Abstract
Mouth provides a congenial environment for the growth of the microorganisms as compared to any other part of the human body by exhibiting an ideal nonshedding surface. Dental plaque happens to be a diverse community of the microorganisms found on the tooth surface. Periodontal disease and the peri-implant disease are specific infections that are originating from these resident microbial species when the balance between the host and the microbial pathogenicity gets disrupted. This review discusses the biofilms in relation to the peri-implant region, factors affecting its presence, and the associated treatment to manage this complex microbial colony. Search Methodology: Electronic search of the medline was done with the search words: Implants and biofilms/dental biofilm formation/microbiology at implant abutment interface/surface free energy/roughness and implant, periimplantitis/local drug delivery and dental implant. Hand search across the journals – clinical oral implant research, implant dentistry, journal of dental research, international journal of oral implantology, journal of prosthetic dentistry, perioodntology 2000, journal of periodontology were performed. The articles included in the review comprised of in vivo studies, in vivo (animal and human) studies, abstracts, review articles.
Keywords: Biofilms, dental implants, peri-implantitis, plaque microbiology
INTRODUCTION
Biofilm is described as relatively undefinable microbial community associated with tooth surface or any hard nonshedding material.[1] Biofilms are ubiquitous and they form on virtually all surfaces immersed in natural aqueous environment, e.g., water pipes, living tissue, tooth surface, implanted medical devices, dental implants, etc., Biofilm adhesion-mediated infections most commonly seen are on the implanted heart valves, venous catheters, vascular prosthesis, fracture fixation devices, breast implants, intraocular lenses, and dental implants.[2] Biofilms consist of one or more communities of microorganisms nonrandomly distributed in a glycocalyx. These biofilms allow the microorganisms to stick and multiply on the surfaces. The interactions among the various bacterial species residing and growing in the biofilm takes place by metabolic exchange, physical contact, exchange of genetic information, signaling molecule-mediated information.[3]
Biofilms formed on the tooth surface is called as dental plaque. Bacteria proliferating in the dental plaque form the main etiologic factors for the majority of the dental ailments, e.g., caries, gingivitis, periodontitis, and periimplantitis. Microbial attack has been cited as the main cause of the dental implant failure.[4] Biofilms are responsible for their association of about 65% of diseases including peri-implantitis and periodontitis.[5] The review addresses the pathogenesis, factors affecting implant biofilm, and the treatment associated.
BIOFILM AND TOOTH
The formation of the microbial complex called biofilm in the oral cavity is a multistage journey.[6] Saliva provides the major source of nutrients to the bacteria. The thin film covering the tooth called as acquired pellicle is derived from the salivary proteins and covers the enamel within seconds after brushing. Proteins and the glycoproteins are the molecules binding to the tooth surface, implants, restorations, etc., These molecules primarily act to promote the adhesion and coaggregation of the oral bacteria. The bacterial adherence to the pellicle is facilitated by the special surface molecules (adhesins) chiefly lectins present on the bacterial cell surface. Further intercellular bacterial adhesion and secretion of the extracellular polysaccharides, e.g., levans, dextrans, form the multilayered bacterial colonies suspended in the polymer matrix. Slow aggregation of the bacterial colonies leads to the formation of the multilayered cell clusters in the polymer matrix.[7] The microbial load in the saliva constitutes about 10[7] bacteria per milliliter.[8] The bacterial cells colonize on the tooth surface within 4 hours of the pellicle formation. The initial colonizers being the Streptococci (S. viridens, S. mitis, S. oralis). The planktonik bacteria that are unable to bind directly to the tooth surface take the aid of their receptors to bind to the cell surfaces of the initial colonizing bacteria and finally on to the tooth surface. This bacterial cell to cell reaction that occurs or the coaggregation happens to be an important mechanism leading to the bacterial colonization and dental biofilm formation. Secondary colonizers bind to the bacteria predominantly comprising of the Actinomyces species, S. mutans, S. sobrinus. The bacteria multiply and co aggregate with the partner species. Fusobacterium nucleatum has the property to co-aggregate with multiple bacteria hence this species is an important link in the dental biofilms bridging the early and the late colonizers.[9] The oral bacteria thrive for their nutrient supply from saliva, gingival crevicular fluid, sugar rich food metabolic products of other bacteria, and food debris. The mature plaque has an inherent “circulatory system.” The plaque begins to behave as a complex microorganism. Metabolic products and evulsed cell wall constituents (lipopolysaccharides, vesicles) activate the host response [Figure 1]. Specialized cell-cell communication is exhibited by the bacteria that coordinate the gene expression. This communication is passed on as signals. Bacteria sense the changes in the local environment (cues) and receive the information of the adjacent population. Specific interspecies communication within the biofilms is mediated through the metabolic exchange, genetic exchange, and the quorom sensing.[10] Quorum sensing is genetically governed chemical communication among bacteria in response to cell density and influence several functions of the bacteria, e.g., virulence, acid tolerance, and the biofilm formation. Two specific signaling molecules have been produced by the oral bacteria. Gram-positive bacteria communicate via small diffusible pepitide channel called as “Competence Stimulating Peptides (CST) and AI-2.” AI-2 (autoinducer-2) is a popular signaling molecule exhibited by both gram-positive and gram-negative bacteria responsible for the quorum sensing.[9,11] The biofilm acts as a barrier for the bacteria against host immunity and the antimicrobial agents. The anaerobic microflora succeeds to occupy the subgingival environment gradually as the plaque starts maturing. Supragingival plaque sets up the stage for the disease process of gingivitis and the subgingival microbial colonies advance the gingivitis to an established form of periodontitis.
Figure 1.
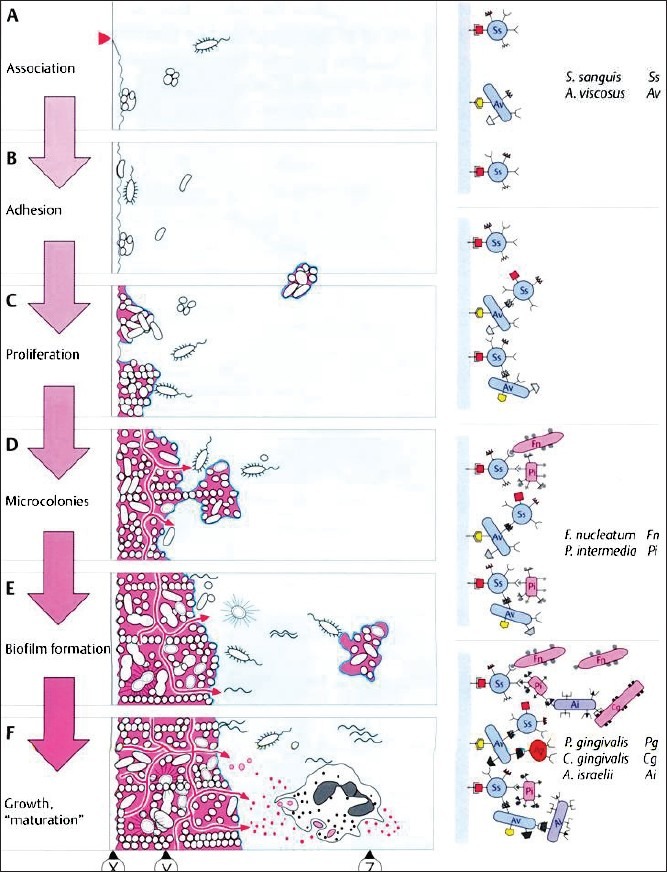
Stages of biofilm formation (ref Wolfe HF. Biofilm plaque formation on tooth and root surfaces. In: Wolfe, H.F. Rateitschak, K.H. (eds). Periodontology, ed 3. Stuttgart: Thieme 2005; 24)
BIOFILM AND IMPLANT
Microbiologic evidence of the first human biofilm-related peri-implant infection comes from the study on plaque samples collected from apical most part of 17 diseased implants. Implants with deeper probing pockets showed a presence of lesser number of coccoid and more levels of the spirochetes.[12] Biofilm formation on dental implants and the teeth follow the similar pattern of microbial colonization.[13] Biofilm formation around natural teeth occurs in minutes and the specific species start colonizing as early as 2-6 hours. The reason attributed possibly lies in the fact that the clean tooth surfaces are likely to have remnants of unattached microbiota that can immediately multiply and provide a favorable surface for the attachment of the late colonizers.[14] The pristine surfaces of the implants lack the desired indigenous microbiota and demand the early colonizers to set the stage for the complex communities to develop.[15] The pellicle starts forming on the implant surface as early as 30 minutes after the implant is exposed in the oral cavity.[16] The acquired pellicle on the dental implants owing to their lower albumin absorption capacity causes a low plaque formation around implants. Early colonizers are predominantly the gram-positive cocci, rods, and actinomyces species.[17] The periodontal pathogens colonizing on the Streptococci (P. gingivalis, P. intermedia, etc) are the causative microorganisms responsible for peri-implantitis and periodontitis [Table 1].[25]
Table 1.
Study data on the effect of dental implant surface properties on biofilm formation
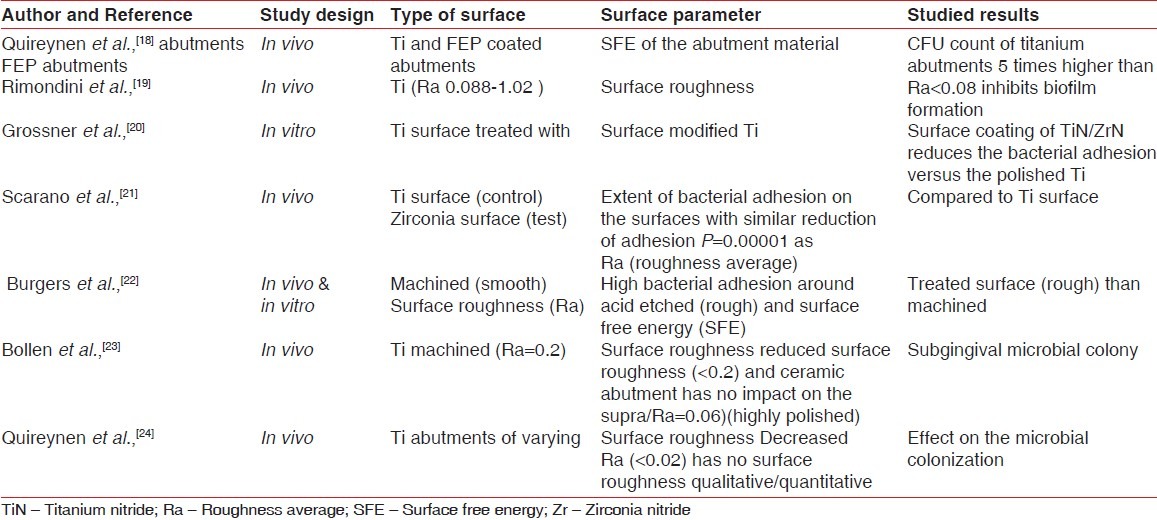
The osseointegration around the dental implant is largely influenced by its surface roughness.[30] However, greater is the surface roughness, higher is the rate of the biofilm formation around the implant.[27] The attachment of the microorganisms to the hard surfaces, i.e., teeth and implants, besides their interactions with the surface components (roughness) also require certain specific characteristics of these interacting surfaces in terms of their wettability/hydrophobicity and surface free energy (SFE). In an in vivo, study a smooth titanium abutment and a sandblasted titanium surface was evaluated for the biofilm accumulation. The results revealed that surface roughening harbored lower percentage of the coccoid cells (64.2%) as compared to the smooth abutments (81%)[28] In yet another previous study, Quireynen in a 96 hour supragingival plaque formation reported a positive relationship between the surface roughness and the plaque growth rate and pathogenicity.[29] These studies highlight an important fact that the surface roughness has a significant contribution for the increased plaque buildup. The bacterial adhesion initially has a weak and reversible binding to the surface before the final irreversible attachment occurs. The possible explanation of the initial weak reversible attachment of the bacteria on the rough surface is attributed to the fact that the bacteria indirectly get a protection against the mechanical shear. An SEM results for an in vitro study on the attachment of the microbes to the different surface morphologies of the titanium discs (grooved, smooth, and rough) revealed a significant bacterial attachment to the rough titanium surface [Table 2].[34]
Table 2.
Studies (in vitro and in vivo data) on the microbiology in relation to the dental implants
SFE/wettability of the surface influences the formation of the biofilm. SFE is defined as the interaction between the forces of adhesion and the forces of cohesion that determine the property of wetting, i.e., spreading of the liquid over the surface.[35] An in vivo study was undertaken on the supra and subgingival microbial plaque samples in patients with implant-supported fixed prosthesis. Two-stage abutments titanium versus coated abutment (Flouroethylene propylene abutment) were studied. A 3 month microbial analysis (phase contrast microscopy, DNA analysis, and colony forming unit (CFU), respectively) for the supragingival and subgingival plaque revealed increased microbial count around FEP-coated abutments. A predominant population of cocci was evident around FEP abutment, whereas the titanium abutments harbored more of spirochetes. CFU count was higher on the titanium abutments than the FEP abutments. The results of the study revealed that SFE of the implant and the abutment material have a vital role in the colonization of the bacteria.[18] An in vivo study done on the titanium discs for evaluation of the effect of the surface roughness and the microbial colonization concluded that a titanium surface with a roughness average Ra <0.088 inhibits the colonization and maturation of the plaque.[19] In vitro study comparing the Ti discs coated with TiN/ZrN (test) with the polished Ti (control) showed decreased bacterial adhesion in the test group.[20] Convincing results were also seen in an in vivo study that aimed at investigating the extent of bacterial adhesion on the two surfaces with similar surface roughness. The conclusion drawn was that zirconium oxide surface has a low bacterial colonization potential than the titanium oxide surface.[21]
Burgers et al. reported a twofold in vivo and in vitro study that aimed at bacterial adhesion on the different textured implant surfaces. In this study, machined and acid-etched titanium specimens (Ra 0.15 and 0.95, respectively) were worn for 12 hours by the subjects. The study aimed to investigate the bacterial growth of S. sanguinis after being incubated in the microbial suspension. The microbial growth was observed with fluorescent techniques. Results revealed a higher bacterial adhesion on the acid etched surface.[22] Surface roughness Ra >0.2 μm leads to increased rate of the biofilm formation and hence acts as the main etiology behind the peri-implant breakdown.[23] However, Ra <0.2 μm has no impact on the supra and subgingival plaque formation.[24] It has further been explored and reported that Ra <0.02 μm has further no quantitative or qualitative effect on the nature of the microflora[36] [Table 3].
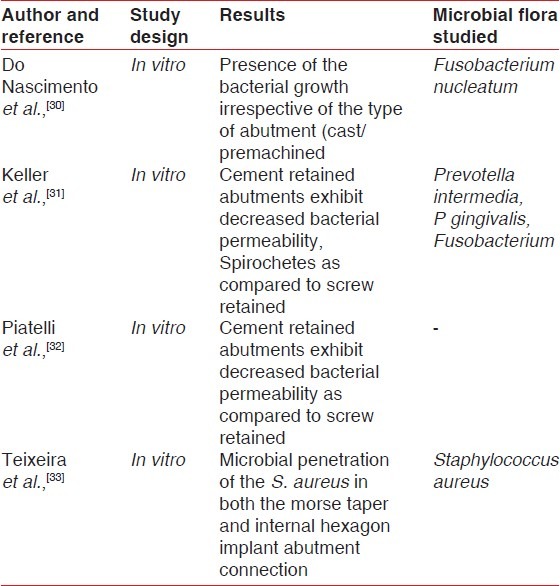
Table 3.

Microbiology of the biofilm around implants
A majority of the studies have pointed out the comparative rates and the composition of the microbiota associated with teeth and implants in health and disease. The microbiota in healthy peri-implant tissues is dominated with gram positive facultative cocci and rods.[37] A classic difference in the microbial profile of the peri-implant microflora in certain in vitro studies reveals affinity of the Staphylococcus aureus for the titanium surface but it isn’t a common microflora around the teeth.
S. aureus has high adhesion for titanium surfaces[38] and has been associated with bleeding on probing and suppuration.[39,40] Several specific adhesins are expressed on the surface of S. aureus that interact with a number of host proteins such as fibrinogen, fibronectin, collagen vironectin, and laminin. These surface adhesions have been referred to as microbial surface components recognizing adhesive matrix molecules (MSCRAMMS). After the placement of the implant, they are coated with the host plasma constituents including extracellular matrix (ECM). The fate of the implant/biomaterial surface may be conceptualized as “race for the surface” involving ECM, host cells and the bacteria. The adhesion mechanism of the S. aureus facilitates their adhesion to the biomaterials and the ECM deposited on the implant surface.[41] Transition from health to disease (peri-implantitis) causes a shift of the microflora from predominantly gram-positive to gram-negative microorganisms. Microflora of the implant in peri-implantitis have a high prevalence of the red and orange complex species as defined by Socransky.[42] This microflora is predominated with the red complex species as P. gingivalis, T. forsythia, and T. denticiola, the orange complex species as F. nucleatum, P. intermedia. Candida albicans has been found to have increased adhesion to titanium implants in certain in vitro studies.[43]
Biofilm at the implant – abutment interface
The two-piece implant consists of an implant abutment junction (IAJ).There is a joint/gap between the implant and abutment referred as the “microgap.” The histologic aspects of this microgap were studied by Ericsson et al., who identified two important microbiologic entities in the implant crestal region: (a) Plaque-associated inflammatory cell infiltrate (PaICT) and (b) implant-associated inflammatory cell infiltrate (IaICT).[44] The microgap has been reported to be as high as 40-60 μm[45] It allows micromovement during function[46] and permits microleakage of fluids congenial for bacterial growth. Several studies have reported the bacterial penetration across the implant abutment interface.[47,48] An in vitro analysis for the possible microleakage at the implant abutment interface was carried out on the implant abutment assemblies in a blood serum media previously inoculated with microorganisms. After 7 days of anaerobic incubation of the partial or completely immersed implants in the medium, the microorganisms from the internal part of the implants were collected and incubated on the blood agar plates under anaerobic conditions. Microorganisms were found in both the assemblies indicating bacterial leakage at the implant abutment interface.[32] The conclusive remarks of the study reveal that the IAJ is a potential source of microbial contamination which affects the health and integrity of the biologic tissues (bone and soft tissue) around the osseointegrated implant.
MICROBIOTA IN EDENTULOUS/PARTIALLY EDENTULOUS/HISTORY OF PERIODONTITIS PATIENTS
Studies have stated that the microbiota colonizing the clinically healthy implant fixtures in fully edentulous subjects are similar to the microbiota associated with the healthy periodontal sites.[49] It was suggested that extraction of all the teeth results in elimination of the P. gingivalis and A. actinomycetemcomitans from the oral microbiota.[50]
In partially edentulous subjects, the developing microbiota around the implants is similar to the naturally occurring teeth. This microflora inhabitate immediately after installation of the implant. 85% of the microflora is identified as gram-positive cocci. Microbial colonization and the subsequent inflammatory reaction in the peri-implant tissues might be analogous to the key events in the pathogenesis of the periodontitis. The literature comparing the microbiota around implants in fully edentulous and partially edentulous mouths stated a high percentage of the black pigment bacteroids, fewer coccoids, and motile rods in a completely edentulous mouth, whereas a high frequency of P. gingivalis and P. intermedia on the implant surface was found in partially edentulous subjects.[51] Microbiota of the remaining teeth serve as the primary source of the putative pathogens and directly influence the fate of the newly incorporated implants.[52]
Microbiota on implants in subjects with the history of the periodontal disease is similar in nature to as found in the periodontal pockets around teeth.[52] It appears imperative to assume that susceptibility to periodontitis may translate to peri-implantitis. Several reviews have reported a history of treated periodontitis as a risk indicator for the implant outcomes with statistically significant results.[50,51] Karousis in his study on the incidence of peri-implantitis in patients with a history of periodontitis reported a high incidence (28.6%) versus the subjects without previous history of periodontitis.[50]
The host response to the biofilms in relation to implants exhibit inflammatory cell infiltrate in the peri-implant mucosa with considerable loss of the collagen with high levels of the B cells and plasma cells.[53]
PREVENTION OF BIOFILM FORMATION
Biofilm formation is an inadvertent phenomenon to occur around the implant. Stringent maintenance therapy is the cornerstone of successful implantology. Systematic monitoring of the clinical and radiographic parameters, i.e., the presence of plaque and calculus, bleeding on probing, probing depths, presence/absence of suppuration, and radiographic evaluation, is important to assure the peri-implant health. Based on the periodic diagnosis, CIST protocol[54] was recommended. The recently introduced PIMI system[55] emphasizes the importance of prognosis while deciding the treatment plan.
Several pure metals, e.g., iron, titanium, nickel, exhibit bacteriostatic property. In an in vitro study done to check the antibacterial property of titanium with amalgam on the microbial strains of S. sangius, S. mitis, A. naeslundi, and Fusobacterium species revealed a weak antibacterial effects of titanium versus gold.[56] However, several orthopedic studies have documented the use of antibiotic coating on the implant surface in order to widen the antibacterial spectrum of titanium.[57] These studies give a new dimension in the possible role of the antibiotic coatings to provide an antibacterial barrier against microbial colony.
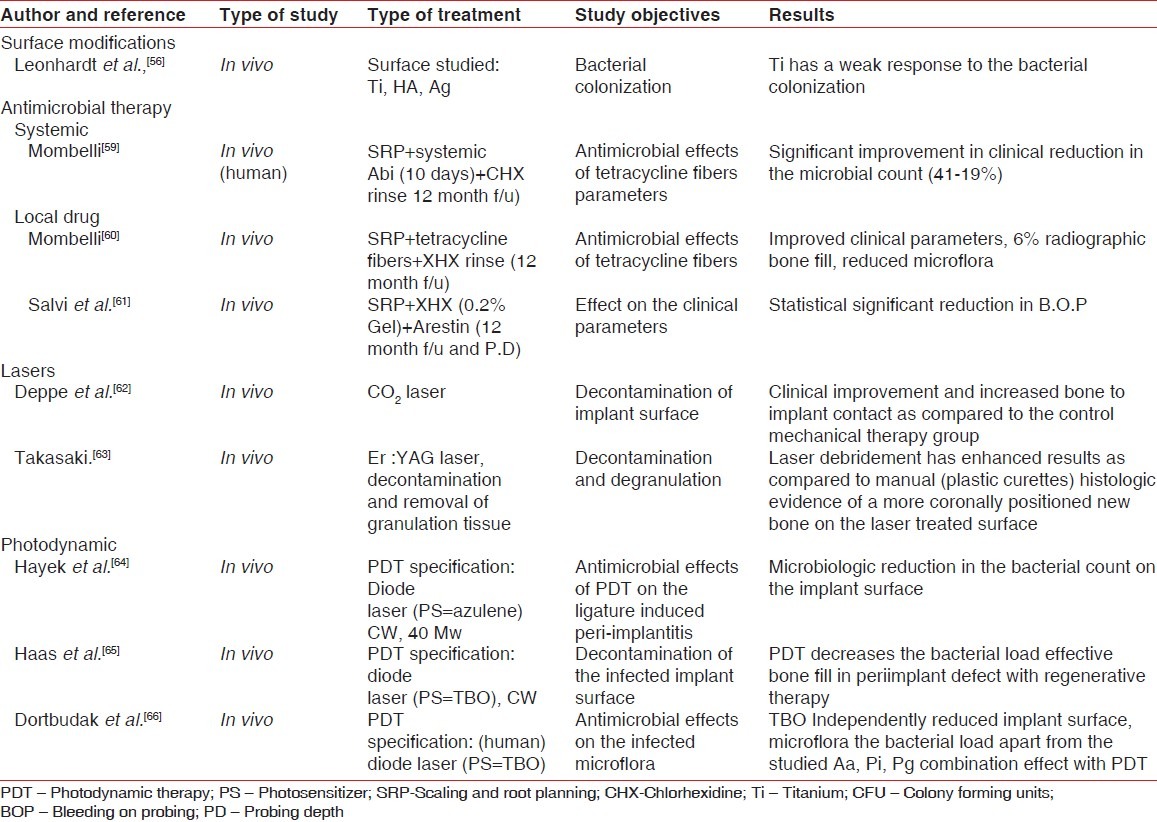
Management of the biofilms has a multilevel approach: (1) Prevention of the microleakage at the IAJ thus limiting/eliminating the biofilm ingress; (2) treatment of the biofilm-related infections. Implant biofilm can lead to infection at two levels: Mucosal level (peri-implant mucositis) described as inflammatory lesion residing in the mucosa and bone level (peri-implantitis) which is explained as inflammatory lesion affecting the supporting tissues.[58] The management of peri-implant infections aim at reduction of inflammation, pathogenic bacterial load and the probing depths. Biofilms related to dental implants are best treated through debridement of the contaminated implant surface (mechanical/laser/photodynamics, etc.,) or the antimicrobial therapy with local or systemic antibiotics [Table 4]. Decontamination of the implant surface is challenging for a predictable treatment outcome. Nonsurgical mechanical therapy has been found effective in reducing the microbial load with enhanced results when combined with the antimicrobial rinse in the peri-implant mucositis lesions.[67] Various systemic local drugs, e.g., minocycline, tetracyclines, have shown promising results by decreasing the levels of the P. gingivalis T. forsythia, A. actinomycetemcomitans.[60,61] Laser-assisted therapy for the management of the biofilm-related infection has also been documented in the literature with satisfactory results.[62] Photodynamic therapy, using low level lasers, has been used to decontaminate the infected implant surfaces. Hayek et al. in an animal study reported an effective reduction of the microbial count of Prevotella species, Strep hemolyticus on subjecting to the photodynamic therapy.[64] Photodynamic therapy and the regenerative periodontal treatment (autogenous bone graft) help in significant regeneration of the peri-implant bone defects.[65] An in vivo study examining the microbial profile of A. actinomycetemcomitans, P. gingivalis, P. intermedia before and after photodynamic therapy on the infected implant surfaces in 15 human subjects revealed a significant reduction in the bacterial species as observed from baseline.[66] IAJ is a vulnerable area for biofilm-related infections. Innovative implant abutment designs have helped reducing the microleakage at the IAJ with the sequential decrease in the microbial growth at the microgap.[68] Platform switch, use of tapered implants deceases or eliminates this probable microbial ingress. Any micro-structured part that is exposed to the oral cavity should be highly polished to generate a anti-plaque adhering surface. The principles of plaque maintenance around the implant are similar to those performed around the teeth with some basic differences. The oral antimicrobial rinse (e.g., chlorhexidine) can be advised as a daily regime for implant patients but fluoride mouth rinses should be avoided with the possible risk of surface damage to the titanium abutments. The plastic-coated scaling tips (ultrasonic and hand scaling) should be used to avoid the risk of surface scratches on the abutment as caused with metal instruments. Light intermittent forces should be applied to the abutment surface while polishing after scaling.
Table 4.
In vitro and in vivo (animal and human) study data on the treatment of biofilm/peri-implant infections
FUTURE PERSPECTIVE
Success of the dental implants lies on a successful osseointegration. The basic principles of biofilm formation are equally applicable in context to implants as they provide favorable grounds for the bacterial adhesion. Future research is required to design implant surfaces that inhibit or reduce the biofilm adhesion. The advent of the antimicrobial photodynamic therapy has added a new dimension in the treatment of the biofilm-related peri-implant infections. However, long-term randomized clinical and microbiological trials are required to fortify the beneficial effects of this therapy in combating the devastating infections caused by the biofilms. Recently, an in vitro study was performed on the principles of electrochemistry with the hypothesis if it can be used as a method to disinfect the implant surface. The conclusive findings for this study were in favor of reduction in the bacterial count. Research needs to be carried out in this dimension on the animal models of peri-implantitis.[69]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wilderer PA, Charakalis WG. Structure and function of biofilms. In: Charakalis PA, editor. structure and function of biofilms. Chichester, UK: JohnWiley; 1989. pp. 5–17. [Google Scholar]
- 2.Costerton JW, Montanaro L. Biofilm in implant infections: Its production and regulation. Int J Artif Organs. 2005;28:1062–8. doi: 10.1177/039139880502801103. [DOI] [PubMed] [Google Scholar]
- 3.Kolenbrander PE, Palmer RJ, Jr, Jakubobics NS. Bacterial interactions and successions during plaque development. Periodontology 2000. 2006;42:47–79. doi: 10.1111/j.1600-0757.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 4.Paquette DW, Brodala N, Williams RC. Risk factors for endosseous dental implant failure. Dent Clin North Am. 2006;50:361–74. doi: 10.1016/j.cden.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Buser D, Merickse-Stern R. Long term evaluation of nonsubmerged ITI implants. Part 1: 8 year life table analysis of a prospective multicenter study with 2359 implants. Clin Oral Implant Res. 1997;8:161–72. doi: 10.1034/j.1600-0501.1997.080302.x. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe HF. In: Biofilm plaque formation on tooth and root surfaces. Wolf HF, Rateitschak KH, editors. Stuttgart: Thieme; Periodontology. pp. 24–30. [Google Scholar]
- 7.Gibbons RJ, Van Houte J. On the formation of dental plaques. J Periodontol. 1973;44:347–60. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- 8.Saxton CA. Scanning electron microscopy study of the formation of dental plaque. Caries Res. 1973;19:111–23. doi: 10.1159/000259835. [DOI] [PubMed] [Google Scholar]
- 9.Kolenbrander PE, Anderson RN, Blehart DS. Communications among oral bacteria. Microbiol Mol Bio Rev. 2002;66:486–05. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hojo K, Nagaoka S, Oshima T. Bacterial interactions in dental biofilm development. J Dent Res. 2009;88:982–90. doi: 10.1177/0022034509346811. [DOI] [PubMed] [Google Scholar]
- 11.Bassler BL, Wright M, Silverman MR. Multiple signaling systems controlling expressions of luminescence in Vibrio harveyii: Sequence and function of genes encoding a secondary sensory pathway. Mol Microbio. 1994;13:273–86. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 12.Rams TE, Link CC. Microbiology of failing dental implants in humans. Electron microscopic observation. J Oral Implantol. 1983;11:93–100. [PubMed] [Google Scholar]
- 13.Kalykakis GK, Nissengard R. Clinical and microbial findings on osseointegrated implants, comparison between partially dentate and edentulous subjects. European J Prosthod Rest Dent. 1998;6:155–9. [PubMed] [Google Scholar]
- 14.Tanner A, Maiden MF, Lee K. Dental implant infections. Clin Infect Dis. 1997;25:S 213–7. doi: 10.1086/516243. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Helmerhorst EJ, Socransky SS. Identification of early microbial colonizers in human dental biofilm. J Appl Microbiol. 2004;97:1311–88. doi: 10.1111/j.1365-2672.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- 16.Furst MM, Salvi GE, Lang NP. Bacterial colonization immediately after installation of oral titanium implants. Clin Oral Implants Res. 2007;18:501–8. doi: 10.1111/j.1600-0501.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg D, Klinger A, Kohavi D. Adsorption of human salivary proteins to titanium powder. Adsorption of human salivary albumin. Biomaterials. 1995;16:1339–43. doi: 10.1016/0142-9612(95)91050-9. [DOI] [PubMed] [Google Scholar]
- 18.Quireynen M, Vander Mei HC, Bollen CM. The influence of surface free energy on supra and subgingival plaque microbiology. An in vivo study on implants. J Periodontol. 1994;65:162–7. doi: 10.1902/jop.1994.65.2.162. [DOI] [PubMed] [Google Scholar]
- 19.Rimondini L, Fare S, Brabilla E. The effect of surface roughness on early in vivo plaque colonization on titanium. J Periodontol. 1997;68:556–62. doi: 10.1902/jop.1997.68.6.556. [DOI] [PubMed] [Google Scholar]
- 20.Grossner-Schreiber B, Greipentrog M. Plaque formation on surface modified dental implants. An in vitro study. Clin Oral Implant Res. 2001;12:543–51. doi: 10.1034/j.1600-0501.2001.120601.x. [DOI] [PubMed] [Google Scholar]
- 21.Scarano A, Piattelli M, Caputi S, Favero GA, Piattelli A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J Periodontol. 2004;75:292–6. doi: 10.1902/jop.2004.75.2.292. [DOI] [PubMed] [Google Scholar]
- 22.Burgers R, Gerlach T, Hahnel S, Schwarz F, Handel G, Gosau M. In vivo and in vitro biofilm formation on two different titanium implant surfaces. Clin Oral Implants Res. 2010;21:156–64. doi: 10.1111/j.1600-0501.2009.01815.x. [DOI] [PubMed] [Google Scholar]
- 23.Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, van Steenberghe D. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin Oral Implants Res. 1996;7:201–11. doi: 10.1034/j.1600-0501.1996.070302.x. [DOI] [PubMed] [Google Scholar]
- 24.Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D. Influence of titanium abutment surface roughness on the plaque accumulation and gingivitis. Short term observation. Int J Oral Maxillofac Implants. 1996;11:169–78. [PubMed] [Google Scholar]
- 25.Mombelli A, Lang NP. Microbial aspects of implant dentistry. Periodontology 2000. 1994;4:74–80. doi: 10.1111/j.1600-0757.1994.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 26.Buser D, Schenk RK, Steinmann S. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25:889–02. doi: 10.1002/jbm.820250708. [DOI] [PubMed] [Google Scholar]
- 27.Teughels W, Van Assche N. Effect of material characteristics and/surface topography on biofilm development. Clin Oral Implants Res. 2006;17:68–81. doi: 10.1111/j.1600-0501.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 28.Quireynen M, VanDerMei HC, Steenberghe VD. An in vivo study of the influence of surface roughness of implants on the microbiology of the supra and subgingival plaque. J Dent Res. 1993;72:1304–9. doi: 10.1177/00220345930720090801. [DOI] [PubMed] [Google Scholar]
- 29.Quireynen M, Marechal M, Steenberghe VD. The influence of surface free energy and surface roughness on early plaque formation. An in vitro study in man. J Clin Periodontol. 1990;17:138–44. doi: 10.1111/j.1600-051x.1990.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 30.do Nascimento C, Barbosa RE, Issa JP, Watanabe E, Ito IY, Albuquerque RF., Jr Bacterial leakage along the implant-abutment interface of premachined or cast components. Int J Oral Maxillofac Surg. 2008;37:177–80. doi: 10.1016/j.ijom.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Keller W, Bragger U, Mombelli A. Periimplant microflora of implants with cemented and screw retained superstructures. Clin Oral Implant Res. 1998;9:209–17. doi: 10.1034/j.1600-0501.1998.090401.x. [DOI] [PubMed] [Google Scholar]
- 32.Piattelli A, Scarano A, Paolantonio M, Assenza B, Leghissa GC, Di Bonaventura G, et al. Fluids and microbial penetration in the internal part of cement-retained versus screw-retained implant-abutment connections. J Periodontol. 2001;72:1146–50. doi: 10.1902/jop.2000.72.9.1146. [DOI] [PubMed] [Google Scholar]
- 33.Teixeira W, Ribeiro RF, Sato S, Pedrazzi V. Microleakage into and from two-stage implants: An in vitro comparative study. Int J Oral Maxillofac Implants. 2011;26:56–62. [PubMed] [Google Scholar]
- 34.Wu-Yaan CD, Egaanhouse KJ, Keller JC. Oral bacterial attachment to titanium surface: A scanning electron microscopic study. J Oral Implantol. 1995:207–13. [PubMed] [Google Scholar]
- 35.Roth TA, Supppayak P. The surface and grain free boundary free energies of pure titanium alloy Ti-6Al-4V. Mat Sci Eng. 1978;35:187–96. [Google Scholar]
- 36.Buser D, Schenk RK, Steinmann S. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25:889–02. doi: 10.1002/jbm.820250708. [DOI] [PubMed] [Google Scholar]
- 37.Mombelli A, Van Oosten MA. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2:145–51. doi: 10.1111/j.1399-302x.1987.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 38.Harris LG, Foster SJ, Richards RG. An introduction to Staphlococcus aureus and techniques for identifying and quatifying S. aureus adhesins in relation to adhesion to biomaterials: Review. Eur Cells Mater. 2002;4:39–60. doi: 10.22203/ecm.v004a04. [DOI] [PubMed] [Google Scholar]
- 39.Renvert S, Lindahl C, Renvert H. Clinical and microbiological analysis of subjects treated with Branemark or Astratech implants: A 7 year follow up study. Clin Oral Implants Res. 2008;19:342–7. doi: 10.1111/j.1600-0501.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- 40.Rams TE, Feik D, Slots J. Staphylococci in human periodontal disease. Oral Microbiol Immunol. 1990;5:29–32. doi: 10.1111/j.1399-302x.1990.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 41.Herrmann M, Lai QJ, Albrecht RM, Mosher DF, Proctor RA. Adhesion of Staphlococcus aureus to surface bound platelets. Role of fibrinogen/fibrin and platelet intergrins. J Infect dis. 1993;167:312–22. doi: 10.1093/infdis/167.2.312. [DOI] [PubMed] [Google Scholar]
- 42.Socransky SS, Haffajee AD, Cugini MA. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 43.Blankenship JR, Mitchell AP. How to build a biofilm: A fungal perspective. Curr Opin Microbiol. 2006;19:342–7. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Ericsson I, Persson LG, Berglundh T. Different types of inflammatory reactions in periimplant soft tissues. J Clin Periodontol. 1995;22:255–61. doi: 10.1111/j.1600-051x.1995.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 45.Steinebrunner L, Wolfart S, Bössmann K, Kern M. In vitro evaluation of bacterial leakage along the implant abutment interface of different implant systems. Int J Oral Maxillofac Implants. 2005;20:875–81. [PubMed] [Google Scholar]
- 46.Engelke W, Decco OA, Rau MJ, Massoni MC, Schwarzwäller W. In vitro evaluation of the horizontal implant micromovement in bone specimen with contact endoscopy. Implant Dent. 2004;13:88–94. doi: 10.1097/01.id.0000116457.03989.01. [DOI] [PubMed] [Google Scholar]
- 47.Quireynen M, Bollen CM, Steenberghe V. Microbial penetration along the implant components of the Branemark system. An in vitro study. Clin Oral Implant Res. 1994;5:239–44. doi: 10.1034/j.1600-0501.1994.050407.x. [DOI] [PubMed] [Google Scholar]
- 48.do Nascimento C, Pedrazzi V, Miani PK, Moreira LD, de Albuquerque RF., Jr Influence of repeated screw tightening on bacterial leakage along the implant-abutment interface. Clin Oral Implants Res. 2009;20:1394–7. doi: 10.1111/j.1600-0501.2009.01769.x. [DOI] [PubMed] [Google Scholar]
- 49.Kalykakis GK, Nissengard R. Clinical and microbial findings on osseointegrated implants, comparison between partially dentate and edentulous subjects. Eur J Prosth Rest Dent. 1998;6:155–9. [PubMed] [Google Scholar]
- 50.Karousis IK, Fourmousis I. A comprehensive and critical review of dental implant prognosis in periodontally compromised partially edentulous patients. Clin Oral Implant Res. 2007;6:669–79. doi: 10.1111/j.1600-0501.2007.01406.x. [DOI] [PubMed] [Google Scholar]
- 51.Karousis IK, Salvi GE, Heitz-Mayfield LJ. Long term prognosis in patients with and without a history of chronic periodontitis: A 10 year prospective cohort study of the ITI dental implant system. Clin Oral Implants Res. 2003;14:329–39. doi: 10.1034/j.1600-0501.000.00934.x. [DOI] [PubMed] [Google Scholar]
- 52.Ong CT, Ivanovski S, Needleman IG. Systematic review of implant outcomes in treated periodontitis subjects. J Clin Periodontol. 2008;35:438–62. doi: 10.1111/j.1600-051X.2008.01207.x. [DOI] [PubMed] [Google Scholar]
- 53.Heitz-Mayfield LJ, Lang NP. Comparative biology of chronic and aggressive periodontitis versus periimplantitis. Periodontology 2000. 2010;53:167–81. doi: 10.1111/j.1600-0757.2010.00348.x. [DOI] [PubMed] [Google Scholar]
- 54.Lang NP, Berglundh T. Consensus statement and recommended clinical procedures regarding implant survival and complications. Int J Oral Maxillofac Implant. 2004;19S:150–4. [PubMed] [Google Scholar]
- 55.Nogueira-Filho G, Iacopino AM, Tenenbaum HC. Prognosis in implant Dentistry: A system for classifying the degree of periimplant mucosal inflammation. J Can Dent Assoc. 2010;77:1–6. [PubMed] [Google Scholar]
- 56.Leonhardt A, Dahlen G. Effect of titanium on selected oral bacterial species in vitro. Eur J Oral Sci. 1995;103:382–7. doi: 10.1111/j.1600-0722.1995.tb01861.x. [DOI] [PubMed] [Google Scholar]
- 57.Antoci V, King SB, Jose B. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J Orthop Res. 2007;25:858–66. doi: 10.1002/jor.20348. [DOI] [PubMed] [Google Scholar]
- 58.Lindhe J, Meyle J. Periimplant diseases: Consensus report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35:282–5. doi: 10.1111/j.1600-051X.2008.01283.x. [DOI] [PubMed] [Google Scholar]
- 59.Mombelli A, Lang NP. Antimicrobial treatment of periimplant infections. Clin Oral Implant Res. 1992;3:162–8. doi: 10.1034/j.1600-0501.1992.030402.x. [DOI] [PubMed] [Google Scholar]
- 60.Mombelli A, Feloutzis A, Bragger U. Treatment of periimplantitis by local delivery of tetracyclines: Clinical, microbiological and radiological results. Clin Oral Implant Res. 2001;14:404–11. doi: 10.1034/j.1600-0501.2001.012004287.x. [DOI] [PubMed] [Google Scholar]
- 61.Salvi GE, Persson GR, Heitz-Mayfield LJ. Adjunctive local antibiotic therapy in the treatment of periimplantitis Clinical and radiographic outcomes. Clin Oral Implants Res. 2007;18:281–5. doi: 10.1111/j.1600-0501.2007.01377.x. [DOI] [PubMed] [Google Scholar]
- 62.Depp H, Horch HH, Henke J. Periimplant care of ailing implants with carbon dioxide laser. Int J Oral Maxillofac Implants. 2001;16:659–67. [PubMed] [Google Scholar]
- 63.Takasaki AA, Aoki A, Mizutani K. Er-YAG laser therapy for periimplant infections: A histologic study. Lasers Med Sci. 2007;22:143–57. doi: 10.1007/s10103-006-0430-x. [DOI] [PubMed] [Google Scholar]
- 64.Hayek RR, Araújo NS, Gioso MA, Ferreira J, Baptista-Sobrinho CA, Yamada AM, et al. Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature induced periimplantitis. J Periodontol. 2005;76:1275–81. doi: 10.1902/jop.2005.76.8.1275. [DOI] [PubMed] [Google Scholar]
- 65.Haas R, Dortbudak O. Elimination of bacteria on different implant surfaces through hotosensitization and soft laser. An in vitro study. Clin Oral Implant Res. 1997;8:249–54. doi: 10.1034/j.1600-0501.1997.080401.x. [DOI] [PubMed] [Google Scholar]
- 66.Dortbudak O, Haas R, Bernhart T. Lethal photosensitization for decontamination of the implant surfaces in the treatment of periimplantitis. Clin Oral Implants Res. 2001;12:104–8. doi: 10.1034/j.1600-0501.2001.012002104.x. [DOI] [PubMed] [Google Scholar]
- 67.Renvert S, Roos-Jansaker AM. Non surgical treatment of periimplant mucositis and periimplantitis. A literature review. J Clin Periodontol. 2008;35:305–15. doi: 10.1111/j.1600-051X.2008.01276.x. [DOI] [PubMed] [Google Scholar]
- 68.Tara B, Taiyeb-Ali, Ong S, Siar C. Influence of abutment design on clinical status of periimplant tissues. Implant Dent. 2009;18:438–46. doi: 10.1097/ID.0b013e3181ad8e7a. [DOI] [PubMed] [Google Scholar]
- 69.Mohn D, Zehnder M, Stark W, Imfeld T. Electrochemical disinfection of dental implants-A proof of concept. PLos One. 2011;6:e157–61. doi: 10.1371/journal.pone.0016157. [DOI] [PMC free article] [PubMed] [Google Scholar]


