Abstract
Implant failure can be divided into early (prior to prosthetic treatment) or late (after prosthetic rehabilitation). Early failure is generally due to interference in the healing process after implant placement. Implants undergoing early failure will show progressive bone loss on radiographs during the healing period (4 to 6 weeks). In the present case report, early progressive bone loss was seen at 6 weeks, after placement of a non-submerged single piece mini implant. Clinical examination revealed peri-implant bleeding on probing and pocket and grade-1 mobility. Treatment protocol included mechanical debridement (plastic curettes), chemical detoxification with supersaturated solution of citric acid, antibiotics and guided bone regeneration therapy using the collagen membrane as guided bone regeneration barrier in combination with bioactive glass as bone grafting material. The 6 month postoperative examination showed complete resolution of the osseous defect, thus suggesting that this technique may hold promise in the treatment of implants undergoing early failure.
Keywords: Bioactive glass, collagen, detoxification, early failure, guided bone regeneration
INTRODUCTION
The placement of dental implant is an effective and predictable treatment modality for replacing a missing tooth. Using implant survival as the indicator of successful clinical outcome, a majority of clinical studies have shown very promising results for dental implants. The success of implant therapy has been reported with a favorable high survival rates ranging between 95% and 99%.[1]
Despite the high success rates and stability of dental implants, failures do occur.[2] Implant failure can be divided into early (prior to prosthetic treatment) or late (after prosthetic rehabilitation).[3] Excessive occlusal stress in conjunction with host characteristics and bacterial-induced marginal bone loss (peri-implantitis) seem to be the major etiologic factors for late losses. On the other hand, operator experience, excessive surgical trauma, bacterial contamination, premature overloading with micromotion, and some local as well as systemic characteristics of the host are believed to be the most common causes of implant failure at an early stage after implant placement.[4]
Most implant failures have been reported in the maxilla, with almost three times as many implant losses as in the mandible.[5] Early failures have been reported to vary between 1.5% and 21%.[3,5–8] Implants undergoing early failure will show progressive bone loss on radiographs during the early stages of healing (4 to 6 weeks). Clinically, signs of infection such as the presence of fistula in the soft tissue covering the implants, purulent discharge on manipulation, bluish red discoloration of surrounding tissue, and mild discomfort at the implant site may also be present. If left untreated, bone loss will continue and implant failure will result. Early failing implants can be successfully managed by mechanical debridement, antimicrobial therapy, and guided bone regeneration (GBR).[9]
GBR[10,11] is a regenerative procedure which is based on the principle of guided tissue regeneration and involves the placement of a barrier membrane to protect the blood clot and create a secluded space around the osseous defect enabling bone regeneration without competition from other tissues. Several barrier membranes and bone grafting materials have been successfully used in different animal and human studies to regenerate the lost bone around the osseointegrated implants.[12] Most recently, many investigations[13–15] have focused on the use of products derived from type-1 and type-3 porcine or bovine collagen in combination with bone substitute like bioactive glass as filling material in the osseous defects.
A case report has been presented here, wherein an early failing implant was salvaged by GBR technique using bioactive glass (Perioglass®) as a defect filling material and resorbable collagen membrane (Haeliguide®) as a barrier membrane.
CASE REPORT
A 35-year-old female patient was referred to the Department of Periodontology and Implantology, Bapuji Dental College and Hospital, Davangere, Karnataka State, for the implant placement in the upper front tooth region. The tooth was removed 6 months back due to excessive mobility. The patient was systemically healthy and non-smoker.
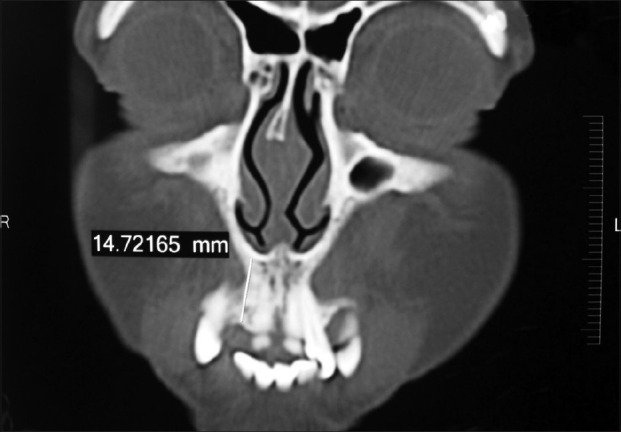
Clinical examination revealed missing upper right lateral incisor (#12) with other neighboring teeth in good health and alignment. The patient was maintaining good oral hygiene and showed keen interest in undergoing implant therapy. A written consent form was submitted by her. Necessary investigations like complete hemogram, intra-oral periapical (IOPA) radiograph [Figure 1], and computed tomographic (C.T.) scan of the edentulous site were done [Figures 2–4]).
Figure 1.
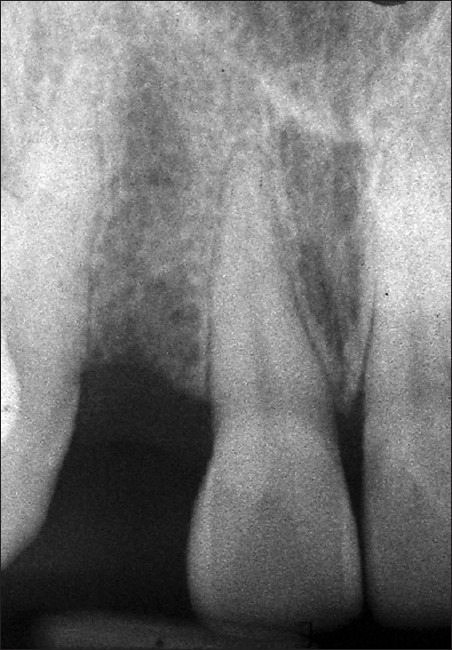
Pre-operative intraoral periapical radiograph of the edentulous site
Figure 2.

Computed tomographic scan showing the labio-palatal width of the available bone at the edentulous site
Figure 4.
Computed tomographic scan showing the height of the available bone at the edentulous site
Figure 3.
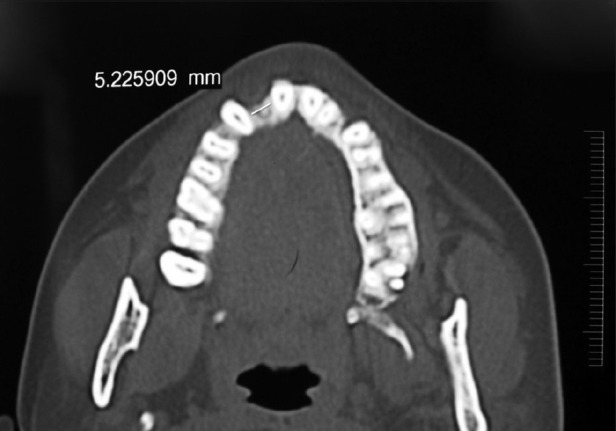
Computed tomographic scan showing the mesio-distal width of the available bone at the edentulous site
C.T. scan measurements revealed inadequate bone volume labio-palatally to accommodate a standard-diameter implant [Figure 2]. The patient did not accept the suggested treatment modalities to augment the deficient labio-palatal bone due to increased expense and prolonged time for several stages, until tooth reconstruction. Therefore, a decision was taken to place a mini implant. The implant used was TRI-N-SR mini implants (Hi-Tech Implants, Herzlia, Israel). This is a threaded, self-tapping, uncoated titanium alloy implants and is available in 10 mm and 13 mm lengths with a fixed diameter of 2.4 mm. The TRI-N-SR mini implant is a single-piece implant with large grit sand blasted and acid etched surface (SLA) and with a smooth polished collar and abutment surface. According to the measurements obtained with the help of C.T. scan, a 10 mm long implant was selected and placed according to the manufacturer's instructions. The insertion torque achieved was only 20 Ncm (indicating less primary stability), and therefore an immediate provisional acrylic restoration was avoided. A decision was made to directly give a final porcelain fused-to-metal (PFM) prosthesis to the patient at the end of 6 weeks, as an early loading protocol. Since it was a one-piece implant, the patient was instructed to follow an appropriate soft diet for 1 week, and avoid putting any kind of excessive load on the implant. The patient reported 10 days after the procedure for the removal of the sutures. The soft tissue healing was satisfactory and there was no clinical mobility of the implant at this time. The patient was now asked to report back at the end of 6 weeks for the fabrication of permanent prosthesis.
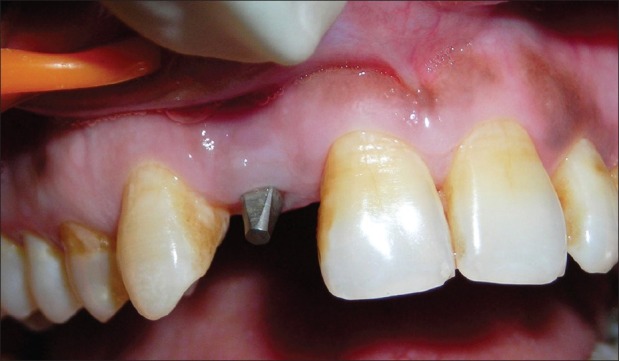
When patient returned at the end of 6 weeks, overall periodontal examination revealed fair oral hygiene according to OHI-S index by Green and Vermillion. A periodontal diagnosis of dental plaque-induced gingival disease – gingivitis associated with dental plaque only without other local contributing factors was arrived at, after thorough clinical examination. However, grade I mobility (according to the Misch's Clinical Implant Mobility Scale) was detected with respect to the implant placed. The Misch's Clinical Implant Mobility Scale uses two rigid instruments to apply a labio-lingual force of approximately 500 g and grades mobility of implant according to the following scale [Table 1]. The peri-implant probing depth was 6 mm on the mesial and 5 mm on distal aspect. Bleeding on probing was also positive. Intraoral periapical (IOPA) radiograph revealed bone loss on both mesial and distal aspects of the implant [Figures 5 and 6]. A clinical diagnosis of failing implant was made and a decision was taken to salvage the failing implant by means of GBR procedure to regenerate the lost bone.
Table 1.
Misch's clinical implant mobility scale

Figure 5.
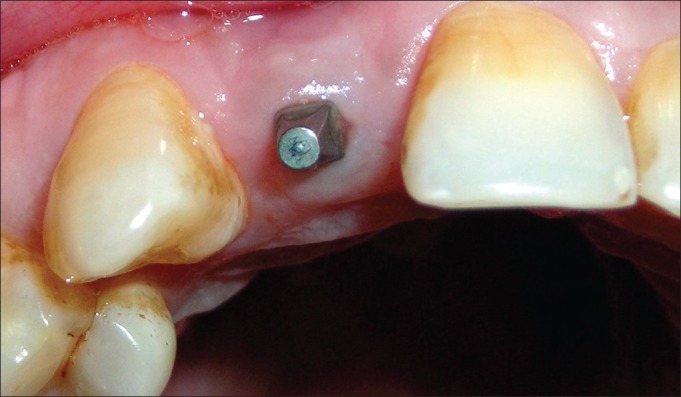
Six-week photograph of the implant
Figure 6.
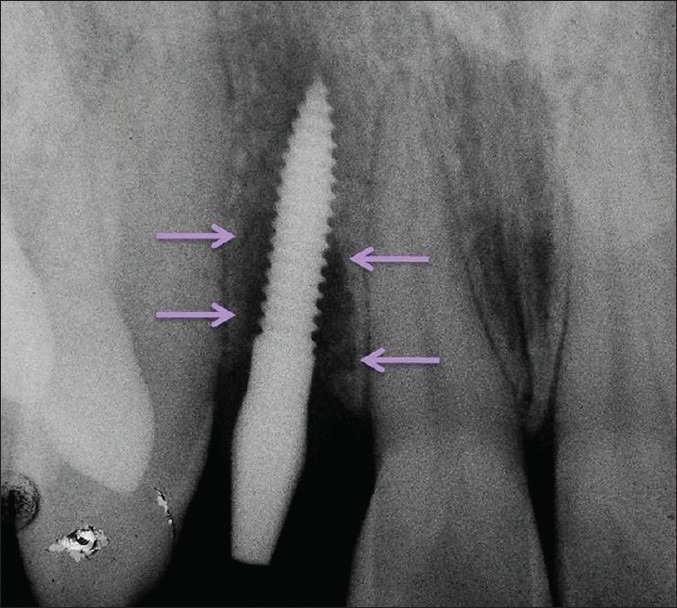
Six-week intraoral periapical radiograph of the implant showing bone loss mesial and distal to the implant
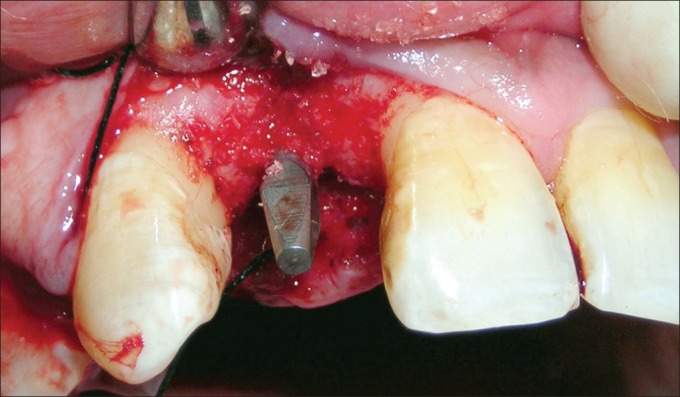
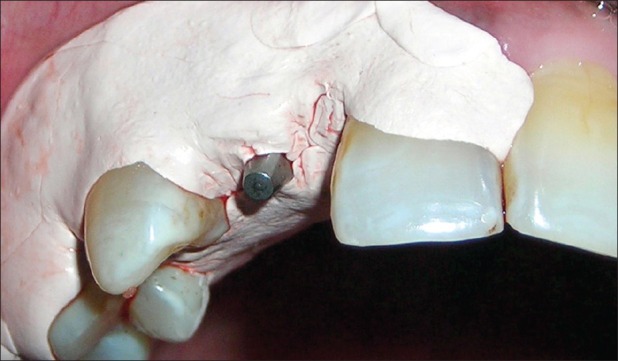
Antibiotic (amoxicillin 500 mg/potassium clavulanate 125 mg tabs, three times daily) was started a day prior to the surgical procedure. Under antibiotic cover, local anesthesia was given, intra-crevicular incisions were placed, and full-thickness mucoperiosteal flap was reflected. The infected granulation tissue was removed from the defect and the implant surfaces were thoroughly debrided using plastic curettes (Straumann® Dental Implant System, Straumann AG, Basel, Switzerland). A circumferential defect with slight buccal bone loss was evident around the implant [Figures 7 and 8]. Next, surface decontamination was done by applying a supersaturated solution of citric acid for 30 s followed by complete rinsing of the site with normal saline [Figure 9].
Figure 7.
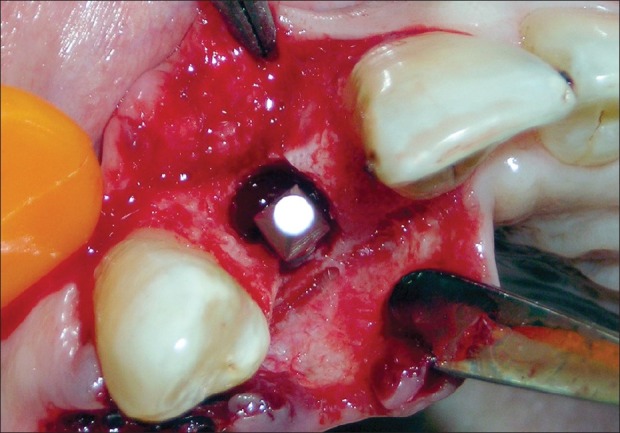
Circumferential defect around the failing implant
Figure 8.
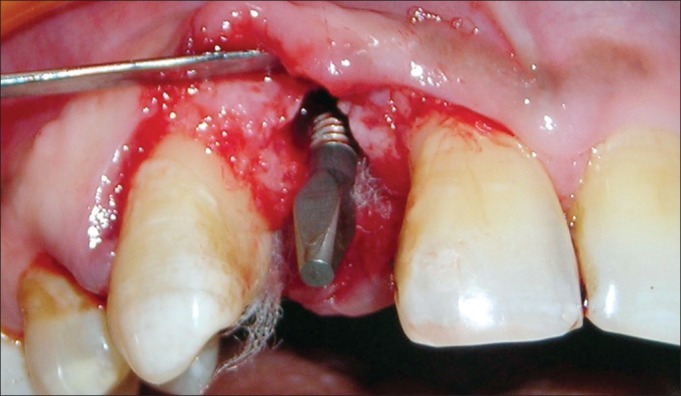
Circumferential defect with slight buccal bone loss around the failing implant
Figure 9.
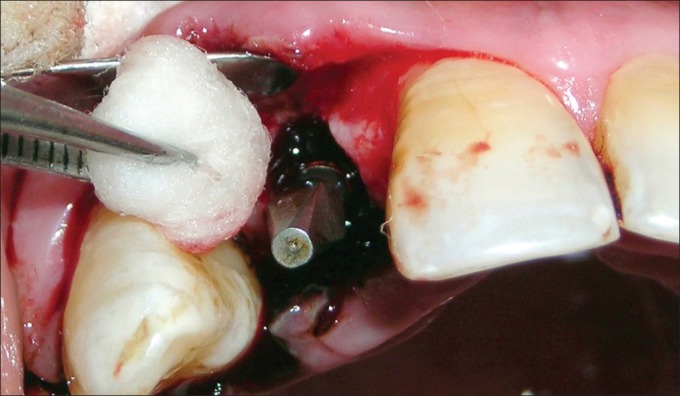
Decontamination by supersaturated solution of citric acid
The peri-implant defect was filled with PerioGlas® (NovaBone Products, LLC, Alachua, Florida, USA) starting from the bottom of the defect [Figures 10 and 11]. However, the graft material was moistened in sterile saline before placement into the defect. Following grafting, the bio-resorbable type-I bovine collagen membrane (Healiguide®, Advanced Biotech Products Pvt. Ltd., Chennai, India) was trimmed and adapted over the entire defect so as to cover 2-3 mm of the surrounding alveolar bone and to ensure stability of the graft material [Figures 12 and 13]. Primary wound closure was achieved, using non-resorbable 3-0 black braided silk sutures (Mersilk®, Ethicon, Johnson and Johnson, India) and the area was covered with periodontal dressing (Coe-pak, G. C. India) for 7 days [Figures 14 and 15]. The patient continued with the antibiotic coverage 1 week postsurgery and was also instructed to rinse twice daily with 0.2% chlorhexidine (Clohex, Dr. Reddy's laboratories ltd., India). The dressing and sutures were removed after 7 days. The patient was asked to continue with 0.2% chlorhexidine for 3 weeks and to avoid brushing this region for the same time period. Regular recall was done, once every month for 6 month period.
Figure 10.
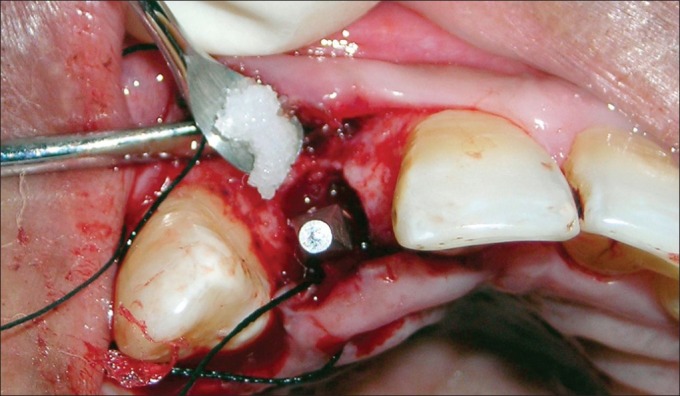
PerioGlas® being carried to the peri-implant defect
Figure 11.
Peri-implant defect filled with PerioGlas®
Figure 12.
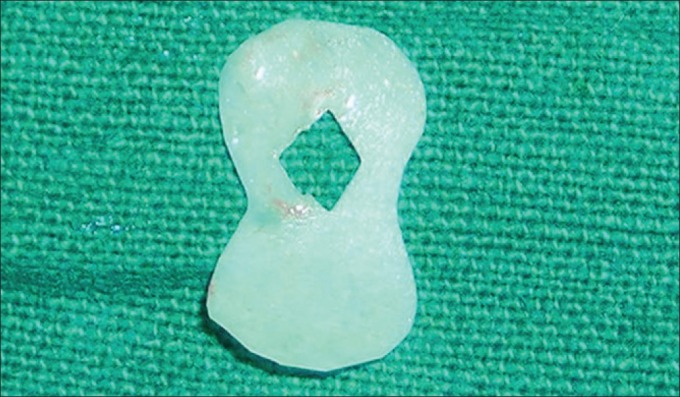
Trimmed and shaped resorbable collagen membrane (Healiguide®)
Figure 13.
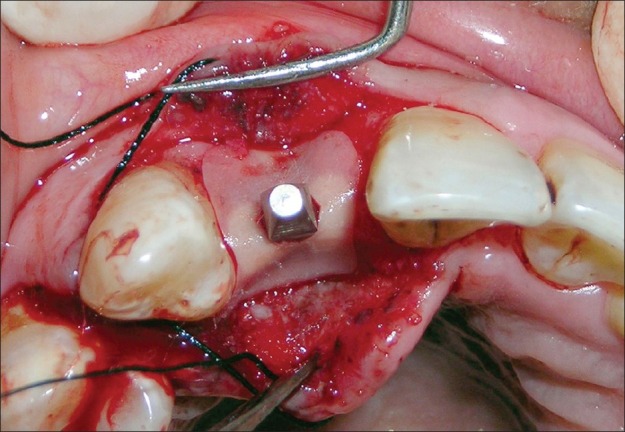
Resorbable collagen membrane (Healiguide®) adapted over the periimplant defect
Figure 14.

Primary wound closure achieved using non-resorbable 3-0 black braided silk sutures
Figure 15.
Surgical area covered with periodontal dressing
At the end of 6 month follow-up, the healing was found to be complete with a clinical evidence of healthy and firm mucosa around the implant [Figure 16] and no sign of clinically detectable implant mobility. Intraoral periapical (IOPA) radiograph showed complete resolution of the treated defect, which may be due to regeneration of bone [Figure 17].
Figure 16.
Six-month follow-up photograph showing complete healing
Figure 17.
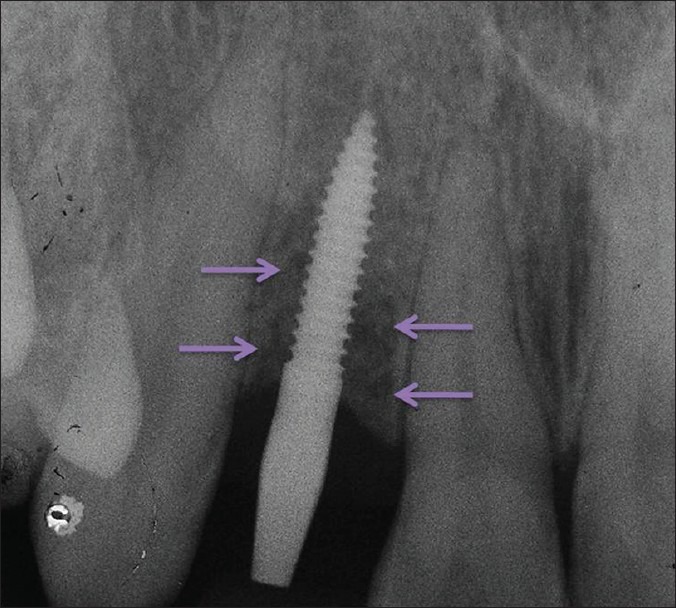
Intraoral periapical radiograph showing complete regeneration of bone and resolution of the treated defect
After obtaining satisfactory clinical outcome, the process of fabrication of definitive prosthesis was taken up. The axial surfaces of the implant were modified using multi-fluted carbide burs under copious irrigation. The reduction of the axial surfaces was kept as minimum as possible. As the abutment was modified intraorally, an impression coping and implant analog could not be used. Hence, a direct pick up impression was made using additional silicone material, without the use of any impression coping, and the cast was poured using type-4 dental stone. Shade selection was also done during this appointment, and finally, the definitive porcelain fused-to-metal prosthesis was fabricated in the laboratory and delivered to the patient [Figures 18–20] with the best satisfaction of the patient.
Figure 18.
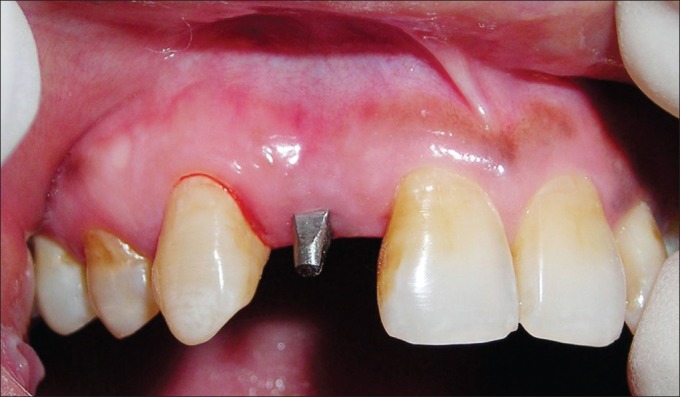
Modified axial surface of the implant abutment
Figure 20.
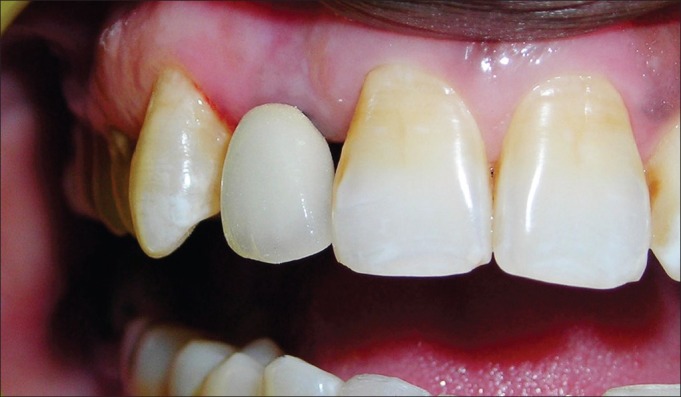
Final porcelain fused to metal prosthesis
Figure 19.
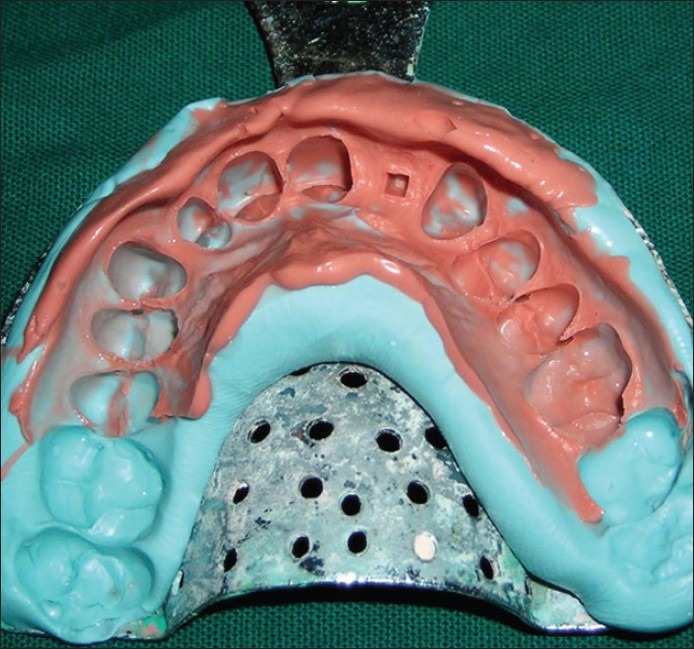
Pick-up impression of the implant abutment
DISCUSSION
The clinical evidence of osseointegration revolutionized the field of dentistry and made implant therapy a viable alternative to the earlier treatments of replacing missing teeth. Osseointegrated implants present predictable, reproducible, and durable results. Despite success which ranges from 95% to 99%,[1] failures do occur.[2]
Implant failures can be classified as Biological failures (related to the biological process), Mechanical failures (fracture of implants, connecting screws, bridge frameworks, coatings, etc.), Iatrogenic failures (nerve damages, wrong alignment of implants, etc.), and Inadequate or insufficient patient adaptation (psychological, aesthetical, and phonetical problems). Biological implant failures can be further classified as Early failure (before loading) and Late failure (after loading).[3]
Factors contributing to implant failures can be divided into Endogenous (systemic and local) and Exogenous (operator-related and biomaterial-related). Proposed etiology for early failure may include inexperienced operator, excessive surgical trauma, bacterial contamination, premature overloading with micromotion during the healing phase, and some local as well as systemic characteristics of the host.[4]
Due to lower bite forces in the anterior region of mouth,[16] esthetic demand and support of scientific literature with successful protocol in mini-implants[17–19] a pre-operative decision was made to give immediate provisional acrylic restoration for 4-6 weeks, followed by final porcelain fused to metal (PFM) prosthesis at the end of 6 weeks. But the insertion torque achieved was only 20 Ncm which indicated less primary stability. Hence, provisional acrylic restoration was avoided and it was decided to directly give a final PFM prosthesis at the end of 6 weeks. However, due to the clinical features of early implant failure at the end of 6 weeks, case was first treated by means of GBR and only at the end of 6 months follow up, PFM prosthesis was fabricated.
The early failing of implant presented here, may be due to early occlusal load on the implant (due to one-piece design) during the initial healing period or excessive surgical trauma, or lack of good initial stability due to unfavorable bone density at the implant site, or increased risk as the patient had a history of periodontitis.[20,21]
Treatment options for early failing implant include mechanical debridement, antimicrobial therapy, and GBR. A case report demonstrated successful management of an early failing implant through antimicrobial therapy and guided tissue regeneration.[11] Similarly, in a recent case series, 18 implants undergoing early failure were treated successfully following the same protocol.[9]
In the case presented here, successful treatment of failing implant was achieved, based on similar guidelines. The surgical treatment allowed complete access for the debridement of the peri-implant defect. Since the bone loss was severe, bone regenerative treatment like GBR using bioactive glass in combination with GTR membrane was attempted. Bioactive glass has been shown to regenerate bone around implants.[15] Surface decontamination allowed for the removal of any residual infection not removed by debridement and made the implant surface conducive to bone regeneration and re-osseointegration.
CONCLUSION
Surgical intervention with mechanical debridement, antimicrobial therapy, and guided bone regeneration may be carried out to salvage an early failing implant. Early detection with early intervention is important to control progressive bone loss around the implant and reverse the fate of implant. The technique mentioned here is not new. Literature is full of various techniques with variable outcomes. Future consideration should focus on proper case selection, avoidance of surgical failure, control of infection during surgery, and long-term follow ups with better study design.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35 w:286–91. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 2.Esposito M, Thomsen P, Ericson LE, Lekholm U. Histopathologic observations on early oral implant failures. Int J Oral Maxillofac Implants. 1999;14:798–810. [PubMed] [Google Scholar]
- 3.Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur J Oral Sci. 1998;106:527–51. doi: 10.1046/j.0909-8836..t01-2-.x. [DOI] [PubMed] [Google Scholar]
- 4.Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (II). Etiopathogenesis. Eur J Oral Sci. 1998;106:721–64. doi: 10.1046/j.0909-8836..t01-6-.x. [DOI] [PubMed] [Google Scholar]
- 5.Friberg B, Jemt T, Lekholm U. Early failures in 4,641 consecutively placed Branemark dental implants: A study from stage 1 surgery to the connection of completed prostheses. Int J Oral Maxillofac Implants. 1991;6:142–6. [PubMed] [Google Scholar]
- 6.Palmqvist S, Sondell K, Swartz B. Implant-supported maxillary overdentures: Outcome in planned and emergency cases. Int J Oral Maxillofac Implants. 1994;9:184–90. [PubMed] [Google Scholar]
- 7.Tinsley D, Watson CJ, Ogden AR. A survey of U.K. centres on implant failures. J Oral Rehabil. 1999;26:14–8. doi: 10.1046/j.1365-2842.1999.00355.x. [DOI] [PubMed] [Google Scholar]
- 8.Kronstrom M, Svenson B, Hellman M, Persson GR. Early implant failures in patients treated with Branemark System titanium dental implants: A retrospective study. Int J Oral Maxillofac Implants. 2001;16:201–7. [PubMed] [Google Scholar]
- 9.Alghamdi AS. Successful treatment of early implant failure: A case series. Clin Implant Dent Relat Res. 2012;14:380–7. doi: 10.1111/j.1708-8208.2009.00267.x. [DOI] [PubMed] [Google Scholar]
- 10.Artzi Z, Tal H, Chweidan H. Bone regeneration for reintegration in peri-implant destruction. (22-3, 26-8).Compend Contin Educ Dent. 1998;19:17–20. [PubMed] [Google Scholar]
- 11.Lehmann B, Bragger U, Hammerle CH, Fourmousis I, Lang NP. Treatment of an early implant failure according to the principles of guided tissue regeneration (GTR) Clin Oral Implants Res. 1992;3:42–8. doi: 10.1034/j.1600-0501.1992.030107.x. [DOI] [PubMed] [Google Scholar]
- 12.Zablotsky MH, Diedrich DL, Meffert RM. Detoxification of endotoxin- contaminated titanium and hydroxyapatite-coated surfaces utilizing various chemotherapeutic and mechanical modalities. Implant Dent. 1992;1:154–8. doi: 10.1097/00008505-199205000-00009. [DOI] [PubMed] [Google Scholar]
- 13.De Smet E, van Steenberghe D, Quirynen M, Naert I. The influence of plaque and/or excessive loading on marginal soft and hard tissue reactions around Brånemark implants: A review of literature and experience. Int J Periodontics Restorative Dent. 2001;21:381–93. [PubMed] [Google Scholar]
- 14.Sahrmann P, Attin T, Schmidlin PR. Regenerative treatment of peri-implantitis using bone substitutes and membrane: A systematic review. Clin Implant Dent Relat Res. 2011;13:46–57. doi: 10.1111/j.1708-8208.2009.00183.x. [DOI] [PubMed] [Google Scholar]
- 15.Veis AA, Dabarakis NN, Parisis NA, Tsirlis AT, Karanikola TG, Printza DV. Bone regeneration around implants using spherical and granular forms of bioactive glass particles. Implant Dent. 2006;15:386–94. doi: 10.1097/01.id.0000243317.57261.86. [DOI] [PubMed] [Google Scholar]
- 16.Helkimo E, Carlson GE, Helkimo M. Bite force and state of dentition. Acta Odontol Sand. 1977;35:297–303. doi: 10.3109/00016357709064128. [DOI] [PubMed] [Google Scholar]
- 17.Mazor Z, Steigmann M, Leshem R, Peleg M. Mini-implants to reconstruct missing teeth in severe deficiencyand small interdental space. Implant dent. 2004;13:336–9. doi: 10.1097/01.id.0000148554.83439.00. [DOI] [PubMed] [Google Scholar]
- 18.Dilek OC, Tezulas E. Treatment of a narrow single tooth edentulous area with mini dental implants; A clinical report. Oral Surg Oral Med Oral Pathol Oral Radiol Endol. 2007;103:e22–5. doi: 10.1016/j.tripleo.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqu AA, Sasovicka M, Goelz M. Use of mini implants for replacement and immediate loading of 2 single tooth restorations: A clinical analysis. J Oral Implantol. 2006;32:82–6. doi: 10.1563/794.1. [DOI] [PubMed] [Google Scholar]
- 20.Koldsland OC, Scheie AA, Aass AM. Prevalence of implant loss and the influence of associated factors. J Periodontol. 2009;80:1069–75. doi: 10.1902/jop.2009.080594. [DOI] [PubMed] [Google Scholar]
- 21.Palma-Carrió C, Maestre-Ferrín L, Peñarrocha-Oltra D, Peñarrocha-Diago MA, Peñarrocha-Diago M. Risk factors associated with early failure of dental implants. A literature review. Med Oral Patol Oral Cir Bucal. 2011;16:e514–7. doi: 10.4317/medoral.16.e514. [DOI] [PubMed] [Google Scholar]


