Background: PDPN is a transmembrane receptor that promotes cell migration, but modifications that regulate its effects are not known.
Results: PKA can phosphorylate PDPN, nonphosphorylatable PDPN promotes cell migration, and phosphomimetic PDPN fails to promote cell migration.
Conclusion: PKA can phosphorylate PDPN to decrease cell migration.
Significance: PDPN effects on cell motility are important for processes including embryonic development and cancer progression.
Keywords: Cancer Biology, Cell Junctions, Cell Migration, Cell Motility, Protein Kinase A (PKA), Contact Normalization, Intercellular Communication
Abstract
Podoplanin (PDPN) is a transmembrane receptor that affects the activities of Rho, ezrin, and other proteins to promote tumor cell motility, invasion, and metastasis. PDPN is found in many types of cancer and may serve as a tumor biomarker and chemotherapeutic target. The intracellular region of PDPN contains only two serines, and these are conserved in mammals including mice and humans. We generated cells from the embryos of homozygous null Pdpn knock-out mice to investigate the relevance of these serines to cell growth and migration on a clear (PDPN-free) background. We report here that one or both of these serines can be phosphorylated by PKA (protein kinase A). We also report that conversion of these serines to nonphosphorylatable alanine residues enhances cell migration, whereas their conversion to phosphomimetic aspartate residues decreases cell migration. These results indicate that PKA can phosphorylate PDPN to decrease cell migration. In addition, we report that PDPN expression in fibroblasts causes them to facilitate the motility and viability of neighboring melanoma cells in coculture. These findings shed new light on how PDPN promotes cell motility, its role in tumorigenesis, and its utility as a functionally relevant biomarker and chemotherapeutic target.
Introduction
PDPN2 (also called OTS8, RTI40, T1α, aggrus, and gp36) is a transmembrane mucin-like protein that augments tumor cell invasion. PDPN is required for proper lung and lymphatic system development during embryogenesis (1–3). However, PDPN expression is also induced by tumor promoters including 12-O-tetradecanoylphorbol-13-acetate, RAS, and Src (4–6) and is found at the invasive front of many types of cancer (7, 8). Indeed, PDPN may serve as a cancer biomarker and effective chemotherapeutic target (8–10).
PDPN regulates the activities of Rho, ezrin, and other proteins linked to the actin cytoskeleton to mediate filopodia formation, cell motility, invasion, and metastasis (8, 11). Some proteins that control the actin cytoskeleton can be modified by phosphorylation to affect cell motility. For example, the adaptor proteins Cas, paxillin, and palladin affect remodeling of the actin cytoskeleton and are phosphorylated as a result of oncogenic kinase signaling cascades to promote or inhibit tumor cell motility (12–15).
PDPN is a unique transmembrane receptor protein that consists of an N-terminal extracellular domain of about 140 amino acids, a transmembrane domain of about 20 amino acids, and a short intracellular C-terminal domain of about 10 amino acids. The intracellular tail of PDPN contains two serine residues that are conserved in all mammals. However, the function of these two intracellular serines has not been elucidated. Here, we report that these serine residues influence the effects of PDPN on cell motility and intercellular interactions between fibroblasts and tumor cells.
EXPERIMENTAL PROCEDURES
Kinase Assays
Phosphorylation of the peptide VVMKKISGRFSP (synthesized by Proteintech, Chicago, IL), containing the entire intracellular region of mouse PDPN (residues 161–172), was measured using a phosphocellulose paper binding assay (16). Reaction mixtures contained 300 μm peptide in 20 mm Tris-HCl (pH 7.4), 10 mm MgCl2, 1 mg/ml bovine serum albumin, 0.25 mm ATP, and [γ-32P]ATP (100 cpm/pmol). The reactions were initiated by the addition of 25 μg/ml PKA (Promega V516A). Aliquots were withdrawn at various time points and quenched by the addition of 10% trichloroacetic acid. The reactions were spotted onto P81 phosphocellulose filters, washed three times with 0.5% phosphoric acid, and analyzed by scintillation counting to determine the picomoles of phosphate transferred.
Generation of Wild Type and Mutant Pdpn Cell Lines
PdpnKo3 cells were isolated from the trunks of homozygous null Pdpn knock-out mice at day 10 postcoitum (1). Cells were maintained in DMEM supplemented with 10% FBS as described previously (15, 17). The cells passed crisis during standard serial passaging, and genotypes were verified by PCR. Pdpn mutant constructs were produced with the QuikChange II XL site-directed mutagenesis kit according to the manufacturer's protocol (Stratagene 200521). The vector pEF4Pdpn encoding full-length wild type mouse PDPN (PdpnWT) (4, 18) was used as a template. Pdpn(S167A/S171A) encoding nonphosphorylatable PDPN (PdpnAA) was generated with the complementary primer pairs 5′-GTTGTTATGAAGAAGATTGCTGGAAGGTTCTCGCC-3′, 5′-GGCGAGAACCTTCCAGCAATCTTCTTCATAACAAC-3′ followed by 5′-TGGAAGGTTCGCGCCCTAAAGAGGGCCCTTCGAAGG-3′, 5′-CCTTCGAAGGGCCCTCTTTAGGGCGCGAACCTTCCA-3′. Pdpn(S167D/S171D) encoding phosphomimetic PDPN (PdpnDD) was generated with the complementary primer pairs 5′-GTTATGAAGAAGATTGATGGAAGGTTCTCGCCCTAAAGAGC-3′, 5′-GCTCTTTAGGGCGAGAACCTTCCATCAATCTTCTTCATAAC-3′ followed by 5′-GATGGAAGGTTCGACCCCTAAAGAGCGTTATGAAGAAGATTGATGG-3′, 5′-CCATCAATCTTCTTCATAACGCTCTTTAGGGGTCGAACCTTCCATC-3′. All constructs were verified by sequencing. PdpnWT, PdpnAA, PdpnDD constructs, and the control vector pEF4/V5HisA (Invitrogen) were cotransfected with pBABEpuro into a single expanded PdpnKo cell clone with Lipofectamine 2000 according to the manufacturer's protocol (Invitrogen 11668-027). Transfectants were selected with Zeocin (InvivoGen ant-zn-1p) and puromycin (Calbiochem 540411). This double transfection/selection technique produces high levels of transgene expression in about 80% or more of surviving cells (4, 15, 19).
Western Blotting
Western blotting was performed as described previously (4, 18). Protein was resolved by SDS-PAGE, transferred to Immobilon-P membranes (Millipore IH1079562), and incubated with antisera specific for PDPN (University of Iowa Developmental Studies Hybridoma Bank 8.1.1), or GAPDH (Santa Cruz Biotechnology A1978). Primary antiserum was recognized by appropriate secondary antiserum conjugated to horseradish peroxidase and detected using enhanced chemiluminescence (Thermo Scientific 32106). After blotting, membranes were stained with India ink to verify equal loading and transfer.
Evaluation of Cell Growth and Migration
Cell growth and migration were evaluated as described previously (4, 15, 18, 19). Briefly, cell growth was measured by plating 10,000 cells/well on standard 24-well tissue culture plates (Falcon 353047) and counting cells at the indicated time points in a Coulter counter. For wound healing migration assays, confluent cell monolayers were scratched, and migration was quantitated as the number of cells that entered a 300 × 200-μm area in the center of the wound at 18 h. For some experiments, the PKA modulators 8-Br-cAMP (Calbiochem 203800) or H-89 (Calbiochem 371963) were added immediately after wounding. For Transwell invasion assays, 600,000 cells were added to each chamber of 6-well Transwell membranes with an 8-μm pore size (Transwell-Clear, Costar 3428), incubated for 24 h, released separately from the top and bottom of the membrane, counted in a Coulter Counter, and quantified as the percentage of cells that migrated from the top of the membrane to the bottom of the membrane.
Video Microscopy
To analyze unopposed movement, PdpnKo cell lines were plated on 35-mm culture dishes at 5% confluence and incubated overnight. To analyze the effects of PdpnKo cells on the movement of melanoma cells in coculture, B16F10 melanoma cells were labeled with 1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate as described (17, 20, 21), plated with unlabeled B16F10 cells or PdpnKo cell lines at a 1:100 ratio on 35-mm culture dishes at 85% confluence, and incubated overnight. Medium was then removed and replaced with fresh medium before dishes were mounted on an INUBG2A-ZILCS with UNIV-35 stage top incubator (Tokai Hit, Shizuoka, Japan) maintained at 37 °C, 5% CO2, and 100% humidity. Images of cells were captured with AxioCam MRm camera at 5-min intervals for 18 (unopposed movement) or 36 h (cocultured melanoma cells) with a 5× CP-Achromat objective on a Zeiss Axiovert 40 CFL inverted microscope or a 10× EC Plan NEOF objective with a Zeiss Axiovert Observer Z1 inverted microscope, respectively. Distance and displacement traveled from the starting point to the end point were measured by tracking the center of the nuclei of motile cells with AxioVision software (version 4.3, Carl Zeiss). The percentage of morphologically intact cells was used to quantitate the survival of cocultured melanoma cells.
Statistical Analysis
Experiments were repeated with similar results and means, errors, and unpaired two-tailed t tests were calculated from representative data sets with Prism (version 4, GraphPad Software).
RESULTS
Phosphorylation of the PDPN Intracellular Tail by PKA
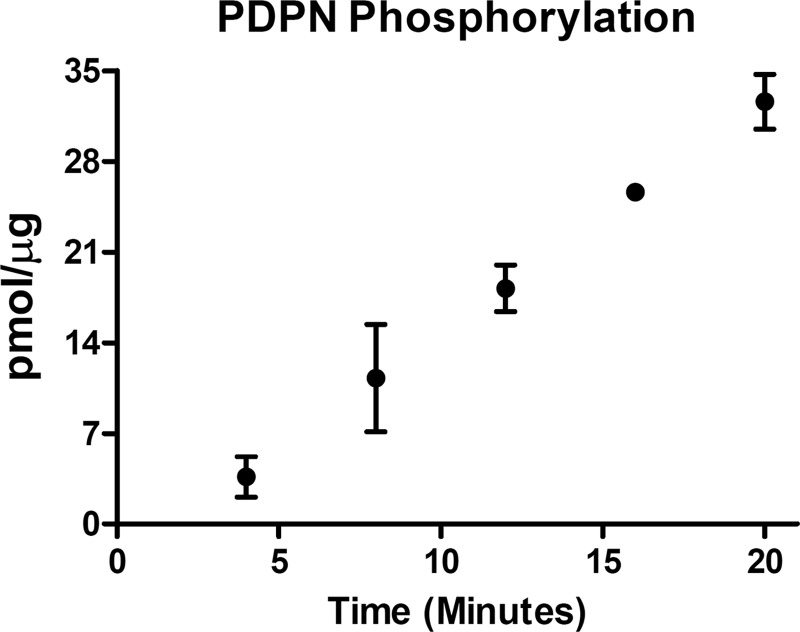
PDPN activity correlates well between humans and mice (22). For example, PDPN expression is strongly induced in human (22, 23) and mouse (5, 24) skin cancers. There are 2 conserved serines in the intracellular region of mouse and human PDPN. These serine residues could potentially serve as phosphorylation sites for PKC or PKA. Using a peptide that corresponds to the entire cytoplasmic portion of PDPN, we performed in vitro kinase assays to evaluate the ability of these kinases to phosphorylate these sites. As shown in Fig. 1, PKA catalyzed the time-dependent phosphorylation of the PDPN cytoplasmic peptide. In contrast, PKC did not phosphorylate the peptide to any significant extent (data not shown).
FIGURE 1.
Phosphorylation of PDPN intracellular tail by PKA. Peptide containing the entire intracellular region of PDPN (VVMKKISGRFSP) was incubated with PKA and [γ-32P]ATP for the indicated time points. Data are shown as the picomoles of phosphate transferred to the PDPN peptide (mean ± S.D., n = 2).
Nonphosphorylated PDPN Enhances Cell Migration
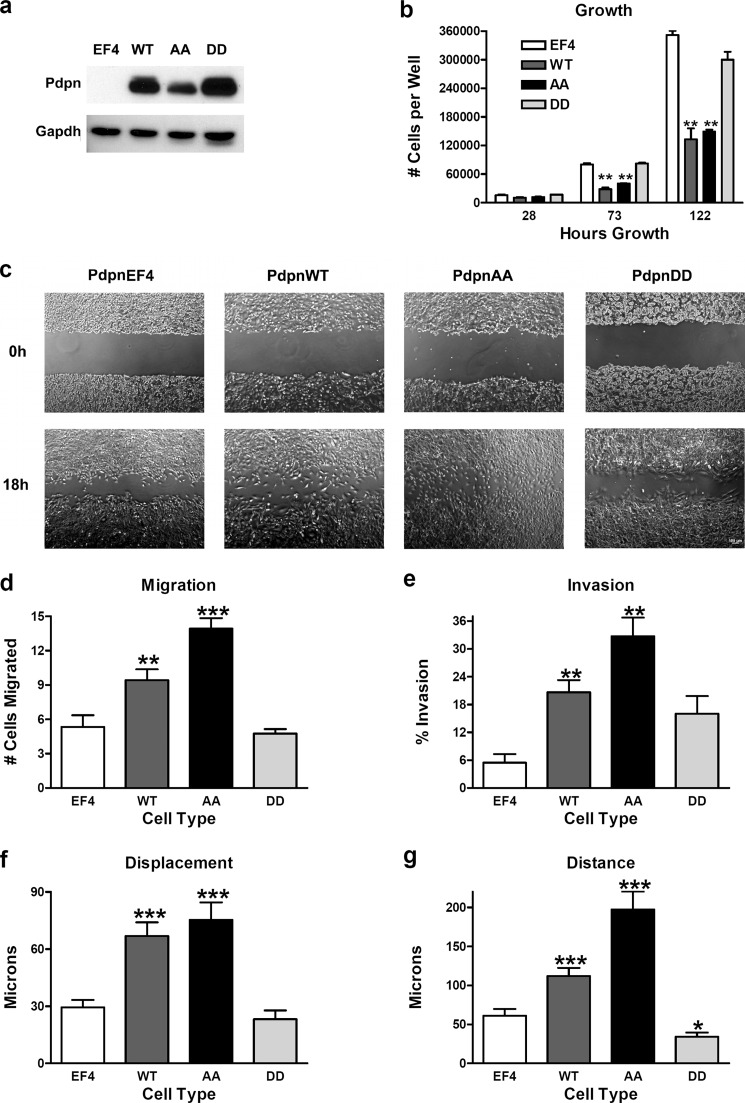
We generated homozygous null Pdpn knock-out mouse embryonic fibroblasts (PdpnKo cells) to study the effects of PDPN modification on cell behavior. The absence of endogenous PDPN background of these cells facilitated evaluation of how modification of PDPN intracellular serines affected cell growth and motility. These cells were stably transfected with constructs encoding wild type PDPN (PdpnWT), nonphosphorylatable PDPN with both intracellular serines mutated to alanine (PdpnAA), phosphomimetic PDPN with both intracellular serines mutated to aspartate (PdpnDD), or empty vectors (PdpnEF4) as controls. Expression of these transgenes was verified by Western blotting as shown in Fig. 2a.
FIGURE 2.
Effect of Pdpn expression on cell growth and motility. a, PDPN and GAPDH were detected by Western blotting of protein (7.5 μg/lane) from homozygous null Pdpn knock-out mouse embryonic fibroblasts transfected with empty parental vector (EF4), wild type Pdpn (WT), Pdpn with both intracellular serines mutated to alanine (AA), or Pdpn with both intracellular serines mutated to aspartate (DD) as indicated. b, cell growth was evaluated by plating 10,000 cells/well on 24-well tissue culture plates and counting at the indicated time points. Data are shown as number of cells/well (mean ± S.D., n = 2). c and d, cell migration was measured by wound healing assays and quantitated as the number of cells that entered a 300 × 200-μm area of in the center of a wound in 18 h (mean ± S.E., n = 12). Bar = 100 μm. e, Transwell invasion assays were performed and quantitated as the percentage of cells that moved from the top of the membrane to the bottom of the membrane in 24 h (mean ± S.E., n = 3). f and g, total displacement (f) and net distance (g) traveled were measured by video microscopy of individual motile cells and presented as micrometers traveled over 18 h (mean ± S.E., n = 20). Single, double, and triple asterisks indicate p < 0.05, p < 0.01, and p < 0.001 when compared with empty vector controls by Student's t test, respectively.
Although PDPN has been shown to promote cell migration, its effects on cell growth and proliferation are less defined (8–10). A role for PDPN in cell growth seemed to emerge on the clear background of homozygous null knock-out cells. As shown in Fig. 2b, expression of wild type (PdpnWT) or nonphosphorylatable (PdpnAA) PDPN appeared to decrease cell growth rates by about 50% when compared with control transfectants (PdpnEF4). Interestingly, this effect was not seen in cells transfected with the phosphomimetic PDPN (PdpnDD) construct.
As expected, wild type PDPN augmented the migration of PdpnKo cells. PdpnWT cells migrated about 50% more than the control transfectants (PdpnEF4) in wound healing migration assays shown in Fig. 2, c and d. However, although expression of the PdpnAA construct did not exceed that of PdpnWT, PdpnAA cells migrated about 40% more than PdpnWT cells in these assays. These results were confirmed by Transwell invasion assays in which PdpnWT cells moved about 4-fold better through 8-μm pores than PdpnEF4 cells, and PdpnAA cells moved about 50% more than the PdpnWT cells (Fig. 2e).
Phosphomimetic PDPN Decreases Cell Migration
Results from wild type (PdpnWT) and nonphosphorylatable (PdpnAA) constructs suggest that PDPN can be phosphorylated to inhibit cell migration. This hypothesis is supported by results from phosphomimetic (PdpnDD) constructs. In contrast to PdpnWT or PdpnAA constructs, phosphomimetic (PdpnDD) Pdpn did not significantly increase cell motility when compared with PdpnEF4 control cells in wound healing migration (Fig. 2, c and d) or Transwell invasion (Fig. 2e) assays. Interestingly, in contrast to wild type or PdpnAA transfectants, PdpnDD transfection did not significantly decrease cell growth when compared with PdpnEF4 controls (Fig. 2b). These results suggest that PDPN signaling may convert some metabolic energy used for cell proliferation into processes needed for motility.
Live Cell Imaging of Individual Cells Confirms That PDPN Serine Residue Modifications Affect Cell Motility
PDPN can promote both collective and individual cell migration in the absence or presence of epithelial-mesenchymal transition (7, 25). Thus, in addition to wound healing and Transwell assays, live cell imaging was used to investigate the effects of PDPN serine modifications on the motility of individual cells in sparse cultures. These assays also allowed direct measurements of total displacement and distance traveled by individual cells over time.
As with wound healing and Transwell assays cells, PDPN transfectants moved better than control cells, and nonphosphorylatable PDPN increased the motility of these cells more than wild type PDPN. PdpnKo cells transfected with PdpnEF4, PdpnWT, or PdpnAA moved at an average displacement speed of 1.6, 3.7, or 4.2 μm/h (Fig. 2f and supplemental Fig. 1, a-c) to travel an average distance of 3.4, 6.2, or 11 μm/h, respectively. In contrast, the movement of cells transfected with phosphomimetic PDPN (PdpnDD) was not significantly different from controls (PdpnEF4) when measured by displacement (1.3 μm/h) and significantly less than controls when measured by distance (1.9 μm/h) (Fig. 2f and supplemental Fig. 1d). These data indicate that PDPN can be phosphorylated to decrease both collective and individual cell motility.
Modulators of PKA Activity Affect Migration of Cells Expressing Wild Type PDPN
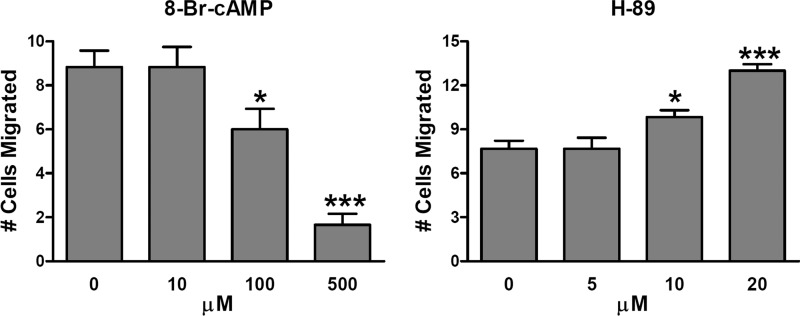
Results from kinase assays and site mutants suggest that PKA activation inhibits PDPN-mediated cell migration. As shown in Fig. 3 and supplemental Fig. 2, the PKA agonist 8-Br-cAMP significantly decreased the migration of cells transfected with wild type PDPN. Conversely, treatment of these cells with the PKA blocker H-89 significantly increased their migration. These data are consistent with the hypothesis that PKA phosphorylates PDPN to decrease cell migration.
FIGURE 3.
Effect of PKA modulators on cell migration. Wound healing experiments were performed on confluent monolayers of PdpnWT cells treated with the PKA agonist 8-Br-cAMP or PKA inhibitor H-89 as indicated. Cell migration was quantitated as the number of cells that entered a 300 × 200-μm area of in the center of a wound in 18 h (mean ± S.E., n = 6). Single and triple asterisks indicate p < 0.05 and p < 0.001 when compared with untreated controls by Student's t test.
PDPN Expression in Fibroblasts Facilitates Neighboring Tumor Cell Migration and Survival
PDPN expression in cancer-associated fibroblasts has been linked to increased tumor invasive and metastatic potential (26, 27). We utilized PdpnKo cells to investigate mechanisms that may underlie the relationship between cancer cells and their neighboring fibroblasts. Melanoma cells that express PDPN as a functionally relevant biomarker and potential chemotherapeutic target were chosen as subjects for this investigation (18).
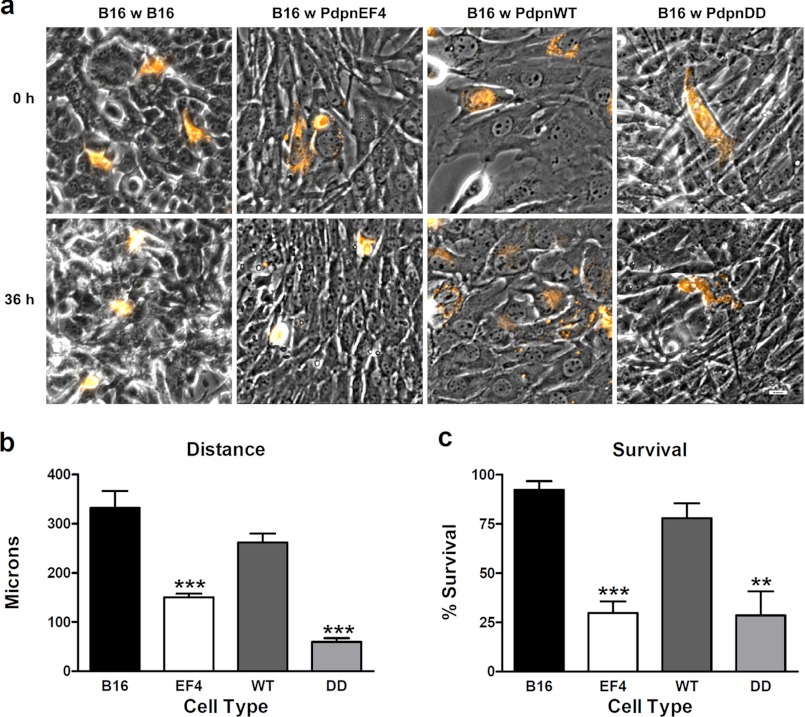
Melanoma cells migrated an average distance of about 9.2 μm/h when surrounded by other melanoma cells (Fig. 4, a and b, and supplemental Fig. 3a). The migration of melanoma cells when surrounded by fibroblasts expressing (PdpnWT cells) was not significantly different from their migration when surrounded by melanoma cells (Fig. 4, a and b, and supplemental Fig. 3b). However, melanoma cell migration was decreased by over 50%, to an average distance of only 4.2 μm/h, when surrounded by PdpnEF4 control cells (Fig. 4, a and b). Thus, fibroblasts expressing PDPN appeared to facilitate the migration of neighboring tumor cells better than PDPN-deficient fibroblasts.
FIGURE 4.
Effect of Pdpn expression on the motility of neighboring melanoma cells. a, movement of 1,1′-dioctadecyl-3,3,3′3′-tetramethylindocarbocyanine perchlorate-labeled B16 melanoma cells cocultured with unlabeled B16 cells or PdpnKo cells transfected wild type Pdpn (WT), phosphomimetic Pdpn (DD), or empty parental vector (EF4) was measured by video microscopy for 36 h (bar = 20 μm). b, migration of individual motile cells was quantitated as micrometers traveled over 36 h (mean ± S.E., n = 5–10). c, the percentage of morphologically intact cells was used to quantitate the survival of cocultured melanoma cells in a 600 × 600-μm field (mean ± S.E., n = 4). Double and triple asterisks indicate p < 0.01 and p < 0.001 when compared with B16 cells cultured with B16 cells by Student's t test, respectively.
In addition to increasing tumor cell motility, PDPN expression in fibroblasts affected the viability of neighboring melanoma cells. As shown in Fig. 4, a and c, about 70% of melanoma cells cocultured with cells lacking Pdpn (PdpnEF4) went through morphological changes indicative of apoptosis (see supplemental Fig. 3c) when compared with about 20% of melanoma cells cocultured with PdpnWT cells or 10% cocultured with other melanoma cells. Also, as shown in Fig. 4, melanoma cells cocultured with fibroblasts expressing phosphomimetic PDPN behaved similarly to those cultured with control transfectants (see supplemental Fig. 3d). Taken together, these data indicate that PDPN expression levels and phosphorylation status in fibroblasts can affect the motility and viability of neighboring tumor cells.
DISCUSSION
PDPN expression enhances the motility and invasion of several transformed cell types including mammary carcinoma (7, 8), glioma (28), and squamous carcinoma cells (29–31). PDPN is found at the invasive front of many tumors, which is consistent with its role in promoting invasion (7, 8). In addition to tumor cells themselves, PDPN is also located on endothelial cells of lymphatic vessels, which can facilitate tumor invasion and metastasis (32, 33). PDPN expression in cancer-associated fibroblasts has also been linked to tumor aggression and poor prognosis of lung cancers (26, 27, 34, 35). Consistent with these findings, our results indicate that PDPN expression in fibroblasts can facilitate migration and survival of neighboring tumor cells in the microenvironment. Further elucidation of events underlying this relationship should lead to a greater understanding of fundamental mechanisms that guide stroma-tumor interactions and how they may be exploited to combat cancer.
Indeed, PDPN presents great potential as a functionally relevant biomarker and chemotherapeutic target (8–10). For example, reagents that target PDPN and a PDPN-interacting partner (tetraspanin CD9) can inhibit lung metastasis of CHO cells transfected with PDPN (36, 37), melanoma tumorigenesis (18), and glioma progression (38, 39) in mice.
A variety of signaling agents and cytokines can induce PDPN expression and cell motility (5, 6, 40). For example, the Src tyrosine kinase utilizes the Cas/BCAR1 adaptor protein to augment PDPN expression to promote transformed cell motility required for malignant cells to break out of their microenvironment to become invasive and metastatic (4, 41).
Elucidating how PDPN promotes cell motility is necessary to understand its role in tumorigenesis and its potential as a chemotherapeutic target. Our findings that PDPN can be phosphorylated to inhibit cell migration and that PDPN expression influences the effects of fibroblasts on neighboring tumor cells shed considerable light on this process. For example, modulators of kinases or phosphatases may be employed to enhance the effects of reagents targeting PDPN to treat a variety of cancers (8–10), and possibly other diseases including psoriasis (40).
Acknowledgments
We thank Tokai Hit for their generous help and consignment of a INUBG2A-ZILCS stage top incubator used for live cell imaging (Tokai Hit, Shizuoka, Japan).
This work was funded in part by grants from the Research Foundation of University of Medicine and Dentistry of New Jersey (UMDNJ) and Sentrimed (to G. S. G.) and National Institutes of Health Grant HL083034 (to M. I. R.) and CA58530 (to W. T. M.).

This article contains supplemental Figs. 1–3.
Throughout this study, the following designations are used: PdpnKo, PDPN knock-out mouse construct; PdpnWT, full-length wild-type mouse PDPN construct; PdpnAA, Pdpn(S167A/S171A) construct encoding nonphosphorylatable PDPN with both intracellular serines mutated to alanine; PdpnDD, Pdpn(S167D/S171D) construct encoding phosphomimetic PDPN with both intracellular serines mutated to aspartate; PdpnEF4, PDPN empty vector control.
- PDPN
- podoplanin
- 8-Br-cAMP
- 8-bromo-cyclic AMP.
REFERENCES
- 1. Ramirez M. I., Millien G., Hinds A., Cao Y., Seldin D. C., Williams M. C. (2003) T1α, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev. Biol. 256, 61–72 [DOI] [PubMed] [Google Scholar]
- 2. Schacht V., Ramirez M. I., Hong Y. K., Hirakawa S., Feng D., Harvey N., Williams M., Dvorak A. M., Dvorak H. F., Oliver G., Detmar M. (2003) T1α/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 22, 3546–3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Millien G., Spira A., Hinds A., Wang J., Williams M. C., Ramirez M. I. (2006) Alterations in gene expression in T1α null lung: a model of deficient alveolar sac development. BMC. Dev. Biol. 6, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shen Y., Chen C. S., Ichikawa H., Goldberg G. S. (2010) SRC induces podoplanin expression to promote cell migration. J. Biol. Chem. 285, 9649–9656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gandarillas A., Scholl F. G., Benito N., Gamallo C., Quintanilla M. (1997) Induction of PA2.26, a cell-surface antigen expressed by active fibroblasts, in mouse epidermal keratinocytes during carcinogenesis. Mol. Carcinog. 20, 10–18 [DOI] [PubMed] [Google Scholar]
- 6. Nose K., Saito H., Kuroki T. (1990) Isolation of a gene sequence induced later by tumor-promoting 12-O-tetradecanoylphorbol-13-acetate in mouse osteoblastic cells (MC3T3-E1) and expressed constitutively in ras-transformed cells. Cell Growth Differ. 1, 511–518 [PubMed] [Google Scholar]
- 7. Wicki A., Lehembre F., Wick N., Hantusch B., Kerjaschki D., Christofori G. (2006) Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell 9, 261–272 [DOI] [PubMed] [Google Scholar]
- 8. Wicki A., Christofori G. (2007) The potential role of podoplanin in tumour invasion. Br. J. Cancer 96, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raica M., Cimpean A. M., Ribatti D. (2008) The role of podoplanin in tumor progression and metastasis. Anticancer Res. 28, 2997–3006 [PubMed] [Google Scholar]
- 10. Astarita J. L., Acton S. E., Turley S. J. (2012) Podoplanin: emerging functions in development, the immune system, and cancer. Front Immunol. 3, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu Y., Khan J., Khanna C., Helman L., Meltzer P. S., Merlino G. (2004) Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat. Med. 10, 175–181 [DOI] [PubMed] [Google Scholar]
- 12. Asano E., Maeda M., Hasegawa H., Ito S., Hyodo T., Yuan H., Takahashi M., Hamaguchi M., Senga T. (2011) Role of palladin phosphorylation by extracellular signal-regulated kinase in cell migration. PLoS One 6, e29338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim L. C., Song L., Haura E. B. (2009) Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 6, 587–595 [DOI] [PubMed] [Google Scholar]
- 14. Brábek J., Constancio S. S., Siesser P. F., Shin N. Y., Pozzi A., Hanks S. K. (2005) Crk-associated substrate tyrosine phosphorylation sites are critical for invasion and metastasis of SRC-transformed cells. Mol. Cancer Res. 3, 307–315 [DOI] [PubMed] [Google Scholar]
- 15. Goldberg G. S., Alexander D. B., Pellicena P., Zhang Z. Y., Tsuda H., Miller W. T. (2003) Src phosphorylates Cas on tyrosine 253 to promote migration of transformed cells. J. Biol. Chem. 278, 46533–46540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Casnellie J. E. (1991) Assay of protein kinases using peptides with basic residues for phosphocellulose binding. Methods Enzymol. 200, 115–120 [DOI] [PubMed] [Google Scholar]
- 17. Alexander D. B., Ichikawa H., Bechberger J. F., Valiunas V., Ohki M., Naus C. C., Kunimoto T., Tsuda H., Miller W. T., Goldberg G. S. (2004) Normal cells control the growth of neighboring transformed cells independent of gap junctional communication and SRC activity. Cancer Res. 64, 1347–1358 [DOI] [PubMed] [Google Scholar]
- 18. Ochoa-Alvarez J. A., Krishnan H., Shen Y., Acharya N. K., Han M., McNulty D. E., Hasegawa H., Hyodo T., Senga T., Geng J. G., Kosciuk M., Shin S. S., Goydos J. S., Temiakov D., Nagele R. G., Goldberg G. S. (2012) Plant lectin can target receptors containing sialic acid, exemplified by podoplanin, to inhibit transformed cell growth and migration. PLoS One 7, e41845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shen Y., Jia Z., Nagele R. G., Ichikawa H., Goldberg G. S. (2006) SRC uses Cas to suppress Fhl1 in order to promote nonanchored growth and migration of tumor cells. Cancer Res. 66, 1543–1552 [DOI] [PubMed] [Google Scholar]
- 20. Goldberg G. S., Martyn K. D., Lau A. F. (1994) A connexin 43 antisense vector reduces the ability of normal cells to inhibit the foci formation of transformed cells. Mol. Carcinog. 11, 106–114 [DOI] [PubMed] [Google Scholar]
- 21. Valiunas V., Bechberger J. F., Naus C. C., Brink P. R., Goldberg G. S. (2005) Nontransformed cells can normalize gap junctional communication with transformed cells. Biochem. Biophys. Res. Commun. 333, 174–179 [DOI] [PubMed] [Google Scholar]
- 22. Schacht V., Dadras S. S., Johnson L. A., Jackson D. G., Hong Y. K., Detmar M. (2005) Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am. J. Pathol. 166, 913–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liang H., Wu H., Giorgadze T. A., Sariya D., Bellucci K. S., Veerappan R., Liegl B., Acs G., Elenitsas R., Shukla S., Youngberg G. A., Coogan P. S., Pasha T., Zhang P. J., Xu X. (2007) Podoplanin is a highly sensitive and specific marker to distinguish primary skin adnexal carcinomas from adenocarcinomas metastatic to skin. Am. J. Surg. Pathol. 31, 304–310 [DOI] [PubMed] [Google Scholar]
- 24. Durchdewald M., Guinea-Viniegra J., Haag D., Riehl A., Lichter P., Hahn M., Wagner E. F., Angel P., Hess J. (2008) Podoplanin is a novel fos target gene in skin carcinogenesis. Cancer Res. 68, 6877–6883 [DOI] [PubMed] [Google Scholar]
- 25. Martín-Villar E., Megías D., Castel S., Yurrita M. M., Vilaró S., Quintanilla M. (2006) Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J. Cell Sci. 119, 4541–4553 [DOI] [PubMed] [Google Scholar]
- 26. Ito S., Ishii G., Hoshino A., Hashimoto H., Neri S., Kuwata T., Higashi M., Nagai K., Ochiai A. (2012) Tumor promoting effect of podoplanin-positive fibroblasts is mediated by enhanced RhoA activity. Biochem. Biophys. Res. Commun. 422, 194–199 [DOI] [PubMed] [Google Scholar]
- 27. Hoshino A., Ishii G., Ito T., Aoyagi K., Ohtaki Y., Nagai K., Sasaki H., Ochiai A. (2011) Podoplanin-positive fibroblasts enhance lung adenocarcinoma tumor formation: podoplanin in fibroblast functions for tumor progression. Cancer Res. 71, 4769–4779 [DOI] [PubMed] [Google Scholar]
- 28. Cortez M. A., Nicoloso M. S., Shimizu M., Rossi S., Gopisetty G., Molina J. R., Carlotti C., Jr., Tirapelli D., Neder L., Brassesco M. S., Scrideli C. A., Tone L. G., Georgescu M. M., Zhang W., Puduvalli V., Calin G. A. (2010) miR-29b and miR-125a regulate podoplanin and suppress invasion in glioblastoma. Genes Chromosomes Cancer 49, 981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martín-Villar E., Scholl F. G., Gamallo C., Yurrita M. M., Muñoz-Guerra M., Cruces J., Quintanilla M. (2005) Characterization of human PA2.26 antigen (T1α-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int. J. Cancer 113, 899–910 [DOI] [PubMed] [Google Scholar]
- 30. Martín-Villar E., Fernández-Muñoz B., Parsons M., Yurrita M. M., Megías D., Pérez-Gómez E., Jones G. E., Quintanilla M. (2010) Podoplanin associates with CD44 to promote directional cell migration. Mol. Biol. Cell 21, 4387–4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Funayama A., Cheng J., Maruyama S., Yamazaki M., Kobayashi T., Syafriadi M., Kundu S., Shingaki S., Saito C., Saku T. (2011) Enhanced expression of podoplanin in oral carcinomas in situ and squamous cell carcinomas. Pathobiology 78, 171–180 [DOI] [PubMed] [Google Scholar]
- 32. Mumprecht V., Detmar M. (2009) Lymphangiogenesis and cancer metastasis. J. Cell Mol. Med. 13, 1405–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mohammed R. A., Martin S. G., Gill M. S., Green A. R., Paish E. C., Ellis I. O. (2007) Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am. J. Surg. Pathol. 31, 1825–1833 [DOI] [PubMed] [Google Scholar]
- 34. Ito M., Ishii G., Nagai K., Maeda R., Nakano Y., Ochiai A. (2012) Prognostic impact of cancer-associated stromal cells in patients with stage I lung adenocarcinoma. Chest 142, 151–158 [DOI] [PubMed] [Google Scholar]
- 35. Kawase A., Ishii G., Nagai K., Ito T., Nagano T., Murata Y., Hishida T., Nishimura M., Yoshida J., Suzuki K., Ochiai A. (2008) Podoplanin expression by cancer associated fibroblasts predicts poor prognosis of lung adenocarcinoma. Int. J. Cancer 123, 1053–1059 [DOI] [PubMed] [Google Scholar]
- 36. Kato Y., Kaneko M. K., Kunita A., Ito H., Kameyama A., Ogasawara S., Matsuura N., Hasegawa Y., Suzuki-Inoue K., Inoue O., Ozaki Y., Narimatsu H. (2008) Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci. 99, 54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakazawa Y., Sato S., Naito M., Kato Y., Mishima K., Arai H., Tsuruo T., Fujita N. (2008) Tetraspanin family member CD9 inhibits Aggrus/podoplanin-induced platelet aggregation and suppresses pulmonary metastasis. Blood 112, 1730–1739 [DOI] [PubMed] [Google Scholar]
- 38. Chandramohan V., Bao X., Kato Kaneko M., Kato Y., Keir S. T., Szafranski S. E., Kuan C. T., Pastan I. H., Bigner D. D. (2013) Recombinant anti-podoplanin (NZ-1) immunotoxin for the treatment of malignant brain tumors. Int. J. Cancer 132, 2339–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kato Y., Vaidyanathan G., Kaneko M. K., Mishima K., Srivastava N., Chandramohan V., Pegram C., Keir S. T., Kuan C. T., Bigner D. D., Zalutsky M. R. (2010) Evaluation of anti-podoplanin rat monoclonal antibody NZ-1 for targeting malignant gliomas. Nucl. Med. Biol. 37, 785–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Honma M., Minami-Hori M., Takahashi H., Iizuka H. (2012) Podoplanin expression in wound and hyperproliferative psoriatic epidermis: regulation by TGF-β and STAT-3 activating cytokines, IFN-γ, IL-6, and IL-22. J. Dermatol. Sci. 65, 134–140 [DOI] [PubMed] [Google Scholar]
- 41. Krishnan H., Miller W. T., Goldberg G. S. (2012) SRC points the way to biomarkers and chemotherapeutic targets. Genes Cancer 3, 426–435 [DOI] [PMC free article] [PubMed] [Google Scholar]