre/Osr2−/−), they were arrested at the lamina/early bud stage (Fig. 5E). These results indicate that the oral epithelial lacking Gpr177 can still respond to odontogenic induction but fail to develop subsequently.
Rescue of Tooth Development in Grp177K14cre Mice by Deletion of the Axin2 Gene
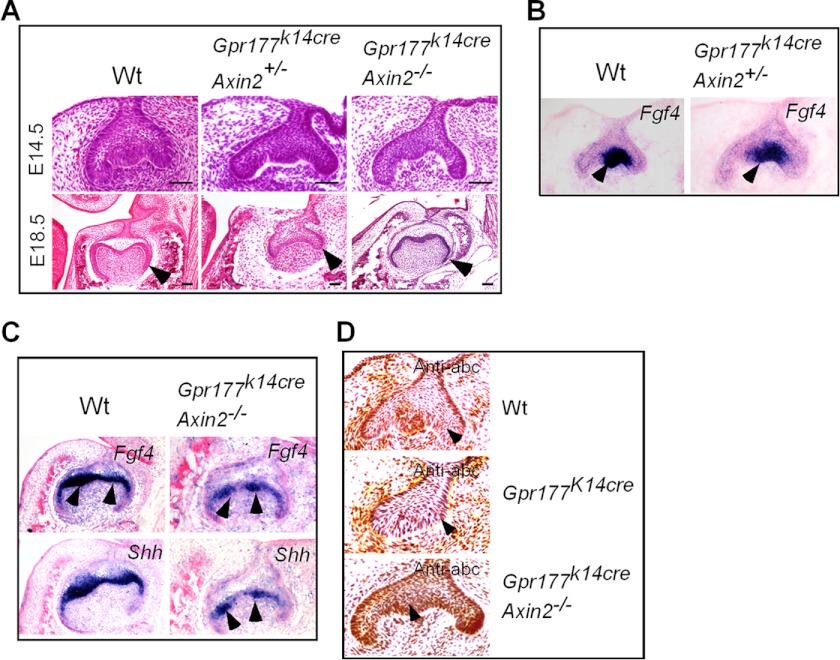
Axin proteins are crucial negative Wnt signaling regulators that target β-catenin for degradation in the absence of Wnt ligands. Because Axin2 is expressed in the developing tooth germ and a lack of Axin2 leads to up-regulation of Wnt canonical signals in a tissue-specific manner (7, 13, 44, 45), we wondered if modulation of Wnt signaling by deletion of Axin2 could alleviate developmental defects of the Gpr177K14cre teeth. We compounded the Axin2 null allele (Axin2LacZ; Ref. 11) onto the Gpr177K14cre background. Histological results showed that in the absence of one Axin2 allele (Gpr177K14cre/Axin2+/−), the tooth defect was indeed alleviated at E14.5 as evidenced by the extensive epithelial folding (Fig. 6A), as compared with the tooth germ in Gpr177K14cre mice at the same stage (Fig. 1N). The formation of a definite enamel knot in the Gpr177K14cre/Axin2+/− tooth at this stage was further confirmed by resumed expression of Fgf4 (Fig. 6B). However, tooth development in Gpr177K14cre/Axin2+/− mice was halted at the cap stage, as manifested at E18.5 (Fig. 6A), indicating that Axin2 haploinsufficiency is not sufficient to compensate for the epithelial deletion of Gpr177 to rescue the tooth developmental defect. Indeed, removal of both alleles of Axin2 further alleviates the developmental defects in the Gpr177 mutant, as demonstrated by comparable morphology of the bell stage tooth between the Gpr177K14cre/Axin−/− mutants and wild type at E18.5 (Fig. 6A). The expression patterns of Fgf4 and Shh in the developing tooth germs of Gpr177K14cre/Axin−/− mice at E16.5, although relatively weaker as compared with the wild type controls, demonstrated the formation of the secondary enamel knots (Fig. 6C). Associated with this rescued tooth development was resumed accumulation of active β-catenin in the dental epithelium, as shown by immunohistochemical staining using anti-active-β-catenin antibody (Fig. 6D). These observations indicate that modulation of canonical Wnt signaling by deletion of Axin2 rescue tooth development by restoring odontogenic program in the dental epithelium in Gpr177K14cre mice.
FIGURE 6.
Reduction of Axin2 dosage partially restored tooth development in Gpr177K14cre mice. A, histological analyses show partial rescue of molar development in Gpr177K14cre mice with a reduced dosage of Axin2. B, shown is resumed Fgf4 expression in the enamel knot of the first molar of an E14.5 Gpr177K14cre embryo with Axin2 haploinsufficiency as compared with the control. Arrowheads point to the enamel knot. C, shown is resumed Fgf4 and Shh expression in the secondary enamel knots of an E16.5 Gpr177K14cre molar lacking Axin2 as compared with a wild type littermate. Arrowheads point to the secondary enamel knot. D, accumulation of active β-catenin, shown by immunohistochemical staining, was restored in the dental epithelium of rescued tooth germ, as compared with wild type control and Gpr177K14cre mutant. Arrowheads point to dental epithelium. Scale bars = 50 μm.
DISCUSSION
Numerous studies have revealed an important role of Wnt canonical signaling in tooth development, from the initiation through the bud, the cap, and differentiation stages (46). The dental epithelium appears to be the major target of canonical Wnt signals, as assessed by Wnt/β-catenin reporter expression (Ref. 16; Fig. 2A). Wnt/β-catenin activity was also reported in dental mesenchymal cells immediately adjacent to the dental epithelium using an Axin2LacZ knock-in allele (44). However, it cannot be ruled out that this activation of Wnt canonical signaling in the dental mesenchyme is a consequence of Axin2 haploinsufficiency. Multiple Wnt ligands are expressed in the developing tooth germ, with most of them in the dental epithelium except Wnt5a (20–22, 46), suggesting that the dental epithelium is the main source of canonical Wnts. Although the epithelial Wnts were thought to function in the dental mesenchyme to form a Wnt-Bmp feedback circuit to regulate early tooth development (19), it remained unknown which tissue compartment is responsible for activation of Wnt/β-catenin signaling in the dental epithelium. Because of overlapped expression of a number of Wnt ligands in the developing tooth, single or double gene inactivation may not reveal real function of Wnt signaling due to potential functional redundancy.
In this study we took advantage of the floxed allele of Gpr177, a recently identified mouse orthologue of Drosophila Wls/Evi/Srt essential for proper sorting and secretion of Wnts, to disrupt Wnt secretion in signal-producing cells in a tissue-specific manner. We found that Gpr177 is expressed in both epithelial and mesenchymal compartments of the early developing tooth. We showed that epithelial inactivation of Gpr177 leads to arrest of tooth development at the early cap stage accompanied by cellular defect and altered gene expression in the dental epithelium but not the mesenchyme. However, ablation of Gpr177 in neural crest-derived tissues including dental mesenchyme does not affect early tooth development but leads to retarded tooth growth from E16.5 on and subsequently delayed odontogenic differentiation, mimicking the tooth defects observed in mice deficient in Wnt5a (47).3 This observation is consistent with the fact that Wnt5a is the only Wnt ligand that is known to be expressed in the dental mesenchyme (22).
The absolute requirement of Wnt/β-catenin signaling in the epithelium for early tooth development has been demonstrated by the epithelial inactivation of β-catenin that results in the arrest of tooth development at the bud stage (16). However, due to the dual role of β-catenin in Wnt signaling and cell adhesion, it cannot be ruled out that an impaired cell adhesion in the absence of β-catenin could also contribute to tooth defect. In our current study, we demonstrate direct physical interaction of Gpr177 with every Wnt ligand that is known to be expressed in the dental epithelium. Certainly, we recognize that this physical interaction was evidenced in HEK 293T cells, which might not hold true in dental epithelial cells. However, the failure of Gpr177K14cre dental epithelium to activate TopGal reporter activity in oral mesenchyme could be attributed to the disruption of Wnt secretion in the dental epithelium. Inactivation of Gpr177 also abolished Wnt/β-catenin signaling in the dental epithelium, indicating an intra-epithelial action of the epithelial Wnts. Together with the observations of unaltered cellular process (such as cell proliferation rate) and retained gene expression patterns in the dental mesenchyme of Gpr177K14cre mice, it appears that the dental epithelial cells are the primary targets of the epithelial Wnts during early tooth development.
The restricted Wnt/β-catenin activity in the tip of the dental epithelium at the bud stage where the enamel knot will form and in the enamel at the late stage implicates a role of Wnt canonical signaling in the formation enamel knot. In addition, Lef, the downstream effector of Wnt canonical signaling, is expressed in the enamel knot and directly activates the expression of Fgf4, a molecular marker of the enamel knot (14). In agreement with this idea is dysregulation of several critical molecules, including Shh, Fgf3, Fgf4, Fgf9, and Bmp4, in the enamel knot of the Gpr177K14cre tooth germ. The expression of these FGFs in the enamel knot was thought to function as mitogen to stimulate cell proliferation in dental epithelium, leading to epithelial folding and formation of cap structure (1, 48, 49). The reduced cell proliferation rate in the dental epithelium of the cap stage Gpr177K14cre tooth germ is apparently the consequence of down-regulated Fgf expression in the enamel knot. Together with aberrant cell apoptosis in the cap stage dental epithelium, these observations indicate that in the absence of epithelial Gpr177, Wnt/β-catenin signaling fails to be activated, leading to formation of a deformed enamel knot that fails to function as a signal center to advance tooth morphogenesis. This conclusion is further supported by the rescue experiments in which deletion of Axin2, which led to resumed accumulation of active β-catenin in the dental epithelium, restored the formation of the enamel knot and advance tooth morphogenesis in Gpr177K14cre mutants.
It was proposed that a Wnt-Bmp feedback circuit between the dental epithelium and mesenchyme regulates early tooth development (19). Indeed, we showed that disruption of this circuit by inhibition of Wnt secretion from the dental epithelium halts tooth development. However, disruption of this circuit seems not to alter the odontogenic program in the dental mesenchyme, as shown by the retained expression of several critical odontogenic genes and the ability of Gpr177K14cre dental mesenchyme to form normal tooth structures when recombined with wild type dental epithelium. However, we cannot rule out the possibility that the K14-Cre allele used in this study did not function efficiently enough to delete Gpr177 completely. On the other hand, it is quite possible that mesenchymally expressed Wnt5a could substitute for the epithelial Wnts to maintain the circuit, because although classified as a non-canonical Wnt ligand, Wnt5a is also capable of activating Wnt/β-catenin signaling depending on receptor context (50). This could also explain the presence of residual TopGal activity in the dental epithelium of E13.5 Gpr177K14cre embryo.
Besides those canonical Wnt ligands, several non-canonical Wnt ligands, such as Wnt-4, -6, and -7b, are also expressed in the dental epithelium during tooth development. Despite that extensive studies have revealed an essential role for Wnt/β-catenin signaling in tooth development, little is known about the potential function of the non-canonical Wnts in the regulation of odontogenesis. Among those non-canonical Wnt ligands expressed in the dental epithelium, Wnt4 was shown to be able to induce Msx1 expression in the dental mesenchyme (51), suggesting an epithelium-to-mesenchyme signaling action of the non-canonical Wnts during tooth morphogenesis. In addition, these Wnts could also elicit non-canonical signaling in an intra-epithelial manner as well. Thus we cannot rule out the possibility that failed secretion of these non-canonical Wnts in the dental epithelium may also contribute to the tooth phenotype observed in Gpr177K14cre mice. However, the expression of Wnt5a, a non-canonical Wnt, in the dental mesenchyme could compensate for the loss of function of these non-canonical Wnts in the absence of Gpr177.
Nevertheless, we conclude that the arrest of tooth development in the absence of epithelial Gpr177 is the consequence of disrupted odontogenic program in the dental epithelium. This point is strengthened by the fact that the wild type dental mesenchyme fails to support formation when recombined with the Gpr177-deficient dental epithelium. Despite loss of odontogenic capability of the dental epithelium in the absence of Gpr177, the oral epithelium lacking Gpr177 could still respond to ectopic odontogenic induction but failed to develop further in the Osr2 mutant background, suggesting that epithelial Wnt is not required for initiation of tooth development but is essential for subsequent development.
In summary, in this study we have demonstrated that Gpr177-mediated Wnt secretion from the dental epithelium is essential for tooth development. Disruption of epithelial Wnt secretion results in the absence of Wnt/β-catenin activity and formation of dysfunctional enamel knot, leading to arrest of tooth development at the early cap stage. The dental epithelial cells are thus the primary targets of epithelium-derived Wnts, indicating the importance of intra-epithelial action of Wnts in early odontogenesis.
This work was supported, in whole or in part, by the National Science Foundation of Zhejiang Province (Z12C120004 and LY12C12002), the National Key Basic Research Program of China (2012CB910402), and the National Nature Science Foundation of China (31100845 and 31201090). This work was also supported in part by start funding from Hangzhou Normal University (to Z. Y. Z), National Institutes of Health Grants DE015654 and CA 10638 (to W. H.) and DE 14044 and DE 17792 (to Y. P. C).
X. Zhu, P. Zhao, Y. Liu, X. Zhang, J. Fu, H.-M. Ivy Yu, M. Qiu, Y. Chen, W. Hsu, and Z. Zhang, unpublished observations.
- BMP
- bone morphogenetic protein.
REFERENCES
- 1. Jernvall J., Thesleff I. (2000) Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech. Dev. 92, 19–29 [DOI] [PubMed] [Google Scholar]
- 2. Jernvall J., Aberg T., Kettunen P., Keränen S., Thesleff I. (1998) The life history of an embryonic signaling center. BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development 125, 161–169 [DOI] [PubMed] [Google Scholar]
- 3. Tucker A., Sharpe P. (2004) The cutting edge of mammalian development. How the embryo makes teeth. Nat. Rev. Genet. 5, 499–508 [DOI] [PubMed] [Google Scholar]
- 4. Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 5. Logan C. Y., Nusse R. (2004) The Wnt signaling pathway in development and disease. Annu. Rev. Cell. Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 6. Zeng L., Fagotto F., Zhang T., Hsu W., Vasicek T. J., Perry W. L., 3rd, Lee J. J., Tilghman S. M., Gumbiner B. M., Costantini F. (1997) The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90, 181–192 [DOI] [PubMed] [Google Scholar]
- 7. Yu H. M., Jerchow B., Sheu T. J., Liu B., Costantini F., Puzas J. E., Birchmeier W., Hsu W. (2005) The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development 132, 1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Amerongen R., Nusse R. (2009) Towards an integrated view of Wnt signaling in development. Development 136, 3205–3214 [DOI] [PubMed] [Google Scholar]
- 9. Mao J., Wang J., Liu B., Pan W., Farr GH, 3rd., Flynn C., Yuan H., Takada S., Kimelman D., Li L., Wu D. (2001) Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7, 801–809 [DOI] [PubMed] [Google Scholar]
- 10. Jho E.-H., Zhang T., Domon C., Joo C. K., Freund J. N., Costantini F. (2002) Wnt/-Catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 22, 1172–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lustig B., Jerchow B., Sachs M., Weiler S., Pietsch T., Karsten U., van de Wetering M., Clevers H., Schlag P. M., Birchmeier W., Behrens J. (2002) Negative feedback loop of Wnt signaling through upregulation of conductin/Axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22, 1184–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeng X., Tamai K., Doble B., Li S., Huang H., Habas R., Okamura H., Woodgett J., He X. (2005) A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438, 873–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qian L., Mahaffey J. P., Alcorn H. L., Anderson K. V. (2011) Tissue-specific roles of Axin2 in the inhibition and activation of Wnt signaling in the mouse embryos. Proc. Natl. Acad. Sci. U.S.A. 108, 8692–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kratochwil K., Galceran J., Tontsch S., Roth W., Grosschedl R. (2002) Fgf4, a direct target of Left and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1−/− mice. Genes Dev. 16, 3173–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Järvinen E., Salazar-Ciudad I., Birchmeier W., Taketo M. M., Jernvall J., Thesleff I. (2006) Continuous tooth generation in mouse is induced by activated epithelial Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. U.S.A. 103, 18627–18632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu F., Chu E. Y., Watt B., Zhang Y., Gallant N. M., Andl T., Yang S. H., Lu M. M., Piccolo S., Schmidt-Ullrich R., Taketo M. M., Morrisey E. E., Atit R., Dlugosz A. A., Millar S. E. (2008) Wnt/β-catenin signaling directs multiple stages of tooth morphogenesis. Dev. Biol. 313, 210–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen J., Lan Y., Baek J. A., Gao Y., Jiang R. (2009) Wnt/β-catenin signaling plays an essential role in activation of odntotogenic mesenchyme during early tooth development. Dev. Biol. 334, 174–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujimori S., Novak H., Weissenböck M., Jussila M., Gonçalves A., Zeller R., Galloway J., Thesleff I., Hartmann C. (2010) Wnt/β-catenin signaling in the dental mesenchyme regulates incisor development by regulating Bmp4. Dev. Biol. 348, 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Connell D. J., Ho J. W., Mammoto T., Turbe-Doan A., O'Connell J. T., Haseley P. S., Koo S., Kamiya N., Ingber D. E., Park P. J., Maas R. L. (2012) A Wnt-Bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Sci. Signal. 5, ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kratochwil K., Dull M., Farinas I., Galceran J., Grosschedl R. (1996) Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 10, 1382–1394 [DOI] [PubMed] [Google Scholar]
- 21. Dassule H. R., McMahon A. P. (1998) Analysis of epithelial mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev. Biol. 202, 215–227 [DOI] [PubMed] [Google Scholar]
- 22. Sarkar L., Sharpe P. T. (1999) Expression of Wnt signaling pathway genes during tooth development. Mech. Dev. 85, 197–200 [DOI] [PubMed] [Google Scholar]
- 23. Andl T., Reddy S. T., Gaddapara T., Millar S. E. (2002) WNT signals are required for the initiation of hair follicle development. Dev. Cell 2, 643–653 [DOI] [PubMed] [Google Scholar]
- 24. Bänziger C., Soldini D., Schütt C., Zipperlen P., Hausmann G., Basler K. (2006) Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125, 509–522 [DOI] [PubMed] [Google Scholar]
- 25. Bartscherer K., Pelte N., Ingelfinger D., Boutros M. (2006) Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125, 523–533 [DOI] [PubMed] [Google Scholar]
- 26. Fu J., Jiang M., Mirando A. J., Yu H. M., Hsu W. (2009) Gpr177/mouse Wntless is required for embryonic axis formation. Proc. Natl. Acad. Sci. U.S.A. 106, 18598–18603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fu J., Ivy Yu H. M., Maruyama T., Mirando A. J., Hsu W. (2011) Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev. Dyn. 240, 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goodman R. M., Thombre S., Firtina Z., Gray D., Betts D., Roebuck J., Spana E. P., Selva E. M. (2006) Sprinter. A novel transmembrane protein required for Wg secretion and signaling. Development 133, 4901–4911 [DOI] [PubMed] [Google Scholar]
- 29. Ching W., Hang H. C., Nusse R. (2008) Lipid-independent secretion of a Drosophila Wnt protein. J. Biol. Chem. 283, 17092–17098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herr P., Basler K. (2012) Porcupine-mediated lipidation is required for Wnt recognition by Wls. Dev. Biol. 361, 392–402 [DOI] [PubMed] [Google Scholar]
- 31. Stefater J. A., 3rd, Lewkowich I., Rao S., Mariggi G., Carpenter A. C., Burr A. R., Fan J., Ajima R., Molkentin J. D., Williams B. O., Wills-Karp M., Pollard J. W., Yamaguchi T., Ferrara N., Gerhardt H., Lang R. A. (2011) Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature 474, 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dassule H. R., Lewis P., Bei M., Maas R., McMahon A. P. (2000) Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127, 4775–4785 [DOI] [PubMed] [Google Scholar]
- 33. Lan Y., Ovitt C. E., Cho E. S., Maltby K. M., Wang Q., Jiang R. (2004) Odd-skipped related 2 (Osr2) encodes a key intrinsic regulator of secondary palate growth and morphogenesis. Development 131, 3207–3216 [DOI] [PubMed] [Google Scholar]
- 34. DasGupta R., Fuchs E. (1999) Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557–4568 [DOI] [PubMed] [Google Scholar]
- 35. Soriano P. (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 [DOI] [PubMed] [Google Scholar]
- 36. Zhang Z., Lan Y., Chai Y., Jiang R. (2009) Antagonistic actions of Msx1 and Osr2 pattern mammalian teeth into single row. Science 323, 1232–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Song Y., Zhang Z., Yu X., Yan M., Zhang X., Gu S., Stuart T., Liu C., Reiser J., Zhang Y., Chen Y. (2006) Application of lentivirus-mediated RNAi in studying gene function in mammalian tooth development. Dev. Dyn. 235, 1334–1344 [DOI] [PubMed] [Google Scholar]
- 38. Zhang Y., Wang S., Song Y., Han J., Chai Y., Chen Y. (2003) Timing of odontogenic neural crest cell migration and tooth-forming capability in mice. Dev. Dyn. 226, 713–718 [DOI] [PubMed] [Google Scholar]
- 39. Zhang Z., Song Y., Zhao X., Zhang X., Fermin C., Chen Y. (2002) Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development 129, 4135–4146 [DOI] [PubMed] [Google Scholar]
- 40. Zhu X. J., Liu X., Jin Q., Cai Y., Yang Y., Zhou T. (2010) The L279P mutation of nuclear distribution gene C (NudC) influences its chaperone activity and lissencephaly protein 1 (LIS1) stability. J. Biol. Chem. 285, 29903–29910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vaahtokari A., Aberg T., Thesleff I. (1996) Apoptosis in the developing tooth. Association with an embryonic signaling center and suppression by EGF and FGF-4. Development 122, 121–129 [DOI] [PubMed] [Google Scholar]
- 42. Thesleff I., Keränen S., Jernvall J. (2001) Enamel knots as signaling centers linking tooth morphogenesis and odontoblast differentiation. Adv. Dent. Res. 15, 14–18 [DOI] [PubMed] [Google Scholar]
- 43. Ishida K., Murofushi M., Nakao K., Morita R., Ogawa M., Tsuji T. (2011) The regulation of tooth morphogenesis is associated with epithelial cell proliferation and the expression of sonic hedgehog through epithelial-mesenchymal interactions. Biochem. Biophys. Res. Commun. 405, 455–461 [DOI] [PubMed] [Google Scholar]
- 44. Lohi M., Tucker A. S., Sharpe P. T. (2010) Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre-and postnatal tooth development. Dev. Dyn. 239, 160–167 [DOI] [PubMed] [Google Scholar]
- 45. Zeng Y. A., Nusse R. (2010) Wnt proteins are self-renewing factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell 6, 568–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu F., Millar S. E. (2010) Wnt/b-catenin signaling in oral tissue development and disease. J. Dent. Res. 89, 318–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin M., Li L., Liu C., Liu H., He F., Yan F., Zhang Y., Chen Y. (2011) Wnt5a regulates growth, patterning, and odontoblast differentiation of developing mouse tooth. Dev. Dyn. 240, 432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Celli G., LaRochelle W. J., Mackem S., Sharp R., Merlino G. (1998) Soluble dominant-negative receptor uncovers roles for fibroblast growth factors in multi-organ induction and patterning. EMBO J. 17, 1642–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jernvall J., Kettunen P., Karavanova I., Martin L. B., Thesleff I. (1994) Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation. Non-dividing cells express growth stimulating Fgf-4 gene. Int. J. Dev. Biol. 38, 463–469 [PubMed] [Google Scholar]
- 50. Mikels A. J., Nusse R. (2006) Purified Wnt5a protein activates or inhibits β-catenin/TCF signaling depending on receptor context. PLoS Biol. 4, e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kettunen P., Løes S., Furmanek T., Fjeld K., Kvinnsland I. H., Behar O., Yagi T., Fujisawa H., Vainio S., Taniguchi M., Luukko K. (2005) Coordination of trigeminal axon navigation and patterning with tooth organ formation. Epithelial-mesenchymal interactions and epithelial Wnt4 and Tgfβ1 regulate semaphoring 3a expression in the dental mesenchyme. Development 132, 323–334 [DOI] [PubMed] [Google Scholar]