Abstract
Background
Brain natriuretic peptide (BNP) and N-terminal pro-brain natriuretic peptide (NT-proBNP) have been shown to be useful biomarkers for the diagnosis of heart failure. Pediatric reference intervals for these analytes have been reported in part. Previous studies lack large numbers in each group, have not covered all age ranges and have not compared results for BNP with NT-proBNP in simultaneously drawn samples.
Methods
We measured BNP in whole blood using the Biosite Triage point-of-care method and plasma NT-proBNP using the Dade RxL Dimension. We assessed between and within-day precision of both methods and after removing outliers employed the Hoffmann approach to calculate pediatric reference intervals over the age range of 0–21 y. We also compared the 2 methods on simultaneously drawn samples.
Results
Reference intervals revealed approximately 20-fold higher 97.5th percentiles for neonates than for children >3 y of age. 97.5th percentiles decreased significantly over the first 3 years of life. As shown by others, the CVs for the automated Dade RxL platform were somewhat lower than those for the POCT method. BNP and NT-proBNP correlated well in simultaneously drawn samples (r =0.947).
Discussion
Reference intervals for BNP and NT-proBNP are far higher in neonates and infants than in children older than three years of age. The reasons for this are unknown but resemble the elevated CK-MBs and troponins also found in neonates, although the 97.5th percentiles for these latter 2 cardiac markers decrease more rapidly to values found in older children by 6 months of age.
Keywords: BNP, NT-proBNP, Pediatric reference intervals
1. Introduction
Congestive heart failure (CHF) is the fourth leading cause of adult hospitalizations in the U.S. [1,2]. CHF is almost always a chronic, long-term condition, although it can sometimes develop suddenly [3]. Failure of both sides of the heart can affect most areas of the body. CHF is traditionally diagnosed by a history and physical examination. Supplementary diagnostic testing includes chest radiography, echocardiography, right-sided heart catheterization and the 6-min walk test. Because a patient’s sudden onset of shortness of breath may be due to any of a variety of causes, clinicians need an inexpensive, simple, rapid and objective test that can be conducted at the point of care to aid diagnosis.
There are 3 major natriuretic peptides (NPs): atrial natriuretic peptide (ANP) synthesized in the atria, brain natriuretic peptide (BNP) synthesized in the ventricles, and C-type natriuretic peptide synthesized in the brain. BNP and NT-proBNP have been studied as biomarkers for the diagnosis, evaluation and management of heart failure. BNP, a cardiac hormone with diuretic, natriuretic and vasorelaxant properties, is secreted by the heart in response to ventricular expansion or pressure overload. The pro form (ProBNP) is cleaved, releasing the 32 amino acid active BNP and an N-terminal piece of 76 amino acids designated NT-proBNP. The concentrations of BNP and NT-proBNP are dependent on both their synthesis and clearance. Both BNP and NT-proBNP are markers of ventricular distension and overload and can serve as early diagnostic tests [4].
Plasma BNP concentrations correlate with elevated end-diastolic pressure, which closely parallels dyspnea in heart failure [4]. This suggests that BNP is uniquely suited to provide accurate profiling in heart failure, increasing as heart failure progresses. A normal BNP or NT-BNP level has a high negative predictive value for heart failure. Ruling out this diagnosis allows the clinician to spare the patient additional invasive and uncomfortable testing (e.g., echocardiogram, right heart catheterization) and to focus on other possible causes of shortness of breath. In patients with elevated BNP or NT-proBNP levels, the degree of elevation correlates with the severity of heart failure as measured by the New York Heart Association classification system [4]. BNP is elevated in patients who have right heart failure, but the degree of elevation is not as great as that associated with left ventricular dysfunction. Research is also being conducted to evaluate the utility of BNP and NT-proBNP for other purposes, including screening for left ventricular (LV) dysfunction and risk stratification in acute ischemic syndromes. Renal function has also been shown to influence the optimal cut-off points of NT-proBNP and BNP for the diagnosis of cardiac-related dyspnoea [5]. Adult reference intervals are available in the literature [6,7] and recently Hammerer-Lercher et al. and Bekker et al. [8,9] demonstrated that NT-proBNP concentrations are markedly higher in the umbilical cord blood of term newborns than in their mothers. Data evaluating BNP and NT-proBNP in children have been reported by Yoshibayashi et al. (BNP, n =58), Mir et al. (NT-proBNP, n =109) and Rauh et al. (NT-proBNP, n =91) [10–13]. All these studies have been performed on very small numbers of children at any one age group. In contrast, we have determined the reference intervals for BNP (n =808) and NT-proBNP (n =1207) with significant numbers for each sex and at each age group.
We measured BNP by the Biosite method (Biosite Inc., San Diego, CA) and NT-proBNP using the Dade RxL Dimension (Dade Behring Inc., Newark, DE). After removing outliers (14) we have employed the Hoffmann approach [15] to obtain the pediatric reference intervals and have plotted the log of the value vs. percent cumulative frequency. A straight line was obtained which deviated from linearity at both ends. This straight line can be extended to provide the 2.5th and 97.5th percentiles for each sex and age range studied [14,15].This approach is well accepted and has been employed by our group for many years [14 –25]. Pediatric reference intervals for BNP and NT-proBNP have not previously been available in the literature.
2. Methods
Patient samples were obtained from left-over specimens and were from an approximate 50/50 mix of inpatient and outpatient specimens. All data except sex, analyte concentration and age were immediately deleted to assure compliance with hospital confidentiality standards. For the reference interval studies, EDTA whole blood was used for the BNP quantification using the Triage Biosite point-of-care method performed according to the manufacturer’s standards. Here all assays were performed within 2 h of blood collection. Reference intervals for NT-proBNP were performed on plasma samples some of which were stored in the freezer (−20 °C) and measured on the Dade RxL Dimension analyzer within 2 days of blood collection. In another study, 160 patients had EDTA samples drawn simultaneously with plasma samples thereby allowing comparison between the methods. In this case all analyses were completed within 2 h of blood collection. Precision data were obtained at 3 concentration levels using BioRad controls.
3. Results
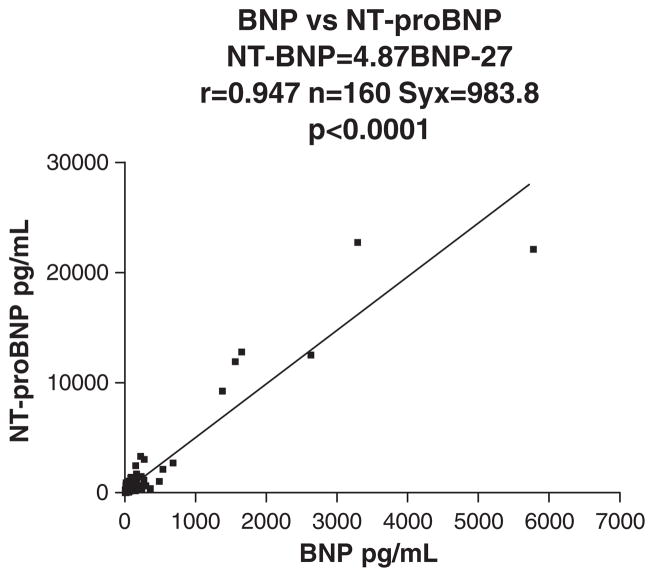
Fig. 1 shows the comparison of the Biosite BNP results with simultaneously drawn plasma samples for measurement of NT-proBNP using the Dade RxL Dimension. Table 1 provides the between and within-day imprecision of the BNP method. Table 2 gives the between and within-day imprecision of NT-proBNP assay. Table 3 shows the 97.5th percentiles for males and females over the age range 0 –21 y for BNP (pg/ml). Table 4 gives the 97.5th percentiles for males and females over the age range 0 –21 y for NT-proBNP (pg/ml).
Fig. 1.
Biosite BNP measurements compared with simultaneously drawn plasma samples for the measurement of NT-proBNP using the Dade RxL Dimension.
Table 1.
Between and within-day imprecision of the BNP method
| Level 1 | Level 2 | Level 3 | |
|---|---|---|---|
| Within-day precision | |||
| CV | 9.5% | 8.9% | 8.5% |
| SD | 21.1 | 87.7 | 256 |
| N | 10 | 10 | 10 |
| Mean pg/ml | 222.2 | 987.1 | 3012.3 |
| Between-day precision | |||
| CV | 5.5% | 14.7% | 13.8% |
| SD | 11.0 | 122.9 | 386.4 |
| N | 10 | 10 | 10 |
| Mean pg/ml | 201.0 | 838.2 | 2807.8 |
Table 2.
Between and within-day imprecision of NT-proBNP assay
| Level 1 | Level 2 | Level 3 | |
|---|---|---|---|
| Within-day precision | |||
| CV | 5.3% | 3.1% | 3.2% |
| SD | 11.6 | 24.6 | 305.1 |
| N | 11 | 11 | 11 |
| Mean pg/ml | 218.2 | 792.8 | 9588 |
| Between-day precision (using a different lot # from above) | |||
| CV | 5.8% | 5.8% | 3.9% |
| SD | 20.0 | 73.1 | 491.9 |
| N | 10 | 10 | 10 |
| Mean pg/ml | 342.5 | 1270 | 12771 |
Table 3.
97.5th percentiles for males and females over the age range 0 –21 years for BNP (pg/ml)
| Age | Sex | 97.5th percentile | n |
|---|---|---|---|
| 0–<31 days | M and F | 1585 | 50 |
| 31–<90 days | M and F | 1259 | 38 |
| 3–<6 months | M and F | 759 | 26 |
| 6 months–<1 y | M and F | 263 | 55 |
| 1–<3 y | M | 173 | 60 |
| 1–<3 y | F | 158 | 51 |
| 3–<10 y | M | 132 | 89 |
| 3–<10 y | F | 120 | 72 |
| 10–<15 y | M | 120 | 91 |
| 10–<15 y | F | 115 | 51 |
| 15–<18 y | M | 100 | 63 |
| 15–<18 y | F | 107 | 66 |
| 18–21 y | M | 110 | 50 |
| 18–21 y | F | 87 | 46 |
Table 4.
97.5th percentiles for males and females over the age range 0 – 21 y for NTproBNP (pg/ml)
| Age | Sex | 97.5th percentile | n |
|---|---|---|---|
| 0–<31 days | M | 28,184 | 46 |
| 0–<31 days | F | 35,481 | 53 |
| 31–<90 days | M | 19,953 | 49 |
| 31–<90 days | F | 15,135 | 45 |
| 3–<6 months | M | 15,849 | 49 |
| 3–<6 months | F | 14,125 | 23 |
| 6 months–<1 y | M | 11,220 | 40 |
| 6 months–<1 y | F | 10,000 | 50 |
| 1–<3 y | M | 5012 | 105 |
| 1–<3 y | F | 2512 | 59 |
| 3–<10 y | M | 1259 | 108 |
| 3–<10 y | F | 1324 | 90 |
| 10–<15 y | M | 1585 | 112 |
| 10–<15 y | F | 1413 | 87 |
| 15–<18 y | M | 1584 | 76 |
| 15–<18 y | F | 1318 | 103 |
| 18–21 y | M | 1600 | 61 |
| 18–21 y | F | 1400 | 51 |
4. Discussion
This study allowed us to compare one of the main point-of-care approaches (Biosite, Triage BNP) for the assessment of potential cardiac failure with one of the most commonly used laboratory platforms (Dade RxL Dimension, NT-proBNP). The correlation between simultaneously drawn whole blood BNP and plasma NT-proBNP is impressive (r =0.947, Fig. 1). Clearly, both systems have acceptable precision (Tables 1 and 2) and, not surprisingly, the CVs for the automated Dade RxL Dimension are lower than the manual and somewhat more subjectively read Biosite POCT approach, which supports the findings of Yeo et al. [6]. The better stability of NT-proBNP over the more labile BNP needs also to be taken into account [6]. These NT-proBNP advantages have to be weighed against the advantage of being able to run the test in the Emergency Room vs. the Main Laboratory with the former providing a somewhat faster turnaround-time. ProBNP is cleaved to give both BNP and NT-proBNP and our studies reflected in Fig. 1 reveal that this splitting resulted in a high correlation between BNP and NT-proBNP levels. NT-proBNP gives results almost 5-fold greater than BNP (slope in Fig. 1 is 4.87). The significant age related effect (20-fold for NT-proBNP and 18-fold for BNP) on the 97.5th percentiles shown in Tables 3 and 4 are similar for both BNP and NT-proBNP. While previous studies had inferred higher concentrations in the newborn period and infants, the n’s used were very small for any sex or age group and consequently could not be used to reliably identify reference intervals [8 –13].We should emphasize that the 0–31 day group is comprised predominantly of premature infants ranging from 25 weeks gestational age to full term infants. It should also be noted that similar age related effects were found for the cardiac markers CPK-MB and troponin [23,25]. Reasons for this are unknown but resemble the increased CK-MBs and troponins also found in neonates although the 97.5th percentiles for these latter cardiac markers decrease more rapidly to values found in older children by around 6 months of age.
This data is supported by the work of Hammerer-Lercher et al. and Bakker et al. [8,9] who showed NT-proBNP to be significantly higher in plasma from cord blood than in maternal plasma. N values in previous studies have been far too small to reliably define reference intervals for males and females at each age group [8–13]. Our data differs from that reported by Rauh et al. [13] and Koch et al. [26]. While both these papers show higher values for BNP and/or NT-proBNP in neonates and agree with our findings in this regard, their values appear to decline fairly rapidly with increasing age. The high levels in the neonate have been conjectured to be possibly due to physiological water loss that occurs in the first week of life [27,28]. Another possible reason is the possibility of peptide clearance by the placenta which ends at birth [27]. A close examination of their [13,26] data reveals that the number of male and female children studied at any one age group are very small with numbers of 1 to 6. This would question the reliability of their reference intervals. In comparison our number of subjects varied from 26 to 91 for BNP and from 23 to 112 for NT-proBNP. In the Hoffmann approach used in this study (and in almost all our previous studies) the top and bottom 20% of the data were automatically deleted. Heart anomalies (congenital heart disease, acquired heart disease such as myocarditis, cardiomyopathy, rheumatic fever, infective endocarditis, Kawaski disease) in pediatrics are found in <2–3% of the pediatric population [27,28] and these data points would have been automatically excluded from our population studied. We plotted the log of the value (BNP or NT-proBNP) vs. the percent cumulative frequency for the central 60% of the data. This resulted in a straight line drawn to the mid-60th percentile of the data. Extension of this line allowed us to calculate the 97.5th percentiles. As indicated approximately 50% of the samples were obtained from outpatients and 50% from inpatients. In our pediatric population only a very small percentage of patients (perhaps 2%) had cardiovascular disease and therefore the samples used were largely from children with normal cardiac function. The 2% abnormal results would clearly have been removed through elimination of the top 20% of the data.
We believe the age related 97.5th percentile data for these 2 important markers published in this manuscript should be helpful to clinicians attempting to assess cardiac failure and left ventricular dysfunction in children.
Acknowledgments
This work was partially funded by the Colaco Foundation and supported by both Biosite and Dade Behring. Supported in part by grant M01 RR 020359 from the National Center for Research Resources, National Institutes of Health, by Public Health Grant PO 1-HD-4067 and by grant 5U10HD047890-03 from NICHD and from the Office of Research on Women’s Health ORWH.
References
- 1.Maisel AS, Zoorob R. American Academy of Family Physicians. 2004. B-type natriuretic peptide in congestive heart failure diagnosis and management. [Google Scholar]
- 2.Zoorob RJ, Campbell JS. Acute dyspnea in the office. Am Fam Physician. 2003;68:1803– 10. [PubMed] [Google Scholar]
- 3.National Guideline Clearinghouse. Complete summary: inpatient management of heart failure. Institute for Clinical Systems Improvement; National Institutes of Health; Updated 2004 Feb. http://www.guideline.gov/summary. Heart failure. Updated 2004 Jun 19. [Google Scholar]
- 4.Elin RJ, Winter WE. Laboratory and clinical aspects of B-type natriuretic peptides. Arch Pathol Lab Med. 2004;128:697– 9. doi: 10.5858/2004-128-697-LACAOB. [DOI] [PubMed] [Google Scholar]
- 5.Chenevier-Gobeaux C, Claessens YE, Voyer S, Desmoulins D, Ekindjian OG. Influence of renal function on N-terminal pro-brain natriuretic peptide (NT-proBNP) in patients admitted for dyspnoea in the Emergency Department: comparison with brain natriureticpeptide (BNP) Clin Chim Acta. 2005;361:167– 75. doi: 10.1016/j.cccn.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Yeo KT, Wu AH, Apple FS, et al. Multicenter evaluation of the Roche NT-proBNP assay and comparison to the Biosite Triage BNP assay. Clin Chim Acta. 2003;338:107– 15. doi: 10.1016/j.cccn.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Shi X, Xu G, Xia T, Song Y, Lin Q. N-terminal-pro-B-type natriuretic peptide (NT-proBNP): reference range for Chinese apparently healthy people and clinical performance in Chinese elderly patients with heart failure. Clin Chim Acta. 2005;360:122– 7. doi: 10.1016/j.cccn.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Hammerer-Lercher A, Mair J, Tews G, Puschendorf B, Sommer R. N-terminal pro-B-type natriuretic peptide concentrations are markedly higher in the umbilical cord blood of newborns than in their mothers. Clin Chem. 2005;51:913–5. doi: 10.1373/clinchem.2004.046557. [DOI] [PubMed] [Google Scholar]
- 9.Bakker J, Gies I, Slavenburg B, Bekers O, Delhaas T, van Dieijen-Visser M. Reference values for N-terminal Pro-B-type natriuretic peptide in umbilical cord blood. Clin Chem. 2004;50:2465. doi: 10.1373/clinchem.2004.040253. [DOI] [PubMed] [Google Scholar]
- 10.Yoshibayashi M, Kamiya T, Saito Y, et al. Plasma brain natriuretic peptide concentrations in healthy children from birth to adolescence: marked and rapid increase after birth. Eur J Endocrinol. 1995;133:207–9. doi: 10.1530/eje.0.1330207. [DOI] [PubMed] [Google Scholar]
- 11.Mir TS, Marohn S, Laer S, Eiselt M, Grllmus O, Weil J. Plasma concentrations of N-terminal pro-brain natriuretic peptide in control children from the neonatal to adolescent period and in children with congestive heart failure. Pediatrics. 2002;110:e76. doi: 10.1542/peds.110.6.e76. [DOI] [PubMed] [Google Scholar]
- 12.Mir TS, Laux R, Hellwege HH, et al. Plasma concentrations of aminoterminal pro atrial natriuretic peptide and aminoterminal pro brain natriuretic peptide in healthy neonates: marked and rapid increase after birth. Pediatrics. 2003;112:896–9. doi: 10.1542/peds.112.4.896. [DOI] [PubMed] [Google Scholar]
- 13.Rauh M, Koch A. Plasma N-terminal Pro-B-type natriuretic peptide concentrations in a control population of infants and children. Clin Chem. 2003;49:1563– 4. doi: 10.1373/49.9.1563. [DOI] [PubMed] [Google Scholar]
- 14.Pediatric reference intervals. 5. Washington, DC: AACC Press; 2005. [Google Scholar]
- 15.Hoffmann RG. Statistics in the practice of medicine. JAMA. 1963;185:864– 73. doi: 10.1001/jama.1963.03060110068020. [DOI] [PubMed] [Google Scholar]
- 16.Porat Soldin O, Miller M, Soldin SJ. Pediatric reference ranges for zinc protoporphyrin. Clin Biochem. 2003;36:21– 5. doi: 10.1016/s0009-9120(02)00405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soldin OP, Hanak B, Soldin SJ. Blood lead concentrations in children: new ranges. Clin Chim Acta. 2003;327:109–13. doi: 10.1016/s0009-8981(02)00333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soldin OP, Bierbower LH, Choi JJ, Thompson-Hoffman S, Soldin SJ. Serum iron, ferritin, transferrin, total iron binding capacity, HS-CRP, LDL cholesterol and magnesium in children; new reference intervals using the Dade dimension clinical chemistry system. Clin Chim Acta. 2004;342:211–7. doi: 10.1016/j.cccn.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soldin OP, Hilakivi-Clarke L, Weiderpass E, Soldin SJ. Trimester-specific reference intervals for thyroxine and triiodothyronine in pregnancy in iodine-sufficient women using isotope dilution tandem mass spectrometry and immunoassays. Clin Chim Acta. 2004;349:181– 9. doi: 10.1016/j.cccn.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084–90. doi: 10.1089/thy.2004.14.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soldin OP, Hoffman EG, Waring MA, Soldin SJ. Pediatric reference intervals for FSH, LH, estradiol, T3, free T3, cortisol, and growth hormone on the DPC IMMULITE 1000. Clin Chim Acta. 2005;355:205–10. doi: 10.1016/j.cccn.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soldin OP, Guo T, Weiderpass E, Tractenberg RE, Hilakivi-Clarke L, Soldin SJ. Steroid hormone levels in pregnancy and 1-year postpartum using isotope dilution tandem mass spectrometry. Fertil Steril. 2005;84:701– 10. doi: 10.1016/j.fertnstert.2005.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quivers ES, Murthy JN, Soldin SJ. The effect of gestational age, birth weight, and disease on troponin I and creatine kinase MB in the first year of life. Clin Biochem. 1999;32:419–21. doi: 10.1016/s0009-9120(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 24.Soldin SJ, Morales A, Albalos F, Lenherr S, Rifai N. Pediatric reference ranges on the Abbott IMx for FSH, LH, prolactin, TSH, T4, T3, free T3, T-Uptake, IgE and ferritin. Clin Biochem. 1995;28:603– 6. doi: 10.1016/0009-9120(95)00038-5. [DOI] [PubMed] [Google Scholar]
- 25.Soldin SJ, Murthy JN, Agarwalla PK, Ojeifo O, Chea J. Pediatric reference ranges for creatine kinase, CKMB, I. Troponin, iron and cortisol. Clin Biochem. 1999;32:77– 80. doi: 10.1016/s0009-9120(98)00084-8. [DOI] [PubMed] [Google Scholar]
- 26.Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89:875–8. doi: 10.1136/heart.89.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasser N, Bar-Oz B, Nir A. Natriuretic peptides and heart disease in infants and children. J Pediatr. 2005;147:248–53. doi: 10.1016/j.jpeds.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 28.Nir A, Nasser N. Clinical value of NT-ProBNP and BNP in pediatric cardiology. J Card Fail. 2005;11:S76–80. doi: 10.1016/j.cardfail.2005.04.009. [Supplement] [DOI] [PubMed] [Google Scholar]