Background: Human β-defensins are predominantly expressed in the male reproductive system, but their functions remain unknown.
Results: Recombinant human DEFB114 rescues human sperm motility and d-GalN-sensitized mice under LPS challenges through its LPS-neutralizing activity.
Conclusion: Recombinant DEFB114 peptides have therapeutic potential for LPS-induced inflammatory diseases.
Significance: This work contributes to unveiling novel functions of human β-defensins that are crucial for addressing their physiological and pathological mechanisms.
Keywords: Defensins, Inflammation, Innate Immunity, Lipopolysaccharide (LPS), Sperm, DEFB114
Abstract
Lipopolysaccharide (LPS) is an important pathological factor involved in serious inflammatory diseases and male reproductive impairments. Emerging evidence demonstrates that antimicrobial peptides possess protective activity in response to LPS-induced inflammation. However, the LPS-binding and/or immunosuppressive activity of β-defensins (DEFBs) has been underestimated. In the present work, we characterized a novel human defensin, DEFB114, which was expressed predominantly in the epididymis and gingival cells at the RNA level. Homogenous recombinant DEFB114 peptides were prepared and characterized using mass spectrometry. DEFB114 protein exhibited a broad spectrum of antimicrobial activity with salt sensitivity against typical pathogenic microbes (i.e. Escherichia coli, Staphylococcus aureus, and Candida albicans). Interestingly, DEFB114 demonstrated novel LPS-binding activity in vitro and inhibited TNF-α release in RAW264.7 cultures through the inhibition of MAPK p42/44 when challenged with LPS. Moreover, DEFB114 could also rescue the LPS-induced reduction of human sperm motility in vitro and protect d-galactosamine-sensitized C57BL/6 mice from LPS-induced lethality in vivo. The protective activity of DEFB114 on RAW264.7, human sperm, and the d-galactosamine-sensitized mice was disulfide bond-dependent because alkylated DEFB114 lost its activity. The low cytotoxicity of the DEFB114 peptide toward human erythrocytes is indicative of its potential therapeutic use in the treatment of LPS-induced inflammation, LPS contamination, and potentially septic shock.
Introduction
The abuse of conventional antibiotics has led to the increasing emergence of resistant pathogenic bacteria, which remain one of the gravest challenges facing modern medicine (1). Moreover, antibiotic treatment could stimulate the release of an excess of lipopolysaccharide (LPS), a major constituent of the outer membrane of Gram-negative bacteria (2) that causes serious sepsis or inflammatory responses. LPS can be opsonized by LPS-binding protein and transferred to CD14 to form a complex that is recognized by Toll-like receptor 4 (TLR-4),5 which is mainly expressed on monocytes and macrophages (3), to activate the immune system. LPS is considered one of the most potent initiators of inflammatory cytokines and a crucial mediator of sepsis and septic shock (2). Therefore, the enhanced release of LPS induced by antibiotics might exacerbate existing inflammation and promote further damage in inflamed areas. In the United States alone, over 600,000 cases of sepsis and 120,000 cases of death are reported on an annual basis (4). To date, no treatment is available to combat sepsis-mediated lethality (5).
In males, 1) neonatal exposure to LPS in rats has an immediate impact on gonocyte genesis in addition to its impairment of the onset of puberty and sexual performance in late adulthood (6); 2) LPS also increases the levels of proinflammatory and oxidative stress markers in the testis (7) and causes apoptosis of germ cells (8); and 3) acting through the LPS receptor TLR-4, which is expressed on the membrane of human and mouse sperm, LPS reduces sperm motility, induces sperm apoptosis, and significantly impairs the potential for fertilization (9). Conversely, molecules that neutralize LPS activity, such as polymyxin B, rescue LPS-induced sperm motility loss and apoptosis via the reduction of LPS binding to TLRs (10). Alternatively, antioxidant and/or anti-inflammatory reagents, such as l-carnitine, also ameliorate proinflammatory and oxidative stress and display protective roles against infertility in severely infected or septic males (7). Thus, molecules with LPS-neutralizing activity and anti-inflammatory effects may have physiological and therapeutic significance. Their common mechanism is also an important clue for the screening of new therapeutic targets.
Fortunately, males have developed an array of natural mechanisms to combat bacteria and LPS. Antimicrobial peptides (AMPs), as ancient evolutionary cationic peptides, protect their hosts against a vast array of microorganisms. The most highlighted human AMPs are cathelicidins and defensins. Males preferentially express β-defensin (DEFB) gene clusters in their reproductive tract, particularly in the epididymis and testis (11). The defensin family is viewed as an effective antimicrobial and immunomodulatory protein family in inflammatory diseases (12). Some human epididymal DEFBs, such as DEFB118 (formerly ESC42), SPAG11E (also known as Bin1b), and DEFB126, possess both antimicrobial and diverse functions in sperm fertility. Moreover, a previous study suggested that defensins might play roles in the protection against LPS-induced inflammation because the intraperitoneal injection of rats with LPS increased the production of Spag11 variants and major defensin expression in the male reproductive tract (13). However, in a rat model of epididymitis, LPS-induced epididymitis decreased the expression of epididymal β-defensins, disrupted SPAG11E expression, and resulted in the impairment of sperm motility (14). The controversial reactions of SPAG11E in response to LPS reflect the complexity of β-defensins in the inflammatory response. The underlying mechanisms remain to be addressed.
It seems that the antimicrobial activity of DEFBs is only the tip of the iceberg in their extensive network of interactions within the immune system and reproductive system. Because of the notion that DEFBs have relatively low LPS neutralization potency (5, 15, 16), the emerging novel LPS-binding capacity of DEFB123 and its ability to inhibit LPS-mediated effects in vitro and in vivo (17) suggest that the LPS neutralization potency of DEFBs might be underestimated. So far, a total of 39 potential human DEFB genes have been identified (18, 19). However, no human DEFB protein products, except for DEFB123 (17), have been tested regarding their LPS-binding capacity and inhibitory effects on LPS-mediated inflammation in vitro and in vivo. DEFBs that are preferentially expressed in the male reproductive tract (11) should be revisited because AMPs are known to modulate the immune response via binding to LPS and blocking LPS-dependent cytokine induction efficiently, exhibiting a therapeutic significance for severe Gram-negative infections and aggressive antibiotic therapies (12, 20).
Recently, we have been interested in a novel human β-defensin, DEFB114 (GenBankTM ID 245928; chromosome 6p21) (18), which was reported to be expressed in the epididymis and saliva according to the records in the BioGPS database. The most predominant expression of DEFB114 RNA is in the epididymis, where it is expressed 2-fold higher than in saliva. Its constitutive mRNA expression in gingival keratinocytes was induced by interleukin (IL)-1β and Candida species (21). Thereby, this peptide was speculated to play a dual role in host defense and fertility. To date, neither the active human DEFB114 peptide nor its functions have been reported. In the present study, we demonstrated that the recombinant human DEFB114 peptide, besides its antimicrobial potential, not only possesses LPS-binding activity and anti-inflammation effects in vitro and in vivo, but could also protect human sperm from motility loss when challenged with LPS. Our data suggest that the LPS-neutralizing and anti-inflammation potency of DEFBs might have been underestimated and needs to be revised carefully. DEFB peptides could also be a natural candidate pool for anti-LPS and anti-inflammation peptide screening.
EXPERIMENTAL PROCEDURES
Expression of DEFB114 in the Human Epididymis
Human epididymis was obtained from an adult male donor who died in an accident. This procedure followed the ethical guidelines of the Institute of Biochemistry and Cell Biology. One of the donor's epididymides was dissected into the caput, corpus, and cauda regions according to their morphological features (22) to extract RNA and proteins. The other epididymis was fixed, paraffin-embedded, and sliced for immunostaining. DEFB114 expression at the RNA level in the human epididymis was confirmed by RT-PCR using the forward primer 5′-ATGAGGATCTTTTACTATCTCC-3′ and reverse primer 5′-AAACATATCATCTTCTTCATA-3′. The PCR program consisted of the following: step 1, denaturation at 94 °C for 5 min; step 2, 25 cycles of reaction (94 °C for 30 s, 57 °C for 30 s, and 72 °C for 45 s); and step 3, 72 °C for 5 min. The DEFB114 protein in the human epididymis of the caput, corpus, and cauda regions was also detected with the purified antibody in Western blotting (1:2000 dilution) and immunohistochemistry staining (1:100 dilution) assays.
Expression and Purification of the Recombinant DEFB114 Peptide
The expression vector pTWIN1 and restriction enzymes were purchased from New England BioLabs Co., Ltd. (Beijing, China). Ex TaqDNA polymerase and T4 DNA ligase were from Takara Co., Ltd. (Dalian, China). Escherichia coli DH5α and BL21 (DE3) strains were employed for subcloning and recombinant protein expression, respectively.
The general approach was as described previously (23). The signal peptide sequence was predicted using SignalP version 3.0. The sequence encoding the mature DEFB114 peptide was amplified and inserted into pTWIN1 in frame with the SapI and PstI sites. The forward and reverse primers were 5′-CAGGCTCTTCTAACGATCGTTGCACC-3′ and 5′-CACCTGCAGTTAAAACATATCATCTTCTTC-3′, respectively. The recombinant constructs were verified by sequencing and transformed into competent E. coli BL21 (DE3) cells. A single colony was cultured in 1 liter of Luria-Bertani (LB) medium containing 100 μg/ml ampicillin with shaking at 37 °C. When the A600 nm reached 0.8–1.0, protein expression was initiated by the addition of 0.3 mm isopropyl 1-thio-β-d-galactopyranoside followed by shaking at 16 °C for 14–16 h. Cells harvested by centrifugation (5,000 × g for 10 min) were resuspended in 30 ml of lysis buffer (20 mm sodium phosphate buffer, 0.5 m NaCl, 0.1 mm EDTA, 0.1% Triton X-100 (v/v), pH 8.5) and lysed by sonication on ice at 200 watts for 5 min. The supernatant was separated by centrifuging at 12,000 × g (4 °C for 30 min) and co-incubated with 10 ml of chitin beads (New England BioLabs) with gentle shaking at 4 °C for 30 min. The standard protocol (New England BioLabs) was applied for the purification of the fusion protein. Briefly, the beads were washed with 20 volumes of washing buffer (20 mm sodium phosphate buffer, 0.5 m NaCl, 0.1 mm EDTA, 0.1% Triton X-100, pH 8.0), followed by the addition of 5 volumes of cleavage buffer (20 mm sodium phosphate buffer, 0.5 m NaCl, 0.1 mm EDTA, pH 5.5, in sterile pyrogen-free water) and subsequent incubation at room temperature overnight. Then 20 μl of the chitin beads loaded with the fusion protein before and after co-incubation with cleavage buffer were added separately to equal volumes of 2× SDS-PAGE loading buffer, boiled in a water bath, and subjected to SDS-PAGE analysis to assess cleavage efficiency. The ratio of the target protein to total proteins was evaluated by analyzing the images of the SDS-polyacrylamide gels using the Gel Image System (TANON Co., Shanghai, China).
The peptides were concentrated and purified using fast protein liquid chromatography (FPLC) system on a Superdex-75 column (GE Healthcare) with elution buffer (20 mm sodium phosphate buffer, 50 mm NaCl, pH 5.5, in sterile pyrogen-free water) at a flow rate of 0.5 ml/min. A Pierce BCA protein assay kit (Fisher) was used for the quantitative analysis of protein concentrations. The recombinant DEFB114 was then analyzed by high performance liquid chromatography (HPLC; Agilent 1200) with a Agilent ZORBAX 300SB-C8 column (4.6 × 150 mm, 5 μm) using mobile phases A and C with a two-step linear gradient of 0–5% C in the first 5.0 min, followed by 5.0–100% C in the next 25 min (mobile phase A, 0.1% trifluoroacetic acid (TFA); mobile phase C, 0.1% TFA, 90% acetonitrile-water).
Matrix-assisted Laser Desorption Ionization Time-of-flight (MALDI-TOF) Mass Spectrometry and LC-MS/MS Analysis
Identification of the recombinant DEFB114 peptide was performed using MALDI-TOF mass spectra recorded on a Bruker Microflex MALDI-TOF MS spectrometer. The sample was added to an equal volume of the matrix (2,5-dihydroxybenzoic acid). The instrument was operated in the positive ion/linear mode with an accelerating voltage of 20 kV.
In order to determine the disulfide connectivity of DEFB114 by mass spectrometry, tryptic map analysis was performed. The buffer of the DEFB114 protein was exchanged to 50 mm NH4HCO3 (pH 7.8), and the protein concentration was adjusted to 1 mg/ml before digestion. Digestion of the DEFB114 protein was conducted by adding modified trypsin to a final protease/protein ratio of 1:50 at 37 °C for 24 h according to the usage information of the sequencing grade modified trypsin (Promega V5113). The products of digestion were centrifuged, and the supernatant was analyzed by an Agilent HPLC system model 1200 with an Agilent ZORBAX Eclipse XDB-C18 column (4.6 × 150 mm, 5 μm). The complex disulfide linkages in the protein were determined by capillary LC-MS/MS using Easy nLC1000 HPLC system and Thermo Q Exactive mass spectrometry (Fisher) in Shanghai Applied ProteinTechnology Co., Ltd.
Reduction and Alkylation of Recombinant DEFB114
Recombinant DEFB114 (20 μm) was reduced with different concentrations of dithiothreitol (DTT; 1, 5, and 20 mm) for 30 min at 37 °C in 10 mm phosphate buffer (pH 7.4) and then alkylated with 30 mm iodoacetamide for 1 h at 25 °C in the dark. The reaction was stopped by adding 30 mm cysteine and incubated for 30 min at 25 °C. The alkylated peptide was then analyzed by mass spectrometry.
To evaluate the effects of the alkylated peptide on cell level and animal models, the modified peptide was concentrated using a Millipore 3-kDa membrane, and buffer exchange was performed by passing it through a Sephadex G-15 column pre-equilibrated with 10 mm phosphate-buffered saline (PBS; pH 7.4). The protein was adjusted to 0.5–1.0 mg/ml and filtered through a sterile 0.2-μm membrane. The sterile sample was stored at 4 °C in the dark before use.
Antimicrobial and Hemolytic Assays
The antimicrobial activity of DEFB114 was determined by using the colony-forming unit (cfu) assay as described previously (24). The culture media for E. coli K12D31, Staphylococcus aureus, and Candida albicans were LB broth, Mueller-Hinton Broth, and YPD, respectively. To assess the salt tolerance of the peptide, 40 μg/ml DEFB114 was incubated with the above strains in the presence or absence of 150 mm NaCl. Individual microbial colonies were counted manually, and antimicrobial activity was calculated as described previously (23). All of the values were the averages of three measurements conducted in triplicate. The peptide concentration at which no viable colonies were formed served as the minimum microbicidal concentration.
The hemolytic activity of DEFB114 was determined as described by Kluver et al. (25) with minor modifications. Synthetic human cathelicidin LL-37 (GL Biochem, Shanghai, China) was used as a positive control (26). The absorbance at 450 nm for saline- and 0.1% Triton X-100-treated erythrocytes served as the 0 and 100% hemolysis controls, respectively. Hemolysis was calculated as described previously (25). All measurements were the means of three experiments conducted in triplicate.
Affinity of DEFB114 for LPS-Sepharose Beads
LPS from E. coli O111:B4 (Sigma-Aldrich) was cross-linked to CNBr-activated Sepharose 6B (GE Healthcare) according to the manufacturer's instructions. The Purpald assay was used to determine the coupling efficiency (27). Purified DEFB114 peptides (2.0 mg) were added to 100 μl of LPS-conjugated beads in binding buffer (10 mm sodium phosphate buffer, pH 7.4). After preincubation at room temperature for 30 min, the suspension was separated by centrifugation at 1,000 × g for 1 min. The beads were eluted with the binding buffer at different NaCl concentrations (0.1, 0.5, 1.0, and 2.0 m) followed by elution with 4 m urea. The eluate was subjected to SDS-PAGE analysis.
The specificity of the affinity of DEFB114 for the LPS beads was determined by adding competitive LPS at different concentrations to the binding buffer. After washing with the binding buffer, the protein bound to the beads was eluted with 4 m urea in the binding buffer and analyzed on 15% SDS-polyacrylamide gels.
Affinity Measurements between LPS and Defensin Proteins by Fortebio's Octet System
Purified protein was diluted or dialyzed in dialysis buffer (20 mm sodium phosphate, pH 7.4) and biotinylated at room temperature for 30 min. The unconjugated biotin was removed by Zeba Spin desalting column (catalogue no. 89882, Fisher). The biotin-conjugated protein was diluted to 50 μg/ml. The sensors (Super Streptavidin) were prewet in dialysis buffer for 15 min prior to use and then were loaded with biotinylated proteins for 15 min. The sensors without loading of biotinylated proteins were used as controls to correct base-line drift. LPS was prepared in a serial dilution (10, 5, 2.5, 1.25, 0.6, and 0.3 μm) in a 96-well plate. The measurements were carried out automatically at room temperature.
Cells and Conditions
Macrophages (RAW264.7) were obtained from the American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 100 units/ml penicillin, 100 mg/ml streptomycin, 2 mm l-glutamine, and 10% fetal calf serum. The cells were grown at 37 °C and 5% CO2 in humidified air. The cells were seeded in 96-well plates at 1.0 × 105 cells/ml or 6-well plates at 2.0 × 106 cells/ml. To explore the effects of DEFB114 on the inflammatory response of macrophages, we followed the protocols that had been utilized in the research of DEFB123 (17) with minor modification. RAW264.7 cells were preincubated with or without DEFB114 in cell culture medium for 30 min before the addition of LPS (E. coli O111:B4, Sigma-Aldrich) and further incubated for 5 h. For MAPK determination experiments, the cells were stimulated for 15 min. Tumor necrosis factor-α (TNF-α) levels per 50 μl of cell culture supernatant were determined using a mouse cytokine enzyme-linked immunosorbent assay (ELISA) kit (Pierce) according to the manufacturer's instructions. For alkylated DEFB114, similar assays were conducted. The corresponding dose-response data were normalized based on the total amount of TNF-α release in relation to medium alone (equal to 100%). All experiments were performed in triplicate.
Western Blot Analysis of MAPK
RAW264.7 cells were preincubated in cell culture medium with or without DEFB114 for 30 min before the addition of LPS (50 ng/ml) (E. coli O111:B4; Sigma-Aldrich). For MAPK determination experiments, the cells were stimulated with LPS for 15 min. The cells were lysed with 2% SDS and incubated in a water bath at 100 °C for 5 min. Supernatants were separated by centrifugation at 12,000 × g for 30 min at 4 °C, and protein concentrations were determined using the BCA method. Thirty micrograms of protein per well were loaded to analyze p44/42 MAPK (Rabbit mAb 4695, 1:10,000; Cell Signaling Technology) and phospho-p44/42 MAPK (rabbit mAb 4370, 1:10,000; Cell Signaling Technology) in comparison with β-actin as a control (rabbit mAb 4970, 1:10,000; Cell Signaling Technology) in Western blots. Goat anti-rabbit IgG-HRP antibody A0545 (1:80,000; Sigma-Aldrich) was the secondary antibody.
Animal Assays
Care and treatment of the animals was based on the institutional animal care policies of Shanghai Institute of Planned Parenthood Research. Male C57BL/6 mice (specific pathogen-free) with body weight ranging from 20 to 22 g were purchased from SIPPR-BK Animal Co., Ltd. (Shanghai, China). d-Galactosamine (d-GalN) and LPS derived from E. coli (strain O111:B4) were purchased from Sigma-Aldrich. All dilutions were conducted in 0.9% NaCl (w/v) in endotoxin-free water. Before injection, either DEFB114 or alkylated DEFB114 was added to the LPS saline solution and incubated at 37 °C for 15 min. C57BL/6 mice were injected intraperitoneally with d-GalN (50 mg/0.2 ml of saline), LPS (100 ng/0.2 ml of saline) with either DEFB114 or alkylated DEFB114, and saline alone (0.2 ml). Deaths due to LPS-induced sepsis were recorded at 10 and 24 h postinjection.
To further evaluate the therapeutic potential of DEFB114, C57BL/6 mice were injected intraperitoneally with 100 ng of LPS on one side and then injected with either DEFB114 or alkylated DEFB114 in 0.5 ml of saline on the opposite side. Concomitantly, all mice were sensitized with sterile d-GalN (50 mg/0.2 ml of saline) solution. Deaths due to LPS induction were recorded at 10 and 24 h postinjection.
Semen Samples and Human Sperm Motility Assays
Semen samples were collected by masturbation after 3–5 days of abstinence, and the sperm parameters were evaluated according to the criteria of the World Health Organization (49) including the volume of semen, sperm concentration, motility, and morphology as well as leukocyte concentration. With informed consent, the semen samples obtained from each individual were tested separately. Semen samples were first liquefied at 37 °C for 30 min. Sperm was collected by centrifugation at 300 × g for 5 min at room temperature and resuspended in human tubal fluid medium containing 10% serum substitute supplement to 2–5 × 106 sperms/ml. Then 200 ng/ml LPS, 10–50 μg/ml DEFB114, or 10–50 μg/ml alkylated DEFB114 were added to the culture medium and mixed gently. The mixture was incubated for 0–6 h at 37 °C under a 5% CO2 atmosphere. At the end of the incubation, sperm motility parameters were measured by a computer-aided semen analysis system (Cyto-S, VideoTesT Co., Saint-Petersburg, Russia).
RESULTS
Expression of DEFB114 in the Human Epididymis
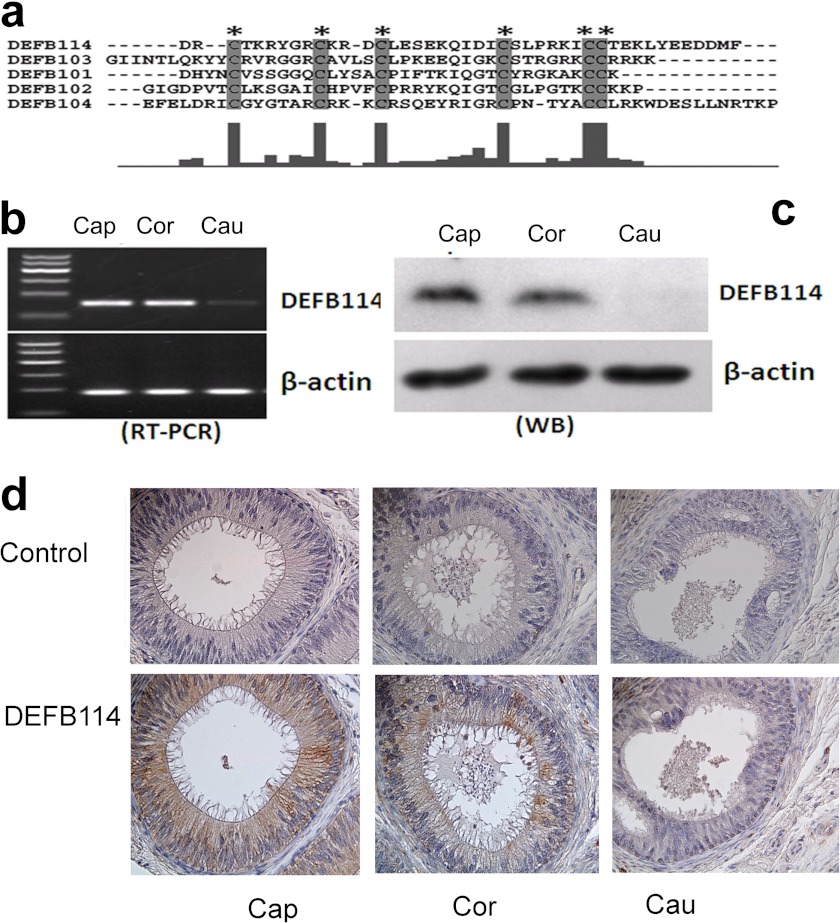
Human DEFB114 has been discovered by using a computational search strategy (18) in the human β-defensin gene clusters. DEFB114 shares conserved cysteine pairing and spacing between cysteine bridges in alignments with typical β-defensins (Fig. 1a). Although DEFB114 expression was detected at the transcriptional level in gingival tissues and keratinocytes (19), data sets from the U133plus2 Affymetrix microarray of a survey across 130 diverse normal human tissues in the BIOGPS database showed that it was most intensively expressed in the epididymis corpus. The relative expression level of DEFB114 in the epididymis is nearly 2-fold higher than in saliva according to the microarray data. No function has been assigned to this gene so far. We identified the expression of DEFB114 mRNA in the caput and corpus regions of the human epididymis by RT-PCR analysis (Fig. 1b). DEFB114 proteins were also detected in the human epididymis by Western blotting and immunohistochemistry staining using a purified antibody raised in our laboratory (Fig. 1, c and d). A band of ∼5 kDa was detected in the caput and corpus, but not in the other portions of the epididymis (Fig. 1c). Immunohistochemistry confirmed that the positive signal was confined to the caput epididymal epithelia and was also present in part of the corpus epididymal epithelia (Fig. 1d). The generation of the antibody followed the protocols described previously (28). The antibody could recognize <5 ng of antigen protein in Western blot assays.
FIGURE 1.
Expression patterns of DEFB114 mRNA and protein in the human epididymis. a, amino acid sequences of five mature human β-defensins (DEFB114, DEFB101, DEFB102, DEFB103, and DEFB104) were aligned using the program ClustalX (48). The identities are indicated by the histograms below, where larger histograms correlate with higher identities. Asterisks and the shaded letters indicate conserved cysteine residues. DEFB114 expression in the caput (Cap), corpus (Cor), and cauda (Cau) regions of the human epididymis was determined by RT-PCR (b) and Western blot (WB) (c). The protein extract was loaded at 50 μg/lane, and the antibody was diluted at 1:1,000. d, immunostaining of the DEFB114 protein in the caput, corpus, and cauda segments of the human epididymis. The brown color in the tissues indicates a high level of DEFB114 expression.
Expression, Purification, and Characterization of Recombinant DEFB114 Protein
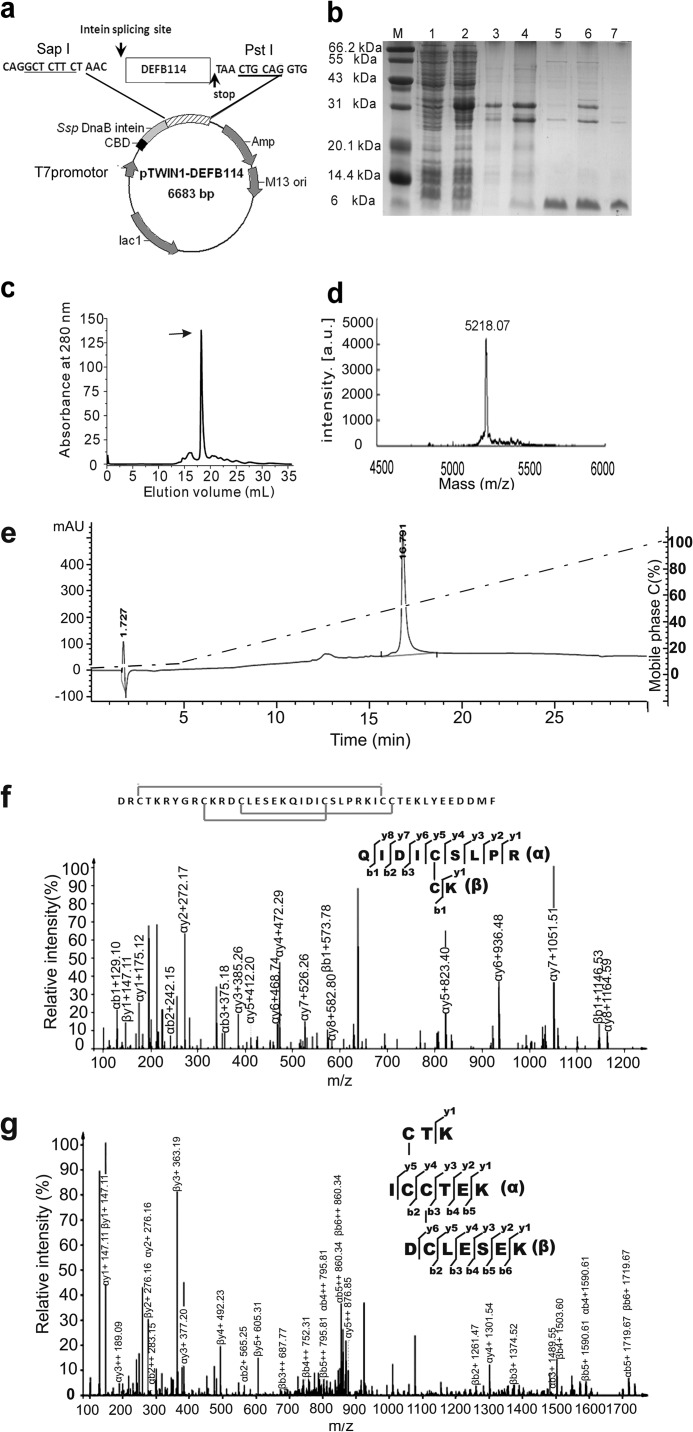
Obtaining active DEFB114 peptide was an essential step to address the roles of DEFB114 in immunity and fertility. In the present study, we used an approach of intein-mediated fusion expression and purification because it is simple, economical, and effective in the study of DEFBs (23, 24), which has been successfully demonstrated in our laboratory. Human DEFB114 was fused with Ssp intein and a chitin-binding domain (Fig. 2a). This construct facilitated the simple and fast purification of DEFB114 after expression by binding to chitin beads and efficient autocatalyzed cleavage to release the mature peptide. The expression, purification, and autocatalyzed cleavage of the fusion protein were analyzed by SDS-PAGE. The efficiency of pH-induced cleavage was ∼40% (Fig. 2b) under optimized conditions. The mature DEFB114 peptides were ultrafiltrated and purified with a Superdex-75 column (GE Healthcare) (Fig. 2c). The yield of the purified DEFB114 peptide was 5–8 mg for 1 liter of LB broth.
FIGURE 2.
Expression, purification, and characterization of the DEFB114 peptide. a, construction of the recombinant plasmid pTWIN1-DEFB114. The coding frame of the mature DFFB114 peptide was inserted into the plasmid pTWIN1 using SapI and PstI. b, SDS-PAGE analysis of DEFB114 expression in E. coli BL21 (DE3). Lanes 1 and 2, cell lysate before and after induction with isopropyl 1-thio-β-d-galactopyranoside, respectively; lanes 3 and 4, DEFB114 fusion protein bound on chitin beads before and after autocleavage, respectively; lanes 5 and 6, released DEFB114 from the chitin beads before and after ultrafiltration, respectively; lane 7, DEFB114 peptides recovered through FPLC. c, purification of DEFB114 by FPLC was monitored at 280 nm. The arrow indicates the peak of purified DEFB114. d, the molecular weight of DEFB114 was determined by mass spectrometry (MALDI-TOF). e, the homogeneity of the purified peptides by FPLC was further analyzed using mobile phases A and C with a two-step linear gradient of 0–5% C in the first 5.0 min, followed by 5.0–100% C in the next 25 min (mobile phase A, 0.1% TFA; mobile phase C, 0.1% TFA, 90% acetonitrile-water). f, tandem analysis of the precursor ion at m/z 646.3278 [M + 2H]2+ showing the dominant fragment ions derived from the tryptic fragment that bears a disulfide bond (Cys10–Cys24) between α and β chains. g, tandem analysis of the precursor ion at m/z 932.8928 [M + 2H]2+ showing the dominant fragment ions derived from the tryptic fragment that bears disulfide bonds (Cys3–Cys31, Cys14–Cys32).
To evaluate the peptide quality, the purified peptide was analyzed with MALDI-TOF mass spectrometry. The construct used allowed the production of mature peptides without extra amino acid residues to avoid possible interference with the properties of DEFB peptides. The measured molecular mass (5,218.07 Da) was consistent with the theoretical value of 5,218.4 Da (Fig. 2d). These data demonstrated that there were no extra vector-derived residues in the DEFB114 peptide, which was in accordance with our previous design.
Because β-defensins have conserved C1-C5, C2-C4, and C3-C6 cysteine pairing patterns (29), characterization of the closely spaced, complex disulfide bond patterns in the DEFB114 peptide was a challenge. We chose capillary LC-MS/MS to identify the complex disulfide bonds of DEFB114 peptides. The DEFB114 peptides and their tryptic products were analyzed by HPLC using an Agilent ZORBAX 300SB-C8 column (Fig. 2e) and an Agilent 20RBAX Eclipse XDB-C18 column, respectively, and the peaks were verified by MALDI-TOF mass spectrometry (supplemental Fig. 1) prior to LC-MS/MS analysis. The tryptic DEFB114 products containing complex disulfide bonds were then analyzed by LC-MS/MS using the Easy nLC1000 HPLC system and Thermo Q Exactive mass spectrometry (Fisher). The ion in the full-scan mass spectrum of one peak was a doubly charged ion at m/z 646.3278 resulting in a measured [M + 2H]2+ of 1,291.6482 Da, which matched for a disulfide-linked pair of peptides (QIDICSLPR and CK). The mass (calculated [M + 2H] = 932.8928 Da) of this ion matches for a complex disulfide-linked fragment (CTK, ICCTEK, and DCLESEK). The MS/MS spectra were manually matched to determine the disulfide bond pairings, and the results demonstrated that the purified tryptic peptides carried correct disulfide bonds (Fig. 2, f and g). The extracted ion chromatogram analysis (supplemental Fig. 2) also demonstrated that the signals of the fragments were reliably related with the disulfide bond pairing patterns (Cys10–Cys24, Cys3–Cys31, Cys14–Cys32).
Antimicrobial Activity and LPS-binding Activity of DEFB114 in Vitro
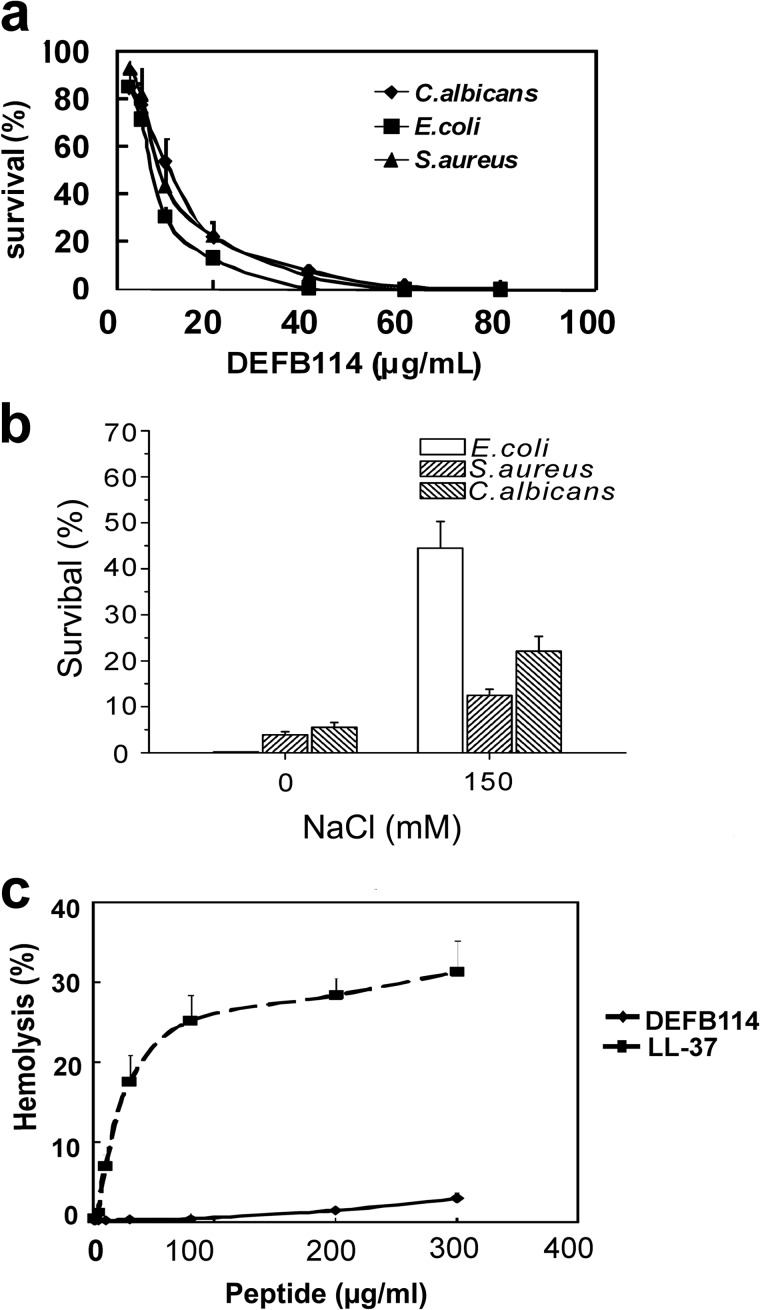
In this study, diverse microbes were used to evaluate the antimicrobial activity of the recombinant DEFB114 peptide. The recombinant DEFB114 peptide exhibited dose-dependent antimicrobial activity against E. coli K12D31, S. aureus, and C. albicans, which are representatives of Gram-negative bacteria, Gram-positive bacteria, and fungi, respectively (Fig. 3a). The minimum microbicidal concentration values were measured to be 45, 50, and 65 μg/ml for the three microbial strains, respectively. As a common feature, the recombinant DEFB114 peptide showed impaired antimicrobial activity with elevated concentrations of NaCl (Fig. 3b). The survival rate of E. coli K12D31 was increased to 44.3% following the addition of 150 mm NaCl. However, the increments were only 8.6 and 16.6% for S. aureus and C. albicans, respectively.
FIGURE 3.
Antimicrobial and hemolytic activity of recombinant DEFB114. a, antimicrobial activity of the DEFB114 peptide against E. coli K12D31, S. aureus (CMCC 26003), and C. albicans (SC 5314) (2.0–4.0 × 105 cfu/ml) was measured using cfu assays. b, salt-sensitive antimicrobial activity of DEFB114 (40 μg/ml) was determined in the absence or presence of 150 mm NaCl. c, concentration-dependent hemolytic effects of the recombinant DEFB114 peptide and LL-37 peptide. Treatment with 0.1% Triton X-100 served as a reference for complete hemolysis. The values represent the averages of three independent measurements with duplicate samples. Error bars, S.E.
Using the active recombinant DEFB114 peptide, we demonstrated that DEFB114 was a typical β-defensin molecule with a broad range of antimicrobial activity and salt sensitivity in vitro. These observations were consistent with the expression of DEFB114 in low salt compartments, such as gingival tissues, keratinocytes, and epididymal epithelium cells. However, recent evidence indicates that the salt sensitivity of DEFBs in vivo may be lower than believed originally (30). To determine whether the recombinant DEFB114 peptide reflects the native peptide in vivo, assays should be conducted in the future, including isolating DEFB114 and confirming its activity in vivo.
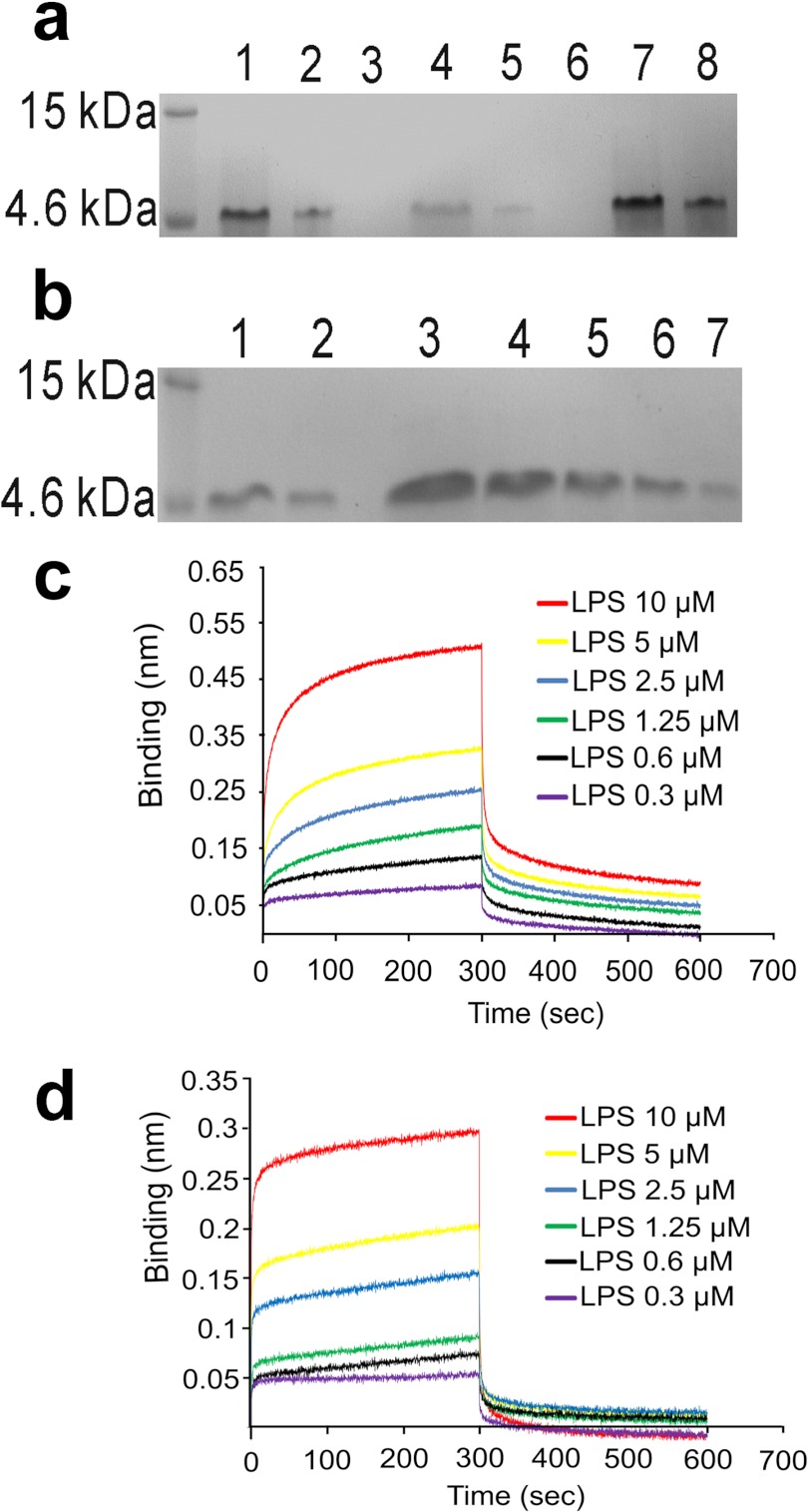
It is known that the antimicrobial activity of AMPs does not necessarily correlate with their affinity for LPS or LPS-neutralizing activity. We thus examined the affinity of DEFB114 for LPS. Recombinant DEFB114 could bind to LPS beads and was subsequently eluted with buffers containing different concentrations of NaCl, whereas the remaining bound peptides were eventually eluted with a binding buffer containing 4 m urea (Fig. 4a). The amount of DEFB114 peptides bound to the beads was decreased with increasing concentrations of free LPS (Fig. 4b). These observations suggested that the binding of DEFB114 to LPS was specific. We then quantitatively evaluated the affinity of DEFB114 for LPS using ForteBio's Octet RED system. Detection technology of biolayer interferometry utilized in this platform is a label-free, fluidics-free, real-time detection based on an optically coated streptavidin biosensor. This technique was based on the measurement of light intensity produced by an interference of two or more light beams. The defensin proteins were biotinylated and fixed on biosensors. LPS was prepared in a serial dilution (10, 5, 2.5, 1.25, 0.6, and 0.3 μm). The measured dissociation constant value (KD) of LPS was 0.44 μm for binding DEFB114 (Fig. 4c) and 368 μm for binding alkylated DEFB114 (Alk-DEFB114) (Fig. 4d). The data implied that DEFB114 possessed a higher affinity for LPS compared with alkylated DEFB114.
FIGURE 4.
LPS-binding activity of DEFB114. a, recombinant DEFB114 bound on LPS beads was eluted with buffers containing variant concentrations of NaCl and 4 m urea and analyzed on a 15% SDS-polyacrylamide gel. Lane 1, DEFB114 prior to loading on the LPS beads; lane 2, flow-through after loading; lanes 3–6, DEFB114 eluted with NaCl concentrations of 0.1, 0.5, 1.0, and 2.0 m, respectively; lanes 7 and 8, DEFB114 eluted with 4 m urea. b, the binding of DEFB114 to LPS beads was attenuated with competitive LPS. The eluates with 4 m urea were analyzed on a 15% SDS-polyacrylamide gel. Lanes 1 and 2, similar to a; lanes 3–7, eluates of DEFB114 with different concentrations of competitive LPS (0.1, 0.2, 0.4, 0.8, and 1.6 mg/ml, respectively). The association and dissociation of LPS for DEFB114 (c) as well as for alk-DEFB114 (d) was monitored by the Octet interferometer (ForteBio).
Inhibitory Activity of DEFB114 on the LPS-mediated Release of TNF-α from RAW264.7 Cells
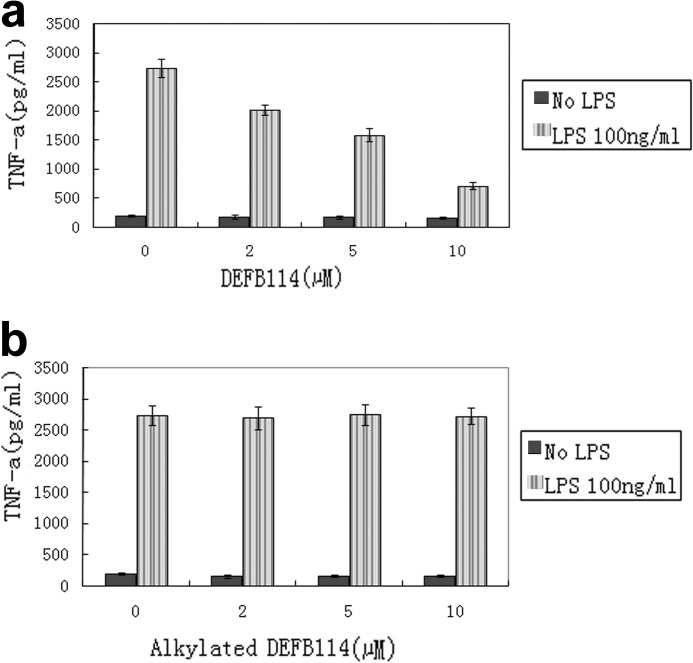
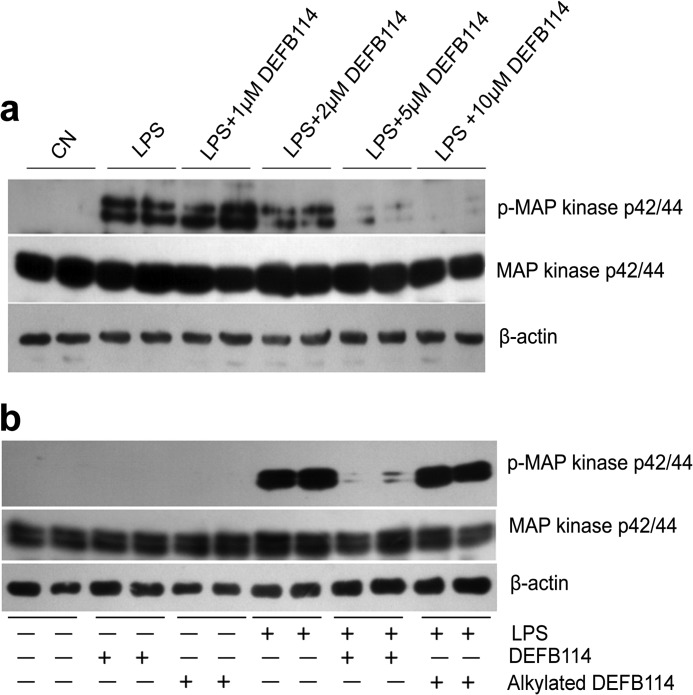
It is unclear whether DEFB114 binding to LPS results in LPS neutralization and inhibition of inflammation. Considering that several AMPs are capable of blocking the release of certain LPS-induced cytokines, such as TNF-α, by interfering with or binding to LPS (31), we measured the TNF-α levels in the supernatant of RAW264.7 cell cultures that had been preincubated with DEFB114 using ELISA after stimulation with LPS. DEFB114 inhibited TNF-α release in a dose-dependent manner compared with alkylated DEFB114 (Fig. 5). To validate this effect, we also examined whether earlier events in LPS signaling were inhibited by the action of DEFB114. By using phospho-specific antibodies to detect phosphorylated kinases (pMAPK42/44), we confirmed that high levels of pMAPK42/44 were abolished if RAW264.7 cells were preincubated with increasing concentrations of DEFB114 when challenged with LPS (Fig. 6a).
FIGURE 5.
DEFB114 attenuates LPS-induced TNF-α release in the RAW264.7 cell model. TNF-α levels in the supernatants of LPS-induced RAW264.7 cell cultures were measured using an ELISA kit. DEFB114 (a) rather than alkylated DEFB114 (b) inhibited the LPS-induced release of TNF-α. Error bars, S.E.
FIGURE 6.
DEFB114 inhibits LPS-mediated MAPK phosphorylation in macrophages (RAW264.7). Macrophages (RAW264.7) were incubated with DEFB114 (or alkylated DEFB114) and then LPS, or left untreated. Cellular proteins were extracted and separated by SDS-PAGE and blotted onto PVDF membranes. LPS signaling via MAPK p42/44 (a) was detected using a phospho-specific antibody. a, decreased intensity of phospho-specific bands (p-MAP kinase p42/44) accompanied with increasing DEFB114 concentrations in comparison with total MAPK p42/44 and actin. Alkylated DEFB114 failed to inhibit the phosphorylation of MAPK p42/44 in comparison with its intact form (b).
Disulfide Bonds Are Essential for DEFB114 to Interfere with the LPS-mediated Effects on RAW264.7 Cells
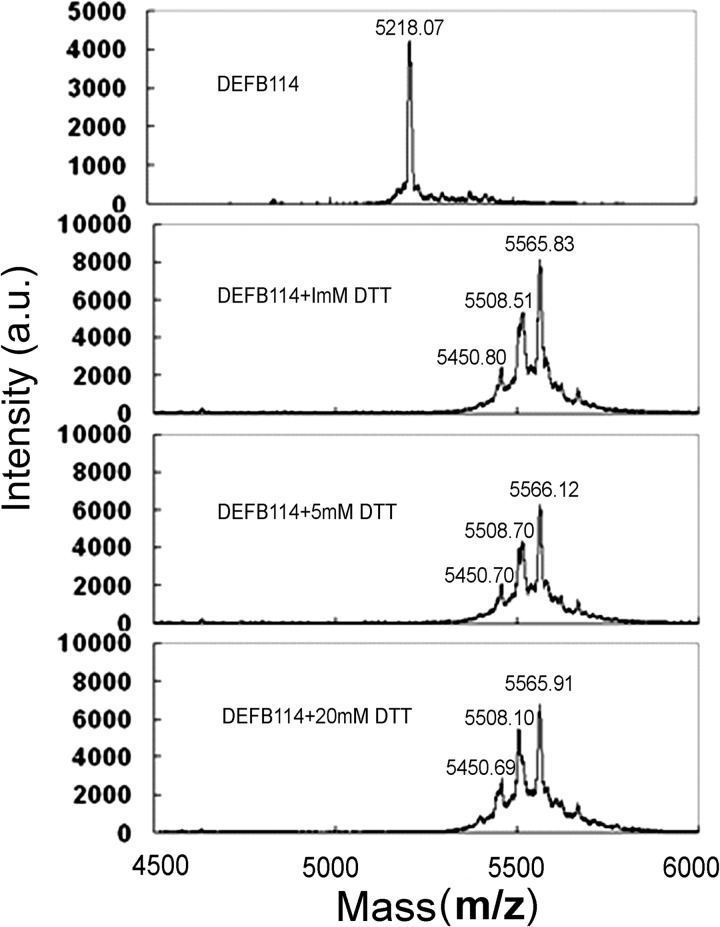
Generally, three intramolecular disulfide bonds in DEFBs play crucial roles to stabilize the three-dimensional structures. The different contributions of these disulfide bonds are related to the antimicrobial activity of different DEFBs (25, 32). The activity of DEFBs on eukaryotic cells is mostly dependent on both disulfide bonds (33) and their overall hydrophobicity (25). To elucidate the essential roles of the disulfide bonds in DEFB114, we reduced DEFB114 with DTT and subsequently alkylated with iodoacetamide. The samples were analyzed by using a MALDI-TOF/TOF mass spectrometer (Applied Biosystems model 4800). A single signal was detected at m/z 5218.07 for purified DEFB114 (Fig. 7). DTT treatment and carboxyamidomethylation of DEFB114 resulted in a single signal corresponding to the completely reduced form (m/z 5566) with all six cysteine residues alkylated and the other two signals corresponding to forms with five (m/z 5508) and four (m/z 5450) alkylated cysteine residues, respectively (Fig. 7). The mass spectrum analysis confirmed that the DEFB114 peptide was reduced and alkylated.
FIGURE 7.
Mass spectrometry analysis of the oxidized and reduced DEFB114 molecules. DEFB114 (20 μm) was incubated with different concentrations of DTT, alkylated, and analyzed by MALDI-TOF MS. DEFB114 in its completely reduced form with all six cysteine residues alkylated (m/z 5566) and the other two forms, corresponding to five (m/z 5508) and four (m/z 5450) alkylated cysteine residues, were also detected. a.u., arbitrary units.
Using the same macrophage model, we tested whether endotoxin-induced MAPK activation was inhibited by the alkylated DEFB114 peptide. We observed that DEFB114, instead of alkylated DEFB114, preincubated with RAW264.7 cells prior to LPS addition, inhibited TNF-α release (Fig. 5) by abolishing the high levels of pMAPK42/44 induced by LPS (Fig. 6b). This observation indicates that the intramolecular disulfide bonds of DEFB114 are essential. The administration of DEFB114 alone had little measurable effect on TNF-α release and inflammatory signaling in the absence of LPS, which was reflected in the lack of a cytokine response in macrophages exposed to different concentrations of DEFB114 (Figs. 5 and 6).
Hemolytic Activity of DEFB114
A concern regarding the development of bioengineered cationic antimicrobial peptides is their potential toxicity to host cells (34). No evident hemolysis was detected after a 1-h exposure of human erythrocytes to DEFB114 peptides at a concentration of 100 μg/ml (Fig. 3c). By contrast, LL-37 exhibited significant hemolytic activity (>20%) at this concentration. Therefore, the treatment of erythrocytes with DEFB114 at concentrations above the minimum microbicidal concentration value resulted in little damage. DEFB114 seems safe and might have the potential to be used in the treatment of LPS-induced sepsis or inflammatory diseases in the future.
DEFB114 Interferes with the LPS-mediated Impairment of Human Sperm Motility
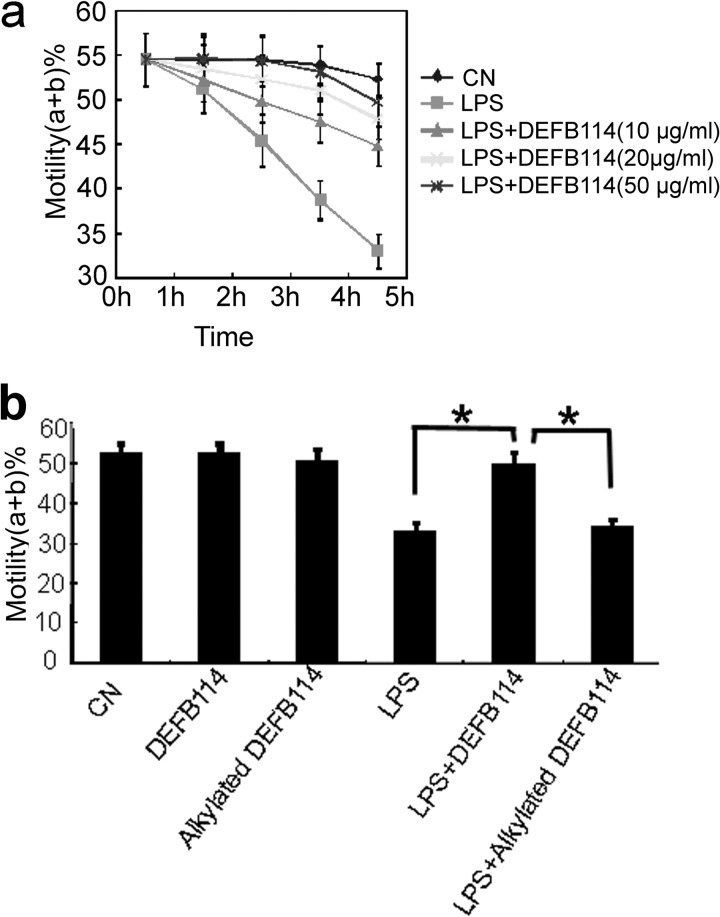
It is known that LPS exposure can reduce sperm motility, induce sperm apoptosis, and significantly impair the potential for fertilization (9). As an epididymal β-defensin with low cytotoxicity, DEFB114 might protect local sperm from inflammation and loss of function via LPS neutralization. Sperm motility, as the most obvious parameter of sperm quality, was measured as an index to evaluate the action of DEFB114 on LPS-challenged human sperm in vitro. DEFB114, rather than alkylated DEFB114, significantly rescued the motility of sperm in vitro, which was impaired by LPS during a 6-h incubation (Fig. 8). The protective effects of DEFB114 were dose-dependent compared with those of LPS addition alone. These data indicate that disulfide bonds are also essential for DEFB114 to interfere with LPS-mediated sperm motility impairment in vitro.
FIGURE 8.
DEFB114 rescues the LPS-induced loss of human sperm motility in a dose- and time-dependent manner. We pretreated and incubated 2.0–5.0 × 106 human sperm cells/ml with combinations of LPS and DEFB114 or with alkylated DEFB114 or left them untreated (CN, control). Progressive sperm motility (a + b%) was measured with CASA after incubation with different doses of the DEFB114 peptide (a). Different effects of DEFB114 and alkylated DEFB114 at the same concentration (50 μg/ml) on sperm motility were compared after 6 h of incubation (b). A significant difference (*, one-way analysis of variance, least significant difference test, p < 0.05, n = 10) was observed between DEFB114 and alkylated DEFB114 regarding their inhibitory effects on the impairment of sperm motility. Error bars, S.D.
DEFB114 Protects d-GalN-sensitized C57BL/6 Mice from LPS-induced Lethality
Although DEFB114 demonstrated LPS neutralization and anti-inflammation effects in cell models in vitro, its protective roles should be confirmed in vivo. Among the β-defensin family members, to our knowledge, only DEFB123 has been shown to have LPS-neutralizing activity in vivo without apparent toxicity (17). Both the observed weak hemolytic activity (Fig. 3c) and apparently lack of toxic effects on sperm (Fig. 8b) implied that DEFB114 was safe. Because no murine orthologs of DEFB114 and DEFB123 have been found, it is impossible to use endogenous murine β-defensin genes. Therefore, we administrated recombinant human DEFB114 into a murine model of acute sepsis to prove its LPS-neutralizing activity following the previously described procedures with minor modifications (17, 35). Groups of C57BL/6 mice were first sensitized with d-GalN to induce high susceptibility to LPS-induced lethality. Then endotoxemia was induced by 100 ng of LPS/mouse. The control groups injected with 50 μg of DEFB114 peptide or alkylated DEFB114 (alk-DEFB114)/mouse (without LPS) showed no lethality during the recording time and in the following 30 days. The group injected with LPS alone resulted in lethality in 13 of 15 mice within 24 h (Table 1). LL-37, which has a high potential to prevent LPS-mediated lethality, as a positive therapeutic control, rescued 14 of 15 mice and 13 of 15 mice within 10 and 24 h, respectively. We preincubated the DEFB114 peptide or alk-DEFB114 with LPS and subsequently injected the solution into the mice (Table 1). Co-incubation of LPS with increasing concentrations of the DEFB114 peptide resulted in the increased survival of the sensitized animals. Preincubation of LPS with 50 μg of DEFB114 rescued 22 of 25 mice, whereas co-incubation with 50 μg of alk-DEFB114 rescued only 4 of 25 mice. There was no significant difference between the survival rates of the alk-DEFB114-treated group and bovine serum albumin (BSA)-treated group within 10 and 24 h (Table 1).
TABLE 1.
DEFB114 blocks LPS-induced lethality in d-GalN-sensitized C57BL/6 mice
Either DEFB114 or alkylated DEFB114 was added to the LPS saline solution and incubated at 37 °C for 15 min. C57BL/6 mice were injected intraperitoneally with d-GalN (50 mg/0.2 ml of saline), LPS (100 ng/0.2 ml of saline) with either DEFB114 or alkylated DEFB114, and saline alone (0.2 ml). Survival animal numbers were recorded at 10 and 24 h postinjection.
| Condition | LPS | DEFB114 | Alk-DEFB114 | LL-37 | BSA | Survivors/total (10 h) | Survival (10 h) | Survivors/total (24 h) | Survival (24 h) |
|---|---|---|---|---|---|---|---|---|---|
| ng | μg | μg | μg | μg | % | % | |||
| Negative control | 100 | 0 | 0 | 0 | 50 | 4/25 | 16 | 4/25 | 16 |
| DEFB114 alone | 0 | 50 | 0 | 0 | 0 | 5/5 | 100 | 5/5 | 100 |
| Alk-DEFB114 alone | 0 | 0 | 50 | 0 | 0 | 5/5 | 100 | 5/5 | 100 |
| Control | 100 | 0 | 0 | 0 | 0 | 2/15 | 13.3 | 2/15 | 13.3 |
| Treatment | 100 | 5 | 0 | 0 | 0 | 8/25 | 32 | 5/25 | 20 |
| 100 | 25 | 0 | 0 | 0 | 17/25 | 68 | 10/25 | 40 | |
| 100 | 50 | 0 | 0 | 0 | 22/25 | 88 | 19/25 | 76 | |
| 100 | 0 | 50 | 0 | 0 | 4/25 | 16 | 4/25 | 16 | |
| Positive control | 100 | 0 | 0 | 10 | 0 | 14/15 | 93.3 | 13/15 | 86.7 |
To further evaluate the therapeutic potency of DEFB114, after intraperitoneal administration of a lethal dose of 100 ng of LPS on one side, we injected either DEFB114 or alkylated DEFB114 on the opposite side of the peritoneum. Concomitantly, all mice were sensitized with d-GalN (50 mg/mouse). DEFB114 showed protective effects by increasing survival rates in the therapeutic assays, as shown in (Table 2).
TABLE 2.
Therapeutic potential of DEFB114 by inhibiting LPS-induced lethality in d-GalN-sensitized C57BL/6 mice
C57BL/6 mice were injected intraperitoneally with 100 ng of LPS on one side and then injected with either DEFB114 or alkylated DEFB114 in 0.5 ml of saline on the opposite side. Concomitantly, all mice were sensitized with sterile d-GalN (50 mg/0.2 ml of saline) solution. Deaths due to LPS induction were recorded at 10 and 24 h postinjection. A series of controls (a, b, c, and d) were included in the experiment. LL-37 served as a positive control.
| Condition | LPS | DEFB114 | Alk-DEFB114 | LL-37 | Experimental group (survivors/total) (10 h) | Survival | Experimental group (survivors/total) (24 h) | Survival |
|---|---|---|---|---|---|---|---|---|
| ng | μg | μg | μg | % | % | |||
| Control a | 0 | 1000 | 0 | 0 | 5/5 | 100 | 5/5 | 100 |
| Control b | 0 | 0 | 1000 | 0 | 5/5 | 100 | 5/5 | 100 |
| Control c | 100 | 0 | 0 | 0 | 1/10 | 10 | 1/10 | 10 |
| Therapeutic | 100 | 100 | 0 | 0 | 2/15 | 13.3 | 2/15 | 13.3 |
| Therapeutic | 100 | 500 | 0 | 0 | 6/15 | 40 | 3/15 | 20 |
| Therapeutic | 100 | 1000 | 0 | 0 | 12/15 | 80 | 10/15 | 66.7 |
| Therapeutic | 100 | 0 | 1000 | 0 | 2/15 | 13.3 | 2/15 | 13.3 |
| Control d | 100 | 0 | 0 | 10 | 5/15 | 33.3 | 4/15 | 26.7 |
| Therapeutic | 100 | 0 | 0 | 50 | 11/15 | 73.3 | 9/15 | 60 |
DISCUSSION
Using an active recombinant peptide, we demonstrated that DEFB114 is a typical β-defensin protein with LPS-neutralizing activity. Although a wide spectrum of antimicrobial activity is a common feature in the DEFB family, both the LPS affinity and neutralizing activity of DEFB114 are novel. So far, among human DEFBs, only DEFB123 has been shown to have LPS-neutralizing activity in vitro and in vivo (17). Whether other human DEFB family members have similar activity will be an important question if this activity is a common feature of DEFBs.
It is more difficult to characterize natural human DEFBs than rodent β-defensins because of ethical problems and the lack of homologs in rodents. Only several human β-defensins have orthologs in rodents because the mammalian β-defensin family has undergone dynamic evolution (36). DEFB114 has no rodent ortholog, which limits the functional assays for DEFB114 by using its endogenous gene in rodents. Moreover, LPS treatment of animals shows different effects on the expression of DEFBs. In the LPS-induced rat model of epididymitis, the expression of β-defensin (Defb) 29, 41, and 42 did not change significantly after LPS treatment, whereas the mRNA levels of the other nine members were significantly decreased, including SPAG11E (14). Another report showed that LPS challenge induced the expression of Spag11 mRNA variants and defensins in the male reproductive tract of rats, followed by a concomitant increase in protein expression (13). These apparent differences might derive from the methods by which LPS was administered (i.e. by intraperitoneal injection or direct injection into the epididymis). The different responses of defensins following LPS treatment reveal the complexity of defensin regulation to some extent. Considering that rodents have no DEFB114 homolog and the roles of antimicrobial responses against infections in the reproductive tract are poorly understood, we chose to observe the protective effects of DEFB114 on the motility of human sperm in vitro. Further, because rodents have no DEFB114 homolog, we simply observed the survival rate of d-GalN-sensitized C57BL/6 mice in different treatment groups to reduce complexity. The fluctuations of endogenous mouse β-defensins potentially have a minimal impact on our observations of the function of DEFB114 because we used an array of control groups.
To our knowledge, DEFB114 is the second β-defensin that possesses both LPS-binding and -neutralizing activity in vivo and in vitro; however, LPS binding potency does not necessarily correlate with neutralizing activity. For example, hBD2 competitively inhibits the interaction between LPS and LPS-binding protein, which initiates and amplifies the LPS signaling (15). However, the anti-inflammatory effect of DEFB103 is not a result of the direct binding of the peptide with LPS (16). Whether DEFB114 has a direct effect on inflammatory signaling pathways should be further examined. Its potential immunosuppressive activity by interfering with inflammatory signaling pathways might be a critical factor to exploit its future clinical use.
Because LPS-induced TNF-α is a key inflammatory cytokine, the toxicity of LPS in d-GalN-sensitized mice actually results from severe liver injury caused by the TNF-α-induced apoptosis of hepatocytes (37). Therefore, it is reasonable to speculate that DEFB114 could provide a defense against LPS by inhibiting the release of TNF-α at the cell level. DEFB114 expression in gingival keratinocytes (19) might exert its effect through a similar mechanism by inhibiting TNF-α release in response to IL-1β and Candida species stimulation (21). Considering that DEFB114 exhibits weak cytotoxicity (∼10 times less than LL-37 at a peptide concentration of 300 μg/ml) in the in vitro assays, the possibility of treating septic shock by inhibiting TNF-α release with DEFB114 administration in vivo should be exploited further to address the underlying mechanism and clinical potency. In addition to LPS binding, the direct interference of DEFB114 in inflammation signaling pathways in the presence of LPS could not be excluded. Among human DEFBs, DEFB103 associates with and rapidly enters macrophages and then inhibits both MyD88 and TRIF signaling pathways, resulting in the transcriptional repression of proinflammatory genes (38). Whether DEFB103 and DEFB114 utilize a similar mechanism to exert their anti-inflammatory activity is an interesting question. The answer will be helpful for identifying similar, but somewhat different, mechanisms of DEFBs and ascertaining the necessary requirements of this family. A comparison of the mechanisms that different DEFB family members may utilize will also facilitate the discovery and combination of new anti-inflammatory targets. Further, the clinical use of DEFB114 is not confined to septic shock. It could be expected that DEFB114 might also have therapeutic potency in other diseases that are characterized by the release of TNF-α.
Although it is still unclear why such a wide range of β-defensins are present in the male reproductive tract, their diverse functions can be briefly classified into three types: their common role in innate immunity by exhibiting antimicrobial activity in the reproductive system (28, 39) and non-reproductive systems (19); sperm maturation-related functions (28, 40, 41); and immunoregulating activity, which has been shown in non-reproductive systems (19). In addition to their expression in reproductive tissues, the majority of human β-defensins are also expressed in non-reproductive tissues (19). As a specific example, DEFB114 is expressed in gingival tissue and keratinocytes, in addition to its expression in the epididymis. Because of its active expression in the epididymis, human DEFB114 might exert inflammation-suppressing activity in the male reproductive tract, although an in vivo study is absent at present, and it is unclear whether this β-defensin has an additional role in sperm maturation.
Furthermore, it is speculated that the potential responses of other cytokines to treatment with DEFB114 under LPS challenge cannot be ignored. Cytokines in the semen have physiological and pathological functions. Men with spinal cord injury have unique semen with normal sperm concentrations but abnormally low sperm motility because their elevated levels of the cytokines IL-1β, IL-6, and TNF-α act at the level of the sperm receptor to inhibit sperm motility (42). TNF-α and IL-6 significantly reduce progressive motility at higher concentrations in a dose- and time-dependent manner (43). In subfertile couples, the levels of TNF-α and IL-1β in the seminal fluid correlate significantly with leukocyte counts and ratios in the same ejaculates as indicators of silent male genital tract infections/inflammation (44). Furthermore, the previous work demonstrated that when mouse bin1b was overexpressed in transgenic mouse models, this β-defensin played a role in the regulation of the inflammatory response in the epididymis by down-regulating IL-1α and IL-1β expression (39). All of these observations suggest that cytokines in the semen are also key factors that affect the function of sperm both physiologically and pathologically. Dysfunction of DEFB114 or any other DEFBs might also interfere with normal cytokine regulation indirectly and cause immunity and fertility problems. Therefore, DEFB114 might have the potency to treat microbe/LPS-contaminated semen by neutralizing LPS and down-regulating inflammatory factors.
Defensins have been considered to be proinflammatory as their expression increases in response to TLR ligands, TNF-α, IL-1β, IFN-γ, PMA, and following infection or injury. They also mediate the innate immune response and modulate inflammation by chemoattracting CD4 T cells and immature dendritic cells through CCR6. However, several lines of evidence support both a pro- and anti-inflammatory role for human DEFBs. They may promote the release of both proinflammatory (IL-6 and IL-18) and anti-inflammatory (IL-10) cytokines and chemokines (45, 46). Emerging evidence suggests that epididymal DEFBs might have complex activity in vitro and in vivo rather than proinflammatory activity alone. Notably, the well known proinflammatory human DEFB4 (hBD2) is not expressed in the epididymis (47). It could be speculated that epididymal DEFBs potentially have anti-inflammatory activity, which might explain, to some extent, why so many DEFBs are specifically expressed in the male reproductive tract where immune tolerance is required.
In conclusion, we identified the recombinant DEFB114 peptide as an active β-defensin. We demonstrated that DEFB114 possessed both LPS-binding and -neutralizing activity. Its activity was dependent on disulfide bonds that stabilize the tertiary structure of the peptide. Both the pleiotropic functions and low hemolytic activity of DEFB114 endow this peptide with therapeutic potential for LPS-induced inflammatory diseases and the impairment of sperm motility.
This work was supported by National Basic Research Program of China Grants 2007CB914304 and 2009CB941702 and Natural Science Foundation of China Grants 31101030, 31170717, 30900233, and 91129713.

This article contains supplemental Figs. 1 and 2.
- TLR
- Toll-like receptor
- AMP
- antimicrobial peptide
- DEFB
- β-defensin
- d-GalN
- d-galactosamine
- alk-DEFB114
- alkylated DEFB114.
REFERENCES
- 1. Peschel A. (2002) How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10, 179–186 [DOI] [PubMed] [Google Scholar]
- 2. Lepper P. M., Held T. K., Schneider E. M., Bölke E., Gerlach H., Trautmann M. (2002) Clinical implications of antibiotic-induced endotoxin release in septic shock. Intensive Care Med. 28, 824–833 [DOI] [PubMed] [Google Scholar]
- 3. Miller S. I., Ernst R. K., Bader M. W. (2005) LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 3, 36–46 [DOI] [PubMed] [Google Scholar]
- 4. Finlay B. B., Hancock R. E. (2004) Can innate immunity be enhanced to treat microbial infections? Nat. Rev. 2, 497–504 [DOI] [PubMed] [Google Scholar]
- 5. Bhattacharjya S. (2010) De novo designed lipopolysaccharide binding peptides. Structure based development of antiendotoxic and antimicrobial drugs. Curr. Med. Chem. 17, 3080–3093 [DOI] [PubMed] [Google Scholar]
- 6. Walker A. K., Hiles S. A., Sominsky L., McLaughlin E. A., Hodgson D. M. (2011) Neonatal lipopolysaccharide exposure impairs sexual development and reproductive success in the Wistar rat. Brain Behav. Immun. 25, 674–684 [DOI] [PubMed] [Google Scholar]
- 7. Abd-Allah A. R., Helal G. K., Al-Yahya A. A., Aleisa A. M., Al-Rejaie S. S., Al-Bakheet S. A. (2009) Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by l-carnitine. Oxid. Med. Cell Longev. 2, 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kajihara T., Okagaki R., Ishihara O. (2006) LPS-induced transient testicular dysfunction accompanied by apoptosis of testicular germ cells in mice. Med. Mol. Morphol. 39, 203–208 [DOI] [PubMed] [Google Scholar]
- 9. Fujita Y., Mihara T., Okazaki T., Shitanaka M., Kushino R., Ikeda C., Negishi H., Liu Z., Richards J. S., Shimada M. (2011) Toll-like receptors (TLR) 2 and 4 on human sperm recognize bacterial endotoxins and mediate apoptosis. Hum. Reprod. 26, 2799–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okazaki T., Mihara T., Fujita Y., Yoshida S., Teshima H., Shimada M. (2010) Polymyxin B neutralizes bacteria-released endotoxin and improves the quality of boar sperm during liquid storage and cryopreservation. Theriogenology 74, 1691–1700 [DOI] [PubMed] [Google Scholar]
- 11. Patil A. A., Cai Y., Sang Y., Blecha F., Zhang G. (2005) Cross-species analysis of the mammalian β-defensin gene family. Presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiol. Genomics 23, 5–17 [DOI] [PubMed] [Google Scholar]
- 12. Guaní-Guerra E., Santos-Mendoza T., Lugo-Reyes S. O., Terán L. M. (2010) Antimicrobial peptides. General overview and clinical implications in human health and disease. Clin. Immunol. 135, 1–11 [DOI] [PubMed] [Google Scholar]
- 13. Biswas B., Yenugu S. (2011) Antimicrobial responses in the male reproductive tract of lipopolysaccharide challenged rats. Am. J. Reprod. Immunol. 65, 557–568 [DOI] [PubMed] [Google Scholar]
- 14. Cao D., Li Y., Yang R., Wang Y., Zhou Y., Diao H., Zhao Y., Zhang Y., Lu J. (2010) Lipopolysaccharide-induced epididymitis disrupts epididymal β-defensin expression and inhibits sperm motility in rats. Biol. Reprod. 83, 1064–1070 [DOI] [PubMed] [Google Scholar]
- 15. Scott M. G., Vreugdenhil A. C., Buurman W. A., Hancock R. E., Gold M. R. (2000) Cutting edge. Cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J. Immunol. 164, 549–553 [DOI] [PubMed] [Google Scholar]
- 16. Semple F., Webb S., Li H. N., Patel H. B., Perretti M., Jackson I. J., Gray M., Davidson D. J., Dorin J. R. (2010) Human β-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur. J. Immunol. 40, 1073–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Motzkus D., Schulz-Maronde S., Heitland A., Schulz A., Forssmann W. G., Jübner M., Maronde E. (2006) The novel β-defensin DEFB123 prevents lipopolysaccharide-mediated effects in vitro and in vivo. FASEB J. 20, 1701–1702 [DOI] [PubMed] [Google Scholar]
- 18. Schutte B. C., Mitros J. P., Bartlett J. A., Walters J. D., Jia H. P., Welsh M. J., Casavant T. L., McCray P. B., Jr. (2002) Discovery of five conserved β-defensin gene clusters using a computational search strategy. Proc. Natl. Acad. Sci. U.S.A. 99, 2129–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pazgier M., Hoover D. M., Yang D., Lu W., Lubkowski J. (2006) Human β-defensins. Cell Mol. Life Sci. 63, 1294–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mookherjee N., Hancock R. E. (2007) Cationic host defence peptides. Innate immune regulatory peptides as a novel approach for treating infections. Cell Mol. Life Sci. 64, 922–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Premratanachai P., Joly S., Johnson G. K., McCray P. B., Jr., Jia H. P., Guthmiller J. M. (2004) Expression and regulation of novel human β-defensins in gingival keratinocytes. Oral Microbiol. Immunol. 19, 111–117 [DOI] [PubMed] [Google Scholar]
- 22. Turner T. T. (1995) On the epididymis and its role in the development of the fertile ejaculate. J. Androl. 16, 292–298 [PubMed] [Google Scholar]
- 23. Dong J., Yu H., Zhang Y., Diao H., Lin D. (2011) Soluble fusion expression and characterization of human β-defensin 3 using a novel approach. Protein Pept. Lett. 18, 1126–1132 [DOI] [PubMed] [Google Scholar]
- 24. Diao H., Guo C., Lin D., Zhang Y. (2007) Intein-mediated expression is an effective approach in the study of β-defensins. Biochem. Biophys. Res. Commun. 357, 840–846 [DOI] [PubMed] [Google Scholar]
- 25. Klüver E., Schulz-Maronde S., Scheid S., Meyer B., Forssmann W. G., Adermann K. (2005) Structure-activity relation of human β-defensin 3. Influence of disulfide bonds and cysteine substitution on antimicrobial activity and cytotoxicity. Biochemistry 44, 9804–9816 [DOI] [PubMed] [Google Scholar]
- 26. Dürr U. H., Sudheendra U. S., Ramamoorthy A. (2006) LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 1758, 1408–1425 [DOI] [PubMed] [Google Scholar]
- 27. Lee C. H., Tsai C. M. (1999) Quantification of bacterial lipopolysaccharides by the purpald assay. Measuring formaldehyde generated from 2-keto-3-deoxyoctonate and heptose at the inner core by periodate oxidation. Anal. Biochem. 267, 161–168 [DOI] [PubMed] [Google Scholar]
- 28. Zhao Y., Diao H., Ni Z., Hu S., Yu H., Zhang Y. (2011) The epididymis-specific antimicrobial peptide β-defensin 15 is required for sperm motility and male fertility in the rat (Rattus norvegicus). Cell Mol. Life Sci. 68, 697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ganz T. (2003) Defensins. Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720 [DOI] [PubMed] [Google Scholar]
- 30. Huang G. T., Zhang H. B., Kim D., Liu L., Ganz T. (2002) A model for antimicrobial gene therapy. Demonstration of human β-defensin 2 antimicrobial activities in vivo. Hum. Gene Ther. 13, 2017–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jerala R., Porro M. (2004) Endotoxin neutralizing peptides. Curr. Top. Med. Chem. 4, 1173–1184 [DOI] [PubMed] [Google Scholar]
- 32. Schroeder B. O., Wu Z., Nuding S., Groscurth S., Marcinowski M., Beisner J., Buchner J., Schaller M., Stange E. F., Wehkamp J. (2011) Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature 469, 419–423 [DOI] [PubMed] [Google Scholar]
- 33. Klüver E., Adermann K., Schulz A. (2006) Synthesis and structure-activity relationship of β-defensins, multi-functional peptides of the immune system. J. Pept. Sci. 12, 243–257 [DOI] [PubMed] [Google Scholar]
- 34. Verma C., Seebah S., Low S. M., Zhou L., Liu S. P., Li J., Beuerman R. W. (2007) Defensins. Antimicrobial peptides for therapeutic development. Biotechnol. J. 2, 1353–1359 [DOI] [PubMed] [Google Scholar]
- 35. Nagaoka I., Hirota S., Niyonsaba F., Hirata M., Adachi Y., Tamura H., Tanaka S., Heumann D. (2002) Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin. Diagn. Lab. Immunol. 9, 972–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Semple C. A., Gautier P., Taylor K., Dorin J. R. (2006) The changing of the guard. Molecular diversity and rapid evolution of β-defensins. Mol. Divers. 10, 575–584 [DOI] [PubMed] [Google Scholar]
- 37. Mignon A., Rouquet N., Fabre M., Martin S., Pagès J. C., Dhainaut J. F., Kahn A., Briand P., Joulin V. (1999) LPS challenge in d-galactosamine-sensitized mice accounts for caspase-dependent fulminant hepatitis, not for septic shock. Am. J. Respir. Crit. Care Med. 159, 1308–1315 [DOI] [PubMed] [Google Scholar]
- 38. Semple F., MacPherson H., Webb S., Cox S. L., Mallin L. J., Tyrrell C., Grimes G. R., Semple C. A., Nix M. A., Millhauser G. L., Dorin J. R. (2011) Human β-defensin 3 affects the activity of pro-inflammatory pathways associated with MyD88 and TRIF. Eur. J. Immunol. 41, 3291–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fei Z., Hu S., Xiao L., Zhou J., Diao H., Yu H., Fang S., Wang Y., Wan Y., Wang W., He Y., Wang C., Xu G., Wang Z., Zhang Y., Fei J. (2012) mBin1b transgenic mice show enhanced resistance to epididymal infection by bacteria challenge. Genes Immun. 13, 445–451 [DOI] [PubMed] [Google Scholar]
- 40. Tollner T. L., Venners S. A., Hollox E. J., Yudin A. I., Liu X., Tang G., Xing H., Kays R. J., Lau T., Overstreet J. W., Xu X., Bevins C. L., Cherr G. N. (2011) A common mutation in the defensin DEFB126 causes impaired sperm function and subfertility. Sci. Transl. Med. 3, 92ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou C. X., Zhang Y. L., Xiao L., Zheng M., Leung K. M., Chan M. Y., Lo P. S., Tsang L. L., Wong H. Y., Ho L. S., Chung Y. W., Chan H. C. (2004) An epididymis-specific β-defensin is important for the initiation of sperm maturation. Nat. Cell Biol. 6, 458–464 [DOI] [PubMed] [Google Scholar]
- 42. Brackett N. L., Cohen D. R., Ibrahim E., Aballa T. C., Lynne C. M. (2007) Neutralization of cytokine activity at the receptor level improves sperm motility in men with spinal cord injuries. J. Androl. 28, 717–721 [DOI] [PubMed] [Google Scholar]
- 43. Lampiao F., du Plessis S. S. (2008) TNF-α and IL-6 affect human sperm function by elevating nitric oxide production. Reprod. Biomed. Online 17, 628–631 [DOI] [PubMed] [Google Scholar]
- 44. Eggert-Kruse W., Kiefer I., Beck C., Demirakca T., Strowitzki T. (2007) Role for tumor necrosis factor α (TNF-α) and interleukin 1-β (IL-1β) determination in seminal plasma during infertility investigation. Fertil. Steril. 87, 810–823 [DOI] [PubMed] [Google Scholar]
- 45. Niyonsaba F., Ushio H., Nagaoka I., Okumura K., Ogawa H. (2005) The human β-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J. Immunol. 175, 1776–1784 [DOI] [PubMed] [Google Scholar]
- 46. Niyonsaba F., Ushio H., Nakano N., Ng W., Sayama K., Hashimoto K., Nagaoka I., Okumura K., Ogawa H. (2007) Antimicrobial peptides human β-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Invest. Dermatol. 127, 594–604 [DOI] [PubMed] [Google Scholar]
- 47. Zhang J. S., Liu Q., Li Y. M., Hall S. H., French F. S., Zhang Y. L. (2006) Genome-wide profiling of segmental-regulated transcriptomes in human epididymis using oligo microarray. Mol. Cell. Endocrinol. 250, 169–177 [DOI] [PubMed] [Google Scholar]
- 48. Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. (1997) The CLUSTAL_X windows interface. Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. World Health Organization (1999) WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction, 4th Ed., Cambridge University Press, Cambridge [Google Scholar]