Abstract
Background:
Treatment of periodontal diseases by nonsurgical debridement has been considered as a gold standard procedure. Various other treatment modalities have been tried and tested to treat periodontal diseases. The aim of this study was to investigate the effect of antioxidant therapy on the progression of periodontal disease as monotherapy and/or as an adjunct to nonsurgical debridement.
Materials and Methods:
70 subjects were divided into three groups, i.e. clinically healthy, gingivitis and periodontitis group on the basis of Community Periodontal Index of Treatment Needs score. Gingivitis and periodontitis groups were further subdivided into three subgroups. At the baseline, periodontal attachment loss was recorded and scaling and root planing was performed in two subgroups. 6 mg antioxidant was administered in three divided doses for 2 weeks. Saliva samples were collected at baseline, 15th day, 30th day and 45th day for evaluation of uric acid levels.
Results:
Uric acid levels were significantly low in patients with more periodontal attachment loss as compared to clinically healthy and gingivitis groups. As the treatment was initiated, significant increase in uric acid levels was observed.
Conclusion:
Rise in salivary antioxidant levels was observed on the administration of antioxidant therapy. Hence, antioxidant therapy can be used as an adjunct to the nonsurgical periodontal therapy.
Keywords: Periodontitis, saliva, uric acid
INTRODUCTION
Periodontal diseases, the most prevalent diseases throughout the world,[1] are predominantly caused by gram-negative, anerobic bacteria present on the tooth root surfaces as a biofilm. Inflamed periodontal tissue produces significant amounts of pro-inflammatory cytokines, mainly IL-1, IL-6, PGE2 and tumor necrosis factor alpha (TNF-α), reactive oxygen species enzymes, proteins, host cells, ions, hormones, and markers of oxidative stress and antioxidant.
Reactive oxygen species (ROS) play crucial roles in normal physiological processes including response to growth factors, the immune response, and apoptotic elimination of damaged cells but are also highly toxic and destructive when generated during the respiratory burst as it represents an important pathogenic mechanism for tissue damage and diseases associated with phagocytic infiltration.[2]
The discovery of the role of free radicals in cancer, diabetes, cardiovascular diseases, autoimmune diseases, neurodegenerative disorders, aging, and other chronic diseases has led to a medical revolution comprising of various antioxidants that is promising a new paradigm of healthcare. Hence, antioxidants are emerging as prophylactic and therapeutic agents. These are the agents, which scavenge free radicals otherwise reactive oxygen species and prevent the damage caused by them. Lycopene, a pigment that gives tomatoes red color, is one of the most potent antioxidants among dietary carotenoids with a singlet-oxygen-quenching ability three times as high as that of ß-carotene and ten times higher than that of α-tocopherol. Dietary intake of tomatoes and tomato products containing lycopene has been shown to be associated with a decreased risk of chronic diseases, such as cancer and cardiovascular disease-tocopherol. Dietary intake of tomatoes and tomato products containing lycopene has been shown to be associated with a decreased risk of chronic diseases, such as cancer and cardiovascular disease.[3]
Various biomarkers apart from clinical appearance have been used to evaluate periodontal destruction which includes enzymes, proteins, host cells, markers of cellular and humoral activity, ions, hormones, and markers of oxidative stress and antioxidants. Uric acid is one of the major antioxidants[4] that is present in saliva and can be used to determine the effect of antioxidant therapy in the treatment of periodontal diseases.
The aim of the present study is to evaluate efficacy of antioxidants in the treatment of chronic gingival and periodontal disease and to compare the efficacy of antioxidant as a monotherapy and as an adjunct in the treatment of gingivitis and chronic periodontitis.
MATERIALS AND METHODS
This study was conducted on subjects between 30 and 60 years of age. 70 subjects were selected from those attending the Department of Periodontics, Pacific Dental College and Hospital, Udaipur, Rajasthan. Subjects were selected on the basis of Community Periodontal Index of Treatment Needs (CPITN). Subjects with systemic diseases like diabetes, cardiovascular diseases, and renal diseases, subjects with a habit of smoking, pan chewing, subjects on medications like antibiotics or antioxidants like vitamin C, vitamin E or β-carotenes within 3 months and pregnant and lactating mothers were excluded from this study.
The proposed study was reviewed by the ethical committee of the institution and clearance was obtained. An informed consent was obtained from each subject before conducting the trial, the form of which is enclosed. The selected subjects were divided into three groups on the basis of CPITN index [Table 1].
Table 1.
Study groups
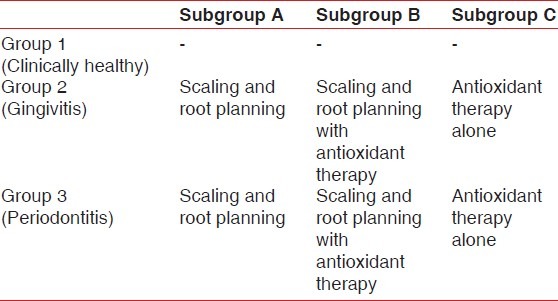
Group I: 10 subjects having clinically healthy gingiva without any signs of inflammation.
Group II: 30 subjects having gingivitis. This group was further divided into three subgroups.
Subgroup IIA: 10 subjects were treated by non-surgical periodontal therapy.
Subgroup IIB: 10 subjects were treated by non-surgical periodontal therapy and were given antioxidants also.
Subgroup IIC: 10 subjects were given antioxidants only.
Group III: 30 subjects suffering from chronic periodontitis. This group was further divided into three subgroups.
Subgroup IIIA: 10 subjects were treated by non-surgical periodontal therapy.
Subgroup IIIB: 10 subjects were treated with non-surgical periodontal therapy and were given antioxidants also.
Subgroup IIIC: 10 subjects were given antioxidants only.
Antioxidant was given in a dosage of 6 mg per day in three divided doses for 2 weeks. Each softgel was composed of lycopene 2000 mcg, zinc 7.5 mg, and selenium 35 mcg.
Collection of saliva sample and uric acid determination
1 ml of unstimulated saliva samples were collected by allowing saliva to passively flow into a sterile test tube after rinsing the mouth with water to wash out the exfoliated cells for all the subjects. Supragingival and subgingival scaling were done after sample collection in Groups II and III subgroup A and B.
The samples of saliva from Group II and III patients were collected again after 15, 30, and 45 days of nonsurgical periodontal treatment.
The uric acid level was evaluated by using a reflotron reflectance photometer at the wavelength of 642 nm.
RESULTS
Russell periodontal index
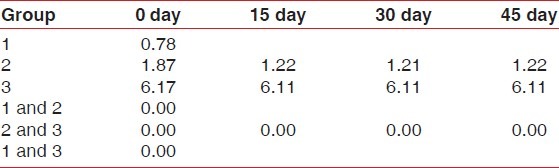
The mean score for patients of Group 1 (clinically healthy) was 0.78±0.26, 1.87±0.27 for Group 2 (gingivitis group), and 6.11±0.58 for Group 3 (periodontitis group). On comparison between 1 and 2, 2 and 3, and 1 and 3 using post hoc test, highly significant difference (P=0.000) was observed on 0 day. Comparison between Groups 2 and 3 was done using the post hoc test. Highly significant difference (P=0.00) was observed on the 15th day. Similar results were observed on both 30th and 45th day.
One-way Anova test was applied to compare the periodontal status between and within the groups on 0, 15th, 30th, and 45th day. Comparison between 0, 15, 30, and 45 days showed a highly significant difference [Table 1].
Uric acid level
Group 1
For patients of Group 1, the mean score on 0 day was 5.19±0.8 and 5.56±0.88 on the 45th day. On comparison between 0 and 45th day using the T test, no significant difference (P=0.15) was observed [Table 2].
Table 2.
Comparison of the Russell periodontal index between all the groups and subgroups on baseline, 15th, 30th, and 45th day
Group 2
For patients of subgroup A, the mean score was 3.16±0.32, subgroup B the mean score was 2.25±0.35, subgroup C the mean score was 2.2±0.26. On comparison between A and B using the post hoc test, no significant difference (P=0.7) was observed while a highly significant difference (P=0.00) was observed between subgroups B & C and A & C using the post hoc test, on 0 day. Similar results were seen on 15th, 30th, and 45th day.
One-way Anova test was applied to compare uric acid levels between and within the subgroups of Group 2 on 0, 15th, 30th, and 45th day [Figure 1]. On comparison of uric acid on 0, 15th, and 30th day, significant difference (P=0.00, 0.001 and 0.009, respectively) was observed. While the uric acid level on 45th day showed non-significant difference (P=0.53).
Figure 1.
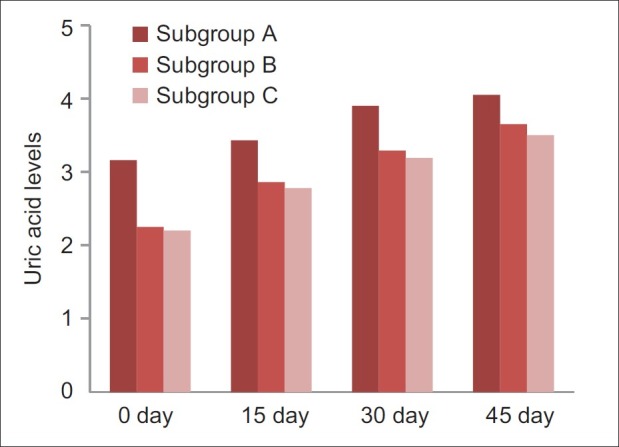
Comparison of uric acid levels between subgroup a, b and c of group 2 on 0, 15th, 30th and 45th day
Group 3
For patients of subgroup A, the mean score was 2.43±0.42, subgroup B the mean score was 2.29±0.4, and subgroup C the mean score was 2.29±0.39. On comparison between A and B, B and C, and A and C using post hoc test, no significant difference (P=0.65, 0.99, and 0.45 respectively) was observed on 0 day.
On comparison between A and B using the post hoc test, no significant difference (P=0.26) was observed. On comparison between B and C and A and C using the post hoc test, significant difference was observed on 15th, 30th, and 45th day.
One-way Anova test was applied to compare uric acid levels between and within the subgroups of Group 3 on 0, 15th, 30th, and 45th day [Figure 2]. On comparison of uric acid on 15th, 30th, and 45th day, significant difference (P=0.042, 0.024, and 0.00, respectively) was observed.
Figure 2.
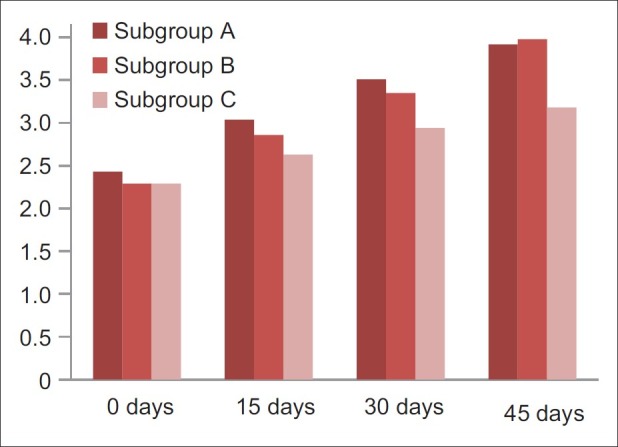
Comparison of uric acid levels between subgroup a, b and c of group 3 on 0, 15th, 30th and 45th day
DISCUSSION
Periodontal diseases are inflammatory diseases which have multifactorial etiology. The inflammatory and immune responses to the bacteria and also viruses that colonize the periodontal and associated tissues involve the systemic circulation and ultimately the peripheral systems of the body. This creates a complex bi-directional series of host - microbial interactions involving cellular and humoral factors and networks of cytokines, chemokines, and growth factors. According to Haffajee, et al.,[5] the primary etiological agent is specific which consists predominantly gram-negative anerobic or facultative bacteria within the subgingival biofilm, but the majority of periodontal tissue destruction is caused by an inappropriate host response to those microorganisms and their products. Detection of markers of tissue destruction can thus be incorporated in diagnosis of periodontal diseases.
Loss of balance between reactive oxygen species and antioxidant defense has also been implicated as an etiologic factor for periodontal diseases. It may manifest as an increase in oxidative stress, reduction of total antioxidant capacity, or decrease in individual antioxidant level. Chapple and Matthews[6] implicated oxidative stress in the pathogenesis of periodontitis.
In this study, significant difference was observed in the periodontal index between healthy, gingivitis, and periodontitis group on 0 day and 15th day. Similar results were also observed in a study by Cugini, et al.[7]
According to Moore, et al.,[4] uric acid is a major antioxidant in saliva and contributes approximately 70% of the total salivary antioxidant capacity. It has been seen that levels of antioxidants vary according to the severity of disease, and similarly uric acid levels also vary according to the severity of periodontal disease. Significant difference was observed in salivary uric acid levels on 0 day between healthy, gingivitis and periodontitis groups.
A decrease in the salivary uric acid levels were observed in the gingivitis and periodontitis groups. Moore, et al.[4] and Chandra, et al.[8] also found similar results in their study.
Diab-Ladki, et al.[9] in a similar study also found that there was a slight decrease in the concentration of three main antioxidants (uric acid, ascorbic acid, and albumin) in saliva with the increase in severity of periodontal disease.
Results of this study showed that there was a significant increase in uric acid levels in all the subgroups A, B, and C [Tables 1 and 3] on 15th, 30th, and 45th day of both gingivitis and periodontitis groups.
Table 3.
Comparison of uric acid levels of Group 1 on 0 and 45th day

Although no significant difference could be observed in uric acid levels between scaling and root planing (subgroup A) and scaling and root planing with antioxidant (subgroup B), an increase in the mean uric acid levels was observed as the time elapsed. This indicates that there is some role of antioxidants in periodontal health.
Chandra, et al.[8] in their study observed that though there was no significant difference in uric acid levels in between groups treated with a combination of oral prophylaxis and antioxidant and group treated with scaling and root planning alone, there was a better response to antioxidant therapy in combination to oral prophylaxis in the gingivitis group. In this study, similar results were obtained in both gingivitis and periodontitis groups.
No significant difference was seen in salivary uric acid levels from 30th to 45th day. This shows that within 45 days, a plateau plasma level of antioxidants was achieved which causes decrease in values of the periodontal index.
CONCLUSION
There was a reduction in periodontal inflammation with an increase in the salivary uric acid levels seen in subgroups treated by antioxidants in both gingivitis and periodontitis groups.
Hence, within the limitations of this study, it can be concluded that antioxidants can be used as an effective adjunct to scaling and root planing for the treatment of gingivitis and periodontitis. To further evaluate long-term effects of antioxidant therapy on periodontal inflammation, more expanded longitudinal studies are required.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ciancio SG. Periodontal medicine: Health policy implications. J Periodontol. 2003;7:1088–95. doi: 10.1902/jop.2003.74.7.1088. [DOI] [PubMed] [Google Scholar]
- 2.Chapple IL. Role of free radicals and antioxidants in the pathogenesis of the inflammatory periodontal diseases. Clin Mol Pathol. 1996;49:M247–55. doi: 10.1136/mp.49.5.m247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal S, Rao AV. Tomato lycopene and its role in chronic diseases. CMAJ. 2000;163:739–44. [PMC free article] [PubMed] [Google Scholar]
- 4.Moore S, Calder KA, Miller NJ, Rice-Evans CA. Antioxidant Activity of Saliva and Periodontal Disease. Free Radic Res. 1994;21:417–25. doi: 10.3109/10715769409056594. [DOI] [PubMed] [Google Scholar]
- 5.Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 6.Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160–32. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 7.Cugini MA, Haffajee AD, Smith C, Kent RL, Jr, Socransky SS. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J Clin Periodontol. 2000;27:30–6. doi: 10.1034/j.1600-051x.2000.027001030.x. [DOI] [PubMed] [Google Scholar]
- 8.Chandra RV, Prabhuji ML, Roopa DA, Ravirajan S, Kishore HC. Efficacy of lycopene in the treatment of gingivitis: A Randomized, Placebo-controlled clinical trial. Oral Health Prev Dent. 2007;5:327–36. [PubMed] [Google Scholar]
- 9.Diab-Ladki R, Pellat B, Chahine R. Decrease in the total antioxidant activity of saliva in patients with periodontal diseases. Clin Oral Investig. 2003;7:103–7. doi: 10.1007/s00784-003-0208-5. [DOI] [PubMed] [Google Scholar]


