Background: The DNA-binding protein Hsf1 transcriptionally activates genes critical to protein homeostasis in all eukaryotes.
Results: Mutations within the Hsf1 N- and C-terminal activation domains or the Mediator Tail module abolish Mediator recruitment to heat shock-induced genes in yeast.
Conclusion: Hsf1 engages in multiple cooperative interactions with Mediator in governing the transcriptional response to heat stress.
Significance: Mediator is a pivotal transcriptional coregulator of Hsf1.
Keywords: Chromatin, Coregulator Transcription, Gene Transcription, Heat Shock Protein, RNA Polymerase II, Yeast Transcription, Activation Domain, Heat Shock Factor 1 (Hsf1), Mediator, S. cerevisiae
Abstract
The evolutionarily conserved Mediator complex is central to the regulation of gene transcription in eukaryotes because it serves as a physical and functional interface between upstream regulators and the Pol II transcriptional machinery. Nonetheless, its role appears to be context-dependent, and the detailed mechanism by which it governs the expression of most genes remains unknown. Here we investigate Mediator involvement in HSP (heat shock protein) gene regulation in the yeast Saccharomyces cerevisiae. We find that in response to thermal upshift, subunits representative of each of the four Mediator modules (Head, Middle, Tail, and Kinase) are rapidly, robustly, and selectively recruited to the promoter regions of HSP genes. Their residence is transient, returning to near-background levels within 90 min. Hsf1 (heat shock factor 1) plays a central role in recruiting Mediator, as indicated by the fact that truncation of either its N- or C-terminal activation domain significantly reduces Mediator occupancy, whereas removal of both activation domains abolishes it. Likewise, ablation of either of two Mediator Tail subunits, Med15 or Med16, reduces Mediator recruitment to HSP promoters, whereas deletion of both abolishes it. Accompanying the loss of Mediator, recruitment of RNA polymerase II is substantially diminished. Interestingly, Mediator antagonizes Hsf1 occupancy of non-induced promoters yet facilitates enhanced Hsf1 association with activated ones. Collectively, our observations indicate that Hsf1, via its dual activation domains, recruits holo-Mediator to HSP promoters in response to acute heat stress through cooperative physical and/or functional interactions with the Tail module.
Introduction
Mediator is a multisubunit complex that is a key co-regulator of the transcription of RNA polymerase II (Pol II)2 genes. It is conserved from yeast to humans and acts as both a physical and functional interface between gene-specific regulatory proteins, transcriptional co-regulators, and general transcription factors (reviewed in Refs. 1–3). Biochemical, genetic, and electron microscopy studies suggest that Mediator exists as a stable core structure composed of distinct domains or modules. These have been termed Head, Middle, and Tail. In addition, a fourth domain, termed Kinase or cyclin-Cdk8, reversibly associates with the core complex (4–6).
Specific functions have been ascribed to each domain. For example, the Head module plays an important role in the assembly and/or stabilization of the transcription initiation complex. Head subunits assemble into a jawlike structure that interacts with the CTD of the largest Pol II subunit, Rpb1, as well as with Rpb3 (7, 8). The flexibility and extended shape of the Head permits interactions with other components of the general transcriptional machinery, including TBP, TFIIB, and TFIIH (8–13). In addition, a direct interaction between the Head subunit Med17 and Rpb3 has been demonstrated in yeast cells, and this interaction is required for Pol II recruitment genome-wide (13). Consistent with this, inactivation of Med17 leads to genome-wide down-regulation of mRNA synthesis (14). The Middle module confers structural integrity to Mediator and may also contact Pol II (5, 15). Dissociation of the Middle module from the core complex (as seen in med19Δ mutants) results in defects in both basal and activated transcription in vitro (16). In addition, genetic analysis indicates that the Middle module has roles in both transcriptional activation and repression in vivo (17–20) and participates in postrecruitment steps in transcription, including promoter escape and Pol II elongation (21). The Tail module is composed of five nonessential subunits and is anchored to the Middle module via the essential subunit, Med14/Rgr1. The Tail, in particular a subcomplex termed the “Triad” (consisting of Med2, Med3, and Med15/Gal11), is a physical target of several yeast activators, including Gal4, Gcn4, Cha4, Pdr1, and Oaf1 (22–27). Med15 is likewise targeted by several metazoan activators, such as the sterol regulatory element-binding protein (reviewed in Ref. 2). The Kinase module is comprised of four nonessential subunits and generally acts as a negative regulator (e.g. see Ref. 28), although it has also been implicated in positive regulation (29, 30). The Kinase subunit Med12 serves as a target for the DNA-bound activator Pdr3 under conditions of mitochondrial stress in yeast (24) and is likewise a target of mammalian activators. Subunits located within the Head and Middle modules have also been implicated as activator targets (reviewed in Ref. 2).
The highly conserved transcriptional activator Hsf1 (heat shock factor 1) regulates the heat shock response in all eukaryotes. It induces the expression of genes encoding heat shock proteins (HSPs), which serve as molecular chaperones that maintain protein homeostasis. Saccharomyces cerevisiae Hsf1 (scHsf1) is essential for the viability of yeast cells at all temperatures (31) and contains a core domain consisting of a winged helix-turn-helix DNA binding domain and an adjacent, coiled-coil trimerization domain (32, 33). In addition, scHsf1 contains two activation domains, one located near its N terminus (termed the N-terminal activator (NTA)) and the other located near its C terminus (termed the C-terminal activator (CTA) (34). Heat-activated scHsf1 has been detected at the promoters of ∼165 genes (35) and has been implicated in the transcriptional activation of >400 genes (36). scHsf1 is of additional interest because it bypasses the requirement for a number of critical general transcription factors, including Kin28 (TFIIH kinase), Taf9 (a subunit of both TFIID and SAGA), TFIIA, and even the CTD of Pol II (reviewed in Ref. 20). In contrast, at least one coactivator is critically required for HSP gene activation, Mediator (20, 21, 37). Mediator is robustly recruited to the HSP gene promoter regions in both yeast (38) and Drosophila, and in the case of Drosophila, this recruitment precedes that of Pol II (39). Biochemical analysis suggests that Drosophila HSF physically interacts with the Head subunit Med17 (39), although whether this is the case in other organisms is unknown.
Despite the importance of Mediator in regulating the yeast heat shock response, little is known of its physical and functional interaction with Hsf1. Here we use a kinetic chromatin immunoprecipitation (ChIP) approach to show that holo-Mediator is rapidly, although transiently, recruited to HSP gene promoters in response to heat shock. We demonstrate that Hsf1 is principally responsible for such recruitment, which is mediated through its N- and C-terminal activation domains. Unlike the case in Drosophila, scHsf1 primarily targets subunits in the Mediator Tail module. A reciprocal role for Mediator in modulating Hsf1 binding is suggested by an occupancy analysis indicating the presence of both antagonistic and cooperative interactions between the two proteins. Our study thus reveals an intimate, two-way interaction between the DNA-binding activator, Hsf1, and the transcriptional coregulator, Mediator, in regulating heat shock gene expression in budding yeast.
EXPERIMENTAL PROCEDURES
Yeast Strains
Yeast strains used in this study were derived from the BY4741 and W303 genetic backgrounds (see Table 1). Tandem affinity purification (TAP)-tagged Med18, Med4, Med3, and CycC strains were constructed by one-step transplacement of the wild-type open reading frame (ORF) with a PCR-amplified DNA fragment bearing C-terminally TAP-tagged gene ORFs. As templates, PCR amplifications employed genomic DNA isolated from strains in the yeast TAP-tagged collection (Open Biosystems, Huntsville, AL). Strain DY2694, bearing rgr1-Δ2, and its isogenic counterpart DY150 were generously provided by David J. Stillman (University of Utah).
TABLE 1.
Yeast strains
| Strain | Genotype | Source/Reference |
|---|---|---|
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Research Genetics |
| med16Δ | BY4741; med16Δ::KANMX | Research Genetics |
| med15Δ | BY4741; med15Δ::KANMX | Research Genetics |
| cdk8Δ | BY4741; cdk8Δ::KANMX | Research Genetics |
| SYK1006 | BY4741; Med18-TAP::HIS3MX10 | This study |
| SYK1007 | BY4741; Med4-TAP::HIS3MX10 | This study |
| SYK1008 | BY4741; Med3-TAP::HIS3MX10 | This study |
| SYK1009 | BY4741; CycC-TAP::HIS3MX10 | This study |
| SYK1010 | med16Δ; Med18-TAP::HIS3MX10 | This study |
| SYK1011 | med16Δ; Med4-TAP::HIS3MX10 | This study |
| SYK1012 | med16Δ; Med3-TAP::HIS3MX10 | This study |
| SYK1013 | med16Δ; CycC-TAP::HIS3MX10 | This study |
| SYK1014 | med15Δ; Med3-TAP::HIS3MX10 | This study |
| SYK1015 | BY4741; med15Δ::KANMX med16Δ::KANMX Med3-TAP::HIS3MX10 | This study |
| SYK1000 | BY4741; med19-1001 | This study |
| S7JJ2a | BY4741; med21-1002 | Jenny Jeon |
| N2JJ1a | BY4741; med10-1001 | Jenny Jeon |
| W303–1A | MATa ade2-1 ura3-1 his3-11,15 trp1-1 leu2-3,112 can1-100 | Rodney Rothstein |
| SYK1016 | W303-1A; hsf1Δ::KANMX Med4-TAP::HIS3MX10 pEVS103 (HSF1+ TRP1 CEN ARS) | This study |
| SYK1017 | SYK1016; MATα pHF312 (HSF1ΔCTA) | This study |
| SYK1018 | SYK1017; pHF35 (HSF1ΔNTA) | This study |
| SYK1019 | SYK1017; pHF71B (HSF1ΔNTA ΔCTA) | This study |
| DY150 | MATa ade2-1 ura3-1 his3-11,15 trp1–1 leu2–3,112 can1–100 | Ref. 45 |
| DY2694 | DY150; rgr1-Δ2::LEU2 | Ref. 45 |
| SLY101 | MATα ade− can1-100 cyh2r his3-11,15 leu2-3,112 trp1-1 ura3 | Ref. 76 |
| HS1004 | SLY101; TRP1+ hsp82-ΔHSE1 hsp82-ΔHSE1/HIS3::URA3 hsp82-ΔHSE1/lacZ::leu2 | Ref. 20 |
Spontaneous ewe suppressor mutations, derived from strain HS1004 (SLY101 background) (20), were transferred to BY4741 by allelic replacement, creating strains termed SYK1000, S7JJ2a, and N2JJ1a, respectively. To generate these strains, we used a modification of the cloning-free PCR-based allele replacement strategy described by Rothstein and colleagues (40). Mutant alleles med19-1001, med21-1002 and med10-1001 were PCR-amplified from genomic DNA and then fused to LEU2 through the use of adaptamers. Resultant PCR fragments were introduced into the BY4741 diploid, and desired Leu+ transformants were screened using genomic PCR. Diploids were then sporulated, and tetrads were dissected; spores containing the ewe mutation were identified based on growth phenotype and confirmed by sequencing analysis (DNA Facility, Iowa State University, Ames, IA). Sequences of these recessive mutations were determined and are as follows: med10-1001, N64D point mutation followed by 94-amino acid C-terminal truncation; med19-1001, 112-amino acid C-terminal truncation; and med21-1002, L76P point mutation (21, 41). cdk8Δ::KAN-MX, med16Δ::KAN-MX, and med15Δ::KAN-MX derivatives of BY4741 were obtained from the ResGen knock-out collection (gift of Kelly Tatchell). med15Δ med16Δ double mutants were constructed by crossing a med16Δ strain with a med15Δ strain, followed by sporulation and tetrad dissection. Double mutant spores were identified by phenotypic analysis and confirmed by genomic PCR.
Hsf1 activation domain mutants were created by transforming a heterozygous W303 diploid, bearing one copy of HSF1 and one copy of hsf1Δ::KAN-MX, with CEN-TRP1-ARS plasmids expressing wild-type Hsf1+ or an Hsf1 mutant derivative. Plasmids employed for this purpose were as follows: pEVS103, Hsf1+; pHF312, Hsf1(1–424) (termed Hsf1ΔCTA); pHF35, Hsf1(40Δ147–833) (termed Hsf1ΔNTA); and pHF71b, Hsf1(40Δ147–583) (termed Hsf1ΔNTA ΔCTA) (34). Plasmids encoding Hsf1 truncation mutants were the gift of Peter Sorger (MIT, Cambridge, MA). The sequence of each Hsf1 mutant was confirmed by PCR amplification followed by sequencing (Retrogen, San Diego, CA). Diploids were then sporulated, and the tetrads were dissected; spores containing the hsf1Δ::KAN-MX allele and the desired Hsf1-expressing plasmid were identified by phenotypic analysis.
Cultivation, Heat Shock, and Recovery Conditions
S. cerevisiae strains were cultivated at 30 °C to early log phase (A600 = 0.3–0.8) in rich YPD broth supplemented with 0.03 mg/ml adenine (YPDA). Induction of heat shock was achieved by an instantaneous 30 °C to 39 °C temperature shift by the addition of an equal volume of prewarmed YPDA medium (50 °C) to the culture (20 ml (reverse transcription (RT)-qPCR assays) or 50 ml (ChIP assays)) and was shaken vigorously in a 39 °C water bath for the times indicated in the figures. For recovery, 15-min heat-shocked cultures were mixed with an equal volume of prechilled (4 °C) YPDA medium to elicit a 39 °C to 23 °C downshift. They were maintained in a vigorously shaking 23 °C water bath for 25 min. To terminate heat shock/recovery, either sodium azide was added to a final concentration of 20 mm (RT-qPCR), or formaldehyde was added to a final concentration of 1% (ChIP).
Chromatin Immunoprecipitation
ChIP was performed essentially as described previously (21). Briefly, 300–350-ml mid-log cell cultures were used in heat shock time course experiments, and 50-ml aliquots were removed at each time point. Cells were cross-linked with 1% formaldehyde and then lysed using glass beads. Lysates were brought up to 2 ml with lysis buffer (50 mm HEPES, pH 7.5, 140 mm NaCl, 1% Triton X-100, 0.1% sodium deoxycholate, and 1 mm EDTA), and chromatin was then sonicated to a mean length of ∼0.5 kb using a Branson 250 sonifier equipped with a microtip. For immunoprecipitation, the equivalent of 500–800 μg of chromatin protein (as quantified by the Bradford assay; typically 100–400 μl) was incubated with the following antibodies: 1 μl of Hsf1 rabbit antiserum (42), 2 μl of anti-TAP antiserum (Open Biosystems), 2 μl of anti- Med17/Srb4 antiserum (gift of Rick Young, Whitehead Institute, MIT), and 1 μl of Rpb1 C-terminal domain (CTD)-specific rabbit antiserum (43). The latter was raised against an Escherichia coli-synthesized GST-mouse CTD fusion polypeptide containing 52 heptad repeats as described previously (43). Protein-DNA cross-links were reversed, and DNA was purified and dissolved in 50 μl of TE (10 mm Tris-HCl, pH 8.0, 0.5 mm EDTA). An aliquot (2–4 μl) of immunoprecipitated DNA was added to each 20-μl real-time PCR performed using RT2 qPCR SYBR Green/ROX Master Mix (SABiosciences, catalog no. 330529) on an Applied Biosystems 7900HT real-time PCR system.
The quantity of DNA present in each IP was determined using a standard curve specific for each amplicon. For TAP ChIP, the background signal arising from beads alone was subtracted. For Hsf1 and Pol II ChIP, the preimmune signal was subtracted from each specific IP signal. Amplicon coordinates, expressed relative to the ATG codon (+1) were as follows: SSA4 promoter, −374 to −291; SSA4 ORF, +815 to +946; SSA4 3′-UTR, +1878 to +2037; HSP104 promoter, −267 to −196; HSP104 ORF, +1223 to +1394; HSP104 3′-UTR, +2612 to +2718; ZPR1 promoter, −299 to −217; ZPR1 ORF, +720 to +809; ZPR1 3′-UTR, +1451 to +1533; HSP82 promoter/upstream activation sequence, −157 to −88 (see Figs. 2 and 3) or −303 to −238 (see Figs. 5–8); HSP82 ORF, +1248 to +1444; HSP82 3′-UTR, +2134 to +2228; HSP12 promoter, −250 to −190; HSP26 promoter, −275 to −196.
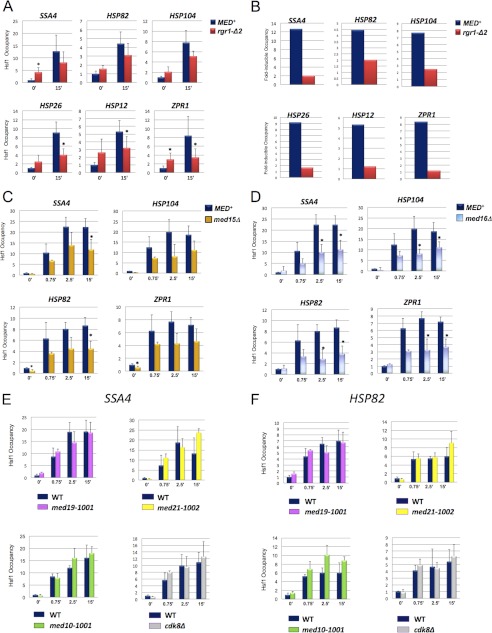
FIGURE 2.
Mutations in Tail subunits Med14, Med15, and Med16 diminish Hsf1 occupancy of heat shock-induced promoters, whereas mutations in the Mediator Head, Middle, and Kinase modules have no effect. A, Hsf1 ChIP analysis of HSP promoters under non-inducing and 15 min heat shock-inducing conditions in isogenic MED+ and med14/rgr1-Δ2 strains DY150 and DY2694. The assay was conducted as in Fig. 1 except that an Hsf1-specific polyclonal antibody was used; preimmune signal was subtracted from each ChIP signal. The abundance of Hsf1 in response to heat shock was quantified relative to its abundance at each promoter under non-heat shock conditions (T = 0 min). Depicted are means ± S.D. (error bars); n ≥ 3. B, dynamic range of Hsf1 occupancy at HSP promoters in MED+ and rgr1-Δ2 strains during the first 15 min of heat shock (data from A). C, Hsf1 occupancy of HSP promoters in isogenic MED+ and med15Δ cells (BY4741 background) either maintained at 30 °C (0 min) or subjected to a 39 °C heat shock for the indicated times. Shown are means ± S.D., n = 3. D, as in C, except isogenic MED+ and med16Δ cells were examined. E and F, as in C, except Hsf1 occupancy of SSA4 and HSP82 promoters was assayed in MED+ and isogenic med19-1001, med10-1001, med21-1002, and cdk8Δ strains. Asterisks signify a significant difference in Hsf1 occupancy between WT and mutant (p < 0.05; two-tailed t test; equal variance).
FIGURE 3.
med16Δ diminishes recruitment of the Tail subunit Med3 to induced HSP promoters while obviating recruitment of subunits representative of the other three modules. A, occupancy of TAP-tagged Head, Middle, Tail and Kinase subunits within the SSA4 promoter in isogenic MED+ and med16Δ cells in a heat shock time course. TAP ChIP analysis was conducted as described in the legend to Fig. 1. Occupancy of each subunit in the MED+ strain at T = 0 min was assigned a value of 1; all other values (MED+ and med16Δ) are normalized to it. Only ChIP values above the beads alone background are shown. Depicted are means ± S.D. (error bars); n = 3. B–D, as in A, except Mediator subunit occupancy at the HSP104, HSP82, and ZPR1 promoters is depicted.
FIGURE 5.
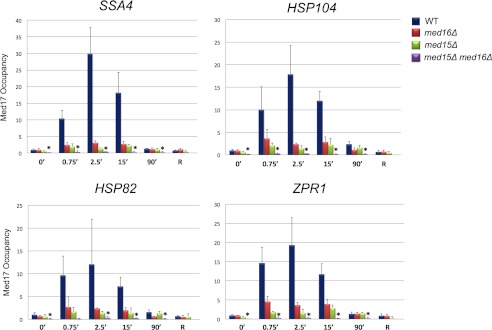
med15Δ and med16Δ single mutations drastically reduce Head subunit Med17 recruitment to induced HSP promoters, whereas the med15Δ med16Δ double mutation eliminates it. Med17 occupancy of HSP promoters in MED+, med16Δ, med15Δ, and med15Δ med16Δ cells in a heat shock time course conducted and analyzed as in Fig. 3. Med17 was detected using a polyclonal anti-Med17/Srb4 antibody; mock IP signal (beads alone) was subtracted from each ChIP signal. Depicted are means ± S.D. (error bars); n = 3. Asterisks signify Med17 occupancy in the double mutant that is significantly less than that observed in either single mutant (p < 0.05; two-tailed t test; equal variance).
FIGURE 6.
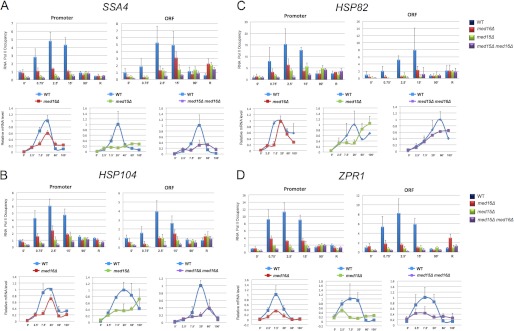
Tail subunit mutations reduce Pol II recruitment to, and mRNA expression of, HSP genes during the early stages of heat shock. A (top panels), Pol II occupancy at the SSA4 promoter and ORF either prior to or for the indicated times following an instantaneous 39 °C heat shock (recovery (R) conducted as in Fig. 1). Pol II abundance was determined by ChIP using a CTD polyclonal antibody; preimmune background was subtracted from each immune signal. Net values for each time point/strain combination were normalized to the MED+ strain at T = 0 min; promoter and ORF were quantified separately. Depicted are means ± S.D. (error bars); n = 3. Bottom panels, RT-qPCR analysis of SSA4 mRNA levels in MED+, med16Δ, med15Δ, and med15Δ med16Δ strains. SSA4 mRNA/SCR1 RNA quotients, normalized to the MED+ 20 min quotient (which was set to 1.0), are shown. Note that SCR1 is a structural RNA (Pol III transcript). Depicted are means ± S.D. (n = 2); each MED+ versus mutant pairwise comparison was conducted separately. B–D, Pol II occupancy at HSP104, HSP82, and ZPR1 promoter and ORF regions and associated mRNA expression levels were determined as in A.
FIGURE 7.
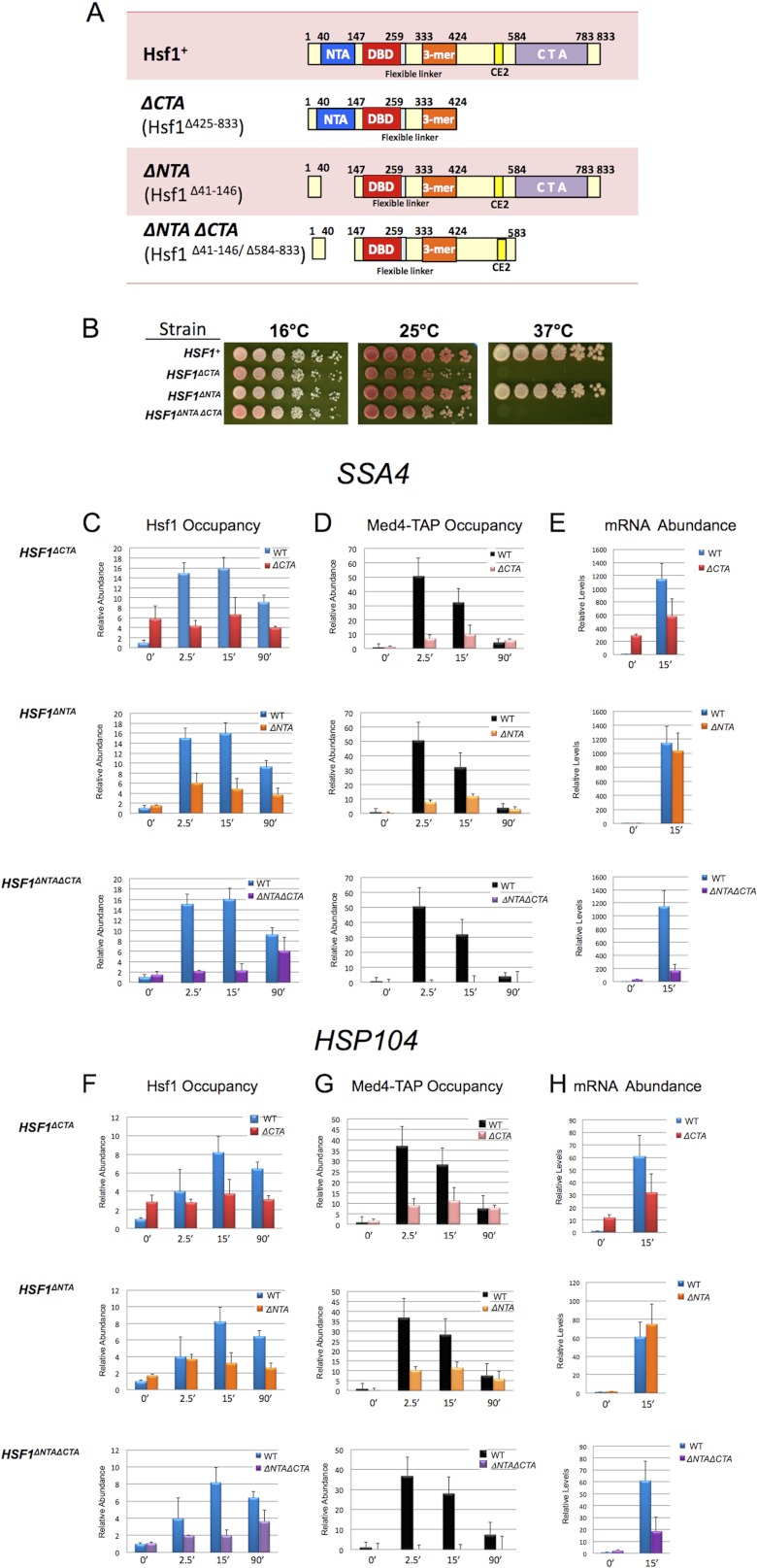
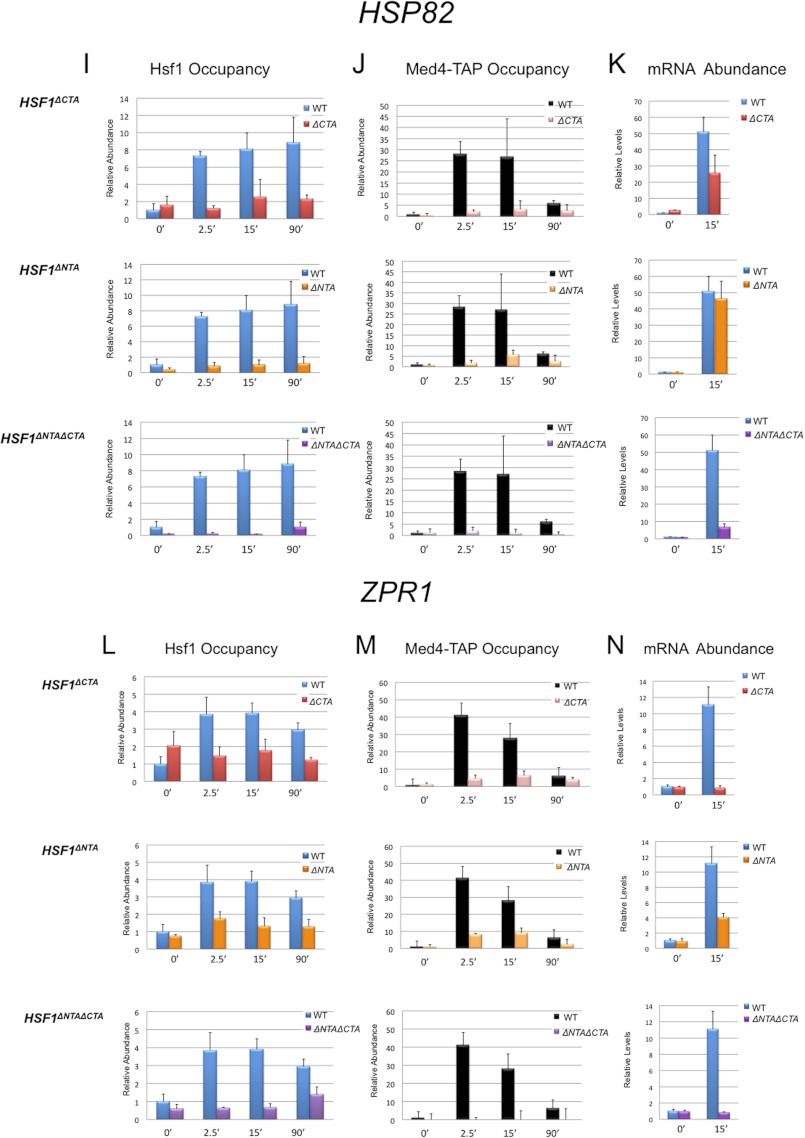
Both N- and C-terminal activators of Hsf1 are required for efficient Mediator recruitment to HSP promoters, whereas either one or the other suffices for transcription. A, domain maps of Hsf1+ and its C- and N-terminally truncated derivatives (DNA-binding domain (DBD), trimerization domain (3-mer), and repression domain (CE2)). B, growth assay of HSF1+, HSF1ΔCTA, HSF1ΔNTA, and HSF1ΔNTA ΔCTA strains. 5-Fold serial dilutions were spotted onto rich medium, and cells were grown at the indicated temperatures for 2–3 days. C and D, Hsf1 and Med4-TAP ChIP analysis of the SSA4 promoter either prior to or following instantaneous heat shock in the indicated isogenic strains, conducted as in Figs. 1 and 2. Means ± S.D. (error bars) are depicted; n = 3. E, RT-qPCR analysis of SSA4 expression under non-inducing and 15-min heat shock-inducing conditions in the indicated isogenic strains. SSA4 mRNA/SCR1 RNA quotients, normalized to the WT 0 min quotient (which was set to 1.0), are shown; means ± S.D. are depicted (n = 2). F–N, factor occupancy and mRNA expression levels of HSP104, HSP82, and ZPR1 analyzed as in C–E.
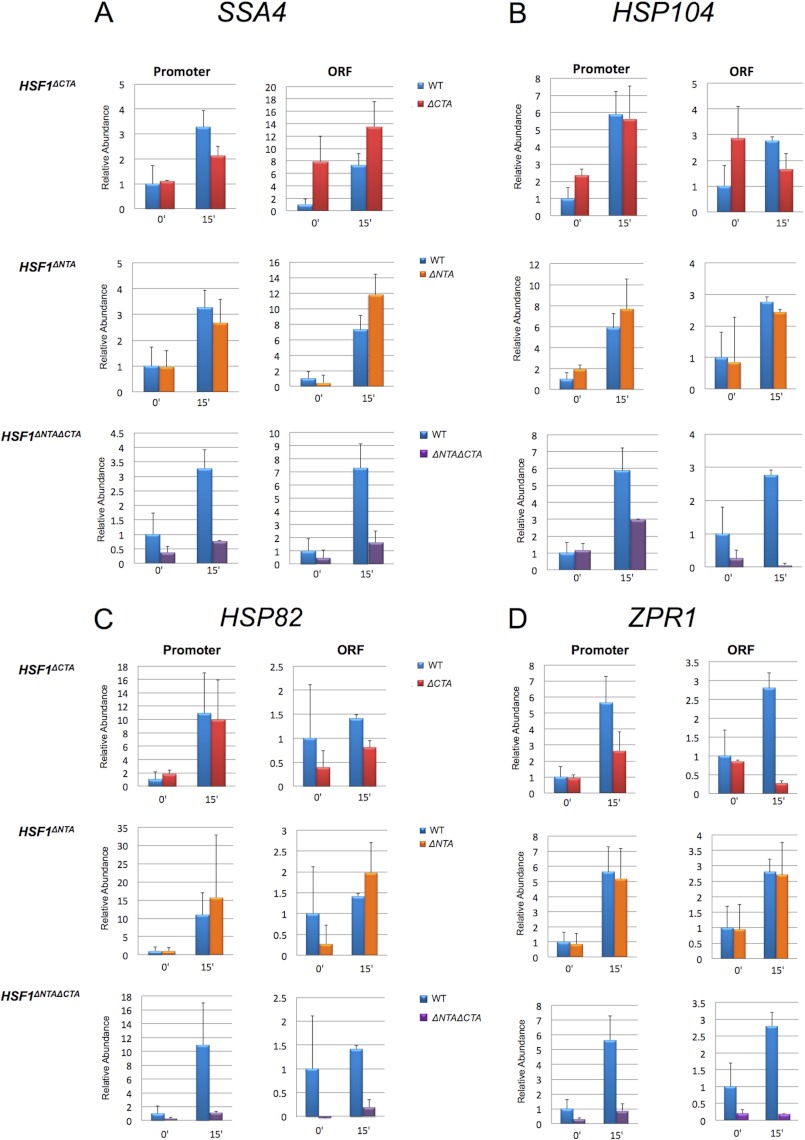
FIGURE 8.
Pol II occupancy of HSP genes in HSF1+, HSF1ΔCTA, HSF1ΔNTA, and HSF1ΔNTAΔCTA strains. A, Pol II occupancy of the SSA4 promoter and ORF either prior to or following a 15-min heat shock. Pol II ChIP analysis was conducted and quantified as described in the legend to Fig. 6. Depicted are means ± S.D. (error bars); n = 3. Note that the extracts used for this analysis were the same as those used for the Hsf1 and Med4-TAP ChIP assays presented in Fig. 7. B–D, Pol II occupancy of the HSP104, HSP82, and ZPR1 promoter and ORF regions as in A.
RT-qPCR Analysis
Cells were cultivated in 150-ml cultures, and 20-ml aliquots were removed for heat shock as described above. RNA was isolated using glass beads, followed by phenol/chloroform extraction. Contaminating genomic DNA was removed from each RNA sample by digestion with TURBO DNA-freeTM (Applied Biosystems, catalog no. AM1907). 2 μg of purified RNA and random primers were used in each cDNA synthesis using the ProtoScript RT-PCR kit (New England Biolabs). Synthesized cDNA was diluted 1:40, and 2–4 μl of diluted cDNA were added to each 20-μl real-time PCR reaction, performed as described above. A standard curve specific for each amplicon was generated using genomic DNA isolated from strain BY4741. The quantity of cDNA present in each sample was determined by subtracting the background signal (without reverse transcriptase), followed by normalization to the SCR1 transcript. Amplicon coordinates were as follows: SSA4, +815 to +946; HSP104, +1223 to +1394; ZPR1, +720 to +809; and HSP82, +2134 to +2228.
Spot Dilution Analysis
Cells were grown to stationary phase in rich YPDA. Cultures were then diluted to a uniform cell density (A600 = 0.5) and transferred to a 96-well microtiter dish. Each sample was then 5-fold serially diluted using sterile, double-distilled water and applied to a solid YPDA plate using a 48-pronged stainless steel applicator. Cells were grown at 16, 25, or 37 °C for 3–6 days.
Western Blot Analysis
Isolation of whole cell extracts was conducted essentially as described (42). Protein concentrations were measured by the Bio-Rad protein assay. Whole cell extracts were separated on 10% SDS-polyacrylamide gels and electroblotted onto nitrocellulose membranes (Bio-Rad). Blotted membranes were incubated with anti-Hsf1 polyclonal antibody, anti-TAP antibody, or anti-Pgk1 antibody (Molecular Probes) and then incubated with horseradish peroxidase-conjugated anti-rabbit (for Hsf1 and TAP) or anti-mouse (for Pgk1) secondary antibodies. Protein bands were visualized using the ECL Plus Western blotting detection reagents (GE Healthcare) and detected and quantified on a Storm 860 PhosphorImager utilizing ImageQuant TL version 2003.02 software. In certain cases, images were detected using x-ray film.
RESULTS
Holo-Mediator Rapidly and Selectively Associates with the Promoter Regions of Hsf1-regulated Genes in Response to Heat Shock
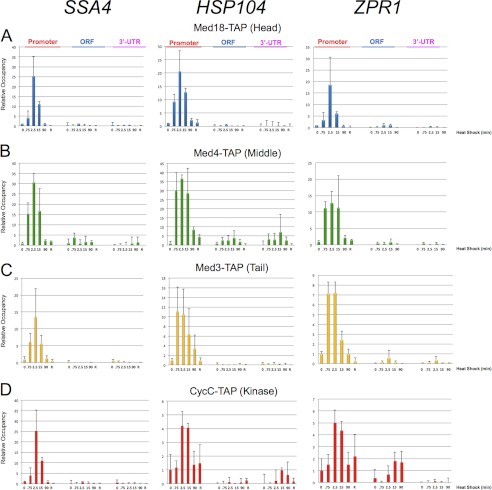
Previous genetic analysis has implicated a role for Mediator in regulating heat shock gene transcription in S. cerevisiae (20, 21, 37). To gain insight into mechanism, we asked if both core Mediator and its reversibly associated Kinase module were recruited to Hsf1-regulated genes in response to heat stress and, if so, how rapidly. We used ChIP-qPCR to measure the occupancy of representative TAP-tagged subunits derived from each of its four modules: Med18 (Head), Med4 (Middle), Med3 (Tail), and CycC (Kinase). Cells bearing TAP-tagged subunits did not elicit a growth phenotype at either normal or elevated temperature (41); therefore, by these criteria, the presence of the C-terminal TAP tag did not affect Mediator function. In response to heat shock, subunits derived from each module were rapidly and robustly recruited to the promoter regions of representative Hsf1 target genes, and their abundance typically peaked within 2.5 min (Fig. 1). By contrast, subunit occupancy within the transcribed regions was insubstantial. Interestingly, following a 90-min heat shock (or a 25-min recovery from acute heat shock), occupancy of each Mediator subunit decayed and returned in most cases to its uninduced level. Therefore, holo-Mediator was robustly and selectively recruited to HSP gene promoter regions in response to heat shock, although its association with these regions was transient and had largely dissipated by 90 min.
FIGURE 1.
Robust yet transient recruitment of holo-Mediator to HSP gene promoter regions. A–D, in vivo cross-linking analysis of representative Head, Middle, Tail, and Kinase subunits at the indicated regions of the SSA4, HSP104, and ZPR1 genes either prior to or for the indicated times following heat shock (30 °C to 39 °C upshift). For recovery (R), cells were heat-shocked at 39 °C for 15 min and then downshifted to 23 °C for 25 min. The indicated subunits were detected using TAP antibody; mock IP signal (beads alone) was subtracted from each ChIP signal. The abundance of each subunit at the promoter, ORF, and 3′-UTR of each gene was quantified relative to its abundance at the promoter at T = 0 min, which was assigned a value of 1. Depicted are means ± S.D. (error bars); n ≥ 3 independent samples.
Mediator Regulates Hsf1 Occupancy at HSP Gene Promoters
Previous studies have shown that Mediator can antagonize the promoter occupancy of transcriptional activators, such as Gcn4 and Msn2, by triggering their displacement and/or proteolytic degradation (e.g. see Refs. 28 and 44). We wished to know if Mediator likewise regulates the promoter occupancy of Hsf1 and addressed this question by conducting an Hsf1 ChIP analysis of Mediator mutants. We began by investigating the role of Med14/Rgr1, an essential subunit that bridges the Tail and Middle modules (6, 45) and that previous work suggests plays a critical role in Hsf1-mediated transcription (20–21, 37). As shown in Fig. 2A, constitutive Hsf1 occupancy of SSA4 and ZPR1 was significantly increased in the context of an rgr1-Δ2 mutation (C-terminal truncation of 336 amino acids) (45), whereas its induced occupancy of ZPR1, HSP12, and HSP26 was significantly diminished. Similar trends were observed at other HSP promoters. Intracellular Hsf1 levels were similar in MED+ and rgr1-Δ2 cells (41), ruling out the possibility that altered levels of Hsf1 contributed to its altered occupancy. Thus, Med14 can antagonize the constitutive DNA binding of Hsf1 yet enhance its induced binding. These two activities substantially increase the dynamic range of Hsf1 occupancy at HSP promoters, as illustrated in Fig. 2B.
We next asked if other Mediator mutations affect Hsf1 occupancy. We suspected that Tail subunits may participate because Mediator isolated from rgr1-Δ2 cells is devoid of Tail subunits Med2, Med3, Med15, and Med16 (45, 46), and an absence of one or more of these could contribute to the rgr1-Δ2 phenotype. We examined the effect of deleting either MED15 or MED16 but observed that neither deletion altered Hsf1 occupancy under non-inducing conditions. However, like the Med14 C-terminal truncation, both med15Δ and med16Δ diminished the increased occupancy of Hsf1 following heat shock (Fig. 2, C and D). These data suggest that the Tail module enhances either the binding and/or retention of heat-activated Hsf1 to promoter DNA. Interestingly, this may be novel to the Tail domain and the bridging subunit Med14 because mutations in subunits of the other domains (Head (med19-1001), Middle (med10-1001 and med21-1002), or Kinase (cdk8Δ)) had no effect on Hsf1 occupancy at any of the four HSP promoters tested, either before or after heat shock (Fig. 2, E and F) (41).
Deletion of either MED15 or MED16 Reduces Mediator Recruitment to HSP Promoters, Whereas Deletion of Both Genes Eliminates It
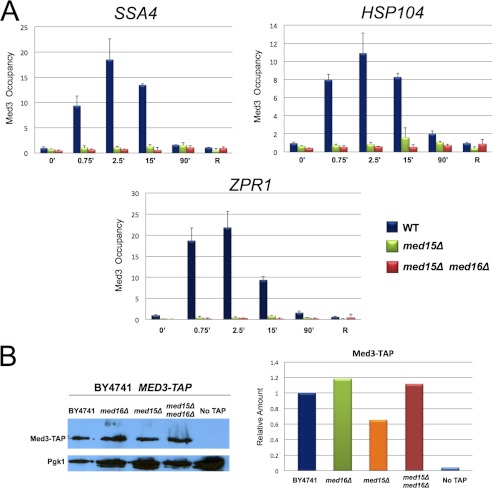
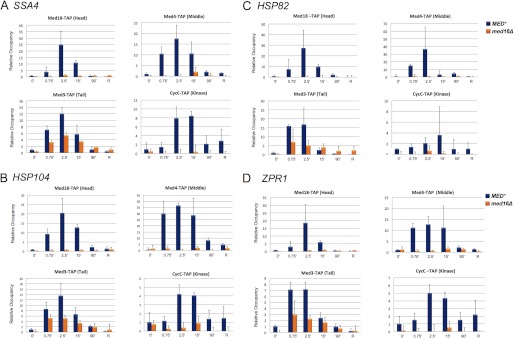
Results presented in Fig. 2, C and D, suggest that Med15 and Med16 engage in either direct or indirect interactions with Hsf1 following heat shock. To gain further insight into their role, we investigated the effect of deleting one or the other subunit (or both) on Mediator recruitment to HSP genes. Previous work has shown that the Med2, Med3, Med15 subcomplex associates less stably with, and may even be lost from, core Mediator in med16Δ cells (6, 26, 45). Therefore, if either Med15 or Med16 is an important target of Hsf1, then deletion of Med16 would be predicted to impair Mediator recruitment. Consistent with this notion, subunits within the Head, Middle, and Kinase modules were recruited very weakly, if at all, to heat-induced HSP promoters in med16Δ cells, as assessed by TAP ChIP (Fig. 3, A–D). On the other hand, the Tail subunit Med3 was recruited to all four tested promoters, although its occupancy was diminished during the early stages of heat shock. As shown in Fig. 4A, the med15Δ mutation had a more drastic effect on Tail recruitment following heat shock because it reduced Med3 occupancy within the SSA4, HSP104, and ZPR1 promoter regions to near-background levels. In most cases, no further decrease was seen in the med15Δ med16Δ double mutant. Reduced recruitment of Med3 to these heat shock-activated promoters in med15Δ, med16Δ, or med15Δ med16Δ cells was unlikely to be a consequence of its diminished intracellular levels (Fig. 4B). Thus, Med15 and Med16 appear to play important roles in Mediator recruitment to activated HSP gene promoters.
FIGURE 4.
med15Δ drastically reduces Tail subunit recruitment to induced HSP promoters, whereas the med15Δ med16Δ double mutation eliminates it. A, Med3-TAP occupancy of the SSA4, HSP104, and ZPR1 promoters in MED+, med15Δ, and med15Δ med16Δ cells in a heat shock time course conducted and analyzed as in Fig. 3. Depicted are means ± S.D. (error bars); n = 3. B, Western blot analysis of Med3-TAP in the same WT, med16Δ, med15Δ, and med15Δ med16Δ strains analyzed above. Pgk1 was used as loading control. Med3-TAP levels were quantified using a Storm 860 PhosphorImager, and the normalized values, relative to Pgk1, are displayed in the adjacent graph.
To strengthen this idea, we examined occupancy of the Head subunit Med17/Srb4 in WT and mutant cells. The presence of Med17 was of special interest, given its central role in genome-wide expression and Pol II recruitment (13, 14) and the fact that it is a principal target of Drosophila HSF (39). In WT cells, Med17 was robustly recruited to heat shock-induced genes, peaking at 2.5 min (Fig. 5) and paralleling observations of the Head subunit Med18 (Fig. 1 and data not shown). Cells depleted of either Med15 or Med16 showed a dramatic reduction in Med17 recruitment, although there was a detectable increase in Med17 occupancy at each HSP promoter in med15Δ and med16Δ cells in response to heat shock (Fig. 5). The reason for the disparity with TAP-tagged Med18, whose occupancy was undetectable in the med16Δ mutant (Fig. 3), is not clear, although it may stem from a higher avidity antibody used in the Med17 ChIP assay. In contrast to the single mutants, recruitment of Med17 in the med15Δ med16Δ double mutant was abolished at all heat shock time points (Fig. 5). These results are consistent with the notion that Med15 and Med16 are independently targeted by Hsf1 (and/or another activator) during heat shock, and their presence is necessary for Mediator recruitment to activated HSP genes. They are also consistent with the strong temperature-sensitive phenotype of med15Δ med16Δ not shared with either single mutant (41).
Med15 and Med16 Are Likewise Pivotal to Pol II Recruitment and May Additionally Regulate Pol II Elongation
The above results implicate a central role for Tail subunits Med15 and Med16 in the recruitment of Mediator to activated HSP gene promoters. If Mediator is required for Pol II recruitment to these genes, then Pol II occupancy would be predicted to mirror that of Mediator in WT, med15Δ, and med16Δ cells. Indeed, in WT cells, the magnitude and kinetics of Pol II recruitment to each promoter correlated with Med17 occupancy (compare Fig. 6 with Fig. 5). Depletion of either Med15 or Med16 substantially reduced Pol II recruitment during the initial stages of heat shock, mirroring the effect on Med17 recruitment. Interestingly, the reduction in Pol II promoter occupancy was not as severe as the reduction in Mediator occupancy (Figs. 3–5), implying that a small fraction of Pol II can be recruited independently of Mediator. Even in the med15Δ med16Δ double mutant, there was a detectable increase in Pol II at all genes examined, although this increased occupancy was not seen until 90 min (Fig. 6, A–D), a time when Mediator association in WT cells generally returned to non-induced levels (Figs. 1 and 3–5). Therefore, in the absence of both Tail subunits, Pol II recruitment to HSP promoters was negligible during the initial 15 min of heat shock, yet nearly normal by 90 min.
We additionally addressed the possibility that the med15Δ and med16Δ mutations affect a postrecruitment step, such as promoter escape or Pol II elongation, in light of our previous work demonstrating such a role for Middle module subunits (21). Indeed, the data are consistent with this idea. Pol II occupancy in WT cells showed a parallel increase within the promoter and ORF of the representative HSP genes (both typically peaking at ∼2.5 min), yet in the med16Δ mutant, Pol II occupancy of promoter and ORF was poorly correlated because ORF occupancy typically lagged promoter occupancy (Fig. 6, ORF graphs). This suggests that in addition to diminished Pol II promoter occupancy, the absence of Med16 perturbed one or more downstream steps. Moreover, the more severe reduction in Pol II ORF occupancy relative to that seen at the promoter for HSP82 and ZPR1 in med15Δ cells could also stem from a defect in a postrecruitment step.
Consistent with the Pol II ChIP analysis, the med16Δ mutation diminished the surge in HSP transcript accumulation during the initial 20 min of heat shock (Fig. 6, A–D, line graphs). In contrast, HSP mRNA levels generally failed to correlate with Pol II ORF occupancy in med15Δ cells, peaking either early (ZPR1) or late (HSP104, HSP82) in the time course. Only in the case of SSA4 did Pol II ORF occupancy show a tight correlation with mRNA level. In theory, the discordant relationship between Pol II density and mRNA levels in med15Δ cells may stem from alterations in one or more post-recruitment steps (e.g. more rapid promoter escape/elongation could lead to diminished Pol II density while simultaneously increasing transcript accumulation). Instead or additionally, it might reflect an indirect effect of med15Δ on mRNA stability, particularly in the case of HSP104 and HSP82. Whatever the basis for these phenotypes, the dominant role played by Med15 in Pol II recruitment and elongation is underscored by the nearly identical Pol II ChIP and RNA phenotypes of med15Δ and med15Δ med16Δ double mutant cells (Fig. 6, A–D).
Hsf1 Recruits Mediator to Induced HSP Genes and Does So Using both N- and C-terminal Activation Domains
We next wished to investigate the role of Hsf1 per se in the recruitment of Mediator to the HSP genes studied here. The promoter regions of these genes typically contain binding sites for other stress-inducible activators, such as Msn2/Msn4, Skn7, and Yap1 (YEASTRACT). In principle, these factors, individually or in combination, could participate in Mediator recruitment and be partially or entirely responsible for the occupancy that we observed. Because Hsf1 is encoded by an essential gene, it could not be deleted. Instead, to address the role of Hsf1, we employed strains bearing engineered deletions of the N- or C-terminal activation domains (activators) of Hsf1 (termed NTA and CTA, respectively) or both, using a plasmid-based strategy (see “Experimental Procedures”). Domain maps of Hsf1+ and the Hsf1 truncated derivatives employed are illustrated in Fig. 7A. Previous work has shown that yeast expressing Hsf1ΔNTA, Hsf1ΔCTA, or Hsf1ΔNTA ΔCTA as the sole source of Hsf1 remains viable (34). In the newly constructed strains, cells expressing Hsf1ΔCTA as their sole source of Hsf1 exhibited a mild growth phenotype at 25 °C and a severe growth phenotype at 37 °C, whereas Hsf1ΔNTA-expressing cells exhibited no phenotype (Fig. 7B). The growth phenotype of the double mutant was indistinguishable from Hsf1ΔCTA. These phenotypes are consistent with previous observations of analogous strains (34).
We initially asked if the mutant proteins could bind DNA in vivo. As shown in Fig. 7, while all three retained DNA-binding activity, their behavior was altered in the context of the native chromatin template (Fig. 7, C, F, I, and L). Ironically, constitutive DNA binding of Hsf1ΔCTA was substantially increased at several genes, most notably SSA4 (6-fold), yet Hsf1ΔCTA exhibited impaired inducible binding and/or retention at each promoter in response to heat shock. On the other hand, occupancy of Hsf1ΔNTA was not typically affected prior to heat shock, although its binding and/or retention was diminished under inducing conditions, and in the case of HSP82, this reduction was substantial, and was seen even prior to heat shock. The occupancy phenotype of the double activation domain mutant resembled that of Hsf1ΔCTA, although the reduction in Hsf1 occupancy was more accentuated. Reduced occupancy of Hsf1 mutants at activated HSP promoters is unlikely to stem from instability of the truncated proteins, as indicated by Western analyses of strains expressing these proteins (data not shown) (34). These results indicate that full in vivo occupancy of Hsf1 requires the function of both activation domains.
We next asked if deletion of one or both activation domains impaired Mediator recruitment. We examined the Middle module subunit, Med4, at HSP promoter regions in cells bearing Hsf1+ or one of its mutant derivatives. In response to heat shock, deletion of either N- or C-terminal activator substantially diminished Med4 occupancy at each promoter over the entire time course (Fig. 7, D, G, J, and M). Notably, removal of both activation domains completely abolished Med4 occupancy, consistent with the idea that Hsf1 plays a primary (and possibly exclusive) role in recruiting Mediator to these promoters. The reduction in Med4 occupancy arising from the loss of either activator argues that the roles of the CTA and NTA are non-overlapping, and that their contributions are synergistic in effecting Mediator recruitment to HSP promoters (see “Discussion”).
Finally, we examined the role of each activation domain on basal and heat-induced expression and, concomitantly, on Pol II occupancy. Because induced occupancy of Med4 was significantly reduced in cells bearing Hsf1 activation mutations, we anticipated that expression of HSP genes would be similarly decreased. However, we found that induced transcript levels were generally diminished much less than Med4 occupancy, just 2-fold in the Hsf1ΔCTA mutant following a 15-min heat shock and not at all in the Hsf1ΔNTA mutant (Fig. 7, E, H, and K). Pol II abundance within promoter regions was likewise less affected than Med4 occupancy following a 15-min heat shock (Fig. 8, A–D). (Note that the same chromatin extracts were employed in the Med4-TAP and Pol II ChIP assays.) The exception to the Med4/mRNA discordance was ZPR1, whose induced transcript levels declined 11-fold in Hsf1ΔCTA and 2-fold in Hsf1ΔNTA (Fig. 7N); a similar reduction in Pol II promoter abundance was also evident, particularly at early time points (data not shown). Deletion of both activators tended to have a synergistic effect on both mRNA levels and Pol II occupancy at all four representative HSP genes, consistent with a predominant role for Hsf1 in promoting the transcription of these genes. Taken together, these results suggest that Mediator is recruited to HSP genes via cooperative interactions with Hsf1 N- and C-terminal activators and that either activation domain alone is capable of only weak recruitment. Despite this, Pol II recruitment and concomitant transcription following acute heat shock is only partially affected by single activation domain mutations. Thus, removal of both Hsf1 activators is necessary for a substantial reduction in Pol II recruitment and HSP gene transcription.
DISCUSSION
We have shown that subunits representative of each Mediator module, including the reversibly associated Kinase module, robustly and rapidly associate with yeast HSP gene promoters in response to acute heat shock. The substantial increase in subunit occupancy (>10-fold over the non-induced level in most cases) is formally consistent with the possibility that multiple Mediator complexes are recruited to each upstream region. On the other hand, the increase in subunit cross-linking may reflect the efficient recruitment of single holo-Mediator complexes to HSP promoters that are by and large unoccupied prior to heat shock. Whichever is the case (and the extent to which one or the other scenario applies may be promoter-dependent), the enhanced occupancy of Mediator is transient because promoter association dissipates following 90-min exposure to elevated temperature or recovery from heat treatment. The basis for this dramatic attenuation is unknown, although it is probably the consequence of an evolutionarily conserved negative feedback mechanism regulating Hsf1 (47, 48). In addition to covalent modification of Hsf1 (or its interaction with inhibitory proteins), recent evidence indicates that most Mediator subunits are phosphorylated under non-stressful conditions and that several, including Med15, are dephosphorylated in response to stress (49). Given that Med15 is a critical target of Hsf1, it is possible that its phosphorylation state is relevant to the attenuation mechanism.
Notably, we observed no appreciable association of Mediator subunits with activated HSP coding regions, consistent with previous analyses of GAL genes (38, 50) but in contrast with a recent study of FLR1, in which Head and Middle subunits were detected within the transcribed region (51), as well as with an earlier analysis suggesting Mediator association with active ORFs (52). The absence of coding region occupancy at HSP genes suggests that the influence of Mediator in governing postrecruitment steps, such as promoter escape and Pol II elongation (this study and Ref. 21), is probably mediated indirectly. One possibility is via CTD phosphorylation, implemented by Cdk8 itself or any of several other CTD kinases whose activity might be influenced by Mediator.
Mediator Recruitment to Heat Shock Genes Occurs through Its Tail Module
A central finding of this study is that Mediator is recruited to HSP genes via direct or indirect interaction between the Tail module and heat shock-activated Hsf1. We discuss below evidence pertaining to Hsf1; here, we discuss evidence concerning the Tail module. First, we found that the med16Δ mutation, which weakens or abolishes the association of the Med2-Med3-Med15 subcomplex (Triad) with core Mediator (6, 26, 45) (see below), eliminates recruitment of Head, Middle, and Kinase subunits to HSP genes yet permits recruitment of the Tail subunit Med3. This observation argues against substantive interactions between Hsf1 and Head, Middle, or Kinase subunits. Because the med16Δ mutation also reduced Med3 recruitment, particularly during the initial stages of heat shock, Med16 itself appears to be a target of Hsf1. Second, we observed that the med15Δ mutation severely reduced both Med3 and Med17 occupancy at each HSP promoter examined. This is consistent with the idea that Med15 is also a target (direct or indirect) of Hsf1 and is probably its principal target. Moreover, the fact that recruitment of Med17 was strongly diminished in both med15Δ and med16Δ single mutants (60–90% reduction) yet was reduced to undetectable levels in the med15Δ med16Δ double mutant argues for non-redundant contributions by Med15 and Med16. Therefore, our data indicate that Med15 is required for the recruitment of the Triad subcomplex present in either med16Δ or WT cells and that both Med15 and Med16 are required for recruitment of Head, Middle, and Kinase modules. Although we have not formally ruled out the possibility that Med2 is also a target of Hsf1, it is unlikely, given that Med2 forms a heterodimeric complex with Med3 (53, 54). Its occupancy would thus be predicted to parallel that of Med3.
The fact that the med15Δ med16Δ double mutant had a more pronounced effect on Med17 occupancy than med16Δ alone is consistent with the idea that a fraction of the Med2-Med3-Med15 Triad associates with Mediator complexes lacking Med16 in vivo, thereby accounting for the stronger effect on Med17 occupancy when both Tail subunits are depleted. Likewise, genome-wide expression changes resulting from deletion of MED2, MED3, MED15 differ from that seen in med16Δ yeast (55). In summary, our data indicate that recruitment of Mediator by Hsf1 requires the Tail module subunits Med15 and Med16. Whether this reflects the fact that Hsf1 physically interacts with these subunits is not addressed by our experiments, although by analogy with other stress-activated factors, it is likely. It has been noted that promoters regulated by stress-induced activators tend to not only require the Tail module for Mediator recruitment but are also SAGA-dependent (27). HSP genes are SAGA-dependent, as indicated by both expression and ChIP assays (56, 57). However, because heat shock promoters also use TFIID upon their robust activation (58), the link between the Mediator Tail and SAGA, at least as it pertains to HSP gene regulation, merits further investigation.
Hsf1 Uses both Its N- and C-terminal Activators to Recruit Mediator to HSP Gene Promoters in Response to Thermal Stress
An important implication of this work is that although multiple activators associate with the upstream activation sequence regions of activated HSP genes, including Hsf1, Msn2/Msn4, Skn7, and Yap1 (59–62), Hsf1 itself is necessary for Mediator recruitment. This conclusion follows from the striking observation that truncation of either Hsf1 activation domain substantially reduced occupancy of Mediator, whereas the removal of both activation domains abolished it. Whether Hsf1 is sufficient for the high level of Mediator recruitment that we observed is unknown; it is formally possible that other DNA-bound factors, including gene-specific regulators, other coactivators, and even the general transcriptional machinery, contribute to this activity. Nonetheless, our observations indicate that in the absence of Hsf1 activation, transcription factors such as Msn2/Msn4, Skn7, and Yap1 do not recruit detectable levels of Mediator in response to heat shock and are consistent with the idea that Mediator is recruited to promoter/upstream activation sequence regions in an activator-specific manner (38). Similarly, an hsfts mutant dramatically reduces Mediator recruitment to heat shock loci in Drosophila (39), suggesting that important aspects of the heat shock activation mechanism are evolutionarily conserved.
Interestingly, at all four HSP genes tested, the NTA and CTA acted synergistically with respect to Mediator recruitment, particularly during the acute phase of heat shock (2.5–15 min). This conclusion follows from the fact that Hsf1 bearing just one activator (Hsf1ΔCTA or Hsf1ΔNTA) recruited Mediator to only 5–33% of the level achieved by Hsf1+ bearing two activation domains. In this regard, Hsf1 may resemble the well characterized Gcn4 activator, whose tandem activation domains target three non-contiguous segments of Med15 in a partially redundant manner (63, 64) and whose N-terminal activation domain additionally targets Med16 (64). Further studies will be necessary to identify the domains within Med15 and Med16 targeted by Hsf1.
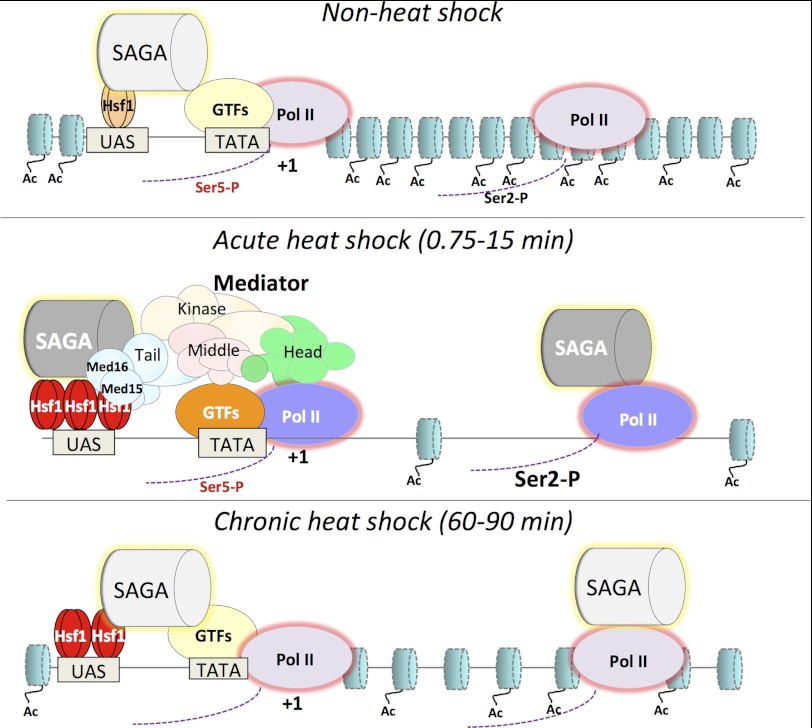
Interestingly, the synergy displayed by the CTA and NTA with respect to Mediator recruitment was less prominent when Pol II recruitment and mRNA expression were assayed. At several of the genes, deletion of the CTA had at most a 2-fold effect, and that of the NTA had virtually none, at 15 min. One explanation for this is that both CTA and NTA promote transcription via recruitment of alternative coactivators, such as SAGA, TFIID, and NuA4, in addition to Mediator. The contribution of these alternative coactivators may be comparatively more important during the chronic stage of heat shock (i.e. 60–90 min), when Mediator has largely dissociated and Hsf1 activity is attenuated. We have shown previously that SAGA is vigorously recruited to HSP82 and other heat shock genes (57), with kinetics of recruitment during the acute phase of heat shock closely resembling those of the core Mediator subunits examined here. Unlike Mediator, however, SAGA generally retains an enhanced level of occupancy (3–7-fold over non-induced levels) during extended exposure to elevated temperature (57). Thus, it is possible that SAGA contributes to Pol II recruitment during chronic heat shock (see Fig. 9 for a summary of factor occupancy at HSP genes under different expression states).
FIGURE 9.
Factor occupancy of a typical yeast HSP gene under non-inducing, acutely inducing, and chronically inducing conditions. The location and abundance of the indicated factors, as inferred from kinetic ChIP assays (this study and Refs. 21, 38, 57, 70, 75), are depicted. The increase in fractional occupancy is symbolized by an increase in the number of symbols (Hsf1, nucleosomes) and/or boldness of font and color. Note that the enhanced promoter occupancy of Hsf1, Mediator, SAGA, Pol II, and general transcription factors (GTFs) in response to heat shock is very rapid and may occur in a nearly simultaneous fashion. Although Mediator occupancy of HSP genes in non-induced and chronically heat-shocked cells is typically <10% of that seen in acutely induced ones (e.g. see Figs. 1 and 5), other factors may be present, albeit at reduced levels, under these conditions (e.g. see Fig. 6) and are so indicated. In addition, whereas Mediator is exclusively recruited to the promoter, SAGA is recruited to both promoter and coding regions (57). Disc-shaped objects, nucleosomes; Ac, hyperacetylated forms of H3 and H4; Ser2-P and Ser5-P, phosphorylated CTD forms of Pol II (phospho-CTD states have not been determined for chronic heat shock conditions).
Mediator Regulates Hsf1 Occupancy Under both Non-inducing and Inducing Conditions
Given that Mediator functions in PIC assembly and Pol II recruitment, activator binding might be expected to occur independently of Mediator, and indeed this has been seen (50, 65). However, our results suggest that in the case of Hsf1, Mediator governs activator occupancy under both non-inducing and inducing conditions. At SSA4, ZPR1, and perhaps other promoters, Mediator antagonizes the constitutive occupancy of Hsf1, whereas at HSP12, HSP26, ZPR1 (and perhaps others), Mediator facilitates induced Hsf1 occupancy. Mediator antagonism of Hsf1 binding is suggested by increased Hsf1 occupancy in the rgr1-Δ2 mutant lacking the C-terminal 336 amino acids of Med14/Rgr1. Moreover, similar to the rgr1-Δ2 effect, in a separate study, we have observed that conditional depletion of Med14 causes a 4-fold increase in Hsf1 abundance at SSA4 and a 2-fold increase at HSP82 and HSP104 under non-inducing conditions.3 How this effect is instigated is currently unknown, but there are several potential mechanisms. First, rgr1-Δ2 might disrupt a hypothetical negative feedback mechanism, in which Mediator-controlled covalent modification of Hsf1 triggers its clearance from promoters via proteasome-mediated degradation, physical displacement, or nuclear export, by analogy with other activators (28, 44, 66). Intriguingly, the CTA may act as a conduit, given that occupancy of Hsf1ΔCTA in MED+ cells was also strongly increased at select HSP promoters. Acidic activation domains have been implicated previously in feedback-regulated degradation of gene-specific activators (67). Second, rgr1-Δ2 might disrupt Hsf1 interactions with Mediator complexes bound to chromatin (68) that act as a “sink” for uninduced activators. In this view, the rgr1-Δ2 form of Mediator might be incapable of binding non-induced Hsf1 monomers, thus leading to their higher effective concentration within the nucleus and concomitant occupancy of HSP promoters as DNA-bound trimers. Similar to the effect we saw with the rgr1-Δ2 mutation, a missense mutation (R617Q) in the Med23 Tail subunit of human Mediator was observed to alter the binding of target transcription activators to the JUN and FOS immediate early gene promoter regions (69). Thus, the role of Mediator in regulating the binding of upstream activators to target promoters has been observed in evolutionarily distant eukaryotes.
In contrast to its antagonistic effect under non-inducing conditions, Mediator and Hsf1 appear to cooperatively bind to Hsf1 target promoters under inducing conditions, thereby stabilizing the Hsf1-DNA interaction. This interpretation is based on reduced Hsf1 occupancy seen in several Tail mutants (rgr1-Δ2, med15Δ, and med16Δ), an effect that appears to be unique to the Tail. In addition, deletion of either N- or C-terminal activator has an equivalent (or greater) effect on Hsf1 occupancy. Together, the data are consistent with the idea that Hsf1, through its two activation domains, physically interacts with Mediator through its Tail module to effect cooperative binding. One way this might occur is via Hsf1 recognition of its target DNA sequences (HSEs) simultaneously with Mediator interaction with one or more components of the PIC shown to be present under non-inducing conditions (this study and Ref. 70). Notably, the Med2-Med3-Med15 Triad (med16Δ context) fails to cooperatively bind; thus, the native Mediator complex may be required to facilitate Hsf1 association.
In summary, our kinetic ChIP analysis indicates the presence of reciprocal cooperative interactions between Hsf1 and Mediator. NTA and CTA cooperate in binding Mediator; likewise, Med15 and Med16 cooperate in binding Hsf1. Moreover, we have found that at HSP gene promoters, Mediator and Pol II recruitment is kinetically indistinguishable (see Fig. 9). This provides an interesting contrast to the HO, GAL, and Gcn4-regulated genes, where Mediator recruitment precedes that of Pol II (71–74). Finally, the findings reported here raise a number of interesting questions. These include identification of the domain(s) within Med15 and Med16 actually targeted by Hsf1; whether such interactions are direct or indirect; the mechanisms underlying Mediator antagonism of Hsf1 under non-inducing conditions and its cooperative binding with Hsf1 following heat shock; and the interplay between Mediator and other transcriptional coactivators in regulating heat shock gene transcription in S. cerevisiae.
Acknowledgments
We thank Peter Sorger for Hsf1 N- and C-terminal truncation constructs; Rick Young for ChIP-grade Med17/Srb4 antibody; David Stillman, Jenny Jeon, and Kelly Tatchell for yeast strains; and Kelly Tatchell, Lucy Robinson, and Larry Meyers for useful discussions.
This work was supported by National Science Foundation Grants MCB-0747227 and MCB-1025025 (to D. S. G.).
Y. Moustafa and D. S. Gross, unpublished observations.
- Pol II
- RNA polymerase II
- NTA
- N-terminal activator
- CTA
- C-terminal activator
- TAP
- tandem affinity purification
- qPCR
- quantitative PCR
- CTD
- C-terminal domain
- ChIP
- chromatin immunoprecipitation.
REFERENCES
- 1. Myers L. C., Kornberg R. D. (2000) Mediator of transcriptional regulation. Annu. Rev. Biochem. 69, 729–749 [DOI] [PubMed] [Google Scholar]
- 2. Malik S., Roeder R. G. (2010) The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat. Rev. Genet. 11, 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ansari S. A., Morse R. H. (2013) Mechanisms of Mediator complex action in transcriptional activation. Cell Mol. Life Sci., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asturias F. J., Jiang Y. W., Myers L. C., Gustafsson C. M., Kornberg R. D. (1999) Conserved structures of mediator and RNA polymerase II holoenzyme. Science 283, 985–987 [DOI] [PubMed] [Google Scholar]
- 5. Davis J. A., Takagi Y., Kornberg R. D., Asturias F. A. (2002) Structure of the yeast RNA polymerase II holoenzyme. Mediator conformation and polymerase interaction. Mol. Cell 10, 409–415 [DOI] [PubMed] [Google Scholar]
- 6. Dotson M. R., Yuan C. X., Roeder R. G., Myers L. C., Gustafsson C. M., Jiang Y. W., Li Y., Kornberg R. D., Asturias F. J. (2000) Structural organization of yeast and mammalian mediator complexes. Proc. Natl. Acad. Sci. U.S.A. 97, 14307–14310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Näär A. M., Taatjes D. J., Zhai W., Nogales E., Tjian R. (2002) Human CRSP interacts with RNA polymerase II CTD and adopts a specific CTD-bound conformation. Genes Dev. 16, 1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larivière L., Plaschka C., Seizl M., Wenzeck L., Kurth F., Cramer P. (2012) Structure of the Mediator head module. Nature 492, 448–451 [DOI] [PubMed] [Google Scholar]
- 9. Cai G., Imasaki T., Takagi Y., Asturias F. J. (2009) Mediator structural conservation and implications for the regulation mechanism. Structure 17, 559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cai G., Imasaki T., Yamada K., Cardelli F., Takagi Y., Asturias F. J. (2010) Mediator head module structure and functional interactions. Nat. Struct. Mol. Biol. 17, 273–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Esnault C., Ghavi-Helm Y., Brun S., Soutourina J., Van Berkum N., Boschiero C., Holstege F., Werner M. (2008) Mediator-dependent recruitment of TFIIH modules in preinitiation complex. Mol. Cell 31, 337–346 [DOI] [PubMed] [Google Scholar]
- 12. Mehta S., Miklos I., Sipiczki M., Sengupta S., Sharma N. (2009) The Med8 mediator subunit interacts with the Rpb4 subunit of RNA polymerase II and Ace2 transcriptional activator in Schizosaccharomyces pombe. FEBS Lett. 583, 3115–3120 [DOI] [PubMed] [Google Scholar]
- 13. Soutourina J., Wydau S., Ambroise Y., Boschiero C., Werner M. (2011) Direct interaction of RNA polymerase II and Mediator required for transcription in vivo. Science 331, 1451–1454 [DOI] [PubMed] [Google Scholar]
- 14. Holstege F. C., Jennings E. G., Wyrick J. J., Lee T. I., Hengartner C. J., Green M. R., Golub T. R., Lander E. S., Young R. A. (1998) Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95, 717–728 [DOI] [PubMed] [Google Scholar]
- 15. Baumli S., Hoeppner S., Cramer P. (2005) A conserved mediator hinge revealed in the structure of the MED7·MED21 (Med7·Srb7) heterodimer. J. Biol. Chem. 280, 18171–18178 [DOI] [PubMed] [Google Scholar]
- 16. Baidoobonso S. M., Guidi B. W., Myers L. C. (2007) Med19(Rox3) regulates intermodule interactions in the Saccharomyces cerevisiae mediator complex. J. Biol. Chem. 282, 5551–5559 [DOI] [PubMed] [Google Scholar]
- 17. Tabtiang R. K., Herskowitz I. (1998) Nuclear proteins Nut1p and Nut2p cooperate to negatively regulate a Swi4p-dependent lacZ reporter gene in Saccharomyces cerevisiae. Mol. Cell Biol. 18, 4707–4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gromöller A., Lehming N. (2000) Srb7p is essential for the activation of a subset of genes. FEBS Lett. 484, 48–54 [DOI] [PubMed] [Google Scholar]
- 19. Gromöller A., Lehming N. (2000) Srb7p is a physical and physiological target of Tup1p. EMBO J. 19, 6845–6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh H., Erkine A. M., Kremer S. B., Duttweiler H. M., Davis D. A., Iqbal J., Gross R. R., Gross D. S. (2006) A functional module of yeast Mediator that governs the dynamic range of heat-shock gene expression. Genetics 172, 2169–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kremer S. B., Kim S., Jeon J. O., Moustafa Y. W., Chen A., Zhao J., Gross D. S. (2012) Role of Mediator in regulating Pol II elongation and nucleosome displacement in Saccharomyces cerevisiae. Genetics 191, 95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fishburn J., Mohibullah N., Hahn S. (2005) Function of a eukaryotic transcription activator during the transcription cycle. Mol. Cell 18, 369–378 [DOI] [PubMed] [Google Scholar]
- 23. Reeves W. M., Hahn S. (2005) Targets of the Gal4 transcription activator in functional transcription complexes. Mol. Cell. Biol. 25, 9092–9102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shahi P., Gulshan K., Näär A. M., Moye-Rowley W. S. (2010) Differential roles of transcriptional mediator subunits in regulation of multidrug resistance gene expression in Saccharomyces cerevisiae. Mol. Biol. Cell 21, 2469–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thakur J. K., Arthanari H., Yang F., Chau K. H., Wagner G., Näär A. M. (2009) Mediator subunit Gal11p/MED15 is required for fatty acid-dependent gene activation by yeast transcription factor Oaf1p. J. Biol. Chem. 284, 4422–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang F., Sumibcay L., Hinnebusch A. G., Swanson M. J. (2004) A triad of subunits from the Gal11/tail domain of Srb mediator is an in vivo target of transcriptional activator Gcn4p. Mol. Cell Biol. 24, 6871–6886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ansari S. A., Ganapathi M., Benschop J. J., Holstege F. C., Wade J. T., Morse R. H. (2012) Distinct role of Mediator tail module in regulation of SAGA-dependent, TATA-containing genes in yeast. EMBO J. 31, 44–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chi Y., Huddleston M. J., Zhang X., Young R. A., Annan R. S., Carr S. A., Deshaies R. J. (2001) Negative regulation of Gcn4 and Msn2 transcription factors by Srb10 cyclin-dependent kinase. Genes Dev. 15, 1078–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Donner A. J., Szostek S., Hoover J. M., Espinosa J. M. (2007) CDK8 is a stimulus-specific positive coregulator of p53 target genes. Mol. Cell 27, 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larschan E., Winston F. (2005) The Saccharomyces cerevisiae Srb8-Srb11 complex functions with the SAGA complex during Gal4-activated transcription. Mol. Cell Biol. 25, 114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sorger P. K., Pelham H. R. (1988) Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell 54, 855–864 [DOI] [PubMed] [Google Scholar]
- 32. Harrison C. J., Bohm A. A., Nelson H. C. (1994) Crystal structure of the DNA binding domain of the heat shock transcription factor. Science 263, 224–227 [DOI] [PubMed] [Google Scholar]
- 33. Sorger P. K., Nelson H. C. (1989) Trimerization of a yeast transcriptional activator via a coiled-coil motif. Cell 59, 807–813 [DOI] [PubMed] [Google Scholar]
- 34. Sorger P. K. (1990) Yeast heat shock factor contains separable transient and sustained response transcriptional activators. Cell 62, 793–805 [DOI] [PubMed] [Google Scholar]
- 35. Hahn J. S., Hu Z., Thiele D. J., Iyer V. R. (2004) Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol. Cell Biol. 24, 5249–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eastmond D. L., Nelson H. C. (2006) Genome-wide analysis reveals new roles for the activation domains of the Saccharomyces cerevisiae heat shock transcription factor (Hsf1) during the transient heat shock response. J. Biol. Chem. 281, 32909–32921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee D.-K., Kim S., Lis J. T. (1999) Different upstream transcriptional activators have distinct co-activator requirements. Genes Dev. 13, 2934–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fan X., Chou D. M., Struhl K. (2006) Activator-specific recruitment of Mediator in vivo. Nat. Struct. Mol. Biol. 13, 117–120 [DOI] [PubMed] [Google Scholar]
- 39. Park J. M., Werner J., Kim J. M., Lis J. T., Kim Y. J. (2001) Mediator, not holoenzyme, is directly recruited to the heat shock promoter by HSF upon heat shock. Mol. Cell 8, 9–19 [DOI] [PubMed] [Google Scholar]
- 40. Erdeniz N., Mortensen U. H., Rothstein R. (1997) Cloning-free PCR-based allele replacement methods. Genome Res. 7, 1174–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim S. (2012) Role of Mediator in Regulating Hsf1-mediated Gene Transcription in Saccharomyces cerevisiae, Ph.D. thesis, Louisiana State University Health Sciences Center, Shreveport, LA [Google Scholar]
- 42. Erkine A. M., Adams C. C., Diken T., Gross D. S. (1996) Heat shock factor gains access to the yeast HSC82 promoter independently of other sequence-specific factors and antagonizes nucleosomal repression of basal and induced transcription. Mol. Cell Biol. 16, 7004–7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Balakrishnan S. K., Gross D. S. (2008) The tumor suppressor p53 associates with gene coding regions and co-traverses with elongating RNA polymerase II in an in vivo model. Oncogene 27, 2661–2672 [DOI] [PubMed] [Google Scholar]
- 44. Rosonina E., Duncan S. M., Manley J. L. (2012) Sumoylation of transcription factor Gcn4 facilitates its Srb10-mediated clearance from promoters in yeast. Genes Dev. 26, 350–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li Y., Bjorklund S., Jiang Y. W., Kim Y. J., Lane W. S., Stillman D. J., Kornberg R. D. (1995) Yeast global transcriptional regulators Sin4 and Rgr1 are components of mediator complex/RNA polymerase II holoenzyme. Proc. Natl. Acad. Sci. U.S.A. 92, 10864–10868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee Y. C., Park J. M., Min S., Han S. J., Kim Y. J. (1999) An activator binding module of yeast RNA polymerase II holoenzyme. Mol. Cell Biol. 19, 2967–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Duina A. A., Kalton H. M., Gaber R. F. (1998) Requirement for Hsp90 and a CyP-40-type cyclophilin in negative regulation of the heat shock response. J. Biol. Chem. 273, 18974–18978 [DOI] [PubMed] [Google Scholar]
- 48. Zou J., Guo Y., Guettouche T., Smith D. F., Voellmy R. (1998) Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 94, 471–480 [DOI] [PubMed] [Google Scholar]
- 49. Miller C., Matic I., Maier K. C., Schwalb B., Roether S., Strässer K., Tresch A., Mann M., Cramer P. (2012) Mediator phosphorylation prevents stress response transcription during non-stress conditions. J. Biol. Chem. 287, 44017–44026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuras L., Borggrefe T., Kornberg R. D. (2003) Association of the Mediator complex with enhancers of active genes. Proc. Natl. Acad. Sci. U.S.A. 100, 13887–13891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Galdieri L., Desai P., Vancura A. (2012) Facilitated assembly of the preinitiation complex by separated tail and head/middle modules of the mediator. J. Mol. Biol. 415, 464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Andrau J. C., van de Pasch L., Lijnzaad P., Bijma T., Koerkamp M. G., van de Peppel J., Werner M., Holstege F. C. (2006) Genome-wide location of the coactivator Mediator. Binding without activation and transient Cdk8 interaction on DNA. Mol. Cell 22, 179–192 [DOI] [PubMed] [Google Scholar]
- 53. Béve J., Hu G. Z., Myers L. C., Balciunas D., Werngren O., Hultenby K., Wibom R., Ronne H., Gustafsson C. M. (2005) The structural and functional role of Med5 in the yeast Mediator tail module. J. Biol. Chem. 280, 41366–41372 [DOI] [PubMed] [Google Scholar]
- 54. Seizl M., Larivière L., Pfaffeneder T., Wenzeck L., Cramer P. (2011) Mediator head subcomplex Med11/22 contains a common helix bundle building block with a specific function in transcription initiation complex stabilization. Nucleic Acids Res. 39, 6291–6304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van de Peppel J., Kettelarij N., van Bakel H., Kockelkorn T. T., van Leenen D., Holstege F. C. (2005) Mediator expression profiling epistasis reveals a signal transduction pathway with antagonistic submodules and highly specific downstream targets. Mol. Cell 19, 511–522 [DOI] [PubMed] [Google Scholar]
- 56. Huisinga K. L., Pugh B. F. (2004) A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol. Cell 13, 573–585 [DOI] [PubMed] [Google Scholar]
- 57. Kremer S. B., Gross D. S. (2009) SAGA and Rpd3 chromatin modification complexes dynamically regulate heat shock gene structure and expression. J. Biol. Chem. 284, 32914–32931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ghosh S., Pugh B. F. (2011) Sequential recruitment of SAGA and TFIID in a genomic response to DNA damage in Saccharomyces cerevisiae. Mol. Cell Biol. 31, 190–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gasch A. P., Spellman P. T., Kao C. M., Carmel-Harel O., Eisen M. B., Storz G., Botstein D., Brown P. O. (2000) Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11, 4241–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Erkine A. M., Magrogan S. F., Sekinger E. A., Gross D. S. (1999) Cooperative binding of heat shock factor to the yeast HSP82 promoter in vivo and in vitro. Mol. Cell Biol. 19, 1627–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Raitt D. C., Johnson A. L., Erkine A. M., Makino K., Morgan B., Gross D. S., Johnston L. H. (2000) The Skn7 response regulator of Saccharomyces cerevisiae interacts with Hsf1 in vivo and is required for the induction of heat shock genes by oxidative stress. Mol. Biol. Cell 11, 2335–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Erkina T. Y., Tschetter P. A., Erkine A. M. (2008) Different requirements of the SWI/SNF complex for robust nucleosome displacement at promoters of heat shock factor and Msn2- and Msn4-regulated heat shock genes. Mol. Cell Biol. 28, 1207–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jedidi I., Zhang F., Qiu H., Stahl S. J., Palmer I., Kaufman J. D., Nadaud P. S., Mukherjee S., Wingfield P. T., Jaroniec C. P., Hinnebusch A. G. (2010) Activator Gcn4 employs multiple segments of Med15/Gal11, including the KIX domain, to recruit Mediator to target genes in vivo. J. Biol. Chem. 285, 2438–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Herbig E., Warfield L., Fish L., Fishburn J., Knutson B. A., Moorefield B., Pacheco D., Hahn S. (2010) Mechanism of Mediator recruitment by tandem Gcn4 activation domains and three Gal11 activator-binding domains. Mol. Cell Biol. 30, 2376–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leroy C., Cormier L., Kuras L. (2006) Independent recruitment of Mediator and SAGA by the activator Met4. Mol. Cell Biol. 26, 3149–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ferdous A., Sikder D., Gillette T., Nalley K., Kodadek T., Johnston S. A. (2007) The role of the proteasomal ATPases and activator monoubiquitylation in regulating Gal4 binding to promoters. Genes Dev. 21, 112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Salghetti S. E., Caudy A. A., Chenoweth J. G., Tansey W. P. (2001) Regulation of transcriptional activation domain function by ubiquitin. Science 293, 1651–1653 [DOI] [PubMed] [Google Scholar]
- 68. Zhu X., Zhang Y., Bjornsdottir G., Liu Z., Quan A., Costanzo M., Dávila López M., Westholm J. O., Ronne H., Boone C., Gustafsson C. M., Myers L. C. (2011) Histone modifications influence Mediator interactions with chromatin. Nucleic Acids Res. 39, 8342–8354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hashimoto S., Boissel S., Zarhrate M., Rio M., Munnich A., Egly J. M., Colleaux L. (2011) MED23 mutation links intellectual disability to dysregulation of immediate early gene expression. Science 333, 1161–1163 [DOI] [PubMed] [Google Scholar]
- 70. Sekinger E. A., Gross D. S. (2001) Silenced chromatin is permissive to activator binding and PIC recruitment. Cell 105, 403–414 [DOI] [PubMed] [Google Scholar]
- 71. Bryant G. O., Ptashne M. (2003) Independent recruitment in vivo by Gal4 of two complexes required for transcription. Mol. Cell 11, 1301–1309 [DOI] [PubMed] [Google Scholar]
- 72. Bhoite L. T., Yu Y., Stillman D. J. (2001) The Swi5 activator recruits the Mediator complex to the HO promoter without RNA polymerase II. Genes Dev. 15, 2457–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cosma M. P., Panizza S., Nasmyth K. (2001) Cdk1 triggers association of RNA polymerase to cell cycle promoters only after recruitment of the mediator by SBF. Mol. Cell 7, 1213–1220 [DOI] [PubMed] [Google Scholar]
- 74. Govind C. K., Yoon S., Qiu H., Govind S., Hinnebusch A. G. (2005) Simultaneous recruitment of coactivators by Gcn4p stimulates multiple steps of transcription in vivo. Mol. Cell Biol. 25, 5626–5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhao J., Herrera-Diaz J., Gross D. S. (2005) Domain-wide displacement of histones by activated heat shock factor occurs independently of Swi/Snf and is not correlated with RNA polymerase II density. Mol. Cell Biol. 25, 8985–8999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lee S., Gross D. S. (1993) Conditional silencing. The HMRE mating-type silencer exerts a rapidly reversible position effect on the yeast HSP82 heat shock gene. Mol. Cell Biol. 13, 727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]